Abstract
Parkinson’s disease (PD) is one of the most common neural degenerative disease, affecting millions of people globally. Great progress has been made in the PD treatment, and one of the most promising one is the stem cell-based therapy. Thus, studies on the differentiation of neural stem cells (NSCs) are important to the advancement in PD therapy. In this study, we used the rat NSCs to elucidate the role of Lithium in the proliferation and differentiation of NSCs by immunostaining against Ki67 and BrdU analysis as well as immunostaining against specific neuronal markers. We concluded that lithium chloride (LiCl) treatment could enhance the proliferation in NSCs and promote the dopaminergic neuronal differentiation of NSCs in vitro. This process was potentially mediated by Wnt signaling pathway. Using the 6-OHDA-induced PD models, we provided evidence to show that LiCl had the capacity to enhance the proliferation in NSCs and differentiation towards dopaminergic neurons in vivo. The beneficial effect of LiCl treatment was further validated by the fact that the motor function as well as learning and memory was improved in the PD models through Rotarod test and Morris water maze analysis. The learning and memory improvement was further supported by the increase in dendrite spine density in PD models receiving LiCl-treated NSCs. Through this study, we concluded that Lithium plays an important role in promoting NSCs’ neuronal differentiation in vitro and improving the symptoms of PD models in vivo. It is of great significance that this work showed the potential application of Lithium in the PD therapy in the future.
Keywords: Lithium, Parkinson’s disease, Neural stem cell, Dopaminergic neuron, Differentiation, Proliferation
Introduction
Neural stem cells (NSCs) are self-renewing and multipotent cells. They have the capacity to generate the main phenotype of the nervous system, primarily including neurons, astrocytes, and oligodendrocytes (Lim and Alvarez-Buylla 2014). This makes NSCs be used for the purposes of both research and clinical applications. In recent years, research on NSCs’ direct differentiation toward specific subtypes of neurons is the hotspot. A bunch of studies reported the generation of dopaminergic neurons from NSCs (Ding et al. 2016; Lee et al. 2016a, b). Due to the fact that degradation of dopaminergic neurons plays key roles in the pathology of Parkinson’s disease (PD), development of stem cell-based therapy is one of the most promising way in the treatment of PD (Zhu et al. 2016). PD is a disorder of the central nervous system that mainly affects the motor system (Keranen et al. 2015). It is estimated that 200 out of 100,000 people worldwide have PD, affecting approximately seven million people globally (Moore et al. 2005). Therefore, development of effective remedies, such as stem cell-based therapy is urgent and critical to the progress of PD treatment, providing hopes to the millions of patients around the world.
Recently, an efficient and simple method to induce the stem cell differentiation was reported. Lithium (Li), the smallest alkali metal with an atomic weight of 6.9, was found to enhance the efficiency of induced pluripotent stem cell-derived neurospheres (Tafreshi et al. 2015), promote the odontoblast differentiation of hair follicle neural crest cells (Shan et al. 2015), modulate the proliferation and differentiation of mesenchymal stem cells (Dong et al. 2015; Tang et al. 2015), prevent neural progenitor cell apoptosis (Cabrera et al. 2014). These studies indicated that Lithium may play function in the NSCs’ differentiation. The knowledge of mechanism underlying the Li’s effect on neural differentiation is still limited. Recently, the mostly widely studied mediators and signaling pathways of lithium action are the glycogen synthase kinase-3 (GSK3) and Wnt/β-catenin pathways (Ferensztajn-Rochowiak and Rybakowski 2016). LiCl could enhance central myelin gene expression in mouse oligodendrocytes mediated by Wnt/β-catenin signaling, participating remyelinating strategy (Meffre et al. 2015). The activation of Wnt signaling by LiCl treatment was dependent on GSK-3β, stimulating mesenchymal stem cell proliferation (Zhu et al. 2014). More studies are still needed to further explore the related signaling pathways mediating Li’s effect.
In this study, we explored the role of lithium chloride (LiCl) in the proliferation and differentiation of rat NSCs. We also found that LiCl could enhance the NSCs’ differentiation towards dopaminergic neurons. In the PD rat models, LiCl treatment also facilitated the proliferation of transplanted NSCs as well as the generation of dopaminergic neurons. More importantly, the motor function and learning/memory was alleviated in PD rats transplanted with LiCl-treated NSCs. These data indicated that Lithium plays roles in the direct differentiation of NSCs to dopaminergic neurons, providing a potential candidate to the PD treatment.
Methods and materials
Animals
Sprague–Dawley rats (weight: 180–220 g) were purchased from Shanghai Silaike Experiment Animal Co., Ltd (Shanghai, China). All procedures of animal handling were carried out in accordance with the protocols approved by the Institutional Animal Care and Use Committee of Center Hospital of the People’s Liberation Army (Reference number CHPLA201310022317).
Cell culture
Neural stem cells were derived from neonatal SD rats. The hippocampal tissues were dissected and digested in 0.25% Trypsin–EDTA. The cells were cultured in DMEM/F12 medium, supplemented with 1% B27, 1% N2, 1% penicillin/streptomycin, 20 ng/ml EGF, and 10 ng/ml bFGF. The medium was half changed every three days. For the in vitro differentiation, the formed neurospheres were placed in 24-well plates coated with 0.1 mg/ml Poly-d-Lysine, and cultured in Neurobasal medium supplemented with 1% B27, 1% N2 and 1% penicillin/streptomycin. For the dopaminergic differentiation, the neurospheres were placed in 24-well plates coated with 0.1 mg/ml Poly-d-Lysine and 10 mg/ml laminin, and cultured in neurobasal medium with 1% B27, 1% N2, 1% penicillin/streptomycin, 20 ng/ml bFGF and 5 μg/ml heparin. All these reagents were purchased from Life Technologies (Carlsbad, CA, USA).
LiCl preparation and treatment
LiCl (purchased from Sigma, St. Louis, MO, USA) was dissolved in purified water as the stock solution at 100 mg/ml. Then, the stock solution was sequentially diluted to 0.01, 0.1, 0.5, 1, 5 and 10 mg/ml. For the LiCl treatment, 1 mg/ml LiCl was added into NSC cultures for the treatment of 48 h.
Immunostaining analysis was used to determine the expression pattern of proliferative cell and neuronal markers in LiCl-treated NSCs. Cells were washed in PBS for three times, and fixed in 2% paraformaldehyde. Then, cells were blocked for 60 min in 0.1% Triton X-100 in phosphate-buffered saline with 5% bovine serum albumin, and then incubated at 4 °C with anti-Ki67 (1:200), anti-MAP2 (1:500), anti-GFAP (1:500), anti-TH (1:500) overnight. All primary antibodies were purchased from Abcam. FITC- or TRITC-conjugated secondary antibodies (ThermoFisher Scientific, Waltham, MA, USA) were applied and incubated for 1 h at room temperature. The slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA). Fluorescent images were taken under a SP5/Leica confocal microscope (Wetzlar, Germany) with LAS AF Lite software.
BrdU analysis
BrdU staining was applied to analyze the proliferation of NSCs in vitro and injected NSCs in vivo. For the in vitro study, BrdU reagent (purchased from Sigma) was added to NSCs and incubated for 2 h, and 1-7 days. Then, cells were fixed in 2% paraformaldehyde for 20 min. After washing three times with phosphate buffered saline (PBS), NSCs were incubated with primary anti-BrdU antibody (purchased from Sigma) at 4 °C overnight and with TRITC-conjugated secondary antibody at room temperature for 1 h. For the in vivo study, 50 mg/kg BrdU was injected into PD models on the day of transplantation. BrdU staining was conducted at the 10th and 30th day after transplantation. Images were taken under a SP5/Leica confocal microscope.
Immunohistochemistry
The sections were washed in PBS, blocked for 60 min in 0.3% Triton X-100 in phosphate-buffered saline with 5% bovine serum albumin, and then incubated at 4 °C with anti-TH, anti-BrdU, and anti-PSD95 antibodies (purchased from Abcam) overnight. FITC- or TRITC-conjugated secondary antibody (ThermoFisher Scientific) was applied to the slides, and incubated for 1 h at room temperature. The Slides were visualized and the images were captured by using SP5/Leica confocal microscope with LAS AF Lite software.
Cell counting
To quantify the immunostaining data, we standardized the counting across all slides. The data for each group were averaged from at least three independent slides. The ratios were calculated by dividing the number of specific antigen positive cells to the total cell number, and the total cell number was based on the DAPI staining.
Real time PCR
To compare the alteration in neuronal and glial marker expression at mRNA level, total RNA of cells with or without the LiCl treatment was isolated using TRIZOL reagent (Life Technologies). Synthesis of cDNA was performed by using one-step RT-PCR kit purchased from Life Technologies. SYBR Green (purchased from Qiagen, Hilden, Germany) RT-PCR amplification and real time fluorescence detection were performed using the PRISM 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA). Relative gene expression was calculated by the ∆∆Ct method. The primer sequences were as follows, β-catenin, 5′-GGCCATATCCACCAGAGTGAA-3′ and 5′-GCCAATGGCTTGGAATGAGA-3′; Cyclin D3, 5′-TGCACATGATTTCCTGGCCT-3′ and 5′-CTGTAGCACAGAGGGCCAAA-3′; GSK-3β, 5′-GACTAAGGTCTTCCGACCCC-3′ and 5′-AAGAGTGCAGGTGTGTCTCG-3′; MAP2, 5′-TGCCACCTGTTTCTCTCCAC-3′ and 5′-TCTTTTGCTTGCTCGGGATT-3′; Tuj1, 5′-CGCCATGTTCAGACGCAAG-3′ and 5′-CTCGGACACCAGGTCGTTCA-3′; GFAP, 5′-CCGACAGCAGGTCCATGTG-3′ and 5′-GTTGCTGGACGCCATTGC-3′; GAPDH, 5′-GGTATCGTGGAAGGACTCATGAC-3′ and 5′-ATGCCAGTGAGCTTCCCGT TCAGC-3′.
Establishment of PD rat model
To evaluate the function of LiCl-treated NSCs in vivo, we established PD models according to the following protocol. The anesthetized rats were secured in a Stoelting stereotaxic instrument. Lesions were made by the unilateral injection of 6-OHDA (purchased from Sigma) into the right MFB (AP: 4.4 mm; L: 1.2 mm; DV: 7.8 mm) and VTA (AP: 4.8 mm; L: 1.0 mm; DV: 7.8 mm). 6-OHDA was administrated through a microsyringe at a rate of 0.5 μl/min. The sham rats have received saline at the same coordinates.
Cell transplantation
Transplantation was performed on the 28th day after lesion. A suspension of cells (5 µl per site, 1 × 104 cells/µl) was injected at a rate of 0.5 µl/min into the VTA region. The syringe was left in situ for 5 min to allow for cell diffusion. For the sham group, the rats were injected with normal saline without cells. For the NSCs group, the suspension contained NSCs treated with laminin/heparin/bFGF (LHF). For the LiCl-NSCs group, the suspension included NSCs treated with LHF and LiCl.
Rotarod test
The motor function was assessed by Rotarod test. Rotational behavior was induced by intraperitoneal injection of apomorphine (0.5 mg/kg). Rats were tested in a session for 30 min at 2, 8 and 12 weeks after transplantation. The rotational speed of 10/min or even higher were considered as the successful PD models.
Morris water maze (MWM)
Learning and memory was evaluated by Morris water maze test. Rats were tested for MWM training at eight weeks after transplantation. Test protocol of Morris water maze was described previously (Vorhees and Williams 2006). Each rat was placed in the water facing the pool wall in one of the four quadrants. For each trial, the rat is allowed to swim within 90 s to find the hidden platform. If unsuccessful within 90 s, the rat is given a score of 90 s and then physically placed on the platform. All rats were allowed a 30 s rest period on the platform. The next trial was initiated immediately after the rest period.
Statistical analysis
All experiments were conducted in triplicate to ensure the consistency of data. In in vitro study, at least 500 cells were counted in each sample. In in vivo study, five rats were in each group. The dendrite spine density was calculated as the number of spines in 30 μm dendrite. All data were presented as mean ± SD. Statistical significance was determined by one-way ANOVA and unpaired Student’s t-tests using SigmaPlot Software (Systat Software, San Jose, CA, USA). A value of p < 0.05 was considered as significantly different.
Results
LiCl promoted the proliferation of rat NSCs in vitro
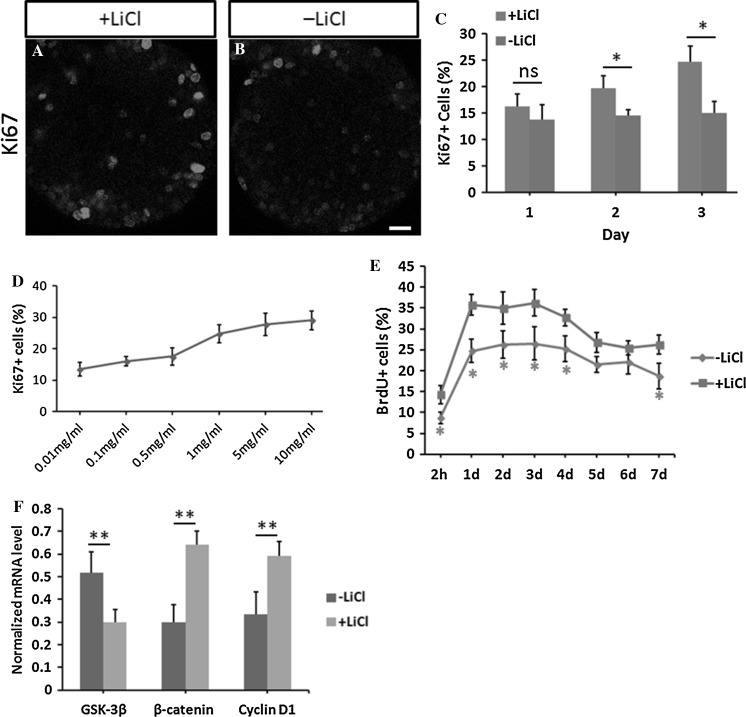
Firstly, we detected whether the treatment of LiCl could affect the proliferation of NSCs by Ki67 immunostaining and BrdU analysis. The results indicated that LiCl enhanced the proliferation of NSCs in vitro. The ratio of Ki67+ cells was increased by 40 ± 3% at three days after LiCl treatment as shown in Fig. 1a–c (p < 0.05). Statistical analysis also showed that the ratio of Ki67+ cells was increased by 40 ± 8% at two days after LiCl treatment (p < 0.05, Fig. 1c). By contrast, we did not observe a significant change at one day after LiCl treatment (p > 0.05, Fig. 1c). Besides, the ratio of Ki67+ cells was gradually increased with the increasing concentration of LiCl, showing a dose-dependent effect (Fig. 1d). The EC50 value of LiCl concentration for ratio of Ki67+ cells was 0.8 mg/ml. Taking the possible cytotoxicity into consideration, we used 1 mg/ml LiCl as the working concentration in all experiments. BrdU analysis data validated the above conclusion. At 2 h and 24 h after adding the BrdU, we found that ratio of BrdU positive cells was increased by 35 ± 9% and 30 ± 7% (p < 0.05, n = 404 cells) in the presence of LiCl, compared to the untreated cells (Fig. 1e). We also observed the significant increase in BrdU+ cells at 2, 3, 4 and 7 days after BrdU incubation (p < 0.05, Fig. 1e). The effect of LiCl to promote the NSCs’ proliferation was still present at the 7th day after BrdU incorporation (Fig. 1e). Furthermore, we determined the involvement of Wnt signaling in LiCl-induced proliferation of NSCs. The mRNA level of GSK-3β was significantly decreased while the level of β-catenin and Cyclin D1 was drastically decreased in LiCl-treated NSCs (p < 0.01, Fig. 1f). Collectively, these data indicated that LiCl treatment enhanced the proliferation in rat NSCs, mediated by Wnt signaling pathway.
Fig. 1.
LiCl promoted the proliferation of rat NSCs. The ratio of Ki67 positive cells in LiCl-treated NSCs (a) was higher than that in the untreated group (b). c Statistical analysis of Ki67+ cells ratio with the treatment of LiCl for 1-3 days. d Ratio of Ki67+ cells in NSCs was raised with the increasing concentration of LiCl. e Statistical analysis showed that the ratio of BrdU+ cells was increased in the LiCl-treated NSCs. Statistical difference was determined by unpaired Student’s t tests. f The mRNA expression level of GSK-3β, β-catenin and Cyclin D1 was altered in the presence of LiCl. *p < 0.05. Scale bar 20 μm
LiCl facilitated the neuronal differentiation of rat NSCs in vitro
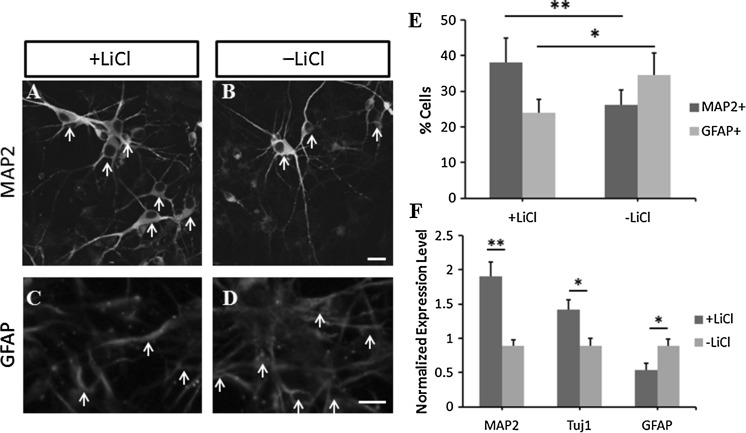
Then, we determined whether the application of LiCl plays a role in NSC’s differentiation. The NSCs grown on laminin-coated plates were differentiated by treating with bFGF, heparin and B27. In the presence of LiCl, we found that the ratio of MAP2+ cells (labeled with white arrows in Fig. 2a, b) differentiated from the NSCs was increased by 46 ± 11% compared to that in LiCl-free cells, indicating the enhancement of mature neuronal differentiation (p < 0.01, n = 704 cells; images are shown in Fig. 2a, b and statistical data are shown in Fig. 2e). By contrast, the ratio of GFAP+ cells was significantly decreased by 29 ± 8% in the presence of LiCl compared to the untreated NSCs (GFAP+ cells were labeled with white arrows in Fig. 2c, d), showing the attenuated capacity to differentiate into glial cells (p < 0.05, n = 612 cells; images are shown in Fig. 2c, d, and statistical data are shown in Fig. 2e). The real time PCR analysis showed similar results. The mRNA level of MAP2 and Tuj1 in LiCl treated differentiated NSCs was increased by 112 ± 17% (p < 0.01) and 62 ± 11% (p < 0.05), compared to that in untreated cells. However, the GFAP expression level was decreased by 39 ± 11% (p < 0.05) in the presence of LiCl (Fig. 2f). These results showed that LiCl enhanced the neuronal differentiation but attenuated glial differentiation in NSCs.
Fig. 2.
LiCl affected the differentiation of NSCs in vitro. LiCl increased MAP2 expression in NSCs (a) compared to that in untreated cells (b). GFAP expression was lower in LiCl-treated NSCs (c) compared to the untreated control (d). e Statistical analysis of change in the number of MAP2+ and GFAP+ cells derived from NSCs with or without LiCl treatment. f The mRNA levels of MAP2 and Tuj1 in NSCs were increased and the level of GFAP was decreased in the presence of LiCl. Statistical difference was determined by unpaired Student’s t tests. *p < 0.05, **p < 0.01. Scale bars in b and d were 10 μm
LiCl promoted the dopaminergic differentiation of NSCs
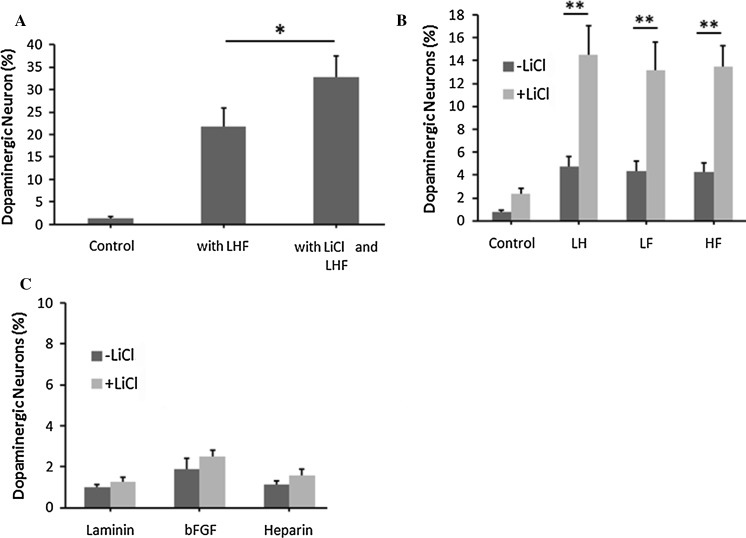
Previous work showed a synergic effect of laminin, heparin, and bFGF on the dopaminergic neuron generation from NSCs (Yu et al. 2007). Thus, we elucidated if LiCl affected dopaminergic differentiation in NSCs. The results indicated that LiCl treatment increased the ratio of dopaminergic neurons by 56 ± 12% (p < 0.05, n = 406 cells) compared to NSCs treated only with laminin (L), heparin (H), and bFGF (F) (Fig. 3a). Furthermore, we found that LiCl also significantly enhanced the capacity of domapinergic differentiation when the NSCs were co-treated with two factors (laminin/heparin, laminin/bFGF, or heparin/bFGF), while laminin/heparin, laminin/bFGF, or heparin/bFGF-treated NSCs generated very limited ratio of dopaminergic neurons (Fig. 3b, p < 0.01). However, the co-application of LiCl with one factor (laminin or heparin or bFGF) did not show significant alteration in the ratio of TH+ neurons (Fig. 3c, p > 0.1). Furthermore, among all the MAP2 positve cells under the treatment of LiCl and LHF, 61 ± 8% cells were positively immunostained with TH, while 27 ± 4% of Tuj1+ cells were co-labeled with TH. Above all, we came to the conclusion that LiCl could promote the generation of dopaminergic neurons from NSCs in vitro.
Fig. 3.
LiCl facilitated the generation of dopaminergic neurons from NSCs. Statistical analysis showed that co-application of LiCl as well as laminin, heparin and bFGF (LHF) (a), co-application of LiCl and LH, LF or HF (b) could increase the ratio of TH+ cells differentiated from NSCs, while co-application with laminin or heparin or bFGF (c) did not significantly enhance differentiation to dopaminergic neurons. Statistical difference was determined by unpaired Student’s t tests. *p < 0.05; **p < 0.01
Dopaminergic neurons induced by LiCl could survive and restore motor function in PD models
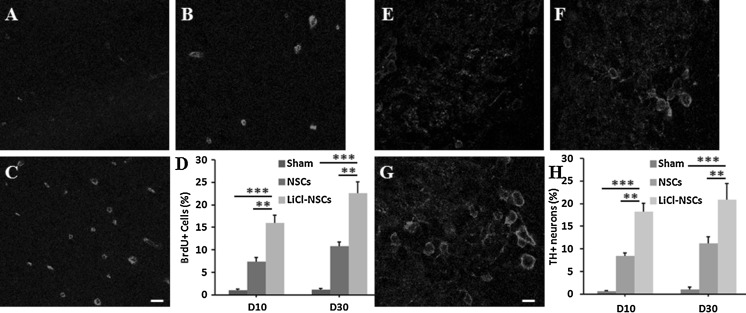
To detect the function of dopaminergic neurons derived from LiCl-treated NSCs, we transplanted those cells into PD rat models. The results indicated that the proliferation in the VTA region was enhanced at both the 10th and 30th day after cell transplantation since 50 ± 11 and 51 ± 7% more BrdU positive cells were observed in LiCl-NSCs transplantation group compared to that in models receiving untreated NSCs (p < 0.01, Fig. 4a–d). Besides, we also found that ratio of TH+ neurons in the VTA region was significantly higher by 51 ± 9 and 46 ± 5% in the PD rats transplanted with LiCl-treated NSCs, compared to the animals transplanted with untreated NSCs (p < 0.01, Fig. 4e–h). Meanwhile, we did not observe apparent appearance of TH+ cells when PD models were injected with LiCl or LHF only, indicating that TH+ cells around the injection site were generated from the exogenous NSCs. These data demonstrated that transplanted LiCl-NSCs in the PD models could survive and proliferate in vivo.
Fig. 4.
LiCl enhanced the proliferation and dopaminergic differentiation in PD models. BrdU staining in sham group (a), NSCs transplanted group (b) and PD animals transplanted with LiCl-treated NSCs (c). d Statistical analysis showed increasing ratio of BrdU+ cells in LiCl-NSCs transplanted rats compared to that in sham control or NSCs transplanted group. TH staining in sham group (e), NSCs transplanted group (f) and PD animals transplanted with LiCl-treated NSCs (g). h Ratio of TH+ cells was increased in LiCl-NSCs transplanted rats compared to that in sham control or NSCs transplanted group. **p < 0.01; ***p < 0.001. Scale bars in c was 20 μm and in g was 10 μm
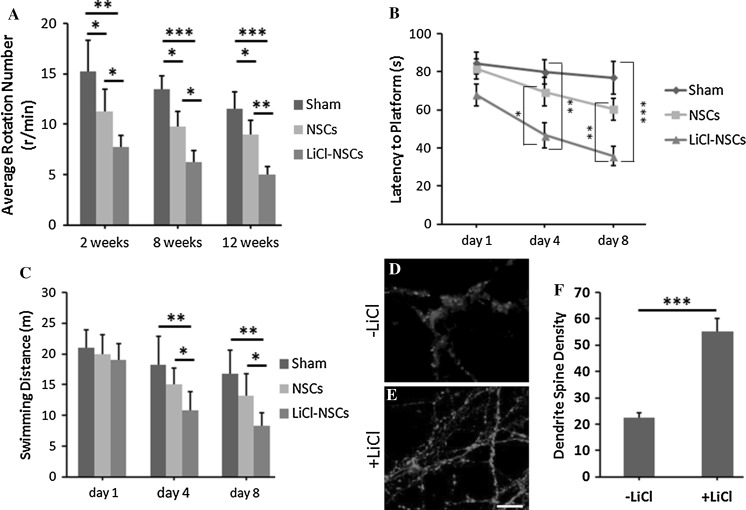
Transplantation with LiCl-treated NSCs could recover the motor function in PD models. The Rotarod test showed that the average rotation numbers were decreased by 48 ± 6% in LiCl-treated NSCs transplanted rats, compared to the saline control rats at 2 weeks after transplantation (Fig. 5a, p < 0.01, n = 4). Furthermore, the average rotation numbers were also reduced by 33 ± 8% in LiCl-treated NSCs transplanted rats in contrast to the rats transplanted with untreated NSCs (Fig. 5a, p < 0.05, n = 4). This beneficial effect was more obvious at 8 and 12 weeks after transplantation. The average rotation number was drastically decreased by 53 ± 9% in rats transplanted with NSCs treated with LiCl compared to that in untreated NSCs transplanted rats (Fig. 5a, p < 0.001, n = 4). Also, the rotation number was significantly decreased by 37 ± 7% in LiCl-NSCs transplanted rats than in the untreated NSCs transplanted models (Fig. 5a, p < 0.05, n = 4). At 12 weeks after operation, the rotation number was decreased by 57 ± 7% in LiCl-NSCs transplanted rats compared to the sham control (Fig. 5a, p < 0.001, n = 4). By contrast to the rats receiving untreated NSCs, the rotation number was reduced by 45 ± 8% (Fig. 5a, p < 0.01, n = 4). This indicated that transplantation with NSCs treated with LiCl could alleviate the motor function in PD models.
Fig. 5.
LiCl application restored motor function and memory in PD models. a The rotation number was decreased in rat transplanted with LiCl-treated NSCs. b Rats receiving LiCl-treated NSCs showed less latency to platform than that in sham and untreated NSCs transplantation group. c The swimming distance was decreased in LiCl-NSCs transplantation group. d–f Dendrite spine density was increased in PD models transplanted with LiCl-treated NSCs (e), compared to the animals with untreated cells (d). Statistical difference was determined by unpaired Student’s t tests and one way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001
The learning and memory improvement was evaluated by the Morris water maze test. At the 4th day after training, the latency to platform was significantly decreased by 41 ± 9% (p < 0.01, n = 4) and 32 ± 5% (p < 0.05, n = 4) in LiCl-NSCs transplanted rats compared to the untransplanted group and rats with untreated NSCs (Fig. 5b). Similar results were obtained at the 8th day after training. The latency to platform was reduced by 53 ± 11% (p < 0.001, n = 4) and 40 ± 7% (p < 0.01, n = 4) in LiCl-NSCs transplanted models when compared to the sham control and models transplanted with untreated NSCs (Fig. 5b). Furthermore, the swimming distance was also reduced by 39 ± 10% (p < 0.01) and 33 ± 9% (p < 0.05) in LiCl-treated NSCs transplantation group, compared to the vehicle group and rats with untreated NSCs (Fig. 5c). The similar result was observed at the 8th day after training (Fig. 5c). Furthermore, we checked the density of dendrite spines around the injection site. The number of PSD95+ punctures was significantly increased by 167 ± 24% in animals transplanted with LiCl-treated NSCs, compared to animals injected with untreated NSCs. This further supported the improvement of learning under the pretreatment of LiCl. Collectively, these data showed that transplantation with LiCl-treated NSCs into the PD models could improve the learning and memory capacity.
Discussion
In the current study, we elucidated the role of Lithium in the proliferation and differentiation of NSCs both in vivo and in vitro. More importantly, the LiCl-treated NSCs enhanced the generation of dopaminergic neurons when transplanted into the PD rat models. These data indicated the potential application of Lithium in the stem cell-based therapy for PD and other neurodegenerative diseases.
Lithium is a mood stabilizer, widely used in the treatment of bipolar disorders. Studies indicated that Lithium treatment prevents neurocognitive deficit (Yazlovitskaya et al. 2006), reduced neural progenitor apoptosis in the hippocampus (Huo et al. 2012), and increases proliferation of hippocampal neural stem/progenitor cells (Zanni et al. 2015). These preclinical data proved that chronic lithium treatment has the capacity to protect hippocampal progenitors from the neurodegeneration through its pro-neurogenic and anti-apoptotic effects. This may explain the results in this study that LiCl could promote the proliferation of NSCs cultures in vitro and in PD model animals after transplantation. The other interesting finding in this work was that LiCl treatment enhanced the differentiation of NSCs. Several studies showed the LiCl plays a role in the differentiation of stem cells, including the odontoblast differentiation of hair follicle neural crest cells (Shan et al. 2015), and differentiation of mesenchymal stem cells to neural cells (Dong et al. 2015). However, limited studies disclosed the correlation between Lithium and differentiation of NSCs. Based on the above-mentioned studies, this work provided supplementary data showing the effect of LiCl on the neuronal differentiation of NSCs. Furthermore, previous work elucidating the neuroprotective effect of Lithium against rotenone-induced injuries in dopaminergic cells provided a clue to predict the role of Lithium in differentiation of NSCs, especially towards dopaminergic cells (Hou et al. 2015). This is consistent with our findings that LiCl-treated NSCs could promote the dopaminergic differentiation in in vitro NSC cultures when co-stimulated with other growth factors (Fig. 3) as well as in PD rat models in vivo after having been transplanted with LiCl-treated NSCs (Fig. 4e–h), indicating the potential clinical application of Lithium in the PD therapy. More importantly, behavior studies showed that injection of LiCl-treated NSCs substantially recover the motor function in PD model animals (Fig. 5), further validating the beneficial effect of LiCl in the recovery of physiological activity. In summary, this study reported a method to simply and efficiently obtain the specific subtypes of neurons from NSCs.
NSCs can differentiate into three neural cell types: neurons, astrocytes and oligodendrocytes (Ming and Song 2011). Microenvironments and signals from extracellular changes (such as immune signals) are important to the skewed differentiation of NSCs to neurons or glias (Molyneaux et al. 2003). In our study, the LiCl-treated NSCs were skewed to differentiate into domapinergic neurons. This is probably due to the secretion of different cytokines and alternation of nearby microenvironment around the infected cells. However, this hypothesis need to be further validated. Furthermore, evaluation of cell survival and function after transplantation is an important factor to determine the clinical effect. However, there is no clear judgment to show the preferential effect of skewed differentiation to neurons or glia since both cell types were applied in transplantation therapy (Quan et al. 2016). As to the recovery of motor function, glial cells transplantation had more beneficial effects compared to the transplantation of neuronal cells according to the above mentioned study (Quan et al. 2016). The future work will elucidate the difference between transplantation of neuronal and glial cells generated from LiCl-treated NSCs.
Until now, there are few studies reporting the underlying mechanism of the effect of Lithium on cell differentiation. One of the well-characterized pathways is Wnt/β-catenin signalling pathway. According to the previous reports, LiCl inhibits GSK-3β, leads to β-catenin stabilization and nuclear translocation and then activates the Wnt/β-catenin signalling pathway (Silva et al. 2010). β-catenin stabilization influences cell differentiation and proliferation, promotes mitotic activity through upregulation of Cyclin D1, a key regulator of cell cycle entry (Liu et al. 2007). Since the conclusion is consistent with our data shown in Fig. 1e, we proposed that this is the possible explanation why LiCl could affect the proliferation and differentiation of NSCs. Another important mechanism for the effect of LiCl is autophagy. LiCl could modulate the autophagy in various neuropsychiatric diseases (Motoi et al. 2014). This is associated with the mammalian target of rapamycin (mTOR)-independent pathway and myo-inositol-1,4,5-trisphosphate (IP3) pathway. The autophagy-enhancing agents like LiCl are able to reduce mitochondrial load (Giordano et al. 2014). All these mechanism studies were not conducted in the current work, and will be the focus in the future study.
Compliance with ethical standards
Conflict of interest
We declared that there is no conflict of interest.
References
- Cabrera O, Dougherty J, Singh S, Swiney BS, Farber NB, Noguchi KK. Lithium protects against glucocorticoid induced neural progenitor cell apoptosis in the developing cerebellum. Brain Res. 2014;1545:54–63. doi: 10.1016/j.brainres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Zhang Z, Ma J, Xia H, Wang Y, Liu Y, Ma Q, Sun T, Liu J. Directed differentiation of postnatal hippocampal neural stem cells generates nuclear receptor related1 protein and tyrosine hydroxylaseexpressing cells. Mol Med Rep. 2016;14:1993–1999. doi: 10.3892/mmr.2016.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong BT, Tu GJ, Han YX, Chen Y. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8:2473–2483. [PMC free article] [PubMed] [Google Scholar]
- Ferensztajn-Rochowiak E, Rybakowski JK. The effect of lithium on hematopoietic, mesenchymal and neural stem cells. Pharmacol Rep. 2016;68:224–230. doi: 10.1016/j.pharep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Giordano S, Darley-Usmar V, Zhang JH. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014;2:82–90. doi: 10.1016/j.redox.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Xiong N, Liu L, Huang J, Han C, Zhang G, Li J, Xu X, Lin Z, Wang T. Lithium protects dopaminergic cells from rotenone toxicity via autophagy enhancement. BMC Neurosci. 2015;16:82. doi: 10.1186/s12868-015-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo K, Sun Y, Li H, Du X, Wang X, Karlsson N, Zhu C, Blomgren K. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol Cell Neurosci. 2012;51:32–42. doi: 10.1016/j.mcn.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Keranen T, Jarvinen H, Laitinen K. Medication information needs of patients with Parkinson’s disease. Mov Disord. 2015;30:S96–S96. [Google Scholar]
- Lee JE, Lim MS, Park JH, Park CH, Koh HC. PTEN promotes dopaminergic neuronal differentiation through regulation of ERK-dependent inhibition of S6K signaling in human neural stem cells. Stem Cells Transl Med. 2016;5:1319–1329. doi: 10.5966/sctm.2015-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Lim MS, Park JH, Park CH, Koh HC. S6K promotes dopaminergic neuronal differentiation through PI3K/Akt/mTOR-dependent signaling pathways in human neural stem cells. Mol Neurobiol. 2016;53:3771–3782. doi: 10.1007/s12035-015-9325-9. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Adult neural stem cells stake their ground. Trends Neurosci. 2014;37:563–571. doi: 10.1016/j.tins.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Yu HMI, Hsu W. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of beta-catenin in proliferation and differentiation. Dev Biol. 2007;301:298–308. doi: 10.1016/j.ydbio.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre D, Massaad C, Grenier J. Lithium chloride stimulates PLP and MBP expression in oligodendrocytes via Wnt/beta-catenin and Akt/CREB pathways. Neuroscience. 2015;284:962–971. doi: 10.1016/j.neuroscience.2014.10.064. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O’Brien W, Raz E, Littman D, Wylie C, et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279–4286. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Motoi Y, Shimada K, Ishiguro K, Hattori N. Lithium and autophagy. ACS Chem Neurosci. 2014;5:434–442. doi: 10.1021/cn500056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Chen J, Zhong Y, Ren WZ. Comparative effect of immature neuronal or glial cell transplantation on motor functional recovery following experimental traumatic brain injury in rats. Exp Ther Med. 2016;12:1671–1680. doi: 10.3892/etm.2016.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Zhou C, Yang R, Yan F, Zhang P, Fu Y, Jiang H. Lithium chloride promotes the odontoblast differentiation of hair follicle neural crest cells by activating Wnt/beta-catenin signaling. Cell Biol Int. 2015;39:35–43. doi: 10.1002/cbin.10340. [DOI] [PubMed] [Google Scholar]
- Silva AK, Yi H, Hayes SH, Seigel GM, Hackam AS. Lithium chloride regulates the proliferation of stem-like cells in retinoblastoma cell lines: a potential role for the canonical Wnt signaling pathway. Mol Vis. 2010;16:36–45. [PMC free article] [PubMed] [Google Scholar]
- Tafreshi AP, Sylvain A, Sun GZ, Herszfeld D, Schulze K, Bernard CCA. Lithium chloride improves the efficiency of induced pluripotent stem cell-derived neurospheres. Biol Chem. 2015;396:923–928. doi: 10.1515/hsz-2014-0261. [DOI] [PubMed] [Google Scholar]
- Tang L, Chen Y, Pei F, Zhang H. Lithium chloride modulates adipogenesis and osteogenesis of human bone marrow-derived mesenchymal stem cells. Cell Physiol Biochem. 2015;37:143–152. doi: 10.1159/000430340. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, Jr, Boone B, Shinohara ET, Hallahan DE. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Gu ST, Huang H, Wen TQ. Combination of bFGF, heparin and laminin induce the generation of dopaminergic neurons from rat neural stem cells both in vitro and in vivo. J Neurol Sci. 2007;255:81–86. doi: 10.1016/j.jns.2007.01.076. [DOI] [PubMed] [Google Scholar]
- Zanni G, Di Martino E, Omelyanenko A, Andang M, Delle U, Elmroth K, Blomgren K. Lithium increases proliferation of hippocampal neural stem/progenitor cells and rescues irradiation-induced cell cycle arrest in vitro. Oncotarget. 2015;6:37083–37097. doi: 10.18632/oncotarget.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Yin J, Guan J, Hu B, Niu X, Jin D, Wang Y, Zhang C. Lithium stimulates human bone marrow derived mesenchymal stem cell proliferation through GSK-3beta-dependent beta-catenin/Wnt pathway activation. FEBS J. 2014;281:5371–5389. doi: 10.1111/febs.13081. [DOI] [PubMed] [Google Scholar]
- Zhu B, Caldwell M, Song B. Development of stem cell-based therapies for Parkinson’s disease. Int J Neurosci. 2016;126:955–962. doi: 10.3109/00207454.2016.1148034. [DOI] [PubMed] [Google Scholar]