Abstract
Urolithiasis is one of the painful multifactorial disorders caused by metabolic abnormalities influencing the composition of body fluids and urine. The bark of Terminalia arjuna (T. arjuna), very well known in Ayurveda for the treatment of cardiovascular diseases, possesses antioxidant and diuretic activity. The present study was undertaken to investigate the antiurolithiatic efficacy of aqueous extract of bark of T. arjuna on oxalate-induced injury to renal tubular epithelial cells. Madin–Darby canine kidney (MDCK) cells were exposed to 2 mM oxalate for 48 h to evaluate the protective effect of T. arjuna aqueous extract on cell viability, CaOx crystal adherence and apoptotic changes caused by oxalate. The results confirmed that oxalate injured MDCK cells were protected by T. arjuna extract. On treatment with a range concentrations, the cell viability increased in a concentration dependent manner. Moreover, the extract prevented the interaction of the calcium oxalate (CaOx) crystals with the cell surface and reduced the number of apoptotic cells. The current data suggests that T. arjuna bark confers a cytoprotective role and based on our results it could be a potential candidate from natural plant sources against urolithiasis.
Keywords: Urolithiasis, Calcium oxalate, Renal epithelial cells, Madin–Darby canine kidney cells, Phytotherapy, Terminalia arjuna
Introduction
Renal stone disease has tormented people throughout the ages. Crystal formation in the kidney is normal and harmless provided these crystals are excreted via the urine from the body. In order for stones to be formed, not only do crystals need to be retained within the kidney, but they must be present at particular sites wherein these crystals can cause ulceration at the papillary surface to form a stone nidus, which further allows for crystal buildup and subsequent stone formation. In addition, renal tubular injury also provides an opportunity for crystal–cell interaction. The crystals that are internalized in the interstitium undergo growth and aggregation, and develop into renal stones (Khan 1995; Tsujihata 2008). The difference between stone formers and non–stone formers is that crystals are retained in kidneys of stone formers and can grow to become full-size stones (Finlayson et al. 1984). Small stones (≤5 mm) often pass without medical intervention, but larger stones (>5 mm) usually must be removed (Verkoelen 2006).
Recurrent and persistent renal stone disease causes substantial morbidity and it has been reported that in patients with congenital errors in endogenous oxalate synthesis, pathologic renal calcifications ultimately may destroy the kidneys (Verkoelen 2006). Till date, there is no permanent cure of urolithiasis. Thus, an adjunct to these conventional methods such as phytotherapy is an area which appears to possess tremendous potential. Medicinal plants have been known for millennia and are being used as a rich source of therapeutic agents worldwide. The use of herbal medicine is further becoming popular owing to the greater toxicity and side effects of allopathic medicines (Verma and Singh 2008). Hence, this study was undertaken to conduct detailed studies to assess the effect T. arjuna on calcium oxalate monohydrate (COM) crystals interaction with cultured renal cells, so as to establish a scientific basis for the anti-urolithiatic property of T. arjuna. The in vitro model system chosen was the MDCK cell line which has been derived from Kidney tubular epithelial cells of Canis familiaris and has been used for conducting useful toxicology studies in the area of urological research.
The plant under study, T. arjuna, belongs to the family Combretaceae and holds a reputed position in Ayurvedic system of medicine (Scassellati-Sforzolini et al. 1999). Experimental and clinical studies revealed the beneficial effects of this plant against various conditions of cardiac dysfunction (Cheng et al. 2002). T. arjuna bark extract has been previously reported to inhibit CaOx crystal precipitation and growth (Chaudhary et al. 2010). In a recent study, the inhibitory potential of T. arjuna was evaluated in vitro on CaOx crystallization and crystal adhesion (Mittal et al. 2015, 2016). In the current study, a reduction of oxalate-induced renal tubular epithelial cell injury was observed by the aqueous extract of T. arjuna.
Materials and methods
Plant
The dried bark of T. arjuna were purchased from Natural Remedies Pvt. Ltd., Bangalore, India. A collection of voucher specimen is available at the company.
Preparation of the aqueous extract of Terminalia arjuna
The dried fine powdered T. arjuna bark was soaked in distilled water for 24 h at 4 °C. The extract was then filtered through muslin cloth followed by centrifugation at 10,000 rpm for 20 min at 4 °C and the filtrate was lyophilized to obtain the dried powder referred to as aqueous extract of T. arjuna bark. This lyophilized powder was stored in labeled sterile bottles and kept at −20 °C (Mittal et al. 2015).
For cell culture studies a stock solution (1000 µg/mL) of the aqueous extract of T. arjuna was dissolved in dimethyl sulfoxide (DMSO, Sigma Aldrich, Mumbai, India) [final concentration of the DMSO in the highest concentration of plant extract tested did not exceed 0.4% (v/v) and did not affect the cell proliferation]. Further dilutions of the stock were done using serum free DMEM (Invitrogen, Bangalore, India) (Dulbecco’s Modified Eagles’s Medium) and filtered by 0.22 µm syringe filter (Moriyama et al. 2007).
Cell lines
Experimental studies were done using in vitro model of MDCK cell line. The cell line was obtained from NCCS (National Centre for Cell Science), Pune, India. All the reagents used for the cell culture experiments were procured from Invitrogen. The cell culture plates were from Thermo Scientific, Bangalore, India.
Cell culture
The cells were maintained as monolayers in DMEM with 2.0 mM l-glutamine adjusted to contain 3.7 g/L sodium bi-carbonate, 4.5 g/L glucose. Medium was supplemented with 1% Penicillin (100 units/mL)-Streptomycin (10,000 μg/mL) and 10% fetal bovine serum. Cells were cultured in 25 cm2 tissue-culture treated flasks at 37 °C and 5% CO2 in humidified chambers (Aggarwal et al. 2010).
Oxalate-induced cell injury
MDCK cells were incubated in DMEM containing 2 mM sodium oxalate in the presence of different concentrations of the aqueous extract for 48 h (Jeong et al. 2005; Moriyama et al. 2007). Cystone drug (Himalaya Herbal Healthcare, Bangalore, India) at a concentration of 40 μg/mL was used as a positive control.
MTT assay
1 × 104 cells/well were seeded into a 96-well microplate and incubated at 37 °C and 5% CO2 in humidified chambers. At 70–80% confluency, the effect of T. arjuna in the presence of oxalate injury was assessed by adding various concentrations (10, 20, 30 and 40 μg/mL) to the cells and incubated for 48 h at 37 °C. At the end of the treatment, 25 μL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reagent (final concentration of 0.5 mg/mL) was added to each well and incubated for 4 h at 37 °C. Supernatant was discarded and 200 μL DMSO was added to each well after the incubation was over to solubilize the formazan product and kept at room temperature for 15–20 min. Absorbance values were determined at a 570 nm test wavelength and a 630 nm reference wavelength to test the cell viability using a microplate reader (Model 680, Bio-Rad, Hercules, CA, USA) (Zhang et al. 2013).
CaOx crystal adhesion
Cells were seeded at a density of 2 × 105 cells/coverslip in a 6-well plate and incubated at 37 °C and 5% CO2 in humidified chambers. At 70–80% confluency, the effect of T. arjuna in the presence of oxalate injury was assessed by adding extract at a concentration of 40 μg/mL to the cells and incubated for 48 h at 37 °C. After the treatment, medium was removed and the cells were fixed with 4% paraformaldehyde for 30 min. After washing twice with 1× PBS, cells were observed under phase contrast and polarization upright microscope (BX53, Olympus Corporation, Tokyo, Japan) at a magnification of 20× to study cell-crystal interactions (Semangoen et al. 2008).
Hoechst 33258 staining
The seeding and treatment schedules were the same as that for CaOx crystal adherence assay. At the end of the treatment, medium was removed and the cells were fixed with 4% paraformaldehyde for 30 min. After washing twice with 1× PBS, cells were stained with 5 μg/mL of Hoechst 33258 dye for 10 min at room temperature in the dark. After washing twice with 1× PBS, stained nuclei were observed under fluorescence upright microscope (BX53, Olympus Corporation) at a magnification of 209 (Allen et al. 2001).
Annexin V/propidium iodide staining
6 × 105 cells were seeded into 60 mm dishes and incubated at 37 °C and 5% CO2 in humidified chambers. At 70–80% confluency, the effect of T. arjuna in the presence of oxalate injury was assessed by treating cells with 40 μg/mL for 48 h at 37 °C. After the treatment, the subsequent procedure followed was in accordance to the instructions of BDPharminogen™ FITC ANNEXIN V Apoptosis Detection Kit 1 (catalogue no. 556547; Becton Dickinson Argentina S.R.L., Vicente Lopez, Prov. de Bs.As., Argentina), where, cell suspension and cells from monolayer were pooled together. The cells were washed with cold 1× PBS twice. The pellet was resuspended in 100 μL of 1× binding buffer and 2 µL of FITC Annexin V and 2 µL of Propidium iodide (PI) were added to it. The cells were gently vortexed and incubated for 15 min at room temperature in the dark. 400 µL of 1× binding buffer was added to each group and then the cells were analyzed by flow cytometry (BD Accuri C6, BD Biosciences).
Detection of Active Caspase-3
The seeding and treatment schedules were the same as that for Annexin V/PI staining. At the end of the treatment, the subsequent procedure followed was in accordance to the instructions of BDPharminogen™ FITC Active Caspase-3 Apoptosis Kit (catalogue no. 550480), where, cell suspension and cells from monolayer were pooled together. Cells were washed with cold PBS twice and resuspended in BD Cytofix/Cytoperm solution. After incubation on ice for 20 min, BD Cytofix/Cytoperm solution was discarded. The cells were washed twice with 1× BD Perm/Wash buffer at room temperature. The cells were then resuspended in the 1× BD Perm/Wash buffer plus 10 µL of antibody and incubated for 30 min at room temperature. The cells were washed with 0.5 mL of 1× BD Perm/Wash buffer and then resuspended in 0.5 mL of 1× BD Perm/Wash buffer for analysis by flow cytometry (BD Accuri C6, BD Biosciences).
Statistical analysis
Statistical procedures were performed with GraphPad Prism software version 6.01. The statistically different groups were identified by one way analysis of variance (ANOVA), followed by Dunnet’s multiple comparison tests. Results were expressed as the mean ± SD. A p value of <0.05 was considered significant. All the experiments were performed three times, each time in triplicate.
Results
MTT assay
The cytoprotective potential of the aqueous extract of T. arjuna towards the oxalate-induced injury to renal tubular epithelial cells, MDCK cells is displayed in Fig. 1. Exposure to oxalate caused injury to the cells which could be ascertained by a concomitant decrease in viability from 100% in control to 24.18 ± 1.17% in MDCK cells. The solvent system or the extract individually had no significant effect on the cells, thereby indicating that there was no cytotoxicity to the cells. However, the injury due to oxalate was significantly reduced in those cells treated with the extract. As the concentration of the extract increased from 10 to 40 μg/mL, the percentage viability improved in a concentration dependent manner. The percentage viability with 10, 20, 30 and 40 μg/mL was 26.64 ± 0.88, 34.98 ± 1.79, 42.7 ± 1.51 and 50.04 ± 1.44% respectively. Cystone treatment also reduced the injury caused by oxalate to renal cells.
Fig. 1.
Effect of aqueous extract of T. arjuna on MDCK cell viability assessed by MTT assay. Data are mean ± SD of three independent observations. ns not significant. ****p < 0.0001 versus untreated control; and ## p < 0.01, ### p < 0.0005 versus oxalate injury control
CaOx crystal adhesion
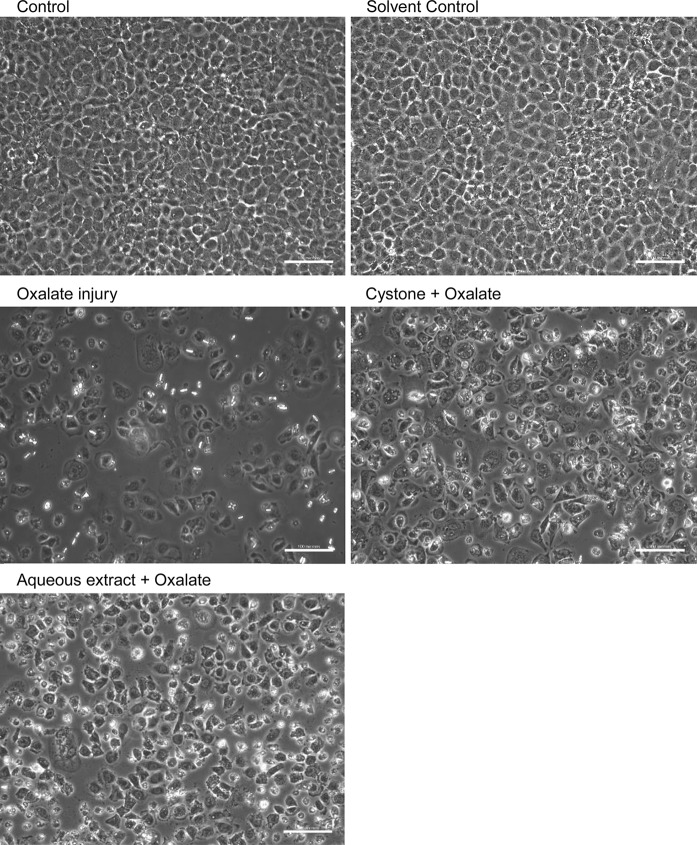
MDCK cells were studied for cell–crystal interactions to demonstrate crystal adhesion on the cell surface as shown in Fig. 2. After incubation of the MDCK cells with oxalate, hexagonal COM crystals were observed. These crystals remained adhered to the cells even after several washes using PBS indicating that CaOx crystals adhered tightly to renal cells leading to the detrimental effects observed in these cells. The cells treated with the solvent system or extract alone, showed healthy cellular morphology, demonstrating that there was no adverse effect. The effect of 40 μg/mL of T. arjuna on oxalate treated MDCK cells was assessed, wherein, more viable cells along with fewer crystals were observed, which could be attributed to the loss of crystal adherence to cells in presence of the extract and thereby causing decreased cell-crystal interaction. Cystone also exhibited cytoprotective effect with more viable cells and loosely bound crystals.
Fig. 2.
Evaluation of T. arjuna extract on CaOx crystal adherence in oxalate induced injury to MDCK cells, visualized under polarization and phase contrast at magnification ×20 and scale bar 100 microns
Hoechst staining
The morphological changes in cell nuclei were determined by staining MDCK cells with Hoechst 33258 dye as shown in Fig. 3. The untreated MDCK cells i.e. control, appeared to be healthy in morphology as they showed intact chromatin, indicating that there was no significant cell death in the untreated cells. The cells treated with the solvent system or extract, showed similar healthy cellular morphology, reflecting no adverse effect to the cells, as can be seen from the inset images from the various treatments. However, following incubation of the MDCK cells with oxalate, a substantial increase in cell death w.r.t. control was observed. The cells showed marked changes in morphology (Fig. 2) including condensed and fragmented chromatin which showed up as bright nuclear fluorescence (Fig. 3, inset image). Representative images of oxalate injured cells with 40 μg/mL of the extract of T. arjuna showed an increase in the number of viable cells with intact cellular membrane and no remarkable nuclear changes, indicating reduced level of cells undergoing apoptosis w.r.t. the oxalate injured cells. A similar trend was also seen in the positive control wherein Cystone also showed a protective role. From these images it was also apparent that oxalate injury led to a reduction in the number of cells, which increased on co treatment with T.arjuna, which reiterated the results seen in Fig. 2.
Fig. 3.
Modulation of apoptosis in oxalate induced injury to MDCK cells by T. arjuna extract, visualized under fluorescence microscopy at magnification ×20 and scale bar 100 microns. Nuclear staining was performed with Hoechst 33258 (blue signal). Inset shows enlargement of areas indicated by white arrows. (Color figure online)
Annexin V/PI staining
Oxalate-induced apoptosis was detected in MDCK cells by staining with Annexin V and PI as depicted in Fig. 4. There was no significant apoptosis and necrosis in the untreated MDCK cells. Treatment of cells with the solvent system and extract did not lead to any significant alteration in cell viability. After incubation of renal cells with oxalate, cell death assay using Annexin V/PI staining showed that percent of cells undergoing apoptosis gradually increased from 1.7% in control to 60.2% in oxalate treated cells (lower right hand quadrants). The protective effect of T. arjuna extract (40 μg/mL) on the oxalate injured renal cells was evident as the number of apoptotic cells was significantly reduced to 33.8%, indicating that protection of the cells from oxalate-induced apoptosis and was comparable to the positive control.
Fig. 4.
Flow cytometry evaluation of apoptosis in oxalate induced injury to MDCK cells, visualized by Annexin V/PI staining
Detection of Active Caspase-3
Oxalate-induced apoptosis in MDCK cells was also assessed by using Anti-Active Caspase-3 antibody staining (Fig. 5). As seen from our previous results the solvent system or extract of T. arjuna did not lead to any adverse effects to renal cells, however renal cells exposed to oxalate, showed that the cells underwent apoptosis which gradually increased from 22.4% (M2) in control to 71.1% (M2) in oxalate treated cells. Treating the oxalate injured cells with T. arjuna led to a significant decrease in the number of apoptotic cells to 57.5%, reiterating that T. arjuna had the ability to diminish oxalate-induced apoptosis.
Fig. 5.
Flow cytometry assessment of apoptosis in oxalate induced injury to MDCK cells, visualized by Anti-Active Caspase-3 antibody staining
Discussion
Evidence suggests that the epithelial cells lining the renal tubules could play an active role in creating the conditions which would allow stones to develop in kidneys. Since these mechanisms are challenging to study in vivo, in vitro models are a good alternative for the detailed study of physiological and cell biological processes linked to renal stone disease (Verkoelen et al. 1997). Exposure to high levels of oxalate and/or COM crystals is injurious to renal epithelial cells and triggers variety of changes in renal epithelial cells, followed by cell injury and cell death (Khan 1995; Miller et al. 2000).
In vitro study with MDCK cells showed that aqueous extract of T. arjuna protected the renal epithelial cells from injury caused by oxalate in a dose-dependent manner. The adhesion of CaOx crystals to the surface of MDCK cells was also studied in the absence and presence of T. arjuna extract, wherein it was observed that when the cells were exposed to oxalate, CaOx crystals adhered tightly to the renal cells with subsequent detrimental effects to the cells. These crystals caused cell damage and cell death which was evident by lesser number of cells seen in the cultures. However, treatment with the extract conferred protection and reduced the injury to the renal cells by disrupting the interaction of CaOx crystals with the surface of the cells which was evident by increase in the number of viable cells and loss of crystal adherence.
We also demonstrated that exposure to oxalate caused induction of apoptosis leading to increased cell death. The morphological changes in cell nuclei were determined by staining cells with Hoechst 33258 dye. The cells treated with oxalate showed apoptotic changes in morphology such as irregular shape, membrane blebbing, apoptotic bodies and condensed and fragmented chromatin. Treatment with T. arjuna lead to cytoprotective potential which was apparent by larger number of viable cells with intact cellular membrane and fewer apoptotic bodies, showing reduced level of apoptosis. This was further confirmed by Annexin V/PI and Anti-Active Caspase-3 antibody staining. When renal cells were injured with oxalate, the number of cells that bound to Annexin V increased, indicating that phosphatidylserine was exposed on the cell luminal surface. This observation is not only consistent with literature which states that exposure to high concentration of oxalate results in the relocation of anionic phospholipids that are normally confined to the inner leaflet of the plasma membrane (Wiessner et al. 1999), but also firmly establishes the role of phosphatidylserine in crystal attachment. T. arjuna treatment significantly reduced the number of apoptotic cells by disrupting the interaction of CaOx crystals with the cells.
Literature has suggested that exposure to high levels of oxalate in vitro (Miller et al. 2000) and in vivo (Sarica et al. 2001) leads to an increase in the abundance of apoptotic renal epithelial cells by a process involving increased oxidant stress (Miller et al. 2000). The generation of ROS causes perturbations in mitochondrial function which are often accompanied by an increase in mitochondrial permeability and a release of pro-apoptotic factors. These factors in turn trigger the activation of cellular caspases that have been linked to apoptotic cell death (Sarica et al. 2001; Petronelli et al. 2001). Caspase-3 is a key protease that is activated during apoptosis. We confirmed that when renal cells were exposed to oxalate, the number of apoptotic cells significantly increased, which were detected by anti-active caspase-3 antibody. Our study shows that treatment with the aqueous extract of T. arjuna reduces the number of apoptotic cells thus proving its ability to protect against oxalate-induced cell injury. Our study provides support to the findings that the action of oxalate at the cell membrane generates lipid signals that act on the mitochondria to elicit an increase in oxidant stress and apoptotic death (Cao et al. 2000).
These data clearly indicate that oxalate and/or CaOx crystals are toxic to renal tubular epithelial cells. Interference with CaOx crystallization and retention to cells, therefore, seems a possible therapeutic strategy for the prevention of recurrent stone disease. The aqueous extract of T. arjuna may contain substances that inhibit the binding of the CaOx crystals to the renal epithelial surface and/or interaction of oxalate ions with calcium ions. Moreover, active biomolecules of T. arjuna by blocking these interactions exhibit cytoprotective role towards oxalate-induced cell injury.
Acknowledgements
This study (SR/SO/HS/132/2010) was financially supported by the Department of Science and Technology (DST), Government of India, New Delhi, India. We would like to thank the Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, Waknaghat, India for providing the necessary facilities to carry out this study.
Funding
The study was funded by Department of Science and Technology (DST), India (SR/SO/HS/132/2010).
Abbreviations
- T. arjuna
Terminalia arjuna
- MDCK
Madin–Darby canine kidney (cell line)
- CaOx
Calcium oxalate
- COM
Calcium oxalate monohydrate
- DMSO
Dimethyl sulfoxide
- DMEM
Dulbecco’s modified Eagles’s medium
- NCCS
National centre for cell science
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
Phosphate buffered saline
- PS
Phosphatidylserine
- PI
Propidium iodide
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aggarwal A, Tandon S, Singla SK, Tandon C. Diminution of oxalate induced renal tubular epithelial cell injury and inhibition of calcium oxalate crystallization in vitro by aqueous extract of Tribulus terrestris. Int Braz J Urol. 2010;36:480–488. doi: 10.1590/S1677-55382010000400011. [DOI] [PubMed] [Google Scholar]
- Allen S, Sotos J, Sylte MJ, Czuprynski CJ. Use of hoechst 33342 staining to detect apoptotic changes in bovine mononuclear phagocytes infected with Mycobacterium avium subsp. paratuberculosis. Clin Diagn Lab Immunol. 2001;8:460–464. doi: 10.1128/CDLI.8.2.460-464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao LC, Honeyman T, Jonassen J, Scheid C. Oxalate-induced ceramide accumulation in Madin–Darby canine kidney and LLC-PK1 cells. Kidney Int. 2000;57:2403–2411. doi: 10.1046/j.1523-1755.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary A, Singla SK, Tandon C. In vitro evaluation of Terminalia arjuna on calcium phosphate and calcium oxalate crystallization. Indian J Pharm Sci. 2010;72:340–345. doi: 10.4103/0250-474X.70480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HY, Lin CC, Lin TC. Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antivir Res. 2002;55:447–455. doi: 10.1016/S0166-3542(02)00077-3. [DOI] [PubMed] [Google Scholar]
- Finlayson B, Khan SR, Hackett RL. Mechanisms of stone formation—an overview. Scanning Electron Microsc. 1984;3:1419–1425. [PubMed] [Google Scholar]
- Jeong BC, Kwak C, Cho KS, Kim BS, Hong SK, Kim JI, Lee C, Kim HH. Apoptosis induced by oxalate in human renal tubular epithelial HK-2 cells. Urol Res. 2005;33:87–92. doi: 10.1007/s00240-004-0451-5. [DOI] [PubMed] [Google Scholar]
- Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995;23:71–79. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- Miller C, Kennington L, Cooney R, Kohjimoto Y, Honeyman T, Pullman J, Jonassen J, Scheid C. Oxalate toxicity in renal epithelial cells: characteristics of apoptosis and necrosis. Toxicol Appl Pharmacol. 2000;162:132–141. doi: 10.1006/taap.1999.8835. [DOI] [PubMed] [Google Scholar]
- Mittal A, Tandon S, Singla SK, Tandon C. In vitro studies reveal antiurolithic effect of Terminalia arjuna using quantitative morphological information from computerized microscopy. Int Braz J Urol. 2015;41:935–944. doi: 10.1590/S1677-5538.IBJU.2014.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A, Tandon S, Singla SK, Tandon C. In vitro inhibition of calcium oxalate crystallization and crystal adherence to renal tubular epithelial cells by Terminalia arjuna. Urolithiasis. 2016;44:117–125. doi: 10.1007/s00240-015-0822-0. [DOI] [PubMed] [Google Scholar]
- Moriyama MT, Miyazawa K, Noda K, Oka M, Tanaka M, Suzuki K. Reduction in oxalate-induced renal tubular epithelial cell injury by an extract from Quercus salicina Blume/Quercus stenophylla Makino. Urol Res. 2007;35:295–300. doi: 10.1007/s00240-007-0114-4. [DOI] [PubMed] [Google Scholar]
- Petronelli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Sarica K, Yagci F, Bakir K, Erbagci A, Erturhan S, Ucak R. Renal tubular injury induced by hyperoxaluria: evaluation of apoptotic changes. Urol Res. 2001;29:34–37. doi: 10.1007/s002400000150. [DOI] [PubMed] [Google Scholar]
- Scassellati-Sforzolini G, Villarini LM, Moretti LM, Marcarelli LM, Pasquini R, Fatigoni C, Kaur LS, Kumar S, Grover IS. Antigenotoxic properties of Terminalia arjuna bark extracts. J Environ Pathol Toxicol Oncol. 1999;18:119–125. [PubMed] [Google Scholar]
- Semangoen T, Sinchaikul S, Chen ST, Thongboonkerd V. Altered proteins in MDCK renal tubular cells in response to calcium oxalate dihydrate crystal adhesion: a proteomics approach. J Proteome Res. 2008;7:2889–2896. doi: 10.1021/pr800113k. [DOI] [PubMed] [Google Scholar]
- Tsujihata M. Mechanism of calcium oxalate renal stone formation and renal tubular cell injury. Int J Urol. 2008;15:115–120. doi: 10.1111/j.1442-2042.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- Verkoelen CF. Crystal retention in renal stone disease: a crucial role for the glycosaminoglycan hyaluronan? J Am Soc Nephrol. 2006;17:1673–1687. doi: 10.1681/ASN.2006010088. [DOI] [PubMed] [Google Scholar]
- Verkoelen CF, Van der Boom BG, Schroder FH, Romijn JC. Cell cultures and nephrolithiasis. World J Urol. 1997;15:229–235. doi: 10.1007/BF01367660. [DOI] [PubMed] [Google Scholar]
- Verma S, Singh SP. Current and future of herbal medicines. Vet World. 2008;1:347–350. doi: 10.5455/vetworld.2008.347-350. [DOI] [Google Scholar]
- Wiessner JH, Hasegawa AT, Hung LY, Mandel NS. Oxalate-induced exposure of phosphatidylserine on the surface of renal epithelial cells in culture. J Am Soc Nephrol. 1999;10:S441–S445. [PubMed] [Google Scholar]
- Zhang L, Jiang F, Chen Y, Luo J, Liu S, Zhang B, Ye Z, Wang W, Liang X, Shi W. Necrostatin-1 attenuates ischemia injury induced cell death in rat tubular cell line NRK-52E through decreased Drp1 expression. Int J Mol Sci. 2013;14:24742–24754. doi: 10.3390/ijms141224742. [DOI] [PMC free article] [PubMed] [Google Scholar]