Abstract
Off-the-shelf availability of human adipose-derived mesenchymal stromal cells (ASCs) for regenerative medicine application requires the development of nontoxic, safe, and efficient protocols for cryopreservation. Favorably, such cell processing protocols should not contain xenogeneic or toxic components, such as fetal bovine serum (FS) and dimethyl sulfoxide (DMSO). The objective of the study was to assess the sensitivity of ASCs to DMSO-free cryopreservation protocol depending on their expansion conditions: conventional, based on the application of FS or xeno-free, using PL as a medium supplement. ASCs expansion was carried out in α-MEM supplemented either with FS or PL. For DMSO- and xeno-free cryopreservation ASCs were pretreated with different concentrations of sucrose during 24 h of culture. Pretreated ASCs were cryopreserved in α-MEM containing 100–300 mM of sucrose with the cooling rate of 1 degree/min. ASCs were tested for survival (Trypan Blue test), viability (MTT test), recovery (Alamar Blue test), proliferation and ability to multilineage differentiation. The optimal concentrations of sucrose for ASCs pretreatment and as an additive in cryoprotective solution, which provided highest cell survival, comprised 100 and 200 mM, correspondingly. Survival and recovery rates of platelet lysate (PL)-expanded ASCs after DMSO-free cryopreservation comprised 59 and 51%, and were higher than in FS-cultured cells. After DMSO-free cryopreservation PL-processed ASCs had a shorter population doubling time and higher capacity for osteogenic differentiation than FS-processed cultures. The described DMSO- and xeno-free processing may form the basis for the development of safe and efficient protocols for manufacturing and banking of ASCs, providing their off-the-shelf availability for regenerative medicine applications.
Keywords: Human adipose-derived mesenchymal stromal cells, DMSO-free cryopreservation, Platelet lysate, Xeno-free expansion, Sucrose pretreatment
Introduction
Adipose-derived mesenchymal stromal cells (ASCs) with their ability of self-renewal, multilineage differentiation potential and immunomodulatory properties open up unique opportunities in regenerative medicine and tissue engineering applications (Barry and Murphy 2004; Gimble et al. 2007; Zuk et al. 2002). In turn, their wide application requires the development of nontoxic, safe and efficient protocols for cryopreservation and banking.
Currently, most ASCs cryopreservation protocols include the application of cell-permeating cryoprotectant dimethyl sulfoxide (DMSO) in combination with fetal bovine/calf serum (FS) (Goh et al. 2007; Marquez-Curtis et al. 2015; Thirumala et al. 2009). However, it has already been confirmed that DMSO exhibits concentration-, temperature- and time-dependent cytotoxicity (Lampugnani et al. 1987; Notman et al. 2006; Reboulleau and Shapiro 1983). The interaction of DMSO with cellular lipids and proteins can lead to structural changes of membrane and cytoskeleton (Lampugnani et al. 1987; Notman et al. 2006). Incubation of fibroblasts for 1 h in 1% DMSO-containing medium has been accompanied by single-strand DNA breaks and alterations in chromatin conformation (Reboulleau and Shapiro 1983). When DMSO has been added to the culture medium it could induce differentiation of multipotent mesenchymal stromal cells (MSCs) towards neuronal and osteogenic lineage (Skorobogatova et al. 2010; Woodbury et al. 2000). Moreover, in patients receiving DMSO-containing cellular products adverse reactions from cardiac, neurological, and gastrointestinal systems have been reported (Rodriguez et al. 2005; Windrum et al. 2005). To overcome these problems, several washing procedures have been developed. However, during recovery from a frozen state, cells become more sensitive to osmotic/mechanical stresses, occurring throughout cryoprotectant removal and centrifugation. The total removal of DMSO and FS from the frozen/thawed cells is costly- and time-consuming process, which leads to a significant loss of cell number (Thirumala et al. 2010). Therefore, the development of effective DMSO-free cryopreservation techniques that will provide high post-thaw cell viability and preserve original morphology, proliferation and differentiation potentials of MSCs is still of great importance.
Sugars are known as the most efficient natural cryoprotectants in a wide variety of biological systems that stabilize and protect cellular membranes and proteins during freezing (Buchanan et al. 2004; Rodrigues et al. 2008). Recently, we have proposed a novel approach (Petrenko et al. 2014) for cryopreservation of human dermal MSCs, which comprised culture of cells in the presence of different sugars (sucrose, raffinose, trehalose) during 24 h prior to freezing (cells pretreatment step), and their following cryopreservation in the presence of the same sugar as a cryoprotectant. Such combined application of sugars on cultivation and cryopreservation stages has enabled us to avoid the inclusion of DMSO during cryopreservation, providing more than 50% survival and metabolic activity of cells and preserving their growth and differentiation potential (Petrenko et al. 2014). These results may represent the initial step towards the development of nontoxic cryopreservation strategy for MSCs, providing their off-the-shelf availability. However, many factors, which may affect the sensitivity of cells to such DMSO-free cryopreservation approach should be considered and additionally studied. We assumed that the source of MSCs and their expansion conditions, which may provide different growth kinetics and changes in cell morphology and metabolic activity, would significantly affect the cryopreservation outcome. Despite a large number of studies showing differences in functional properties of MSCs, derived from various tissues, the distinctions in their cryosensitivity depending on the tissue of origin are not well described.
From the point of regenerative medicine, the adaptation of cell manufacturing process (isolation, expansion, and banking) towards clinical grade Good Manufacturing Practice (cGMP) standards is required. ASCs are typically cultured for both experimental and clinical settings in the presence of FS, which provides vital nutrients and growth factors for cell growth and stabilizes cell membrane, reduces the extracellular ice formation, prevents excessive concentration of solutes and minimizes cell dehydration to a tolerable degree at cryopreservation. However, due to xenogeneic origin FS introduces the risk of viral, prion, zoonose contaminations and may cause the immune response of organism after injection (Lange et al. 2007), that necessitates the exclusion of FS application from all stages of cell production, including expansion and cryopreservation.
Currently, human platelet lysate (PL) represents the most promising natural alternative to FS in supporting MSCs expansion and maintaining their potential for further multilineage differentiation (Doucet et al. 2005; Horn et al. 2010; Astori et al. 2016). PL contains a broad spectrum of coagulation and growth factors, adhesion molecules, protease inhibitors, and proteoglycans, which can be released from α-granules after platelet activation by physical or physiological methods (Astori et al. 2016). Growth-promoting activity of PL towards MSCs is explained by the complex effect of cytokines, mainly platelet-derived growth factor-AB/BB (PDGF-AB/BB), basic fibroblast growth factor (bFGF), and transforming growth factor-beta1 (TGF-β1). It has been shown by Doucet et al. (2005), that the inhibition of these cytokines by neutralizing antibodies reduced MSCs proliferation for about 75%. In addition to these three essential components, epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor-1 (HGF-1) and nerve growth factor-β (NGF-β), contained in PL are also very important to provide its overall biologic activity (Bernardo et al. 2007; Doucet et al. 2005; Gottipamula et al. 2012; Kinzebach et al. 2013; Reinisch et al. 2007). Up to now, different aspects of PL production, quality control, safety and activity in vitro and in vivo have been extensively reviewed (Bernardo et al. 2007; Blande et al. 2009; Gottipamula et al. 2012). However, little is known about the effect of these FS-free culture conditions on the sensitivity of cells to cryopreservation process.
The objective of the study was to assess the sensitivity of ASCs to DMSO-free cryopreservation protocol depending on their expansion conditions: conventional, based on the application of FS or xeno-free, using PL as a medium supplement.
Materials and methods
Preparation of human platelet lysate
Platelet lysate was prepared from whole blood units (500 ml) of 10 different adult donors, received from the Kharkov Regional Blood Service Center. The contents of transfusion bags were transferred to 50 ml tubes and centrifuged at 680 g for 15 min. After centrifugation plasma with platelets was separated from erythrocytes and leukocytes and submitted to rapid centrifugation (2400 g, 20 min). Obtained platelet concentrates (~ 0.5–1 × 1010 platelets/ml) were lysed by three freeze/thawing cycles and stored at −20 °C. Before addition to a culture medium, aliquots were thawed, centrifuged, and sterile filtered through 0.22 µm filter. To compensate individual differences in the level of growth factors, PL donations from ten donors were pooled together.
Cell isolation and culture
ASCs were isolated from lipoaspirate of adult patients, after receiving the written consent of informed healthy volunteer donors, in accordance with the recommendations of the World Medical Association Declaration of Helsinki. The study was approved by the Institute for Problems of Cryobiology and Cryomedicine Ethical Committee for the usage of biological material for research purposes. ASCs were obtained by collagenase digestion according to the previously described method (Petrenko and Petrenko 2012).
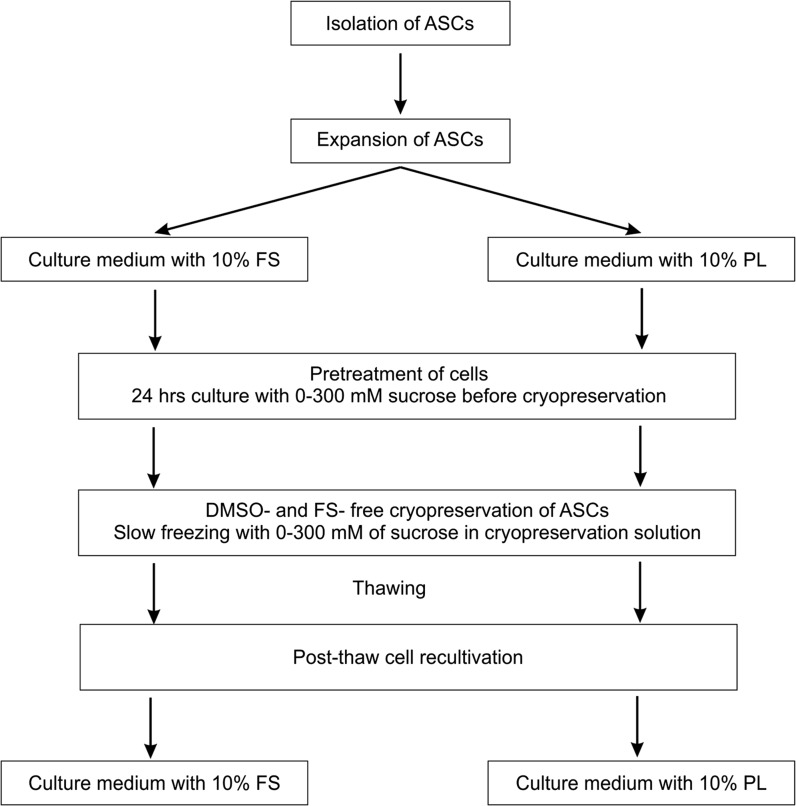
ASCs were cultured in T75 adhesive polystyrene cell culture flasks (S = 75 cm2, TPP, Trasadingen, Switzerland) at 37 °C, 5% CO2, and 95% humidity in Minimal Essential Medium-α modification (α-MEM, Sigma-Aldrich, St. Louis, MO, USA), containing 50 μ/ml penicillin (Biowest, Nuaillé, France), 50 μg/ml streptomycin (Biowest, France) and 0.2 mM l-glutamine (Sigma-Aldrich, USA) as well as 10% FS (PAA, Pasching, Austria) or 10% PL. Medium containing 10% PL was additionally supplemented with 2 μ/ml of heparin (StemCell Technologies, Vancouver, Canada). Complete medium changes were performed every 3–4 days. When cells reached 70–80% confluence, they were detached with 0.05% Trypsin/0.02% EDTA (Biowest, France) at 37 °C. Trypsin was inactivated by the addition of PL- or FS-containing medium, correspondingly. Suspensions of ASCs were centrifuged at 350 g for 6 min and resuspended in fresh medium. Then cells were counted using a Neubauer hemocytometer according to manufacturing instructions and replated at a ratio of 1:3. ASCs at passages 4–7 were used for experiments described below (Fig. 1).
Fig. 1.
Experimental design. ASCs adipose-derived mesenchymal stromal cells; FS fetal bovine serum; PL human platelet lysate, DMSO dimethyl sulphoxide
Cell pretreatment and cryopreservation
For cell pretreatment, sucrose in concentrations 50, 100, 200 or 300 mM was added to basic culture medium containing 10% FS or 10% PL. Pretreatment procedure was performed by 24 h cell culture with sucrose at 37 °C and 5% CO2 prior to cryopreservation.
Pretreated ASCs were detached and resuspended in 1 ml cryopreservation solution. The cryopreservation solution consisted of α-MEM, supplemented with 100, 200, or 300 mM of sucrose. After 5 min incubation cell suspensions were placed into 1 ml cryovials (NUNC, Thermo Fisher Scientific, Waltham, MA, USA) and cryopreserved with the cooling rate of 1 degree/min down to −80 °C with following plunging into liquid nitrogen. Cryopreserved samples (1 × 106 cells/cryovial) were stored in liquid nitrogen at −196 °C for at least 3 weeks and thawed in a water bath at 37 °C before further studies. Cells frozen without any cryoprotectants have been used as a negative control. ASCs cryopreserved in medium, containing 10% DMSO and 20% FS served as a positive control group.
Assessment of post-thaw cell survival and metabolic activity
For accurate assessment of the cryopreservation efficiency, the overall post-thaw properties of cells were studied using several methods on different stages of cell recovery process (Fig. 1).
Survival. Following cryopreservation, ASCs were thawed and immediately tested for cell survival using Trypan blue plasma membrane integrity assay. Briefly, cell suspension was diluted 1:1 with 0.4% Trypan blue solution (Sigma-Aldrich, USA) in PBS and counted in Neubauer hemocytometer, using standard protocol. Survival rate was expressed as a percentage of viable unstained cells in suspension related to the total number of cells counted in a hemocytometer (n = 5).
Viability. To test cell metabolic activity after thawing, MTT test was performed. Briefly, the 0.5 ml of cell suspension was placed in a conical tube and supplemented with 50 µl of FS and 50 µl of 5 mg/ml solution of redox indicator MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2h-tetrazolium] bromide (Sigma-Aldrich, USA). After 2 h of incubation at 37 °C viability of thawed cells was assessed in Neubauer hemocytometer, according to standard protocol and expressed as a percentage of ASCs accumulated intracellular formazan (viable cells) to the total number of cells in the chamber (n = 5).
Recovery. ASCs from the same sample before and after cryopreservation were plated at a density of 5 × 103 cells/cm2 into standard 96-well culture plates (TPP, Switzerland) and cultured at 37 °C, 5% CO2, and 95% humidity. Recovery of cryopreserved ASCs after 24 h of recultivation was evaluated by Alamar Blue test (AB) (Serotec Ltd, Bio-Rad, Raleigh, NC, USA). Briefly, ASCs were incubated for 3 h with culture medium containing 10% AB. Reduced AB solution was collected with medium change and the fluorescence level of AB was assessed by TECAN GENios microplate reader (Tecan Genios; Tecan, Grödig, Austria) with an excitation wavelength of 550 nm and an emission wavelength of 590 nm. The difference in fluorescence between experimental and blank sample (without cells) was used as AB value (n = 5). The recovery rate was counted as a percent ratio between AB values of cryopreserved and non-cryopreserved cells.
Cell proliferation assays
For the determination of cell proliferation, ASCs at a density 5 × 103 /cm2 were plated into standard adherent T25 flasks (TPP, Switzerland) in culture medium supplemented with 10% FS or 10% PL (n = 5). On the 5th day of culture, cells were harvested by trypsinization and counted using a Neubauer hemocytometer.
Population doubling time (PDT) at each passage (n = 5) was calculated using the formula: t/3.32 × (lgNt–lgN0), where N0 and Nt represent the initial cell number and the final cell number, respectively, while t is the time interval between N0 and Nt.
Flow cytometry analysis
Immunophenotyping of cells harvested from expansion cultures at passage 4 (n = 4) was performed using the following monoclonal antibodies (mAbs): CD29-PE (Serotec, USA), CD34-FITC (Dako, Glostrup, Denmark), CD45-PE (Serotec, USA), CD73-PE (BD Biosciences, UK), CD90-FITC (Serotec, UK), CD105-FITC (Serotec, Kidlington, UK). All antibodies were titrated before analysis. Trypsinized cells were resuspended in PBS and centrifuged at 200 g for 10 min. 5 µl of mAbs were added to each sample containing 50 µl of cell suspension in PBS (3 × 105 cells). After 30 min incubation at room temperature in the dark, cells were washed twice with PBS and analyzed using flow cytometer FACS Calibur (BD Biosciences, Oxford, UK). Each measurement was prepared from the single cell population, using minimum 20,000 events per sample. Debris and cell clumps were gated out, according to FSC/SSC parameters. Data analysis was prepared by WinMDI 2.8 software.
Adipogenic differentiation
Adipogenic differentiation medium consisted of α-MEM, supplemented with 10% FS and following adipogenic stimulants: 0.5 mM 3-isobutyl-1-methyl-xanthine (Sigma-Aldrich, USA), 1 μM dexamethasone (Sigma-Aldrich, USA), 10 μg/ml insulin (Sigma-Aldrich, USA), and 100 μM indomethacin (Sigma-Aldrich, USA). Complete medium changes were performed every 3–4 days. After 21 days of culture with adipogenic supplements, cells were fixed in 4% buffered formalin for 30 min at 4 °C and stained with Nile Red (1 µg/ml in PBS) solution (Sigma-Aldrich, USA) according to the manufacturer’s instructions. Then, cultures were additionally stained for 5 min with 4′,6-diamidino-phenylindole-2 (DAPI, 0.1 μg/ml, Sigma-Aldrich, USA), washed in PBS and assessed with fluorescent microscope CETI EpiFluor (CETI, Seraing, Belgium). Images of stained cells were captured from at least 10 independent microscopic fields and analyzed using ImageJ 1.50b software. The efficiency of adipogenic differentiation was determined as a percentage of Nile Red-positive cells to the total number of cells in the same microscopic field, assessed by direct counting of all cell nuclei stained with DAPI.
Osteogenic differentiation
Osteogenic differentiation medium consisted of α-MEM, supplemented with 10% FS and osteogenic inductors: 0.2 mM ascorbic acid (Sigma-Aldrich, USA), 10 mM β-glycerolphosphate (Sigma-Aldrich, USA), and 1 μM dexamethasone (Sigma-Aldrich, USA). Complete medium changes were performed every 3–4 days. Following 21 days of culture, ASCs were fixed in 4% buffered formalin for 30 min at 4 °C. Alkaline phosphatase expression was assessed using Fast Blue RR Salts/Naphtol kit (Sigma-Aldrich, USA) during 30 min at room temperature in the dark.
Then, cultures were additionally stained for 5 min with 4′,6-diamidino-phenylindole-2 (DAPI, 0.1 μg/ml, Sigma-Aldrich, USA), washed in PBS and assessed with fluorescent microscope CETI EpiFluor (CETI, Belgium). Images of stained cells were captured from at least 10 independent microscopic fields and analyzed using ImageJ 1.50b software. The efficiency of osteogenic differentiation was determined as a percentage of alkaline phosphatase-positive cells to the total number of cells in the same microscopic field, assessed by direct counting of all cell nuclei stained with DAPI.
Statistical analysis
Data were presented as mean ± SD with n indicating the number of independent experiments. Each experiment was repeated in triplicate. Statistical evaluation was performed using one-way analysis of variance (ANOVA), followed by the Bonferroni post hoc test. P < 0.05 was considered statistically significant. All analyses were made using Past version 3.0 statistical software package (Hammer et al. 2001).
Results
Effect of sucrose pretreatment approach on the ASCs survival after DMSO-free cryopreservation
As we have previously reported (Petrenko et al. 2014), inclusion of sugars into incubation medium for 24 h prior to cryopreservation (pretreatment) with their obligatory presence in freezing solution significantly increased post-thaw viability of human dermal MSCs in the absence of DMSO. Examination of wide range of sucrose concentrations has revealed that 200 mM for pretreatment and 300 mM for addition into cryopreservation solution were the most effective, improving the survival of dermal MSCs from 5 ± 1 (without sucrose) to 53 ± 5% (Petrenko et al. 2014). We assumed that protective effect of sucrose is not limited only to the derma-derived cells, but at least extends to MSCs from other sources. However, cryopreservation of ASCs using the pretreatment approach successful for dermal MSCs provided survival and metabolic activity of only 19 ± 2% of cells (Table 1), that caused the need for the additional examination of optimal sucrose concentrations able to preserve ASCs.
Table 1.
Survival rate of ASCs cryopreserved with different sucrose concentrations
| Pretreatment | |||
|---|---|---|---|
| 50 mM sucrose | 100 mM sucrose | 200 mM sucrose | |
| Cryopreservation medium | |||
| 100 mM sucrose | 28 ± 3 | 30 ± 4 | 21 ± 5 |
| 200 mM sucrose | 39 ± 2* | 45 ± 5*# | 31 ± 4* |
| 300 mM sucrose | 27 ± 5 | 33 ± 3 | 19 ± 2 |
Pretreatment procedure was performed by 24 h cell culture with sucrose before cryopreservation. The basic composition of cryopreservation solution was basic culture medium supplemented with 100, 200, or 300 mM of sucrose. Cell survival was evaluated by Trypan blue test and expressed as a percentage of unstained cells related to the total number of cells. The data (n = 5) were presented as the mean ± SD
* significant (P < 0.05) as compared to groups with 100 and 300 mM sucrose in cryopreservation medium; # significant (P < 0.05) as compared to groups with 50 and 200 mM sucrose for pretreatment
Cryopreservation of ASCs in α-MEM without pretreatment caused the death of 96 ± 3% of cells (negative control). Pretreatment of ASCs with low concentrations of sucrose (50 and 100 mM) provided better survival rates (P < 0.05), compared with the 200 mM concentration. Culture of cells in the presence of 300 mM sucrose led to substantial changes in their morphology and viability due to the increase of the osmotic pressure. The dome-shaped interrelation between survival rate of cells and applied sucrose concentrations for pretreatment was revealed. Screening of optimal sucrose concentration in cryopreservation medium showed that the most effective concentration was 200 mM. When increasing or decreasing the sucrose concentration, the average value of cell survival was significantly lower, compared to 200 mM group (P < 0.05). The best survival rates (45 ± 5%) were obtained by combined application of 100 mM sucrose for cells pretreatment and the inclusion of 200 mM of the sugar into cryopreservation medium, without the addition of DMSO.
Expansion of ASCs in PL-supplemented medium improves post-thaw cell survival and viability
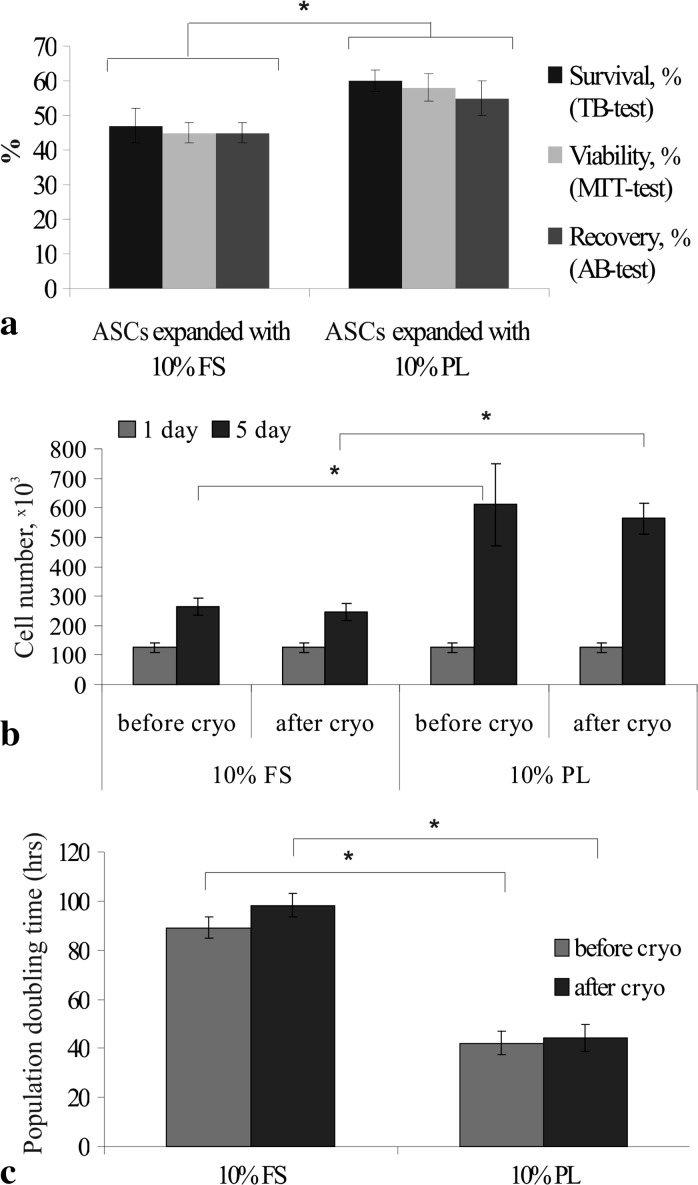
We then compared the effect of pre-cryopreservation expansion conditions on cell response on cryopreservation procedure. ASCs cultured in medium supplemented either with FS or human PL were cryopreserved using DMSO-free protocol described in materials and methods section. In each group the post-thaw recultivation of ASCs was performed in the corresponding FS- or PL-supplemented medium (Fig. 1). Immediately after thawing the survival rate of PL-cultured ASCs assessed by trypan blue staining was significantly higher than after expansion in FS-supplemented medium and comprised 60 ± 3% (Fig. 2a). MTT test performed 2 h after thawing revealed that viability of ASCs cultured in FS-containing medium was approximately 45 ± 3%, whereas viability of PL-expanded cells reached 58 ± 4% (Fig. 2a). ASCs expanded both in FS- and PL-containing media after 24 h of recultivation were able for attachment and spreading. After 24 h of post-thaw recultivation, the recovery of cryopreserved ASCs assessed by AB test was significantly higher in PL groups (55 ± 5%), compared to FS group (45 ± 3%) (Fig. 2a). Thus, the expansion of ASCs in PL-supplemented medium prior to cryopreservation promoted better survival, viability and recovery rates of cells in DMSO-free conditions. However, these parameters were lower, compared to positive control values (cryopreserved in the presence of 10% DMSO and 20% FS), where survival, viability and recovery rates comprised 89 ± 3, 85 ± 3, and 81 ± 5%, respectively.
Fig. 2.
Characteristics of ASCs after DMSO- and FS-free cryopreservation: a—post-thaw survival, viability and recovery of ASCs expanded in FS- or PL-containing media (%); b—total number of ASCs cultured in FS- or PL-containing media before and after DMSO- and FS-free cryopreservation; c—population doubling time of ASCs in FS- or PL-containing media before and after DMSO- and FS-free cryopreservation (hours). The data (n = 5) are presented as the mean ± SD (* P < 0.05)
After 5 days of post-thaw recultivation in the presence of PL, the cell number increased 4.5–4.6 times (P < 0.05) (Fig. 2b). In FS cultures proliferation rate was significantly lower, the cell number increased 1.9–2.1 times (P < 0.05).
PDT of cryopreserved ASCs, assessed during four passages of post-thaw recultivation did not significantly differ from their fresh counterparts and comprised 98.3 ± 4.9 versus 89.2 ± 4.3 h for FS-cultured ASCs and 44.2 ± 5.6 versus 42.0 ± 4.8 h for PL-cultured ASCs (Fig. 2c).
To confirm that ASCs preserved their immunophenotype and differentiation properties after cryopreservation and post-thaw expansion the corresponding analysis was performed. During flow cytometry studies it was revealed that cryopreserved ASCs from both groups were positive (>95%) for CD73, CD90, CD105, and negative (<5%) for CD45 antigens and this immunophenotype did not differ from the cells, processed without cryopreservation.
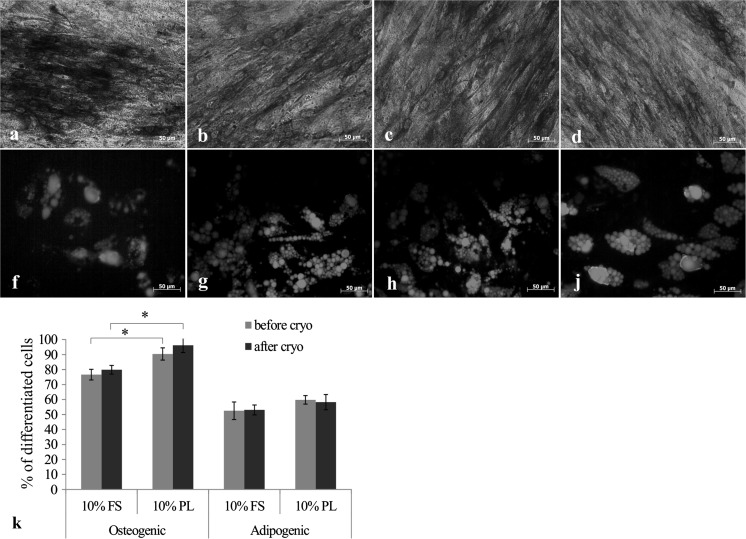
As shown in Fig. 3 a–d, when cultured in osteogenic medium most of the cells changed morphology and expressed alkaline phosphatase (ALP), which is an early marker of osteogenesis. The quantitative evaluation of differentiation efficiency revealed that the percentage of ALP-positive cells was significantly higher in PL cultures (76 ± 4% in FS group vs 90 ± 3% in PL group).
Fig. 3.
In vitro differentiation towards osteogenic (a–d) and adipogenic (f–j) lineages of ASCs before and after DMSO- and FS-free cryopreservation and post-thaw expansion in FS- or PL-supplemented medium: a, f—ASCs expanded using 10% FS before cryopreservation; b, g—ASCs expanded using 10% FS after cryopreservation; c, h—ASCs expanded using 10% PL before cryopreservation; d, j—ASCs expanded using 10% PL after cryopreservation. Expression of alkaline phosphatase was assessed by Fast Blue staining (a–d); Nile Red staining of intracellular lipids (f–j). The efficiency of differentiation was determined as a percentage of specific marker-positive cells to the total number of cells (k). The results (n = 5) are expressed as the mean ± SD (* P < 0.05)
During adipogenic differentiation ASCs of both groups (FS and PL) accumulated intracellular lipids positively stained with Nile Red (Fig. 3 f–j). The efficiency of lipid droplets formation in PL group cultures was at the same level as in FS group. No significant difference was found between cryopreserved and non-cryopreserved ASCs in their ability to multilineage differentiation towards osteogenic and adipogenic lineages.
Discussion
Cryopreservation can provide off-the-shelf availability of cells for further experimental or clinical application; enable quality control and standardization of the cellular product (Marquez-Curtis et al. 2015). Conventional cryopreservation techniques based on DMSO application ensure high cell survival after thawing but limit their therapeutic applicability by introducing the risk of undesirable side effects (Rodríguez et al. 2005; Windrum et al. 2005). Numerous strategies aimed to reduce DMSO concentration or completely remove it from cryopreservation solution have been already published (Thirumala et al. 2010; Shivakumar et al. 2015). Positive results have been achieved by using the cocktails of different polymers (polyvinylpyrrolidone, ethylene glycol, hydroxyethyl starch or carboxylated poly-l-lysine) in conjunction with the programmed freezing protocols (Matsumura et al. 2013; Thirumala et al. 2010; Shivakumar et al. 2015). However, these methods either require the application of special processing equipment and reagents or possess the similar to DMSO drawbacks and should be removed prior to clinical application.
Previously, we have proposed a DMSO- and serum-free cryopreservation protocol for human dermal MSCs (Petrenko et al. 2014) comprising pretreatment of cells with sugars during culture combined with their presence in cryopreservation medium. Unlike most cryoprotectants, sugars are used in relatively small concentrations and thus do not need to be removed from cells after thawing. Such benefit significantly decreases laboratory processing time and prevents cell loss, which usually occurs during washing. Although sugars have been widely used for many years in research studies involving freeze-drying and cryopreservation, the precise mechanism of their protecting action remains to be determined. According to a classic concept, sugar transport across animal cell membranes is restricted to monosaccharides. Thus, disaccharides and trisaccharides were considered only as additional extracellular cryoprotectants. During cryopreservation, their presence only outside the cells has been shown to be insufficient for obtaining satisfactory results. Sugar is required in both the intra- and extracellular compartments (Motta et al. 2014). It has been known that intracellular sugar can interact with proteins and lipids, stabilizing cell membranes and preventing their phase transition during freeze–thawing (Oliver et al. 2004; Campbell and Brockbank 2012). Several methods have been proposed to transport saccharides across the cell plasma membrane, including genetic engineering, microinjection, electroporation or the application of liposomes (Motta et al. 2014).
Recent publications have shown trehalose uptake by animal cells under normal conditions by a fluid-phase endocytosis mechanism (Oliver et al. 2004). After overnight incubation of cells with trehalose, its concentration inside the cells was approximately 13–20 mM. The metabolic activity of trehalose-loaded cells was slightly decreased compared to untreated cells but reached the normal level during the following culture.
In our previous study (Petrenko et al. 2014) pretreatment approach based on cell culture in medium containing different sugars (sucrose, trehalose or raffinose) might have allowed them to penetrate into cells. We have confirmed that application of sugars on both pretreatment and cryopreservation stages significantly increased cell viability. In the concentrations of 200 mM for pretreatment and 300 mM in cryopreservation medium sucrose, trehalose or raffinose supplementation provided about 50% viability of dermal MSCs.
While different sugars acted almost equally during previous studies, sucrose was chosen in the present research as the most attractive due to a low price and very high availability. Intriguingly, in the present study the optimal sucrose concentrations for the preservation of ASCs were 100 mM lower at each step of the protocol, compared to dermal MSCs, shown previously. The obtained results showed that there is a different response of MSCs isolated from various sources on the cryopreservation procedure. Despite the fact that both types of cells have similar phenotypic and differentiation properties, some differences in their gene expression, differentiation ability, anti-inflammatory and angiogenic potential have been revealed (Blasi et al. 2011; Jääger et al. 2012). Our results suggest that human ASCs may be more susceptible to osmotic changes resulting from pretreatment, freeze-thaw and post-thaw dilution than dermal MSCs. In contrast, when comparing viability of rat bone marrow, dental pulp and adipose tissue-derived MSCs after cryopreservation with 10% DMSO and 90% FS, the highest level of post-thaw cell recovery has been obtained for adipose tissue-derived cells (Davies et al. 2014). Taking together, these data indicate that MSCs isolated from different tissues do not respond equally to cryopreservation, which should be additionally studied.
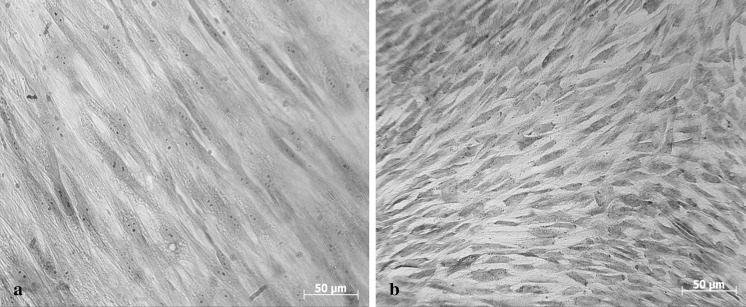
Next, to reveal whether the xeno-free expansion conditions affect the cryosensitivity of ASCs, starting from primary culture, ASCs were expanded in human PL-supplemented medium. Control ASCs were cultured in FS-supplemented medium. It is interesting that ASCs expanded in PL after cryopreservation demonstrated higher survival and viability rates than cells expanded in FS. The differences may be explained by the cell size and permeability of cryoprotecting agents and water across the cell membrane. The surface-to-volume (S/V) ratio determines the time constant of the heat and mass transfer into and out of the cells (Liu et al. 2011). Water flows out from the smaller cells with high S/V ratio faster than from the bigger cells, which reduces the chance of intracellular ice formation during freezing (Dumont et al. 2004). In the present study, we registered that PL-cultured ASCs were two times smaller and less stretched compared with FS-cultured cells. PL-expanded cells had a much smaller size (approximately in 2 times) than ASCs expanded with FS, and their post-thaw viability was better. The average cell size varied from 47 to 60 µm in FS cultures and from 25 to 29 µm in PL cultures (Fig. 4). Similar results have been obtained by Griffiths et al. (2013) for bone marrow-derived MSCs. In particular, MSCs maintained in PL were significantly smaller in cell size (forward scatter) and exhibited less internal complexity (side scatter) than MSCs maintained in FS. It has been demonstrated that smaller size of MSCs, in general, could indicate that these cells have a higher potential for cell therapy, such as a higher differentiation capacity (Haasters et al. 2009; Naaijkens et al. 2012).
Fig. 4.
The morphology of ASCs during culture in FS- (a) and PL- (b) supplemented medium
In general, the presented DMSO-free/xeno-free ASCs preparation process allows preserving about 60% of cells after cryopreservation in the absence of DMSO and FS. Further cell recovery during culture in PL-supplemented medium improved proliferation and allowed replacing damaged cells in a shorter time period compared with FS. The proliferation rate of ASCs cultured with PL was significantly higher compared with those cultured with FS. Numerous reports have been also indicated that human PL supports expansion of MSCs even better than xenogeneic FS, including shortening the population doubling time and enriching the cell number (Blande et al. 2009; Gottipamula et al. 2012; Naaijkens et al. 2012). This could be explained by the effect of cytokine spectrum contained in PL (Doucet et al. 2005; Kinzebach et al. 2013) that is capable of handling a wide range of cellular responses from migration and mitogenesis up to differentiation. We also demonstrated that PL promoted ASCs differentiation into osteogenic direction. The efficiency of osteogenic differentiation was by 15% higher in PL-cultured ASCs compared with FS. Such impact of human PL might be supported by bone morphogenetic protein 2, 4 and 6 (BMP 2, BMP 4 and BMP 6), interleukin-1 (IL-1), osteonectin, platelet factor-4 (PF-4), insulin-like growth factor-1 (IGF-1), EGF, TGF-β1, bFGF and PDGF, which are known to mediate osteoinductive effects (Chevallier et al. 2010; Zaky et al. 2008). Obtained data are in line with those reported by Xia et al. (2011) and Horn et al. (2010) for bone marrow-derived MSCs. In contrast, Gruber et al. (2004) have shown that platelet growth factors released by calcium and thrombin stimulation decreased osteogenic differentiation. Consequently, human PL offers a number of advantages for the expansion of ASCs over typical FS supplemented medium.
The described DMSO- and xeno-free processing may push forward the development of safe and efficient cGMP-compliant protocols for manufacturing and banking of ASCs, providing their off-the-shelf availability for regenerative medicine applications.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schäfer R, Sella S, Rodeghiero F. Platelet lysate as a substitute for animal serum for the ex vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016;7:93. doi: 10.1186/s13287-016-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, Del Fante C, Novara F, de Silvestri A, Amendola G, Zuffardi O, Maccario R, Locatelli F. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol. 2007;211:121–130. doi: 10.1002/jcp.20911. [DOI] [PubMed] [Google Scholar]
- Blande IS, Bassaneze V, Lavini-Ramos C, Fae KC, Kalil J, Miyakawa AA, Schettert IT, Krieger JE. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion. 2009;49:2680–2685. doi: 10.1111/j.1537-2995.2009.02346.x. [DOI] [PubMed] [Google Scholar]
- Blasi A, Martino C, Balducci L, Saldarelli M, Soleti A, Navone SE, Canzi L, Cristini S, Invernici G, Parati EA, Alessandri G. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell. 2011;3:5. doi: 10.1186/2045-824X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan SS, Gross SA, Acker JP, Toner M, Carpenter JF, Pyatt DW. Cryopreservation of stem cells using trehalose: evaluation of the method using a human hematopoietic cell line. Stem Cells Dev. 2004;13:295–305. doi: 10.1089/154732804323099226. [DOI] [PubMed] [Google Scholar]
- Campbell LH, Brockbank KG. Culturing with trehalose produces viable endothelial cells after cryopreservation. Cryobiology. 2012;64:240–244. doi: 10.1016/j.cryobiol.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Chevallier N, Anagnostou F, Zilber S, Bodivit G, Maurin S, Barrault A, Bierling P, Hernigou P, Layrolle P, Rouard H. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2010;31:270–278. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology. 2014;69:342–347. doi: 10.1016/j.cryobiol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, Lataillade JJ. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- Dumont F, Marechal PA, Gervais P. Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl Environ Microbiol. 2004;70:268–272. doi: 10.1128/AEM.70.1.268-272.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1:322–324. doi: 10.1002/term.35. [DOI] [PubMed] [Google Scholar]
- Gottipamula S, Sharma A, Krishnamurthy S, Majumdar AS, Seetharam RN. Human platelet lysate is an alternative to fetal bovine serum for large-scale expansion of bone marrow-derived mesenchymal stromal cells. Biotechnol Lett. 2012;34:1367–1374. doi: 10.1007/s10529-012-0893-8. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Baraniak PR, Copland IB, Nerem RM, McDevitt TC. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15:1469–1483. doi: 10.1016/j.jcyt.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer M, Watzek G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004;15:29–35. doi: 10.1080/09537100310001643999. [DOI] [PubMed] [Google Scholar]
- Haasters F, Prall WC, Anz D, Bourquin C, Pautke C, Endres S, Mutschler W, Docheva D, Schieker M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214:759–767. doi: 10.1111/j.1469-7580.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9–18. [Google Scholar]
- Horn P, Bokermann G, Cholewa D, Bork S, Walenda T, Koch C, Drescher W, Hutschenreuther G, Zenke M, Ho AD, Wagner W. Impact of individual platelet lysates on isolation and growth of human mesenchymal stromal cells. Cytotherapy. 2010;12:888–898. doi: 10.3109/14653249.2010.501788. [DOI] [PubMed] [Google Scholar]
- Jääger K, Islam S, Zajac P, Linnarsson S, Neuman T. RNA-seq analysis reveals different dynamics of differentiation of human dermis- and adipose-derived stromal stem cells. PLoS ONE. 2012;7:e38833. doi: 10.1371/journal.pone.0038833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzebach S, Dietz L, Klüter H, Thierse H-J, Bieback K. Functional and differential proteomic analyses to identify platelet derived factors affecting ex vivo expansion of mesenchymal stromal cells. BMC Cell Biol. 2013;14:48. doi: 10.1186/1471-2121-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Pedenovi M, Niewiarowski A, Casali B, Donati MB, Corbascio GC, Marchisio PC. Effects of dimethyl sulfoxide (DMSO) on microfilament organization, cellular adhesion, and growth of cultured mouse B16 melanoma cells. Exp Cell Res. 1987;172:385–396. doi: 10.1016/0014-4827(87)90396-X. [DOI] [PubMed] [Google Scholar]
- Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213:18–26. doi: 10.1002/jcp.21081. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu X, Ma XH, Liu J, Cui ZF. Effect of various freezing solutions on cryopreservation of mesenchymal stem cells from different animal species. CryoLetters. 2011;32:425–435. [PubMed] [Google Scholar]
- Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71:181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Hayashi F, Nagashima T, Hyon SH. Long-term cryopreservation of human mesenchymal stem cells using carboxylated poly-l-lysine without the addition of proteins or dimethyl sulfoxide. J Biomater Sci Polym Ed. 2013;24:1484–1497. doi: 10.1080/09205063.2013.771318. [DOI] [PubMed] [Google Scholar]
- Motta JP, Paraguassú-Braga FH, Bouzas LF, Porto LC. Evaluation of intracellular and extracellular trehalose as a cryoprotectant of stem cells obtained from umbilical cord blood. Cryobiology. 2014;68:343–348. doi: 10.1016/j.cryobiol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Naaijkens BA, Niessen HWM, Prins H-J, Krijnen PA, Kokhuis TJ, de Jong N, van Hinsbergh VW, Kamp O, Helder MN, Musters RJ, van Dijk A, Juffermans LJ. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012;348:119–130. doi: 10.1007/s00441-012-1360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notman R, Noro M, O’Malley B, Anwar J. Molecular basis for dimethylsulfoxide (DMSO) action on lipid membranes. J Am Chem Soc. 2006;128:13982–13983. doi: 10.1021/ja063363t. [DOI] [PubMed] [Google Scholar]
- Oliver AE, Jamil K, Crowe JH, Tablin F. Loading human mesenchymal stem cells with trehalose by fluid-phase endocytosis. Cell Preserv Technol. 2004;2:35–49. doi: 10.1089/153834404322708745. [DOI] [Google Scholar]
- Petrenko YA, Petrenko AY. Phenotypical properties and ability to multilineage differentiation of adipose tissue stromal cells during subculturing. Cytol Genet. 2012;46:36–40. doi: 10.3103/S0095452712010070. [DOI] [PubMed] [Google Scholar]
- Petrenko YA, Rogulska OY, Mutsenko VV, Petrenko AY. A sugar pretreatment as a new approach to the DMSO- and xeno-free cryopreservation of human mesenchymal stromal cells. CryoLetters. 2014;35:239–246. [PubMed] [Google Scholar]
- Reboulleau CP, Shapiro HS. Chemical inducers of differentiation cause conformational changes in the chromatin and deoxyribonucleic acid of murine erythroleukemia cells. Biochemistry. 1983;22:4512–4517. doi: 10.1021/bi00288a025. [DOI] [PubMed] [Google Scholar]
- Reinisch A, Bartmann C, Rohde E, Schallmoser K, Bjelic-Radisic V, Lanzer G, Linkesch W, Strunk D. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen Med. 2007;2:371–382. doi: 10.2217/17460751.2.4.371. [DOI] [PubMed] [Google Scholar]
- Rodrigues JP, Paraguassú-Braga FH, Carvalho L, Abdelhay E, Bouzas LF, Porto LC. Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56:144–151. doi: 10.1016/j.cryobiol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez L, Velasco B, García J, Martín-Henao GA. Evaluation of an automated cell processing device to reduce the dimethyl sulfoxide from hematopoietic grafts after thawing. Transfusion. 2005;45:1391–1397. doi: 10.1111/j.1537-2995.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- Shivakumar SB, Bharti D, Jang SJ, Hwang SC, Park JK, Shin JK, Byun JH, Park BW, Rho GJ. Cryopreservation of human Wharton’s Jelly-derived mesenchymal stem cells following controlled rate freezing protocol using different cryoprotectants; a comparative study. Int J Stem Cells. 2015;8:155–169. doi: 10.15283/ijsc.2015.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorobogatova NG, Novikov AN, Fuller BJ, Petrenko AY. Importance of a three-stage cooling regime and induced ice nucleation during cryopreservation on colony-forming potential and differentiation in mesenchymal stem progenitor cells from human fetal liver. CryoLetters. 2010;31:371–379. [PubMed] [Google Scholar]
- Thirumala S, Goebel W, Woods E. Clinical grade adult stem cell banking. Organogenesis. 2009;5:143–514. doi: 10.4161/org.5.3.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumala S, Gimble JM, Devireddy RV. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med. 2010;4:224–232. doi: 10.1002/term.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrum P, Morris TCM, Drake MB, Niederwieser D, Ruutu T. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transpl. 2005;36:601–603. doi: 10.1038/sj.bmt.1705100. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Xia W, Li H, Wang Z, Xu R, Fu Y, Zhang X, Ye X, Huang Y, Xiang AP, Yu W. Human platelet lysate supports ex vivo expansion and enhances osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35:639–643. doi: 10.1042/CBI20100361. [DOI] [PubMed] [Google Scholar]
- Zaky SH, Ottonello A, Strada P, Cancedda R, Mastrogiacomo M. Platelet lysate favours in vitro expansion of human bone marrow stromal cells for bone and cartilage engineering. J Tissue Eng Regen Med. 2008;2:472–481. doi: 10.1002/term.119. [DOI] [PubMed] [Google Scholar]
- Zuk P, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]