Abstract
Two dimensional (2D) cell culture systems lack the ability to mimic in vivo conditions resulting in limitations for preclinical cell-based drug and toxicity screening assays and modelling tumor biology. Alternatively, 3D cell culture systems mimic the specificity of native tissue with better physiological integrity. In this regard, microfluidic chips have gained wide applicability for in vitro 3D cancer cell studies. The aim of this research was to develop a 3D biomimetic model comprising culture of breast cancer cells in butterfly-shaped microchip to determine the cytotoxicity of carnosic acid and doxorubicin on both estrogen dependent (MCF-7) and independent (MDA-MB231) breast cancer cells along with healthy mammary epithelial cells (MCF-10A) in 2D, 3D Matrigel™ and butterfly-shaped microchip environment. According to the developed mimetic model, carnosic acid exhibited a higher cytotoxicity towards MDA-MB 231, while doxorubicin was more effective against MCF-7. Although the cell viabilities were higher in comparison to 2D and 3D cell culture systems, the responses of the investigated molecules were different in the microchips based on the molecular weight and structural complexity indicating the importance of biomimicry in a physiologically relevant matrix.
Keywords: Microfluidics, Microchip, Breast cancer, Carnosic acid, Doxorubicin
Introduction
Microfluidic platforms provide physiologically relevant settings, offer more realistic microenvironment for facilitating the study of cell invasion and chemotaxis (Ozdil et al. 2014; Håkanson et al. 2014; Yildiz-Ozturk and Yesil-Celiktas 2015) and are promising approaches to three-dimensional (3D) cell culture which can reveal improved biological models and functionality while reducing the required volumes of cell culture medium and thus reducing the cost of the experimental design (Sung and Beebe 2014; Thoma et al. 2014). The tumor microenvironment plays a critical role in driving cancer progression and the tumor progresses with its microenvironment to drive invasion and metastasis. As microfluidic platforms could be easily adapted for parallel testing of the potential efficacy of chemotherapeutic agents, the applicability of 3D in vitro microfluidic platforms becomes more attractive (Hockemeyer et al. 2014; Wu et al. 2014).
New assays are designed for culturing multiple different cell lines and/or tissue samples under controlled user-defined conditions by microfluidic culture platforms where integration of more than two cell types with biological 3D gels and controlled conditions will allow for improved organotypic culture models. In order to provide 3D structures, hydrogels are used to cell trapping, embedment and encapsulation (Elliott and Yuan 2011). An adequate confinement of biopolymeric matrices in microfluidic devices provides the possibility of controlling cell microenvironments in 3D subjected to a constant gradient of biomolecular signals (Baudoin et al. 2011). For that, tumor extracts rich in extracellular matrix proteins are used to mimic extra cellular matrix (ECM) and support cell differentiation in microfluidics systems (Dolega et al. 2015). Matrigel™ (BD Biosciences), a basement membrane matrix, is the well-known commercial product that has a complex mixture of multiple proteins and associated molecules found in ECM. It is the trade name for gelatinous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells and consists of several common ECM proteins including laminin, collagen and entactin, as well as various growth factors (Knight and Przyborski 2015). Gel-partitioned microfluidic systems enable patterning cells within 3D matrices, and tuning their environment through controlled addition/removal of soluble factors. This technique provides controlled spatial distribution of cells within 3D matrices and application of gradients of soluble factors across the Matrigel™. These properties make this technique a powerful and versatile tool for modeling in vivo cellular microenvironments (Wong et al. 2008).
Studies indicate that purified components from rosemary such as carnosic acid and its extract display significant growth inhibitory activity on a variety of human cancers, especially human breast cancers (Yesil-Celiktas et al. 2010; Omri et al. 2012). For breast cancer, 3D heterotypic cell culture models that are more representative of the different types of primary breast tumors and the metastatic settings are required especially for investigating the structural and stromal microenvironment and drug response (Weigelt et al. 2014). In vitro disease models as used in microfluidic devices were comprehensively reviewed in the literature (Ghaemmaghami et al. 2012; Kim et al. 2014; Sung and Beebe 2014). Modelling cancer in 3D systems attracts great attendance due to the lack of well-defined disease models especially to study metastasis and angiogenesis. Breast cancer is often modeled using multicellular tumor spheroids by using extracellular matrix components like Matrigel™ to obtain 3D cellular masses. In addition to this, microfluidic devices were used to detect circulating tumor cells by using several markers especially for early breast cancer detection (He et al. 2009).
Within the scope of this study, a breast cancer cell model was developed to investigate the effect of carnosic acid towards estrogen dependent and independent human breast cancer cells (MCF-7 and MDA-MB231) and healthy mammary epithelial cells (MCF-10) along with the invasion and migration of cells determined in 96 well plates as 2D cell culture system, Matrigel™ formed 3D culture system and butterfly-shaped microfluidic chips.
Materials and methods
Chemicals and reagents
Carnosic acid (93%) (A7781) was purchased from A.G. Scientific (San Diego, CA, USA). Doxorubicin hydrochloride was purchased from Bedford Laboratories TM (Bedford, OH, USA). MCF-7, MDA-MB-231 and MCF-10A cells were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). The following reagents and chemicals were obtained from the respective suppliers: Roswell Park Memorial Institute 1640 (RPMI 1640) medium, Leibovitz’s L-15 medium, Dulbecco’s modified Eagle’s medium/Nutrient Mixture Ham’s F12 (DMEM/F12), fetal bovine serum (FBS), l-glutamine, horse serum, streptomycin, and penicillin (Biochrom, Berlin, Germany); epidermal growth factor (EGF) (E9644), hydrocortisone (H0888) insulin (SI9278), lectin (Phytohemagglutinin PHA-M) (L8902) (Sigma, St. Louis, MO, USA).
Preparation of carnosic acid solution
Carnosic acid (93%) (A7781) was dissolved in methanol to a final concentration of 1 μg/μL and diluted in cell culture medium. Subsequent dilutions were made in appropriate cell culture medium (40 μg/mL; 20 μg/mL; 10 μg/mL; 5 μg/mL; 2.5 μg/mL) to determine the inhibitory concentration (IC) values.
Cell culture
Breast cancer cells, MCF-7 breast cancer adenocarcinoma, estrogen receptor dependent, and MDA-MB 231, breast cancer adenocarcinoma estrogen receptor independent, were used. As a control, MCF-10A, a non-cancerous mammary epithelial breast cell line was used. MCF-7 cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine; MDA-MB 231 cells were cultured in Leibovitz’s L-15 medium; supplemented with 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. MCF-10A cells were cultured in DMEM/F12 medium supplemented with horse serum 5%, 20 ng/mL EGF, 0.5 µg/mL hydrocortisone, 10 µg/mL insulin, 100 ng/mL lectin, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine; MDA-MB 231 cells were cultured in a humidified incubator with atmospheric CO2, MCF-7 and MCF-10A cells were cultured in a humidified incubator with 5% CO2 at 37 °C. All cell lines were routinely tested for contamination by mycoplasma on regular basis.
Cells in exponential growth phase of cancer and healthy breast cancer cells were counted by using Trypan blue in microscope (Axio Vert.A1, Carl Zeiss, Oberkochen, Germany). In order to make a comparison 6 × 103 cells/well were injected into both the 96 well plate and butterfly-shaped microfluidic chip in respect to the effect of applied drug on the cells. The seeded 96 well plate and microfluidic chip were maintained at 37 °C, 5% CO2 in a fully humidified incubator overnight for proper cell attachment and spreading.
The butterfly-shaped microchip
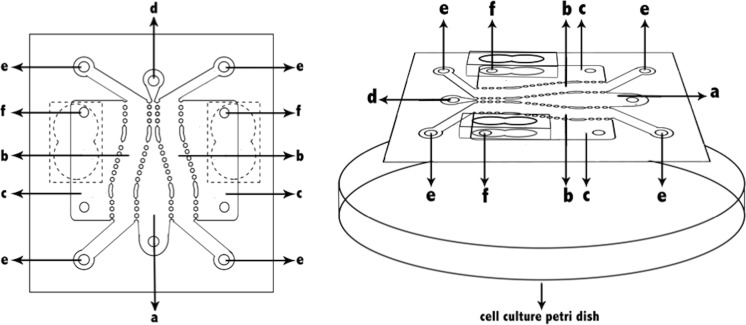
An image of the butterfly-shaped microchip is shown in Fig. 1. The microchip was fabricated by polymerizing PDMS on silicon prepared with photolithography (UV lithography) techniques. The optically transparent surface and the structure was bonded using UV treatment (Ozdil et al. 2014). The butterfly microchips used in this study were supplied from Izmir Institute of Technology (Pesen-Okvur 2015). The structure on the optically transparent glass surface of the microchip is polydimethylsiloxane (PDMS). The butterfly-shaped microchip (Fig. 1) consists of one main channel (a), two test channels (b) separated by gaps. The width of the main channel can be constant at different regions and increases in steps along its length in one direction and in the middle of the test channels (b), two fluid reservoirs (c) each neighboring a test channel (b), two main channel openings (d), four test channel openings (e), and four fluid reservoir openings (f).
Fig. 1.
Design of the butterfly-shaped microchip. The parts of microchip a main channel, b test channel, c fluid reservoir, d main channel opening, e test channel opening, f fluid channel opening
The width of the main channel is between 1 and 3.5 mm, whereas the widths of the test channels are 1 and 2.5 mm. The main channel (a), test channels (b) and fluid reservoirs (f) of the microchip consist of openings (d, e, f) that allow for loading of cell culture medium, physiological buffer solution, one or more biological molecule or chemical, cell loaded matrix, cell free matrix or a combination and allow the exit of air or previously loaded material. Fluid reservoirs consist of a single wide fluid reservoir opening which can be as large as the fluid reservoir allowing the fluids to be easily changed.
In order to provide 3D cell culture microenvironment, different matrices such as matrigel, collagen, laminin, agarose, polyacrylamide can be used in the main channel, test channels and fluid reservoirs separated from each other by arrays of gaps. Incorporating existing arrays of gaps instead of continuous walls allow factors in a channel or fluid reservoirs to pass into other channels.
The cell culture assays in butterfly-shaped microchip were carried out in the following order. Firstly, carnosic acid in DMEM cell culture medium was loaded to the main channel. Then healthy mammary epithelial cells embedded in matrigel were loaded into the test channel on one side of the main channel, breast cancer cells embedded in matrigel were loaded in the other test channel. Lastly, culture medium was loaded into the fluid reservoirs.
Dose-dependent cytotoxic activity in 2D culture systems
The cytotoxic activity of carnosic acid on MCF-7 and MDA-MB 231 cell lines were determined by MTT assay in 2D culture systems on 96 well plates. Briefly, cells in exponential growth phase were counted and 6 × 103 cells/well were cultured in 96 well plates overnight. After that, carnosic acid at different concentrations from 40 to 2.5 μg/mL was added for 24 and 48 h. To determine the effect of methanol on cells, 0.1% methanol was used and, in addition, as a positive control for the inhibition of cells doxorubicin (Adriamycin; Bedford Laboratories), a chemotherapy reagent, was used. Weber et al. (2013) investigated interaction of the chemotherapeutic drug doxorubicin in MCF-7 human breast cancer cells and a 2 μM (12 μg/mL) doxorubicin was used as a control for cell inhibition studies. So, doxorubicin was used at 2 μM dose for all cell lines in order to compare cytotoxicity of carnosic acid.
Cell proliferation after 48 h was determined by MTT assay. Briefly, the cell culture medium was removed and 0.5 mg/mL MTT solution was added to each well which was diluted in fresh cell culture medium and incubated 4 h at 37 °C, 5% CO2 in a humidified cell culture incubator. After that, the medium was removed and DMSO was added to dissolve blue formazan crystals and the quantities of blue formazan product was determined by a microplate reader at 570–690 nm (SpectraMax 190, Molecular Devices, Sunnyvale, CA, USA). For the cells, strong correlations between numbers of cells present and amounts of MTT formazan product were observed. The data were obtained from three independent assays, using three wells for each assay. Cytotoxicity was determined according to percent cell viability. Percentage survival was determined as per the formula (absorbance of drug-treated cells/absorbance of control cells) ×100 and compared with control, untreated cells regarded as 100%.
Three dimensional (3D) culture systems in microchips and drug administration
The operation of the butterfly-shaped microfluidic chip for drug-induced apoptosis and anti-proliferation included cell seeding, cell culture, drug stimulation, cell cytotoxicity assay and microscopic examination. Prior to cell culture, the channels of the microfluidic chips were sequentially washed with 70% ethanol and ultrapure water and then baked at 80 °C overnight to restore hydrophobicity for preventing the hydrogel leakage from the PDMS microfluidic device (Ozdil et al. 2014). Combinations of estrogen dependent breast cancer cell line (MCF-7) and healthy mammary epithelial cells (MCF-10A) and estrogen independent breast cancer cell line (MDA-MB 231) and healthy mammary epithelial cells (MCF-10A) were cultured in either channel of butterfly shaped microchips for carnosic acid and doxorubicin administrations. In order to mimic 3D culture, Matrigel™ (BD Biosciences, San Jose, CA, USA) was used which in turn created some challenges as it is a biological product indicating that it may vary between lots and optimization was required for 3D staining as well as extracting cells from 3D matrices (Lee et al. 2007). There are several techniques to get to a 3D culture system. In this study, cells in exponential growth phase were counted and after mixing with Matrigel™ at a ratio of 1:1 (v:v), 103 cells/microreactor channel were cultured in both right and left butterfly-shaped microchannels (Fig. 1). Then, carnosic acid (93%) (A7781) and doxorubicin were added to the central channel of the microchips in order to investigate the effect on breast cancer and healthy epithelial cells. Subsequently, four microchips were placed in a fully humidified incubator at 37 °C, 5% CO2 and 95% room air. After 24 and 48 h incubation periods, all cells were visualized by microscopy in order to observe cell membranes and migration of cells towards applied drug.
Cell count in butterfly-shaped microchip
After carnosic acid and doxorubicin administration in butterfly-shaped microchips, cell count and calculation of microscopic image area were carried out by using ImageJ program in order to investigate the effect of administered drugs towards breast cancer and healthy mammary epithelial cells. ImageJ is an open source image processing program for multidimensional image data with a focus on scientific imaging, where cells that have healthy membrane structures were counted. The area of microscope image was calculated with straight line tool in ImageJ program.
Statistics
Statistical analysis was performed with Prism 5.0 (Graphpad, San Diego, CA, USA). An unpaired Student’s t test was used to determine the significance of the results comparing the experimental groups, p values <0.05 were considered statistically significant. Data are presented as mean values ±SEM (standard error of the mean).
Results and discussion
Inhibitory effects of carnosic acid in 2D and 3D cell culture systems
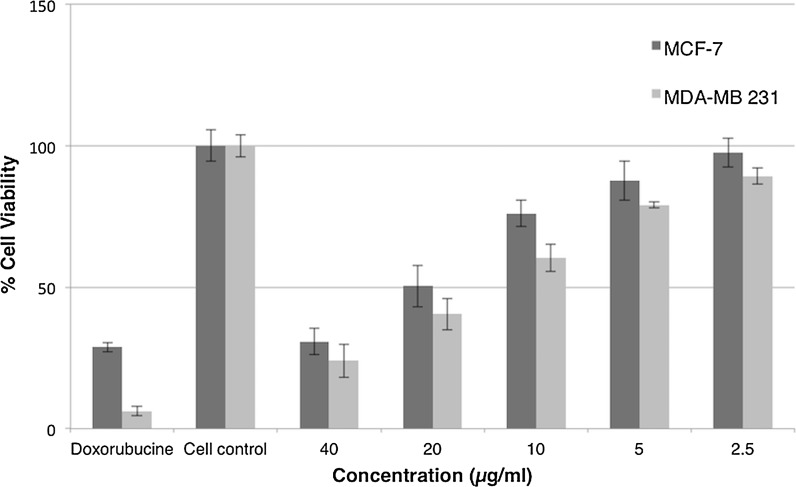
Considering chemotherapy reagent doxorubicin, the effect of carnosic acid on cell viability of estrogen dependent and independent human breast cancer cells (MCF-7 and MDA-MB231) and healthy mammary epithelial cells (MCF-10A) was assessed by MTT assay. Principally dose-dependent cytotoxicity assay was carried out in 2D cell culture systems on 96 well plates. In order to determine IC50 values, MCF-7 and MDA-MB-231 cancer cell lines were treated with doses ranging between 2.5 and 40 μg/mL for 48 h of treatments (Fig. 2). Based on the results, IC50 values of carnosic acid were 8.5 and 14.02 µg/mL for MDA-MB 231 and MCF-7 cell lines, respectively, which indicates the selectivity of carnosic acid for estrogen independent breast cancer cells. Since we have not anticipated drug dose that is required for inhibition of cells in 3D cell culture systems in matrigel doped butterfly-shaped microchips, we have decided to apply a 30–40% higher dose in 3D cell culture system in compliance with the determined IC50 values in 2D cell culture system due to the fact that 3D cell culture environment would favor tumor progression and therefore cancer cells would become more resistant to drug molecules. Consequently, the concentrations of carnosic acid were used as 15 µg/mL (45 µM) and 20 µg/mL (60 µM) in microchips for MDA-MB 231 and MCF-7, MCF-10A cell lines, respectively.
Fig. 2.
Dose-dependent cytotoxic activity of carnosic acid on estrogen dependent (MCF-7) and independent breast cancer (MDA-MB 231). Each cell type was incubated with carnosic acid for 48 h at 37 °C, and subjected to MTT assays to measure % cell viability. The data were obtained from three independent assays using three wells for each assay
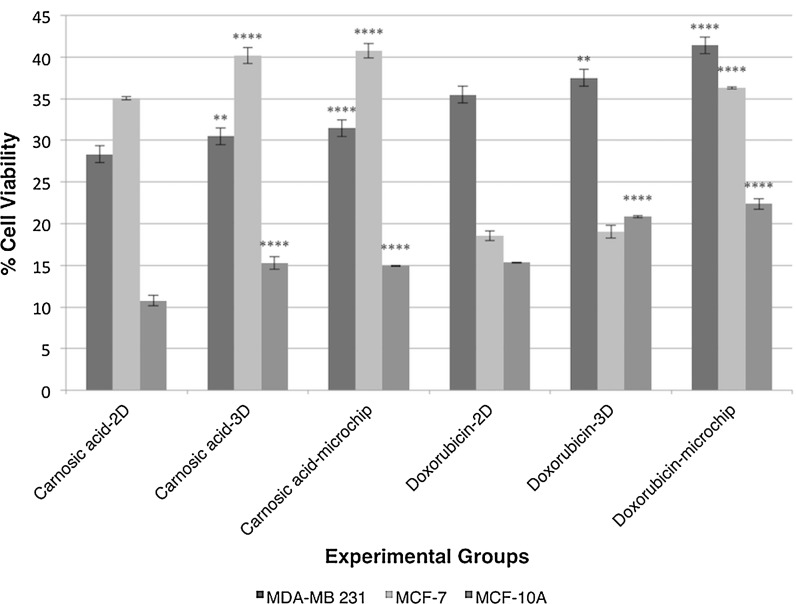
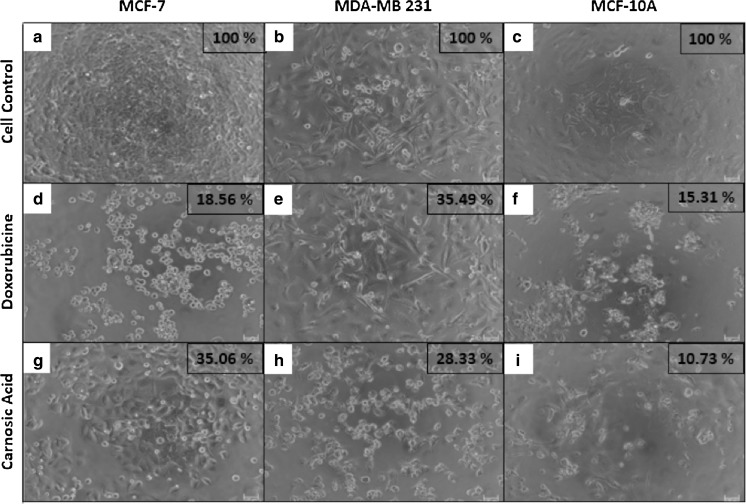
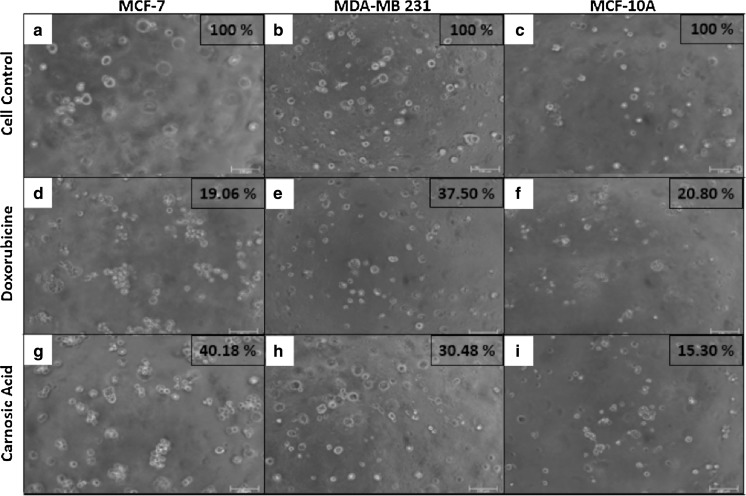
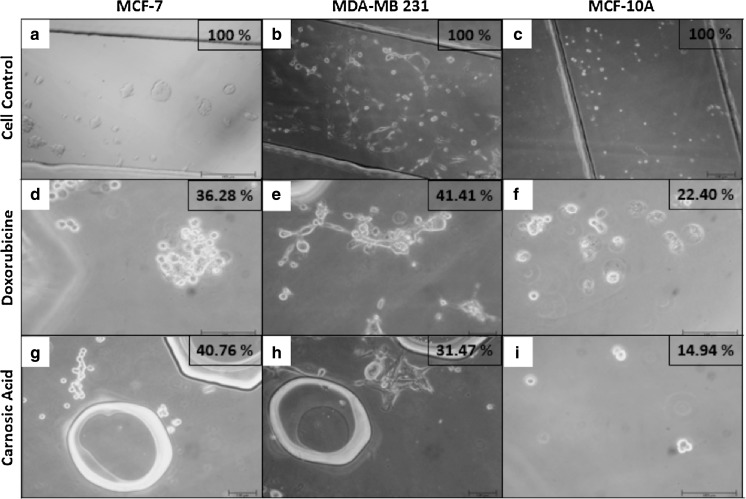
Cell viability values of breast cancer and healthy mammary cell lines are depicted in Fig. 3 for carnosic acid and doxorubicin administrations in 2D, 3D and microfluidic cell cultures. Furthermore, all cell groups cultured in 2D, 3D and butterfly-shaped microchip with Matrigel were observed using an inverted phase-contrast microscope and representative micrographs showing cell morphologies in each group with cell viability results determined by MTT assay and cell count in butterfly-shaped microchip are presented in Figs. 4, 5, 6. Images were taken in order to compare control groups (Fig. 4a–c) with those of drug administered groups. After treating the cells with chemotherapeutic agent doxorubicin in 2D culture system, MCF-7 and MCF-10A cells showed similar behavior as their sizes diminished and displayed an aggregate like phenotype with cell viabilities of 18.56 and 15.31%, respectively (Fig. 4d, f), however the viability of MDA-MB 231 cells treated with doxorubicin was 35.49% in 2D culture systems (Fig. 4e). On the other hand, after treating the cells with carnosic acid, cell viabilities were 28.33, 35.06 and 10.73% for MDA-MB 231, MCF-7 and MCF-10A, respectively in 2D culture systems (Fig. 4g–i). Likewise, after doxorubicin treatment in 3D culture system, similar trends were observed in 2D culture systems as apoptotic cell death was started in MCF-7 and MCF-10A cells with cell viabilities of 19.06 and 20.80%, respectively (Fig. 5d, f), whereas the viability of MDA-MB 231 cells was 37.50% in 3D culture systems (Fig. 5e). Similar observations were reported for cisplatin induced cytotoxicity for 2D and 3D cultured MCF-7 cells (Ravi et al. 2016). As for matrigel doped butterfly-shaped microchip, the cell viabilities were 41.41, 36.28 and 22.40% after treating the MDA-MB 231, MCF-7 and MCF-10A cells with doxorubicin, whereas cell viabilities were 31.47, 40.76 and 14.94%, respectively, after carnosic acid treatment. Generally the cell viabilities were higher in microchips compared to 2D and 3D cell culture systems due to the resistance generated by the cells against the applied drug molecule.
Fig. 3.
Cell viability values of breast cancer and healthy breast cell lines for carnosic acid and doxorubicin administrations in 2D, 3D and microfluidic cell cultures. Significant differences between 2D cell culture groups and the experimental groups are represented in the graph as two asterisks, p < 0.01; four asterisks, p < 0.0001
Fig. 4.
Images of doxorubucin and carnosic acid treated cells in 96 well plates after 48 h and cell viability rates determined by MTT assay (Scale bar 100 µm)
Fig. 5.
Images of doxorubucin and carnosic acid treated cells in 3D Matrigel™ coated 96 well plates after 48 h and cell viability rates determined by MTT assay (Scale bar 100 µm)
Fig. 6.
Images of cross sectional area of butterfly-shaped microchip after treatment of doxorubicin and carnosic acid within 48 h (Scale bar 100 µm)
Overall, the results indicated that all the cells exhibited higher viabilities after doxorubicin and carnosic acid treatments in 3D culture system compared to the 2D system, indicating the importance of mimicry in a physiologically relevant matrix. Indeed, cell attachment occurs on one side of the cell in 2D cell culture systems, whereas cell attachment can be observed around the entire surface of the cell in 3D cell culture systems (Baker and Chen 2012). For all cell lines, carnosic acid and doxorubicin treatments in 2D versus 3D and butterfly shaped microchips significantly lowered the cell viabilities except for MCF-7 cells where no significant difference was observed for doxorubicin treatments between 2D and 3D culture systems (p > 0.05) and also no difference for carnosic acid treatments between 3D culture system and microchip (p > 0.05). Furthermore, cell viabilities after doxorubicin treatment in butterfly shaped microchips were significantly higher than in 2D culture systems for MDA-MB 231, MCF-7 (p < 0.0001) and MCF-10A (p < 0.05) (Fig. 3). The principle differences in the microenvironment of 2D and 3D cell culture systems affected a variety of cellular behaviors such as cell morphology, polarity, gene and protein expression, along with attachment, viability, proliferation, migration, differentiation, response to stimuli, cellular metabolism and overall function (Antoni et al. 2015; Hickman et al. 2014). Controlling large number of cells and real time observations are more convenient in 2D cell culture systems, whereas 3D culture conditions can better mimic the real cellular environment. The development of cell patterning, 2D and 3D cell culture systems, gradient generation, integration and automation continue to advance, but the generation of microenvironments representing the complex and diverse range of conditions encountered in living systems remains as a challenge that requires extensive further research (Tehranirokh et al. 2013).
Morphologies of cells in butterfly shaped microchips
Microfluidic chips have ease of integration into a single device and the potential for parallel processing makes it ideal for drug discovery (Yesil-Celiktas 2014). In regards to elicite potential candidates for cancer treatment, these platforms provide valuable information from cancer cells in response to various drugs with minimal sample amounts and less time. There are a variety of microfluidic chip designs used for developing 3D breast cancer models and in one of these research studies, Saadi et al. (2006) developed a parallel-gradient microfluidic chemotaxis chamber that can generate soluble gradients of epidermal growth factor side by side in order to examine the migration of human metastatic breast cancer cell line MDA-MB-231 in various conditions. Komen et al. (2008) developed two microfluidic devices made of PDMS to determine the chemosensitiviy of estrogen dependent MCF-7 cells in response to drug staurosporine. Another microfluidic device was developed by Song et al. (2010) consisting of flow channels and eight circular PDMS chambers for evaluation of apoptosis and proliferation of MCF-7 breast cancer cells treated with mitomycin C and tamoxifen. In another study, Hwang et al. (2013) introduced the multilevel microfluidic device as a new platform for micro 3D cell culture in order to test the effect of estrogen receptor antagonist drug, tamoxifen, on the growth of ER positive MCF-7 cells. Continuing with various designs, Zheng et al. (2014) constructed a microchip with Y-shaped PDMS channel in order to generated chemical gradient of epidermal growth factor to induce the chemotaxis of MDA-MB 231 cells, whereas the device designed by Patra et al. (2014) consisted of two PDMS layers in order to culture spheroids of human umbilical vein endothelial cells within the spheroids of controlled sizes and suitable for live cell imaging and provide ideal disease model for drug screening and fundamental studies. In another study, Rios-Mondragon et al. (2012) designed a microfluidic device with three 300 µm-wide channels in order to investigate the interaction of tamoxifen-treated human breast cancer MCF-7 cells with their neighboring counterparts by exploiting laminar flow patterning for selective drug delivery. Furthermore, Thierry et al. (2010) developed PDMS based disposable microfluidic device for capturing circulating tumor cells, where Herceptin was covalently immobilized for detection of cells with a high efficiency in overexpressing HER2 protein (human epidermal growth factor receptor 2), thereby developing a fast and reliable methodology to determine HER2 status for breast cancer patients with metastases.
In our study, we have utilized a butterfly-shaped microchip consisting of a central channel in which carnosic acid was fed, two channels at both sides of the central channel for feeding cells with matrigel and two reservoirs at both sides of the cell channels in which nutrient medium was fed in order to investigate the cytotoxic effect of carnosic acid and doxorubicin towards MDA-MB 231 and MCF-7 and healthy mammary epithelial cell line, MCF-10A. The possible chemoattractant properties of carnosic acid and doxorubicin were of interest as well, where a chemical concentration gradient was generated by addition of these compounds. Breast cancer cells and healthy mammary epithelial cells migrated towards the central chamber in the presence of carnosic acid (Fig. 6g–i), whereas they moved away from the central chamber in the presence of doxorubicin (Fig. 6d–f). The butterfly-shaped microchip consists of one main channel (a), two test channels (b) separated by gaps (Fig. 1). There are two channels at both sides of the central channel for feeding cells with matrigel and two reservoirs at both sides of the cell channels in which nutrient medium was fed. We had observed that more cells got near to the gaps of main central channel in the presence of carnosic acid during microscopic examination. Contrary to these, in the presence of doxorubicin in the central chamber, cancer and healthy cells come into existence in the middle of the test channels that cell lines fed with matrigel or away from the gaps of main central channel. As a consequence, these results demonstrate that carnosic acid has a chemotactic ability within the generated concentration gradient fields. The concentration gradient of biomolecules results in responses such as directed cell migration, which is a key biological process involved in a wide range of physiological and pathological phenomena (Kunze et al. 2014) and particularly migration towards a soluble chemoattractant is usually defined as chemotaxis which can have a significant impact on developmental and regenerative processes (Moreno-Arotzena et al. 2014).
Apart from reporting chemotactic ability of carnosic acid, another significant issue was the differences observed in responses from the microchips based on the chemical structure and complexity of the molecules of interest. Carnosic acid has a typical o-diphenol and is easily oxidized (Masuda et al. 2001). While carnosic acid (C20H28O4) consists of natural benzenediol abietane diterpene, doxorubicin (C27H29NO11) has a tetracyclic ring with the sugar daunosamine attached by a glycosidic linkage. According to the chemical structures, doxorubicin is more complex and has a higher molecular weight in comparison to carnosic acid. In this regard, it was hypothesized that the molecule with a simpler chemical structure and lower molecular weight diffuses more easily to the cells, exhibiting a significant influence and a higher inhibition capability both in 3D culture system and the microchip. Indeed, cytotoxicities of carnosic acid were similar in 3D culture system and microchip considering three of the cell lines. However, the responses of these three cell lines in microchips were different in the presence of doxorubicin supporting our hypothesis where contribution of diffusion phenomena is more realistically mimicked in the microfluidic systems when dealing with high molecular weight and complex structured compounds.
Conclusion
The studies in recent years demonstrated that traditional 2D cell culture systems may provide inaccurate data regarding the predicted response of cancer cells to chemotherapeutics and not mimic the 3D cell microenvironments. Therefore, there is a considerable interest in developing 3D in vitro microfluidic cell culture platforms to provide biomimetic microenvironments by including hydrogels and generating chemical gradients that direct cellular processes such as cell migration. This research study focused on the investigation of the cytotoxic effect of carnosic acid and doxorubicin on breast cancer and healthy mammary epithelial cells using microfluidic chips along with 2D and 3D cell culture systems. Overall, carnosic acid exhibited a higher cytotoxicity towards estrogen independent breast cancer cell line MDA-MB 231, while doxorubicin was more effective against estrogen dependent counterpart, MCF-7. According to the developed mimetic model, the cell viabilities were higher in comparison to 2D and 3D cell culture systems but the responses of the investigated molecules were different in the microchips based on the molecular weight and structural complexity. Application of such mimetic models will contribute to advancing basic research and increasing the predictive accuracy of potential drug molecules which may accelerate the translation of novel therapeutics to the clinic, possibly decreasing the use of animal models.
Acknowledgements
Access to the Animal Cell and Tissue Engineering and Novel Fluidic Technologies Laboratories of Ege University, Department of Bioengineering is highly appreciated.
References
- Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo International journal of molecular sciences. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Chen CS. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin R, Griscom L, Prot JM, Legallais C, Leclerc E. Behavior of HepG2/C3A cell cultures in a microfluidic bioreactor. Biochem Eng J. 2011;53:172–181. doi: 10.1016/j.bej.2010.10.007. [DOI] [Google Scholar]
- Dolega ME, Abeille F, Picollet-D’hahan N, Gidrol X. Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials. 2015;52:347–357. doi: 10.1016/j.biomaterials.2015.02.042. [DOI] [PubMed] [Google Scholar]
- Elliott NT, Yuan F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci. 2011;100:59–74. doi: 10.1002/jps.22257. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A. Biomimetic tissues on a chip for drug discovery. Drug Discov Today. 2012;17:173–181. doi: 10.1016/j.drudis.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkanson M, Cukierman E, Charnley M. Miniaturized pre-clinical cancer models as research and diagnostic tools. Adv Drug Deliv Rev. 2014;69:52–66. doi: 10.1016/j.addr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Reboud J, Ji H, Lee C, Long Y. Development of microfluidic device and system for breast cancer cell fluorescence detection. J Vac Sci Technol, B. 2009;27:1295–1298. doi: 10.1116/1.3049529. [DOI] [Google Scholar]
- Hickman JA, Graeser R, de Hoogt R, Vidic S, Brito C, Gutekunst M, van der Kuip H. Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnol J. 2014;9:1115–1128. doi: 10.1002/biot.201300492. [DOI] [PubMed] [Google Scholar]
- Hockemeyer K, et al. Engineered three-dimensional microfluidic device for interrogating cell-cell interactions in the tumor microenvironment. Biomicrofluidics. 2014;8:044105. doi: 10.1063/1.4890330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H, Park J, Shin C, Do Y, Cho Y-K. Three dimensional multicellular co-cultures and anti-cancer drug assays in rapid prototyped multilevel microfluidic devices. Biomed Microdevices. 2013;15:627–634. doi: 10.1007/s10544-012-9733-9. [DOI] [PubMed] [Google Scholar]
- Kim D, Wu X, Young AT, Haynes CL. Microfluidics-based in vivo mimetic systems for the study of cellular biology. Acc Chem Res. 2014;47:1165–1173. doi: 10.1021/ar4002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat. 2015;227:746–756. doi: 10.1111/joa.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komen J, Wolbers F, Franke HR, Andersson H, Vermes I, van den Berg A. Viability analysis and apoptosis induction of breast cancer cells in a microfluidic device: effect of cytostatic drugs. Biomed Microdevices. 2008;10:727–737. doi: 10.1007/s10544-008-9184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze A, Pushkarsky I, Kittur H, Di Carlo D. Research highlights: measuring and manipulating cell migration. Lab Chip. 2014;14:4117–4121. doi: 10.1039/C4LC90091J. [DOI] [Google Scholar]
- Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Inaba Y, Takeda Y. Antioxidant mechanism of carnosic acid: structural identification of two oxidation products. J Agric Food Chem. 2001;49:5560–5565. doi: 10.1021/jf010693i. [DOI] [PubMed] [Google Scholar]
- Moreno-Arotzena O, Mendoza G, Cóndor M, Rüberg T, García-Aznar J. Inducing chemotactic and haptotactic cues in microfluidic devices for three-dimensional in vitro assays. Biomicrofluidics. 2014;8:064122. doi: 10.1063/1.4903948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri AEL, Han J, Abdrabbah MB, Isoda H. Down regulation effect of Rosmarinus officinalis polyphenols on cellular stress proteins in rat pheochromocytoma PC12 cells. Cytotechnology. 2012;64:231–240. doi: 10.1007/s10616-011-9352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdil B, Onal S, Oruc T, Okvur DP. Fabrication of 3D controlled in vitro microenvironments. MethodsX. 2014;1:60–66. doi: 10.1016/j.mex.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra B, et al. Migration and vascular lumen formation of endothelial cells in cancer cell spheroids of various sizes. Biomicrofluidics. 2014;8:052109. doi: 10.1063/1.4895568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesen-Okvur D (2015) Microfluidic device for investigation of distance dependent interactions in cell biology Patent No WO 2015/052034 A1, World Intellectual Property Organization
- Ravi M, Kaviya SR, Paramesh V. Culture phases, cytotoxicity and protein expressions of agarose hydrogel induced Sp2/0, A549, MCF-7 cell line 3D cultures. Cytotechnology. 2016;68:429–441. doi: 10.1007/s10616-014-9795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Mondragon I, Wang X, Gerdes H-H. Spatio-temporal analysis of tamoxifen-induced bystander effects in breast cancer cells using microfluidics. Biomicrofluidics. 2012;6:024128. doi: 10.1063/1.4726349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi W, Wang S-J, Lin F, Jeon NL. A parallel-gradient microfluidic chamber for quantitative analysis of breast cancer cell chemotaxis. Biomed Microdevices. 2006;8:109–118. doi: 10.1007/s10544-006-7706-6. [DOI] [PubMed] [Google Scholar]
- Song H, Chen T, Zhang B, Ma Y, Wang Z. An integrated microfluidic cell array for apoptosis and proliferation analysis induction of breast cancer cells. Biomicrofluidics. 2010;4:044104. doi: 10.1063/1.3497376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KE, Beebe DJ. Microfluidic 3D models of cancer. Adv Drug Deliv Rev. 2014;79:68–78. doi: 10.1016/j.addr.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranirokh M, Kouzani AZ, Francis PS, Kanwar JR. Microfluidic devices for cell cultivation and proliferation. Biomicrofluidics. 2013;7:051502. doi: 10.1063/1.4826935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B, Kurkuri M, Shi JY, Lwin LEMP, Palms D. Herceptin functionalized microfluidic polydimethylsiloxane devices for the capture of human epidermal growth factor receptor 2 positive circulating breast cancer cells. Biomicrofluidics. 2010;4:032205. doi: 10.1063/1.3480573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev. 2014;69:29–41. doi: 10.1016/j.addr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Weber P, Wagner M, Schneckenburger H. Cholesterol dependent uptake and interaction of doxorubicin in MCF-7 breast cancer cells. Int J Mol Sci. 2013;14:8358–8366. doi: 10.3390/ijms14048358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Ghajar CM, Bissell MJ. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv Drug Deliv Rev. 2014;69:42–51. doi: 10.1016/j.addr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Perez-Castillejos R, Love JC, Whitesides GM. Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials. 2008;29:1853–1861. doi: 10.1016/j.biomaterials.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, et al. A compact microfluidic system for cell migration studies. Biomed Microdevices. 2014;16:521–528. doi: 10.1007/s10544-014-9854-4. [DOI] [PubMed] [Google Scholar]
- Yesil-Celiktas O. Patenting trends in enzyme related microfluidic applications. Biochem Eng J. 2014;92:53–62. doi: 10.1016/j.bej.2014.06.017. [DOI] [Google Scholar]
- Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr. 2010;65:158–163. doi: 10.1007/s11130-010-0166-4. [DOI] [PubMed] [Google Scholar]
- Yildiz-Ozturk E, Yesil-Celiktas O. Diffusion phenomena of cells and biomolecules in microfluidic devices. Biomicrofluidics. 2015;9:052606. doi: 10.1063/1.4923263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, et al. An on-chip study on the influence of geometrical confinement and chemical gradient on cell polarity. Biomicrofluidics. 2014;8:052010. doi: 10.1063/1.4898209. [DOI] [PMC free article] [PubMed] [Google Scholar]