Abstract
Abstract
Engineered titanium dioxide nanoparticles (TiO2 NPs) are extensively used in cosmetic, pharmaceutical and other industries globally due to their unique properties, which has raised concern for biosafety. Genotoxicity assessment is an important part of biosafety evaluation; we report in vitro cytogenetic assays for NPs considering their unique physicochemical characteristics to fill the gap of laboratory data regarding biological safety along with mechanistic study for mode of interaction of NP with genetic material. Comet and chromosome aberration assay (CA assay) using short-term human peripheral blood cultures following exposure to TiO2 NPs; along with physicochemical parameters for stability of nano form in cultures; and DNA binding activity were carried out. The dynamic light scattering and zeta potential measurements revealed mono dispersion in media. The fluorescence spectroscopy for binding affinity of TiO2 NPs and human genomic DNA showed binding constant (Kb), 4.158 × 106 M−1 indicating strong binding affinity and negative ΔG0 value suggesting spontaneous DNA binding supporting its genotoxic potential. Following in vitro exposure to TiO2 NPs for 24 h, the cultures were analyzed for comet and CA assays, which showed significant results (p < 0.05) for % DNA intensity in tail, Olive Tail Moment and frequency of Chromosomal aberrations (CA) at 75 and 125 μM but not at 25 μM.
Graphical Abstract
Keywords: TiO2 nanoparticles, In vitro genotoxicity, Chromosomal aberration assay, Comet assay, DNA binding, Fluorescence spectroscopy
Introduction
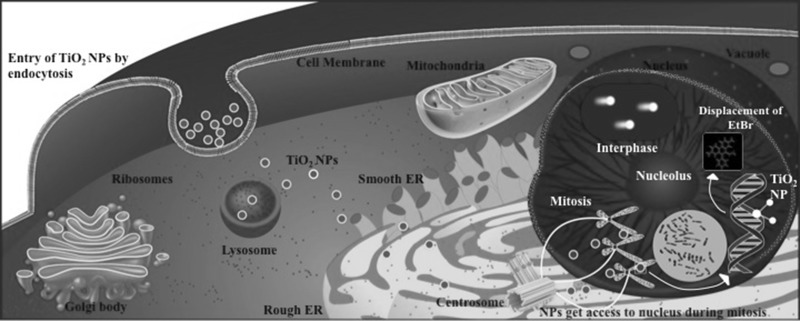
Recent developments in the field of nanotechnology have raised concern over impact on human health and environment. The small size of NPs (defined as having at least one dimension <100 nm) renders unique properties making them preferred in industrial and commercial usage. However, the same properties that contribute to their wide applications are also responsible for their toxicity. As size reduces, there is an increase in surface area to volume ratio, which leads to an increase in chemical and biological activity. Since human population is highly exposed to NPs, study of their potential health hazards becomes extremely important. The term nanotoxicology coined by Donaldson et al., established a new category to address the gaps in knowledge and issues specific for NPs (Donaldson et al. 2004). Engineered TiO2 NPs are one of the highest manufactured and most widely used nanomaterials globally. The global production of TiO2 NPs was around 2000 tons, worth $70 million in 2005, which increased to 5000 tons in 2010, and the trend is expected to continue (US EPA 2009; Landsiedel et al. 2010). TiO2 is widely used as a pigment due to its high refractive index and excellent light scattering properties, making it useful in applications that require white opacity and brightness, while TiO2 in their nano form provide UV attenuation and transparency by reducing the scattering of visible light. Around 70% of TiO2 is used as pigments in paints and the rest in food, plastic, enamels and paper industry. It is also used in sunscreens, cosmetics, as a food and pharmaceutical additive, in dental pastes and in oral capsules. A recent study reported that food grade TiO2 (E171) consists of approximately 36% particles being less than 100 nm in at least one dimension (Weir et al. 2012). Thus increased production, has led to concern regarding the exposure of TiO2 NPs. The environmental or occupational exposure of TiO2, regardless of exposure route, was considered to be harmless as it is a biologically inert compound (Ophus et al. 1979; Lindenschmidt et al. 1990). Despite several studies conducted to address the safety concerns of TiO2, our understanding of biological effects and potential risks of the widely present nano form of TiO2 has not kept pace with the rapid increase in (intended and unintended) exposure of human and environment to NPs. Genotoxicity is the capability of a chemical to alter the genetic material of the cell, and is one of the earliest effects of most carcinogens (Xie et al. 2011). In vitro assays of genotoxicity measure different types of genetic damage (e.g., Structural Chromosomal breakage and DNA strand breakage) induced by a test compound. Capacity to induce genetic damage is important for a potential carcinogen and thus is essential for cancer risk assessment. In addition to somatic cells, the genetic damage in the germ cells can lead to genetic disease or reproductive toxicity, influencing the next generation. Identifying various modes of DNA interaction will help optimize the choices of test conditions and easier extrapolation of genotoxicity test results to human risks (Landsiedel et al. 2009). TiO2 NPs induced genotoxicity can be of two kinds; primary genotoxicity which is in the absence of inflammation, and secondary genotoxicity which is due to the generation of reactive oxygen Species (ROS) during inflammation, and its reaction with DNA. The primary genotoxicity can be of two types: direct primary genotoxicity, in which the NPs enter the nucleus, interact with the DNA and disturb the replication by inhibiting it. Indirect primary genotoxicity includes effect of ROS generated by cell components, interaction of NPs with nuclear proteins, inhibition of antioxidant defense, disturbance of cell cycle check points or due to the toxic ions released from soluble or poorly soluble NPs (Li and Zhao 2013; Golbamaki et al. 2015; Petersen and Nelson 2010; Park et al. 2011; Auffan et al. 2009; De Marzi et al. 2013; Wang et al. 2012; Barnes et al. 2008; Vevers and Jha 2008; Sharma et al. 2012; AshaRani et al. 2009; Merhi et al. 2012; Ong et al. 2014). With wide spread human exposure, NPs can enter into the body via respiratory, oral, or dermal routes; and cause DNA damage in direct and indirect ways. Entry of TiO2 NPs into the nucleus can occur during mitosis when the nuclear membrane disappears. Here it can interact directly and cause damage to DNA (Fröhlich 2012; Magdolenova et al. 2012). Despite the International Agency for Research on Cancer (IARC) classifying TiO2 as a Group 2B carcinogen (possibly carcinogenic to humans) on the basis of sufficient evidence of carcinogenicity in experimental animals, debate regarding its genotoxicity still continues due to inconsistent results (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2006; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2010). This is due to unique physicochemical characteristics of NPs that require to be considered while performing current genotoxicity assays in which stability of nano form of test compound and related parameters are addressed. With increasing toxicity concerns, there is an urgent need for guidelines, recommendations, and regulations from regulatory agencies. Major concerns in laboratory methods for cancer risk assessment are; characterization, definition, level of permitted usage, and most importantly labeling of product and thereby notifying the consumer regarding the presence of NPs in the product. Physicochemical properties such as shape, surface area, surface charge, surface chemistry, chemical composition, agglomeration, size, and crystal structure influence the toxicity of NPs, therefore characterization needs to be carried out. Agglomeration of NPs in culture media is of important concern when size as well as duration and dose dependent toxicity of NPs is the focus of study. The state of dispersion depends on the extent to which NPs are agglomerated in the media, hence, characterization of NPs in relevant media is important for assessment of toxicity (Powers et al. 2007). Human peripheral blood lymphocytes are used routinely as highly sensitive indicator for in vitro and in vivo induced structural chromosomal aberrations (OECD 2014). However, there are very few reports involving human lymphocytes to assess the genotoxic effect of TiO2 NPs; majority are with comet assay, and only one includes chromosome aberration assay (Khan et al. 2015; Ghosh et al. 2010, 2013; Tavares et al. 2014; Catalán et al. 2012; Hackenberg et al. 2011; Gopalan et al. 2009; Turkez 2011; Kang et al. 2008). To assess possible clastogenicity we carried out both: Chromosomal aberration assay and Comet assay following treatment of human lymphocytes with TiO2 NPs at 25, 75 and 125 μM in vitro. To the best of our knowledge, we report here the first study including both, Chromosome aberration and Comet assays at low dose exposure for 24 h. Keeping in mind the widespread use of TiO2 NPs and insufficient information regarding its genotoxicity, we have also attempted mechanistic analysis by studying its binding affinity with DNA using fluorescence titration method. The extent of dispersion and stability of TiO2 NPs in water and RPMI-1640 cell culture media was assessed by Dynamic Light Scattering and Zeta Potential measurements; the results of which supported our previous study where dispersion of NPs was found to be better in media than water (Patel et al. 2016). Since, research in the field of in vitro TiO2 NPs toxicity with proper characterization and effect of low dose exposure in terms of genotoxicity and carcinogenicity is important, we report the DNA damaging potential of TiO2 NPs in their ‘nano’ mono-dispersed form in human lymphocyte cultures at 25, 75 and 125 μM when exposed for 24 h by Chromosome aberration assay and Comet assay. Our results showed a dose dependent increase in frequency of chromosomal breakage and DNA damage by in vitro chromosomal aberration and comet assays, respectively, after 24 h of exposure.
Materials and methods
Chemicals/materials
TiO2 NPs were procured from Sigma Aldrich (Cat. no. 634662) (Bangalore, India) which had a primary diameter of less than 100 nm. Methanol and glacial acetic acid of analytical grade were procured from Merck (Mumbai, India). Complete growth medium; RPMI-1640 HiKaryoXL™ -AL165A; low melting point agarose, agarose, sodium chloride, sodium EDTA, Trizma base, 1% Triton X-100, DMSO, EDTA and Ethidium bromide, were purchased from Himedia (Mumbai, India). Human genomic DNA was isolated from normal WBCs of peripheral blood as per manufacturer’s protocol Qiagen’s kit (Cat. no. 51104) (New Delhi, India).
Methods
Nanoparticle characterization
X-ray diffraction pattern of TiO2 NPs was recorded using an X-ray diffractometer using Cu Kα radiation of wavelength λ = 0.1541 nm in the scan range 2θ = 20–80°. TiO2 NPs were suspended in deionized water (Milli-Q) to get a 5 mM stock solution and dispersed by sonication for 10 min. The optical absorption spectrum of TiO2 was recorded using UV–Vis spectrophotometer by Agilent Technologies Cary 60, from 200 to 800 nm. The hydrodynamic particle size, polydispersity index (PDI) and Zeta potential was measured with a Zetasizer (Nano ZS, Malvern Instruments Ltd., Malvern, UK), in water and RPMI cell culture medium. The refraction index used for the dispersant medium was 1.33 (Malvern Instruments Ltd. 2013; Kathiravan and Renganathan 2009).
Study of extent of DNA binding by fluorescence measurements
Binding of TiO2 NPs to human DNA was studied using extrinsic fluorescence quench titration method. Fluorescence measurements were performed on a Cary Eclipse fluorescence Spectrophotometer (Agilent Technologies, Richardson, TX, USA) equipped with thermostated cell holder for temperature control. 1 cm path length fluorescence cuvette was used for experiments and all fluorescence measurements were recorded at 25 °C. Ethidium bromide (EtBr) was used as fluorescence stain. Intercalation of EtBr between the base pairs of DNA leads to significant increase in fluorescence. 2 mL sample of human DNA in 1X phosphate buffer saline (PBS) (pH 7.4) containing 5 µM EtBr was titrated with different concentrations of TiO2 NPs. The mixture was kept for 2–3 min before each measurement. Excitation slit and emission slit were set as 5 nm and the averaging time was 0.1 s. Fluorescence emission spectra were recorded in the range 500–720 nm at the excitation wavelength of 471 nm. The fluorescence quenching data were analyzed according to the Stern–Volmer equation.
where F0 and F are the fluorescence intensities in the absence and presence of different concentrations of TiO2 nanoparticles, respectively. KSV is the Stern–Volmer quenching constant, which was obtained from the slope of the plots F0/F versus [TiO2] (Rahban et al. 2010).
Determination of binding constant and number of binding sites
The binding constant Kb and number of binding sites (n) for binding of TiO2 NPs to DNA were determined using the modified Stern–Volmer Eq. (Kathiravan et al. 2009).
Human peripheral blood lymphocyte culture for chromosome aberration assay
Human peripheral blood lymphocyte from the whole blood of healthy volunteers was used as a test system in this study after approval of the institutional ethical committee. The blood donor was selected after informed consent and taking detailed history to ensure healthy status and past excluding exposure to drug, radiation, and infection etc., known confounding factors. The protocol was as per the OECD guideline (OECD 2014). The heparinized blood (1.0 ml) was cultured in 10 ml of RPMI-1640 culture medium by incubation at 37 °C for 72 h. At the 48th hour, cultures were exposed to 25, 75 and 125 μM TiO2 NPs, and Mitomycin-C (positive control). Colchicine (0.3 mg/ml) was added at 70th hour and cells were harvested at the 72th hour by hypotonic treatment (0.5% KCl, 20 min) and fixed with Carnoy’s fixative 3:1 (methanol: acetic acid) with multiple changes till clear pellet was obtained. Slides were prepared by air-dry method and stained with 4% Giemsa for 6–8 min in Sorensen buffer (pH 7.0). Slides were coded before microscopic analysis and 200 metaphases were scored per experiment (100 cells/replicate culture). The scoring criteria for CA was as described by Savage (1999) and OECD guidelines (OECD 2014). For statistical analysis, various kinds of structural aberrations (Chromatid gap, Chromatid break, Chromosome gap, Chromosome break) were considered. Analysis of Variance (ANOVA) was applied to examine whether percentage of cells with aberrations were statistically significant affected by the in vitro exposure to TiO2 NPs in comparison with the untreated control culture. The results were considered significant if the p value was <0.05.
Comet assay
The alkaline single cell gel electrophoresis or Comet assay was performed using the standard protocol with slight modifications using whole blood culture (Singh et al. 1988; http://www.cometassayindia.org/protocol%20for%20comet%20assay.pdf) Heparinized human peripheral blood (1.0 ml) was cultured in 10 ml of RPMI-1640 culture medium, and incubated at 37 °C for 48 h. Cultures were exposed to 25, 75 and 125 μM TiO2 NPs along with MMC as a positive control for 24 h before performing the Comet assay on harvested cells. To visualize and quantify DNA damage, 40× magnification was used in a fluorescence microscope connected to a CCD camera and an image analysis system (Tritek Comet score 1.5.2.6 software, Sumerduck, VA, USA ). 50 randomly selected cells were analyzed per sample. The parameters used to evaluate DNA damage were percentage of DNA in the tail and Olive Tail Moment. Statistical analysis was done using Graph Pad Prism software Version 6.0 (Graph Pad Inc.). The statistical significance for all experiments was analyzed by one-way ANOVA to assess if there is significant difference in DNA damage between the control and treated cultures. Results were considered to be significant if the p value was < 0.05.
Results
TiO2 NPs characterization
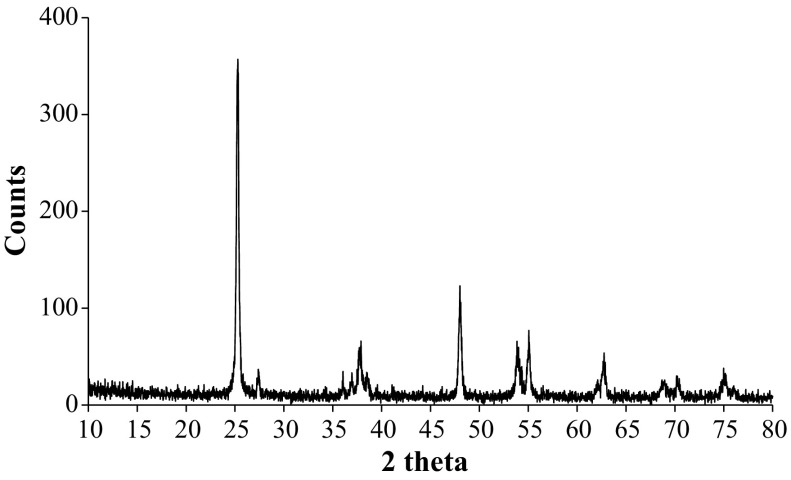
X-ray diffraction analysis depicted mixture of anatase and rutile in our TiO2 NP as can be seen by the peaks specific to both types of nanoparticles (Fig. 1). Since there is definite line broadening of the XRD peaks, we confirm that the material consists of particles in nano range. We determined peak intensity and full-width at half-maximum (FWHM). The diffraction peaks located at 25.2914°, 37.8874° and 48.0129° are characteristic of Anatase while 27.3772° and 36.0446° correspond to Rutile phase of TiO2 NP. All peaks are in good agreement with the standard spectrum (JCPDS—Joint Committee on Powder Diffraction Standards), (JCPDS no: 21-1272, Anatase and JCPDS no: 21-1276, Rutile). By using Debye–Scherrer’s formula the calculated average size of the TiO2 NPs was 20.25 nm (Table 1) (Tables 1, 2). A study earlier reported 21 nm size by Transmission Electron Microscopy for similar TiO2 NPs (Mao et al. 2015).
where, λ is wavelength of X-ray (0.1540 nm), β is FWHM (full width at half maximum), θ is diffraction angle, and D is particle diameter size.
Fig. 1.
XRD pattern for TiO2 NPs
Table 1.
Physical characteristics of TiO2 NPs
| Appearance* | Melting point* | BET (Brunauer–Emmett–Teller)*(surface area) | XRD (X-ray diffraction)* | Average particle size (XRD) | Trace metal basis* |
|---|---|---|---|---|---|
| Form: powder Color: white |
>350° | <100 nm | <50 nm | 20.25 nm | 99.5% |
* Details by the manufacturer
Table 2.
Zeta potential values (mV) of TiO2 NPs when dispersed in water and RPMI-1640 complete growth medium
| Concentration of TiO2 nanoparticles (μM) | Water (at 0 h) (mV) | In RPMI medium (at 0 h) (mV) | In RPMI medium (after 24 h) (mV) |
|---|---|---|---|
| 25 | −10.1 | −5 | −7.01 |
| 75 | −4.75 | −7.31 | −7.44 |
| 125 | −8.05 | −6.61 | −7.98 |
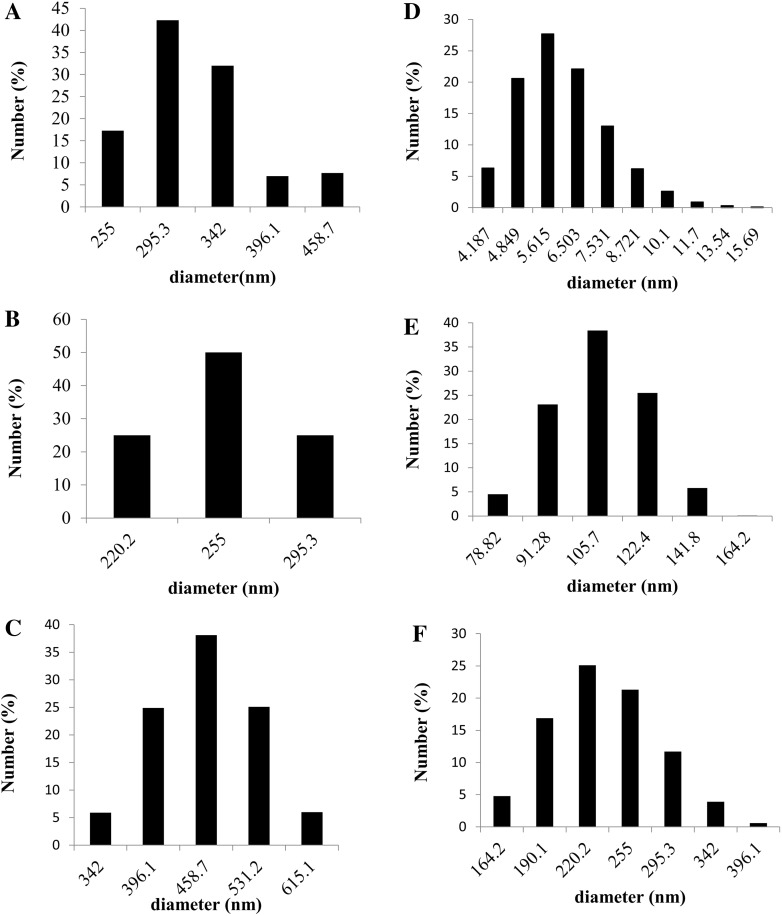
Dispersion and stability studies of TiO2 NPs in water and RPMI-1640 culture media were performed by Dynamic Light scattering and Zeta Potential analysis. Particle size distribution by number revealed that hydrodynamic diameter of TiO2 NPs was in a higher range (255–650 nm) as compared to in medium (4–350 nm), as shown in Fig. 2. RPMI-1640 culture medium is a complex and a complete growth medium containing Fetal Bovine Serum (FBS). Earlier reports have explained the role of FBS as a dispersing agent contributing to the stability of cell culture medium (Ji et al. 2010; Allouni et al. 2009). In addition to size the decrease of Poly dispersity index (PdI) in RPMI medium (0.6–0.9), in comparison to TiO2 NPs in water (1.0) showed that the size distribution of nanoparticles became narrower in presence of the proteins in RPMI medium. Zeta potential of the TiO2 NPs (25, 75, and 125 μM) was in the range of −7.0 to 8.0 mV in RPMI-1640 medium, indicating the surface of TiO2 is negatively charged in culture medium, whereas the range was lower in water (−8 to −10.0 mV) (Table 2). This may also be due to absorption of proteins on the NP surface. Our results thereby showed, mixing TiO2 NPs in RPMI medium enhanced its dispersion, as proteins get adsorbed on their surface reducing the hydrodynamic diameter.
Fig. 2.
Particle size distribution by number of TiO2 NPs dispersed in water and RPMI-1640 complete growth medium
DNA binding by fluorescence measurements (quenching study)
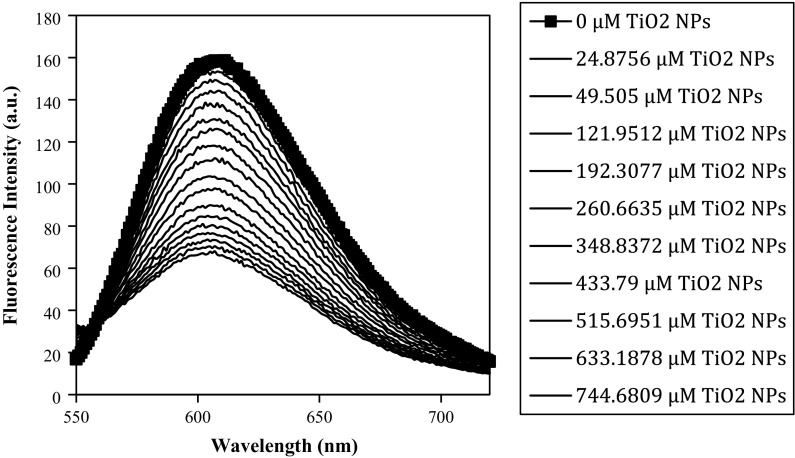
Fluorescence titration method is most commonly used to study molecular interactions between metal ions and DNA. EtBr fluorescence increases when it goes from a polar to a nonpolar environment because of the decrease in the intersystem crossing lifetimes. The planar phenenthridine ring structure of EtBr intercalates between adjacent base pairs on DNA double helix, forming soluble complexes and thus exhibiting a substantial increase in fluorescence intensity. EtBr interacts with DNA in two ways (a) through intercalation between the planar bases of DNA, and (b) by an electrostatic interaction between the cationic EtBr and anionic phosphate groups on the surface of DNA. The electrostatic mode of binding is most apparent at low salt and high dye concentrations (Rahban et al. 2010). No significant fluorescence was observed for the TiO2 NPs at 25 °C in the presence and/or absence of DNA. Therefore, EtBr displacement assay was performed to investigate the binding between TiO2 NPs and DNA. Fluorescence titration of the solution containing DNA intercalated EtBr with increasing concentration of TiO2 NPs at 25 °C showed quenching of fluorescence emission spectra (Fig. 3) suggesting competitive mode of binding. Here, quenching of fluorescence emission spectra may be because of displacement of EtBr between the planar bases of DNA by TiO2 NPs, suggesting intercalative mode of binding for TiO2 NPs.
Fig. 3.
Fluorescence emission spectra of intercalated Ethidium bromide and human genomic DNA incubated with increasing concentrations of TiO2 nanoparticle at 37 °C
Fluorescence quenching of DNA intercalated EtBr by TiO2 NPs was analyzed by the Stern–Volmer equation:
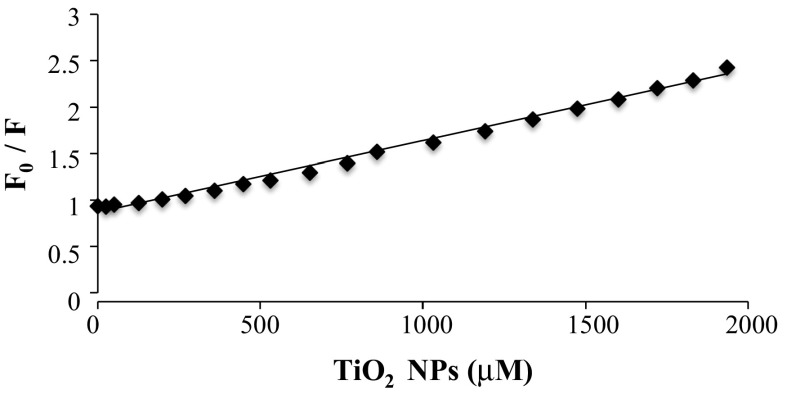
where F0 and F are the fluorescence intensities in the absence and presence of increasing concentrations of TiO2 NPs, respectively. K SV is the Stern–Volmer quenching constant, which was obtained from the slope of the plots F0/F versus [TiO2], as shown in Fig. 4. The plot showed that within the preferred range of TiO2 NPs concentration, the results exhibited a good agreement (R2 = 0.9928) with the linear Stern–Volmer equation. For TiO2 NPs, the Ksv value is 7.688 × 102 M−1 at 25 °C suggesting good binding affinity to DNA. The rate constant of the quenching process (Kq) can be calculated by the equation
where KSV is the Stern–Volmer quenching constant and τ0 is the average lifetime of DNA, s (Shahabadi et al. 2012). Therefore, the quenching constant (Kq) for TiO2 is 7.688 × 1010 . The results revealed that the value of Kq was greater than the maximum collision quenching constant of biomolecules (2 × 1010 M−1 s−1), which indicated that the fluorescence quenching of TiO2 was initiated by complex formation between DNA and TiO2 NPs (Ranjbar et al. 2013).
Fig. 4.
Stern Volmer plot for fluorescence DNA quenching by TiO2 NPs
Determination of binding constant and binding stoichiometry
The binding parameters, association or binding constant (Kb) and binding stoichiometry (n) were calculated using modified Stern–Volmer equation:
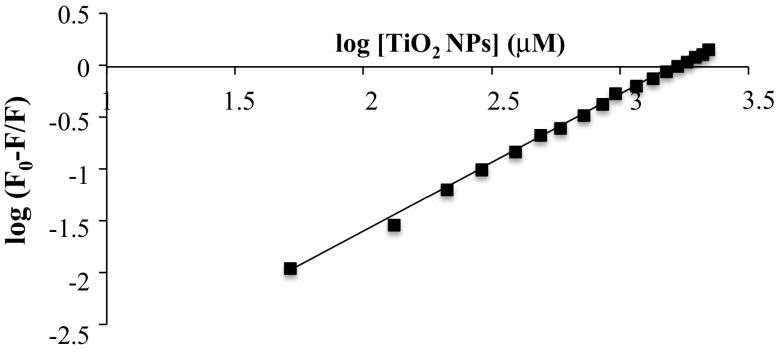
where F0 and F are the fluorescence intensities in the absence and presence of different concentrations of TiO2 NPs. As shown in Fig. 5, a plot of log [(F0−F)/F] versus log [TiO2] gives a straight line whose slope equals to n and the intercept on Y-axis equals to log Kb. The value of binding constant shows the binding affinity between TiO2 NPs and DNA double helix. The higher the Kb value is, the stronger is the interaction. The value of association constant (Kb) was 4.158 × 106 M−1 (R2 = 0.9981) which represents strong binding affinity of TiO2 NPs for DNA. Binding stoichiometry (n) is 1.322 suggesting the ratio of TiO2 NPs to DNA double helix in the complex. The association constant (Kb) thus determined was used to calculate the standard free energy change ∆G0 for the binding of TiO2 NPs to DNA using the equation
where ΔG0 is standard free energy change, R is gas constant = 1.98 × 10−3 kcal mol−1 deg−10, T is the absolute temperature (298 K) and Kb is the association constant. The value of ΔG0 was −8.9987 kcal/mol, suggesting spontaneous binding between TiO2 NPs and DNA double helix.
Fig. 5.
The modified Stern–Volmer plot for DNA quenching by TiO2 NPs
Chromosome aberration assay
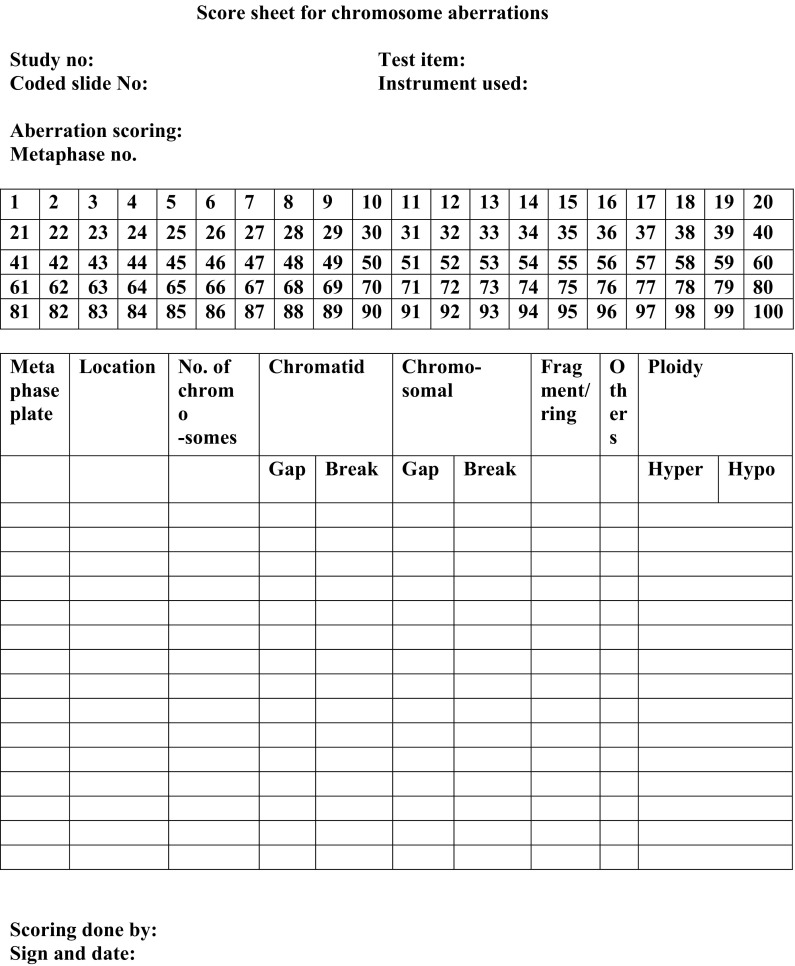
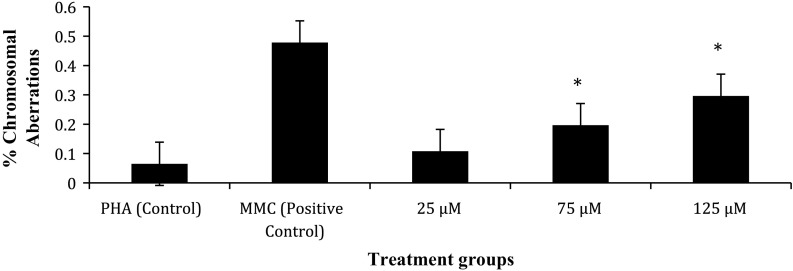
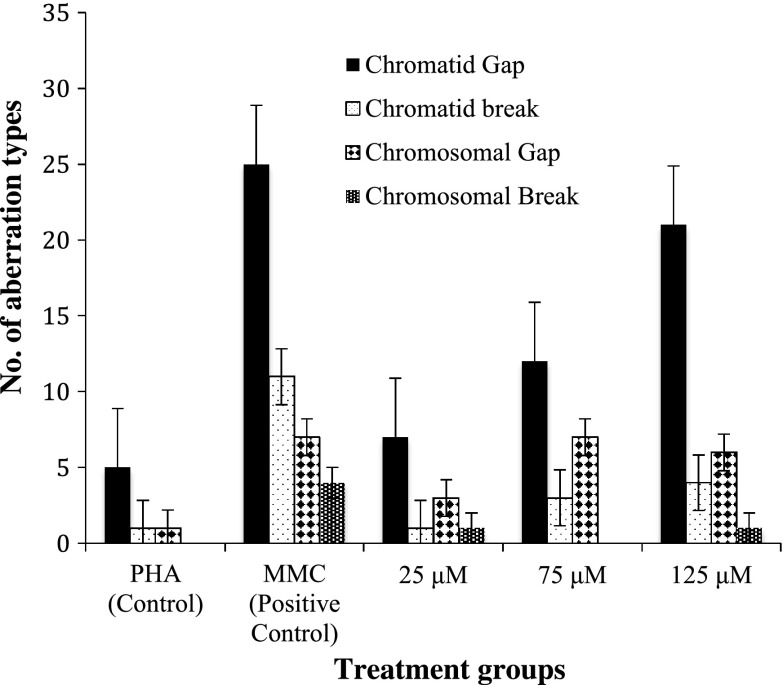
Table 3 shows the frequency of chromosome aberrations in metaphase following exposure to TiO2 NPs during in vitro short-term cultures of human peripheral blood. The in vitro exposure to TiO2 NPs significantly increased the percentage of structural Chromosomal Aberrations (Shown in Fig. 10). A sample of the scoring sheet for Chromosome aberration assay is shown in Fig. 11. As compared with control, 75 and 125 μM TiO2 NP showed significant increase in chromosomal aberrations (p < 0.05), whereas at 25 μM concentration there was no statistically significant difference in induced frequency of CA (Fig. 6). It is important to note that there was a dose dependent increase in the percentage of structural aberrations (Fig. 7) after exposure to nanoparticles for 24 h and the data were found to be statistically significant (p < 0.05).
Table 3.
The frequency of % chromosome aberrations in metaphase following exposure to TiO2 NPs during in vitro short term cultures of human peripheral blood
| Treatment groups | % frequency of structural aberrations | |||
|---|---|---|---|---|
| Chromatid type | Chromosome type | |||
| Gap | Break | Gap | Break | |
| Untreated control | 5 | 1 | 1 | 0 |
| MMC (positive control) | 25 | 11 | 7 | 4 |
| 25 μM | 7 | 1 | 3 | 1 |
| 75 μM | 12 | 3 | 7 | 0 |
| 125 μM | 21 | 4 | 6 | 1 |
Fig. 10.
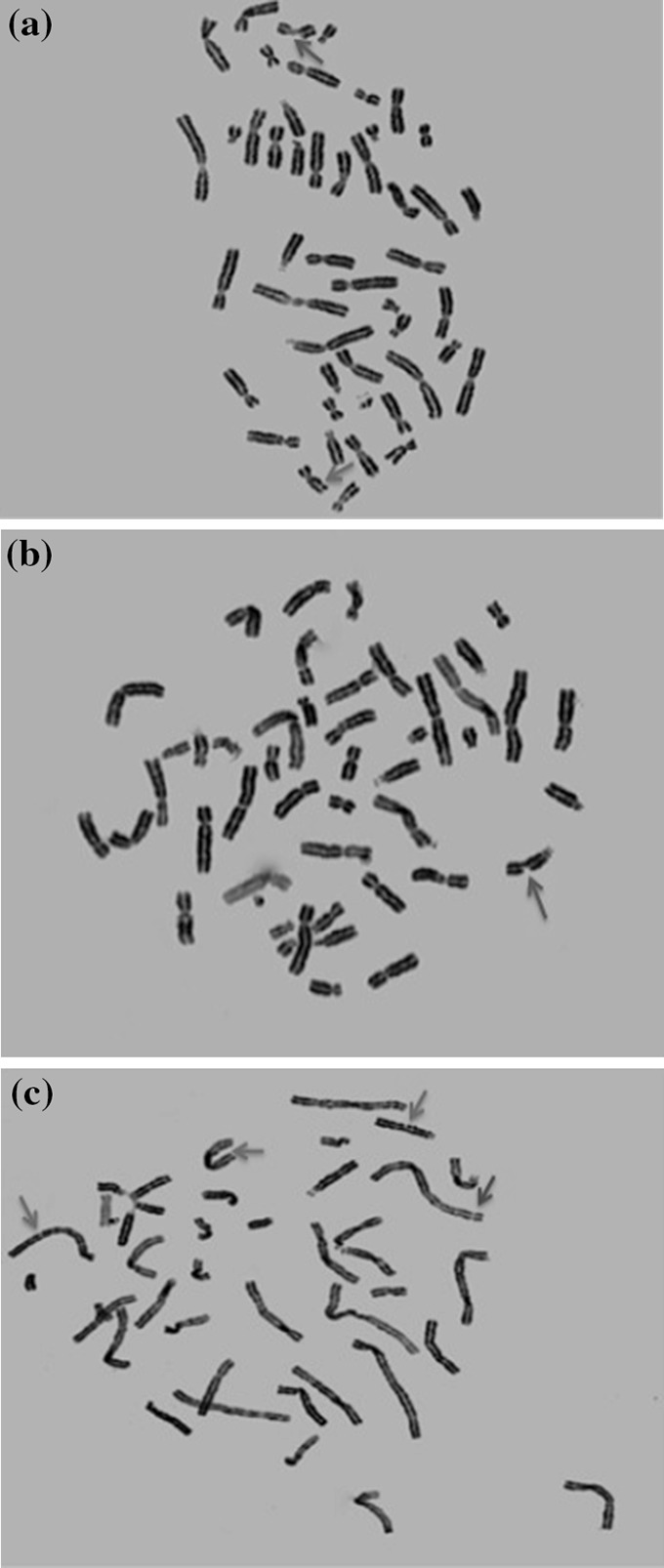
Photomicrographs of metaphase chromosomes. Arrows representing the aberrations are shown in green (chromatid gap), blue (chromatid break), and red (chromosomal break). a Single chromatid gap and break each. b Single chromatid break. c 3 chromatid gaps and 1 chromosomal break
Fig. 11.
Scoring sheet for Chromosome Aberration Assay
Fig. 6.
The % frequency of chromosomal aberrations in human lymphocytes following in vitro exposure to TiO2 NPs and controls in short term cultures
Fig. 7.
The different types of chromosome aberrations in metaphase stage of human lymphocytes following in vitro exposure to TiO2 NPs and controls in short term cultures
Comet assay
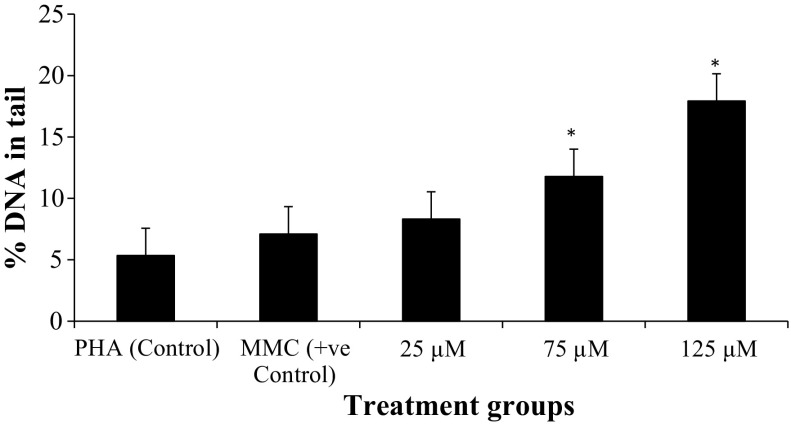
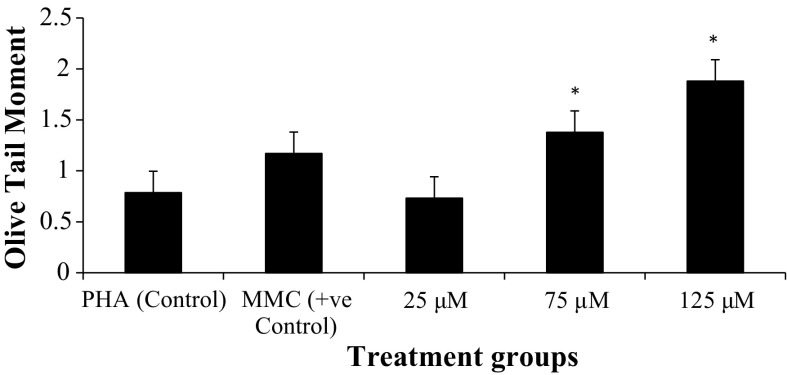
The genotoxicity of TiO2 NPs on human peripheral blood lymphocytes was evaluated using comet assay (Fig. 12). In vitro exposure to TiO2 NPs significantly increased DNA damage, which was quantified as Olive Tail Moment and percentage of DNA in the tail, as shown in Table 4. As compared to control, 75, and 125 μM TiO2 NPs showed significant increase (p < 0.05) in percentage of DNA in the tail (% Tail DNA) and Olive Tail Moment (μm), whereas at 25 μM, data were statistically significant for % Tail DNA only, but not for % Olive Tail Moment (Figs. 8, 9).
Fig. 12.
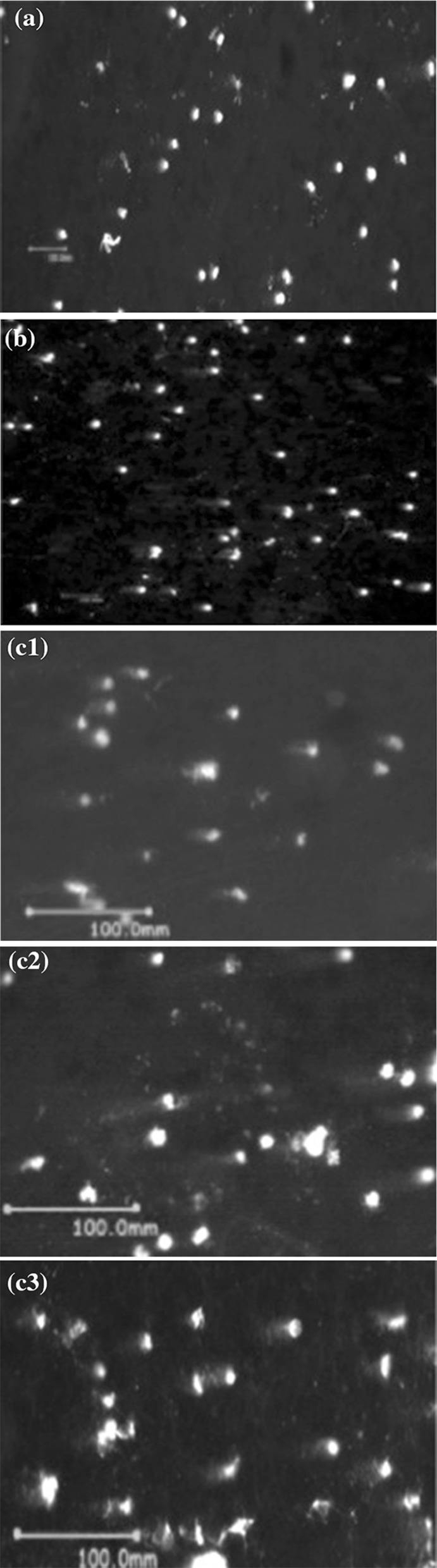
Representative comet assay images showing DNA damage in terms of tail intensity a untreated control; b positive control; c1, c2, c3 cultures treated with 25, 75 and 125 μM of TiO2 NPs respectively
Table 4.
DNA strand breakage (reported in terms of % DNA intensity in tail and Olive Tail Moment (OTM)) following 24-hour exposure to TiO2 NPs during in vitro short-term cultures of human peripheral blood
| Treatment groups | % DNA intensity in tail (Mean ± SD) | OTM (μm) (Mean ± SD) |
|---|---|---|
| PHA (control) | 5.34 ± 2.98 | 0.78 ± 0.37 |
| MMC (+ve control) | 7.09 ± 4.70 | 1.17 ± 0.72 |
| 25 μM | 8.31 ± 5.31 | 0.73 ± 0.44 |
| 75 μM | 11.78 ± 5.65 | 1.37 ± 0.85 |
| 125 μM | 17.92 ± 7.57 | 1.87 ± 0.86 |
Fig. 8.
Percentage tail DNA damage in human lymphocytes following in vitro exposure to TiO2 NPs and controls of short-term cultures
Fig. 9.
Olive Tail Moment (μm) in human lymphocytes following in vitro exposure to TiO2 NPs and controls in short term cultures
Discussion
The objective of this study was to assess the genotoxicity of TiO2 NPs in short-term peripheral blood lymphocyte culture using in vitro cytogenetic endpoints (Chromosome aberration and Comet assays). Since mono dispersion of NPs in the culture media is a prerequisite for its proper in vitro assessment of biological safety, Dynamic Light Scattering and Zeta Potential analysis was done to study the stability of nano form. The interaction of TiO2 NPs with human genomic DNA was investigated by Fluorescence spectroscopy.
Earlier studies have shown the influence of physicochemical properties of NPs on cellular response (Podila and Brown 2013). Particle size, surface charge, agglomeration, and dispersity of NPs can have a dramatic effect on the cellular response due to altered cellular uptake, bioavailability and toxicological response (Magdolenova et al. 2014). Moreover; size, shape, zeta potential, surface area, length, chemical composition and surface attachments (attachment of additional proteins to the surface of NPs when suspended in cell culture medium) of NPs have known to influence their toxicity thereby emphasizing the need of characterization of the physicochemical properties (Oberdörster et al. 2005). Dispersion of nanoparticles determines the extent of agglomeration and therefore size distribution is highly dependent on the state of dispersion of the system (Powers et al. 2007). Since TiO2 NPs tend to agglomerate, dispersion study needs to be carried out to ensure bioavailability of nano form in in vitro culture (Trouiller et al. 2009). Due to the small size and charge, the probability of internalization of TiO2 NPs into cells and thereby interaction with biomolecules (DNA) is very high. Damage to the genetic material is known to trigger induction or promotion of carcinogenesis; therefore an important aspect of evaluating the genotoxicity of NPs includes its DNA damaging potential (Doak et al. 2012). Since most human carcinogens are clastogens, an increase in the chromosomal aberrations has been linked with carcinogenicity (Bonassi et al. 2008). Assessment of in vitro genotoxicity was carried out by the Chromosome aberration assay and the single cell gel electrophoresis (comet) assay. Although TiO2 NPs are one of the components in pharmaceuticals and cosmetic products like sunscreens with the approval of the US-FDA, the concerns regarding their toxicity still prevail (Hackenberg et al. 2010). Moreover, NIOSH recently reported that nano sized TiO2 is a potential carcinogen that acts through a secondary genotoxicity mechanism related to particle size and surface area (Woodruff et al. 2012).
The systematic study of physicochemical properties of NPs was done by assessing stability of TiO2 NPs in dispersion medium, in addition to the binding affinity of TiO2 NPs with human genomic DNA. Our DNA binding study showed that TiO2 NPs quenched fluorescence of DNA and the binding constant obtained by the modified Stern–Volmer plot as shown in Fig. 5 was 4.158 × 106 M−1 indicating its strong binding capacity with DNA. Two studies have previously shown a direct chemical interaction between TiO2 NPs and DNA through the DNA phosphate group (Patel et al. 2016; Zhu et al. 2007).
Despite their widespread applications, and efforts to study the toxicological profile of TiO2 NPs, concerns regarding their effect on DNA still prevail. Genotoxicity testing of NPs can be carried out in vitro and in vivo. In vitro studies using various cell lines have demonstrated genotoxic effects of TiO2 NPs (Landsiedel et al. 2009; Catalán et al. 2012; Singh et al. 2009). It has been reported that exposure to nano TiO2 at the genetic level could interfere with cell cycle progression at mitosis leading to chromosomal instability while at the molecular level it activated the DNA damaging check points and accumulation of tumor suppressor protein p53 (the main regulator of the cellular response to DNA damage (Huang et al. 2009; Kang et al. 2008). Although many studies report genotoxicity of TiO2 NPs, there are few studies that show negative results, which could be due to different cytogenetic endpoints, cell types, doses, NP sizes, forms, and experimental conditions used in the study (Trouiller et al. 2009; Chen et al. 2014).
The present work focused on the genotoxicity of TiO2 NPs using Chromosomal aberration assay, one of the in vitro methods recommended by OECD guidelines for novel drug molecules and any compound for human safety assessment (Warheit and Donner 2010). Recently OECD guidelines emphasized on the need for specific adaptations to the existing protocol of Chromosome Aberration assay for manufactured nano-materials (OECD 2014). Chromosome aberration assay detects the structural and numerical aberrations in proliferating cells in vitro, arrested in metaphase stage of cell division whereas Comet assay determines single and double stranded DNA damage in individual interphase cells in vitro at baseline and induced by the exposure to test compound (Kumar et al. 2014). Thus a comprehensive assessment of TiO2 NP induced genetic damage was done in vitro. It is important to assess the degree of DNA damage in interphase cells that may or may not divide further due to lethal effect of exposure. However, with regard to testing carcinogenic potential, it is more important to see if there is increase in number of cells carrying sub-lethal genetic damage, which is in the scope of in vitro cytogenetic endpoints for genotoxicity. Despite many genotoxicity studies of TiO2 NPs reported, very few have focused on its mechanistic aspect (Catalán et al. 2012; Warheit et al. 2007; Theogaraj et al. 2007). Moreover, there also are very few reports regarding in vitro DNA damage using human peripheral blood (Khan et al. 2015; Ghosh et al. 2010, 2013; Tavares et al. 2014; Catalán et al. 2012; Hackenberg et al. 2011; Gopalan et al. 2009; Turkez 2011; Kang et al. 2008)
In our study, human peripheral blood lymphocyte cultures were exposed to TiO2 NPs at the 48th hour, and harvested at the 72nd hour. Considering the average cell cycle duration 18 h, TiO2 NPs were added at 48th hour after culture initiation, when cells were dividing asynchronously (Barch et al. 1997). After the addition of TiO2 NPs, cells needed to undergo S phase, in order to show aberrations. Studies have shown that chromosomal aberration is best detected when cells in culture are in their first mitotic division. A harvesting time of 1.5 cell cycles is considered to be optimum for detecting clastogens since asymmetrical structural chromosome aberrations prevent unlimited division, and therefore harvesting cells when they are in first division is crucial (Loveday et al. 1989). The cells with chromosome damage are more likely to experience a delay in the cell cycle and so highly damaged cells will reach metaphase more slowly than the cells with less aberrations, a possible reason for aberrations increasing with time. The results of Comet assay combined with CA assay will take into account both, aberrant cells in interphase as well as metaphase (Clare 2012).
Therefore, at the time of harvesting, most cells will be in their first cell cycle division after exposure to TiO2 NPs. The type of structural chromosome aberrations observed at metaphase reflects the duplication status of chromosomes in the treated cell. Chromatid type aberrations are mostly spontaneous and termed as primary aberrations resulting from chromosomal instability while the chromosome type aberrations are formed when chromatids with unrepaired aberrations duplicate following interphase. These are termed as derived aberrations. Savage in his earlier studies reported that chromatid aberrations were formed due to interference or an abnormality in chromatin replication visible after mitosis. Although lesions into chromatin could be produced at all stages of life cycle, very few aberration causing agents could produce actual structural changes in G1 stage giving rise to Chromosome type changes, while changes in S and G2 phase led to Chromatid type aberrations (Savage 1999). Since most of the chromosome aberration inducing agents are S phase dependent, the cells with unrepaired lesions from G1 or G2 formed Chromatid type aberrations.
The results of chromosome aberration assay as shown in Fig. 6 indicated the clastogenic potential of TiO2 NPs, which can be related to the strong binding capacity of TiO2 NPs with DNA as shown by the fluorescence measurements in Fig. 3. This result supports the work done in our previous study (Patel et al. 2016). Exposure to TiO2 NPs for 24 h in in vitro short-term culture induced a significant and dose dependent increase in frequency of structural chromosomal aberrations as compared to controls (p < 0.05). Moreover, a significant increase in Chromatid type and Chromosomal type aberrations was observed for 75 and 125 μM when compared to control (p < 0.0001), which was not significantly higher for 25 μM TiO2 NP concentration. The results include gaps as an indicator of DNA damage. An earlier study has reported gaps have biological significance because they could increase the risk of loss of genetic material or could reorganize the gene expression due to their susceptibility to breakage (Paz-y-Miño et al. 2002). Higher level of Chromatid type of aberrations than Chromosome type were observed as seen in Table 3 suggesting that most of the cells were exposed to NPs in G2/M phase.
An earlier study reported that uptake of NPs is influenced by the cell cycle phase and the extent of uptake of NPs was G2/M > S > G0/G1. In G2/M phase the cells have not divided if it is shorter than one cell cycle, whereas in S phase, cells accumulated NPs after cell division and in G0/G1 phase cells would have just divided and therefore have no time to internalize new NPs (Kim et al. 2011; Kansara et al. 2015). Based on the published reports regarding cellular uptake and cell cycle phases and results of our present study, we would like to hypothesize that TiO2 NPs accumulated and induced Chromatid aberrations in the cells during the G2/M phase of cell cycle.
The Comet assay is a widely used method to detect single stranded DNA breaks at an alkaline pH in individual interphase cells (Singh et al. 2009; Olive et al. 1990). Amongst 24 in vitro studies for assessment of TiO2 NP genotoxicity reported with Comet assay, 17 report positive results (Chen et al. 2014). The results of our study show TiO2 NPs induce a dose-dependent increase in Olive Tail Moment and % tail DNA intensity (p < 0.05). The DNA damage in interphase cells detected with Comet assay takes into account the DNA interaction with NPs that can influence the replication of DNA physically and chemically by binding to DNA.
The in vitro genotoxicity of TiO2 NPs can result from its capacity to induce DNA damage directly by binding or indirectly by inducing ROS generation and imbalance of oxidative stress. The present report indicates that TiO2 NPs interact directly with DNA.
Physicochemical characterization is a prerequisite for assessing the toxicity of nanoparticles therefore X-ray diffraction was done for study of structure; in addition to Dynamic Light Scattering and Zeta Potential for determining size and stability of TiO2 NPs, respectively, in water and RPMI medium (Dhawan and Sharma 2010). Using the Debye–Scherrer equation for XRD data, the average particle size was found to be 20.25 nm, which is similar to the size obtained by TEM.
The dispersion medium of NPs influences size, contact area and morphology of NP agglomerates, therefore the corresponding NP size, stability and Surface Potential (Charge) were studied in water and RPMI medium, which helped determine extent of agglomeration, mono dispersity and stability of TiO2 NPs. Since, preparation of stable and mono dispersed suspension is important to ensure reproducibility, reliability and relevance of NP risk and assessment studies it has been suggested that selection of dose should be done in a way that it does not exceed a limit that enhances agglomeration (Ji et al. 2010; Magdolenova et al. 2014; Nel et al. 2009; Taurozzi et al. 2013).
Our results show TiO2 NPs tend to agglomerate more in water than in RPMI cell culture medium. Previous studies have shown that serum proteins present in the media help in stabilizing the NPs and prevent them from agglomeration in the cell culture medium (Ji et al. 2010; Allouni et al. 2009; Tedja et al. 2012). Thus, we can conclude that the serum containing FBS stabilizes NPs and provides better dispersion in RPMI medium than in water (Schulze et al. 2007). NPs have a tendency to agglomerate in solution because of van der Waal’s forces; hence we observed an increase in hydrodynamic diameter in water, as compared to in RPMI medium.
Colloidal stability of TiO2 NP in a RPMI culture medium is influenced by particle concentration, ionic strength and presence of proteins (Allouni et al. 2009). Kumar et al. reported that electrostatic hydrogen binding and hydrophilic interactions affected the uptake and dispersity of NPs in addition to adsorption of proteins on surface of NPs (Kumar et al. 2011; Mandzy et al. 2005). NPs are dispersed in the medium due to their electrostatic and surface charges. As the surface charge value skews towards zero, there is reduction in the repulsive forces between particles that keeps them dispersed, which eventually leads to particles getting agglomerated and settling down due to gravitational force (Magdolenova et al. 2014; Marucco et al. 2015).
We carried out Zeta potential analysis to evaluate the stability of colloidal suspensions. A comparison of the Zeta potential values of TiO2 NPs was done when dispersed in water and RPMI culture medium. The 25 and 125 μM showed decrease in Zeta potential values in water, however, 75 μM showed opposite trend. This result indicates that slightly higher zeta potential of NPs in RPMI medium thus indicating increased stability. Adsorption of proteins onto NP results in formation of protein corona around it, introducing steric hinderance between particles and thereby prevents agglomeration, increasing the stability of NPs in cell culture medium (Marucco et al. 2015). Reports suggest divalent cations (mainly calcium) binding is a mechanism whereby serum proteins in medium adsorb to TiO2 (Ellingsen 1991; Klinger et al. 1997).
Studies have suggested clathrin mediated endocytosis as the mode of entry of TiO2 NPs into cytoplasm, and the accumulation of TiO2 NPs in the nucleus (Fröhlich 2012; Hackenberg et al. 2010; Shukla et al. 2011). Chromosomal aberration assay results show that majority aberrations are of chromatid type, indicating NP interaction with genetic material during G2/M phase with an increased possibility of direct contact with DNA during mitosis when the nucleus envelope breaks down. Earlier studies have reported that NPs having a high affinity for DNA and which can bind to it are capable of strongly inhibiting DNA replication in addition to changing the normal conformation of DNA molecules, leading to genotoxicity (Li and Zhao 2013; Li et al. 2013; Zhang et al. 2011).
Results obtained by the present study indicate that TiO2 NPs are clastogenic causing DNA damage and are therefore genotoxic to human lymphocyte cultures at 25, 75 and 125 μM, when exposed for 24 h. Although there exists DNA repair mechanisms, if erroneous repair and extensive DNA damage due to NPs takes place, there can be a possibility of mutation that would eventually lead to cell transformation, indicating genotoxicity testing is imperative for nanoparticles (Huang et al. 2009).
Conclusion/summary
We report in vitro genotoxicity of TiO2 NPs at 25, 75 and 125 μM in short term cultures of human peripheral blood using Chromosomal aberration assay and Comet assay specifically adapted for nanoparticles as a test compound, along with the mechanistic study.
The frequency of chromosomal breakage and DNA damage in terms of % tail DNA intensity and Olive Tail moment at 75 and 125 μM concentrations was found to be significantly higher as compared to control. The nano form of TiO2 NP in culture was observed by dispersion and stability studies, which revealed better dispersion in RPMI medium as compared to water. The mode of genotoxicity was found to be due to direct effect, based on the in vitro DNA binding study that showed strong binding affinity of TiO2 NPs with human genomic DNA, in addition to negative free energy value, which indicated spontaneous binding. Further in vivo studies are required for assessing genotoxicity potential at comparable concentrations and physicochemical parameters of NPs.
Acknowledgements
We would like to thank Nirma University for the financial support.
Appendix
Contributor Information
Suhani Patel, Email: suhanipalkhiwala@gmail.com.
Palak Patel, Email: palak608@gmail.com.
Sonal R. Bakshi, Phone: 91-79-30642755, Email: sonal.bakshi@nirmauni.ac.in
References
- Allouni ZE, Cimpan MR, Hol PJ, Skodvin T, Gjerdet NR. Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Surf B. 2009;68:83–87. doi: 10.1016/j.colsurfb.2008.09.014. [DOI] [PubMed] [Google Scholar]
- AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- Auffan M, Rose J, Orsiere T, De Meo M, Thill A, Zeyons O, et al. CeO2 nanoparticles induce DNA damage towards human dermal fibroblasts in vitro. Nanotoxicology. 2009;3:161–171. doi: 10.1080/17435390902788086. [DOI] [Google Scholar]
- Barch MJ, Knutsen T, Spurbeck JL. Association of genetic technologists. The AGT cytogenetics laboratory manual. 3. Philadelphia: Lippincott-Raven Publishers; 1997. [Google Scholar]
- Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J, Leśniak A, et al. Reproducible cornet assay of amorphous silica nanoparticles detects no genotoxicity. Nano Lett. 2008;8:3069–3074. doi: 10.1021/nl801661w. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Norppa H, Ceppi M, Strömberg U, Vermeulen R, Znaor A, et al. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22.358 subjects in 11 countries. Carcinogenesis. 2008;29:1178–1183. doi: 10.1093/carcin/bgn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán J, Järventaus H, Vippola M, Savolainen K, Norppa H. Induction of chromosomal aberrations by carbon nanotubes and titanium dioxide nanoparticles in human lymphocytes in vitro. Nanotoxicology. 2012;6:825–836. doi: 10.3109/17435390.2011.625130. [DOI] [PubMed] [Google Scholar]
- Chen T, Yan J, Li Y. Genotoxicity of titanium dioxide nanoparticles. J Food Drug Anal. 2014;22:95–104. doi: 10.1016/j.jfda.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare G. The in vitro mammalian chromosome aberration test. Methods Mol Biol. 2012;817:69–91. doi: 10.1007/978-1-61779-421-6_5. [DOI] [PubMed] [Google Scholar]
- De Marzi L, Monaco A, De Lapuente J, Ramos D, Borras M, Di Gioacchino M, et al. Cytotoxicity and genotoxicity of ceria nanoparticles on different cell lines in vitro. Int J Mol Sci. 2013;14:3065–3077. doi: 10.3390/ijms14023065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan A, Sharma V. Toxicity assessment of nanomaterials: methods and challenges. Anal Bioanal Chem. 2010;398:589–605. doi: 10.1007/s00216-010-3996-x. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Bajpayee M, Pandey AK, Parmar D Protocol for the Single Cell Gel Electrophoresis/Comet assay for rapid genotoxicity assessment. Ind Toxicol Res Centre. http://www.cometassayindia.org/protocol%20for%20comet%20assay.pdf
- Doak SH, Manshian B, Jenkins GJ, Singh N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat Res Genet Toxicol Environ Mutagen. 2012;745:104–111. doi: 10.1016/j.mrgentox.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ. Nanotoxicology. J Occup Environ Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen JE. A study on the mechanism of protein adsorption to TiO2. Biomaterials. 1991;12:593–596. doi: 10.1016/0142-9612(91)90057-H. [DOI] [PubMed] [Google Scholar]
- Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Bandyopadhyay M, Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 2010;81:1253–1262. doi: 10.1016/j.chemosphere.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Chakraborty A, Mukherjee A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J Appl Toxicol. 2013;33:1097–1110. doi: 10.1002/jat.2863. [DOI] [PubMed] [Google Scholar]
- Golbamaki N, Rasulev B, Cassano A, Marchese Robinson RL, Benfenati E, Leszczynski J, et al. Genotoxicity of metal oxide nanomaterials: review of recent data and discussion of possible mechanisms. Nanoscale. 2015;7:2154–2198. doi: 10.1039/C4NR06670G. [DOI] [PubMed] [Google Scholar]
- Gopalan RC, Osman IF, Amani A, De Matas M, Anderson D. The effect of zinc oxide and titanium dioxide nanoparticles in the Comet assay with UVA photoactivation of human sperm and lymphocytes. Nanotoxicology. 2009;3:33–39. doi: 10.1080/17435390802596456. [DOI] [Google Scholar]
- Hackenberg S, Friehs G, Froelich K, Ginzkey C, Koehler C, Scherzed A, et al. Intracellular distribution, geno- and cytotoxic effects of nanosized titanium dioxide particles in the anatase crystal phase on human nasal mucosa cells. Toxicol Lett. 2010;195:9–14. doi: 10.1016/j.toxlet.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Hackenberg S, Friehs G, Kessler M, Froelich K, Ginzkey C, Koehler C, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2011;52:264–268. doi: 10.1002/em.20615. [DOI] [PubMed] [Google Scholar]
- Huang S, Chueh PJ, Lin YW, Shih TS, Chuang SM. Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposure. Toxicol Appl Pharmacol. 2009;241:182–194. doi: 10.1016/j.taap.2009.08.013. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. IARC Monogr Eval Carcinog Risks Hum. 2006;86:1–294. [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Carbon black, titanium dioxide, and talc. lyon (FR): International Agency for Research on Cancer; 2010. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 93. https://www.ncbi.nlm.nih.gov/books/NBK326521/ [PMC free article] [PubMed]
- Ji Z, Jin X, George S, Xia T, Meng H, Wang X, Suarez E, Zhang H, Hoek EM, Godwin H, Nel AE, Zink JI. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ Sci Technol. 2010;44:7309–7314. doi: 10.1021/es100417s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Kim BM, Lee YJ, Chung HW. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- Kansara K, Patel P, Shah D, Shukla RK, Singh S, Kumar A, et al. TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ Mol Mutagen. 2015;56:204–217. doi: 10.1002/em.21925. [DOI] [PubMed] [Google Scholar]
- Kathiravan A, Renganathan R. Photosensitization of colloidal TiO2 nanoparticles with phycocyanin pigment. J Colloid Interface Sci. 2009;335:196–202. doi: 10.1016/j.jcis.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Kathiravan A, Anandan S, Renganathan R. Interaction of colloidal TiO2 with human serum albumin: a fluorescence quenching study. Colloids Surf A Physicochem Eng Asp. 2009;333:91–95. doi: 10.1016/j.colsurfa.2008.09.027. [DOI] [Google Scholar]
- Khan M, Naqvi AH, Masood A. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol Rep. 2015;2:765–774. doi: 10.1016/j.toxrep.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Åberg C, Salvati A, Dawson KA. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat Nanotechnol. 2011;7:62–68. doi: 10.1038/nnano.2011.191. [DOI] [PubMed] [Google Scholar]
- Klinger A, Steinberg D, Kohavi D, Sela MN. Mechanism of adsorption of human albumin to titanium in vitro. Biomed Mater. 1997;36:387–392. doi: 10.1002/(SICI)1097-4636(19970905)36:3<387::AID-JBM13>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kumar A, Pandey AK, Singh SS, Shanker R, Dhawan A. Cellular uptake and mutagenic potential of metal oxide nanoparticles in bacterial cells. Chemosphere. 2011;83:1124–1132. doi: 10.1016/j.chemosphere.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Kumar A, Khan S, Dhawan A. Metal oxide nanoparticles elicit genotoxic responses in mammalian cells: a critical review. In: Misra A, Bellare JR, editors. Nanoscience and technology for mankind. Allahabad: National Academy of Sciences; 2014. pp. 160–194. [Google Scholar]
- Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations-many questions, some answers. Mutat Res. 2009;681:241–258. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Ma-Hock L, Kroll A, Hahn D, Schnekenburger J, Wiench K, et al. Testing metal-oxide nanomaterials for human safety. Adv Mater. 2010;22:2601–2627. doi: 10.1002/adma.200902658. [DOI] [PubMed] [Google Scholar]
- Li K, Zhao X, Hammer KB, Du S, Chen Y. Nanoparticles inhibit DNA replication by binding to DNA: modeling and experimental validation. ACS Nano. 2013;7:9664–9674. doi: 10.1021/nn402472k. [DOI] [PubMed] [Google Scholar]
- Li KG, Zhang W, Chen YS. Quantum dot binding to DNA: single molecule imaging with atomic force microscopy. Biotechnol J. 2013;8:110–116. doi: 10.1002/biot.201200155. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt RC, Driscoll KE, Perkins MA, Higgins JM, Maurer JK, Belfiore KA. The comparison of a fibrogenic and two nonfibrogenic dusts by bronchoalveolar lavage. Toxicol Appl Pharmacol. 1990;102:268–281. doi: 10.1016/0041-008X(90)90026-Q. [DOI] [PubMed] [Google Scholar]
- Loveday KS, Lugo MH, Resnick MA. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro: II. Results with 20 chemicals. Environ Mol Mutagen. 1989;13:60–94. doi: 10.1002/em.2850130108. [DOI] [PubMed] [Google Scholar]
- Magdolenova Z, Bilaničová D, Pojana G, Fjellsbø LM, Hudecova A, Hasplova K, et al. Impact of agglomeration and different dispersions of titanium dioxide nanoparticles on the human related in vitro cytotoxicity and genotoxicity. J Environ Monit. 2012;14:455–464. doi: 10.1039/c2em10746e. [DOI] [PubMed] [Google Scholar]
- Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8:233–278. doi: 10.3109/17435390.2013.773464. [DOI] [PubMed] [Google Scholar]
- Malvern Instruments Ltd. (2013) Introducing the Zetasizer nano. Zetasizer nano user manual. p. 2(1–21). http://www.chem.uci.edu/~dmitryf/manuals/Malvern%20Zetasizer%20ZS%20DLS%20user%20manual.pdf
- Mandzy N, Grulke E, Druffel T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005;160:121–126. doi: 10.1016/j.powtec.2005.08.020. [DOI] [Google Scholar]
- Mao Z, Xu B, Ji X, Zhou K, Zhang X, Chen M, et al. Titanium dioxide nanoparticles alter cellular morphology via disturbing the microtubule dynamics. Nanoscale. 2015;7:8466–8475. doi: 10.1039/C5NR01448D. [DOI] [PubMed] [Google Scholar]
- Marucco A, Catalano F, Fenoglio I, Turci F, Martra G, Fubini B. Possible chemical source of discrepancy between in vitro and in vivo tests in nanotoxicology caused by strong adsorption of buffer components. Chem Res Toxicol. 2015;28:87–91. doi: 10.1021/tx500366a. [DOI] [PubMed] [Google Scholar]
- Merhi M, Dombu CY, Brient A, Chang J, Platel A, Le Curieux F, et al. Study of serum interaction with a cationic nanoparticle: implications for in vitro endocytosis, cytotoxicity and genotoxicity. Int J Pharm. 2012;423:37–44. doi: 10.1016/j.ijpharm.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD . Test No. 473: in vitro mammalian chromosome aberration test. Paris: OECD Publishing; 2014. [Google Scholar]
- Olive PL, Banáth JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res. 1990;122:86–94. doi: 10.2307/3577587. [DOI] [PubMed] [Google Scholar]
- Ong KJ, MacCormack TJ, Clark RJ, Ede JD, Ortega VA, Felix LC, et al. Widespread nanoparticle-assay interference: implications for nanotoxicity testing. PLoS ONE. 2014;9:e90650. doi: 10.1371/journal.pone.0090650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophus EM, Rode L, Gylseth B, Nicholson DG, Saeed K. Analysis of titanium pigments in human lung tissue. Scand J Work Environ Health. 1979;5:290–296. doi: 10.5271/sjweh.3104. [DOI] [PubMed] [Google Scholar]
- Park MV, Verharen HW, Zwart E, Hernandez LG, van Benthem J, Elsaesser A, et al. Genotoxicity evaluation of amorphous silica nanoparticles of different sizes using the micronucleus and the plasmid lacZ gene mutation assay. Nanotoxicology. 2011;5:168–181. doi: 10.3109/17435390.2010.506016. [DOI] [PubMed] [Google Scholar]
- Patel S, Patel P, Undre SB, Pandya SR, Singh M, Bakshi S. DNA binding and dispersion activities of titanium dioxide nanoparticles with UV/vis spectrophotometry, fluorescence spectroscopy and physicochemical analysis at physiological temperature. J Mol Liq. 2016;213:304–311. doi: 10.1016/j.molliq.2015.11.002. [DOI] [Google Scholar]
- Paz-y-Miño C, Dávalos MV, Sánchez ME, Arévalo M, Leone PE. Should gaps be included in chromosomal aberration analysis? Evidence based on the comet assay. Mutat Res. 2002;516:57–61. doi: 10.1016/S1383-5718(02)00021-9. [DOI] [PubMed] [Google Scholar]
- Petersen EJ, Nelson BC. Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal Bioanal Chem. 2010;398:613–650. doi: 10.1007/s00216-010-3881-7. [DOI] [PubMed] [Google Scholar]
- Podila R, Brown JM. Toxicity of engineered nanomaterials: a physicochemical perspective. J Biochem Mol Toxicol. 2013;27:50–55. doi: 10.1002/jbt.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KW, Palazuelos M, Moudgil BM, Roberts SM. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology. 2007;1:42–51. doi: 10.1080/17435390701314902. [DOI] [Google Scholar]
- Rahban M, Divsalar A, Saboury AA, Golestani A. Nanotoxicity and spectroscopy studies of silver nanoparticle: calf thymus DNA and K562 as targets. J Phys Chem C. 2010;114:5798–5803. doi: 10.1021/jp910656g. [DOI] [Google Scholar]
- Ranjbar S, Shokoohinia Y, Ghobadi S, Bijari N, Gholamzadeh S, Moradi N, et al. Studies of the interaction between isoimperatorin and human serum albumin by multispectroscopic method: identification of possible binding site of the compound using esterase activity of the protein. Sci World J. 2013;2013:305081. doi: 10.1155/2013/305081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JRK. An introduction to chromosomal aberrations. In: Huret J-L, editor. Atlas of genetics and cytogenetics in oncology and haematology. 2. Vandœuvre-lès-Nancy: INIST-CNRS; 1999. pp. 110–115. [Google Scholar]
- Schulze C, Schulze C, Kroll A, Schulze C, Kroll A, Lehr C-M. Not ready to use—overcoming pitfalls when dispersing nanoparticles in physiological media. Nanotoxicology. 2007;2:51–61. doi: 10.1080/17435390802018378. [DOI] [Google Scholar]
- Shahabadi N, Ghasemian Z, Hadidi S. Binding studies of a new water-soluble iron(III) Schiff base complex to DNA using multispectroscopic methods. Bioinorg Chem Appl. 2012;2012:126451. doi: 10.1155/2012/126451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Singh P, Pandey AK, Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat Res. 2012;745:84–91. doi: 10.1016/j.mrgentox.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro. 2011;25:231–241. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, et al. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Taurozzi JS, Hackley VA, Wiesner MR. A standardised approach for the dispersion of titanium dioxide nanoparticles in biological media. Nanotoxicology. 2013;7:389–401. doi: 10.3109/17435390.2012.665506. [DOI] [PubMed] [Google Scholar]
- Tavares AM, Louro H, Antunes S, Quarré S, Simar S, De Temmerman PJ, et al. Genotoxicity evaluation of nanosized titanium dioxide, synthetic amorphous silica and multi-walled carbon nanotubes in human lymphocytes. Toxicol In Vitro. 2014;28:60–69. doi: 10.1016/j.tiv.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Tedja R, Lim M, Amal R, Marquis C. Effects of serum adsorption on cellular uptake profile and consequent impact of titanium dioxide nanoparticles on human lung cell lines. ACS Nano. 2012;6:4083–4093. doi: 10.1021/nn3004845. [DOI] [PubMed] [Google Scholar]
- Theogaraj E, Riley S, Hughes L, Maier M, Kirkland D. An investigation of the photo-clastogenic potential of ultrafine titanium dioxide particles. Mutat Res. 2007;634:205–219. doi: 10.1016/j.mrgentox.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009;69:8784–8789. doi: 10.1158/0008-5472.CAN-09-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H. The role of ascorbic acid on titanium dioxide-induced genetic damage assessed by the comet assay and cytogenetic tests. Exp Toxicol Pathol. 2011;63:453–457. doi: 10.1016/j.etp.2010.03.004. [DOI] [PubMed] [Google Scholar]
- US EPA (2009) External Review Draft-Nanomaterial Case Studies: Nanoscale Titanium Dioxide in Water Treatment and in Topical Sunscreen; EPA/600/R-09/057. https://nepis.epa.gov/Exe/ZyPDF.cgi/P100N7OP.PDF?Dockey=P100N7OP.PDF
- Vevers WF, Jha AN. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology. 2008;17:410–420. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li N, Zhao J, White JC, Qu P, Xing B. CuO nanoparticle interaction with human epithelial cells: cellular uptake, location, export, and genotoxicity. Chem Res Toxicol. 2012;25:1512–1521. doi: 10.1021/tx3002093. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Donner EM. Rationale of genotoxicity testing of nanomaterials: regulatory requirements and appropriateness of available OECD test guidelines. Nanotoxicology. 2010;4:409–413. doi: 10.3109/17435390.2010.485704. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RS, Li Y, Yan J, Bishop M, Jones MY, Watanabe F, et al. Genotoxicity evaluation of titanium dioxide nanoparticles using the Ames test and Comet assay. J Appl Toxicol. 2012;32:934–943. doi: 10.1002/jat.2781. [DOI] [PubMed] [Google Scholar]
- Xie H, Mason MM, Wise JP., Sr Genotoxicity of metal nanoparticles. Rev Environ Health. 2011;26:251–268. doi: 10.1515/REVEH.2011.033. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yao Y, Chen YS. Imaging and quantifying the morphology and nanoelectrical properties of quantum dot nanoparticles interacting with DNA. J Phys Chem C. 2011;115:599–606. doi: 10.1021/jp107676h. [DOI] [Google Scholar]
- Zhu R-R, Wang S-L, Zhang R, Sun X-Y, Yao S-D. A novel toxicological evaluation of TiO2 nanoparticles on DNA structure. Chin J Chem. 2007;25:958–961. doi: 10.1002/cjoc.200790186. [DOI] [Google Scholar]