Abstract
Island evolution may be expected to involve fast initial morphological divergence followed by stasis. We tested this model using the dental phenotype of modern and ancient common voles (Microtus arvalis), introduced onto the Orkney archipelago (Scotland) from continental Europe some 5000 years ago. First, we investigated phenotypic divergence of Orkney and continental European populations and assessed climatic influences. Second, phenotypic differentiation among Orkney populations was tested against geography, time, and neutral genetic patterns. Finally, we examined evolutionary change along a time series for the Orkney Mainland. Molar gigantism and anterior‐lobe hypertrophy evolved rapidly in Orkney voles following introduction, without any transitional forms detected. Founder events and adaptation appear to explain this initial rapid evolution. Idiosyncrasy in dental features among different island populations of Orkney voles is also likely the result of local founder events following Neolithic translocation around the archipelago. However, against our initial expectations, a second marked phenotypic shift occurred between the 4th and 12th centuries AD, associated with increased pastoral farming and introduction of competitors (mice and rats) and terrestrial predators (foxes and cats). These results indicate that human agency can generate a more complex pattern of morphological evolution than might be expected in island rodents.
Keywords: Dispersal, evolutionary rate, geometric morphometrics, island evolution, tooth shape, zooarchaeology
Alien species on islands, whether they arrive with humans or by natural dispersal (Williamson 1981), can show rapid changes, in which evolutionary and ecological processes overlap (Lambrinos 2004; Bradshaw and Holzapfel 2006; Kinnison and Hairston 2007). Both founder events and adaptation to specific insular conditions should promote rapid initial divergence, with subsequent stasis once the population reaches a demographic equilibrium and its local ecological optimum (Sondaar 2000; Millien 2006; Nagorsen and Cardini 2009). However, many islands suffer human‐induced disturbances (destruction of habitat, introduction of species) that may also promote rapid phenotypic changes (Palumbi 2001; Price et al. 2003; Pigliucci et al. 2006; Carroll et al. 2007; Ghalambor et al. 2007; Hendry et al. 2008). In Europe the bioarchaeological record reveals that island systems were subjected to substantial anthropogenic disturbance through the early to middle Holocene, when the first (Neolithic) farmers spread across Eurasia (Cherry 1990; Blondel and Vigne 1993; Schüle 1993; Vigne 1999). A phenotypic stasis for introduced animals in these insular contexts is, therefore, unlikely. Instead, many phenotypic changes should be expected to maintain fitness in a dynamic environment in which directions of selection driven by human activities fluctuated.
Testing these ideas requires documenting phenotypic change through time. However, most studies of phenotypic evolution following island colonization make inferences solely from present‐day populations (e.g., Clegg et al. 2008) or on the basis of laboratory (e.g., Templeton 1996) or field experiments (e.g., Losos et al. 1997). In their study of natural colonization, it is notable that Nargosen and Cardini (2009) did compare 30 subfossil specimens of an endemic insular marmot (Marmota vancouverensis) with its extant insular and continental relatives. They found that most of the divergence occurred soon after the island became isolated during the Pleistocene (with subsequent morphological stasis continuing to the present day), supporting the ecological “optimum model.” However, this model remains largely untested in situations in which human introduction and human impact to the environment occurred, a fact largely due to the lack of continuous subfossil records documenting each step of insular evolution.
In this article, our objective is to examine morphological change through time in populations of the Orkney vole, Microtus arvalis orcadensis (Major 1905)—an endemic subspecies of the common vole Microtus arvalis (Pallas 1778)—introduced to the Orkney archipelago (Fig. 1) by Neolithic farmers around 5000 years ago, from a source outside the British Isles (Martínková et al. 2013). Large samples of archaeological Orkney voles, as well as good palaeo‐environmental records revealing anthropization of the Orkney archipelago (Bunting 1994, 1996), provided an important opportunity to investigate the pace of evolutionary change in this insular rodent over the last 5000 years, within the context of an island environment impacted by humans.
Figure 1.
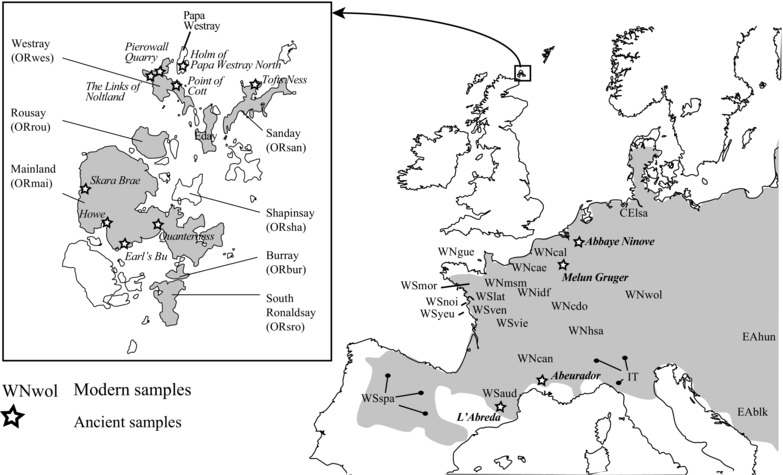
Localization of the modern samples (abbreviated group names) and ancient samples (stars with site names) of Microtus arvalis in continental Europe and Orkney (insert) distribution area of the species (gray shading; Shenbrot and Krasnov 2005).
Present‐day Orkney voles are morphologically characterized by a large body size—twice that of their continental European cousins—and differing in many other skeletal traits and pelage (Berry 1996). Considerable morphological diversity has been described among the different island populations of the archipelago (Fig. 1). For instance, those from islands in the northern part of the archipelago (Westray and Sanday) show a darker pelage and a molar morphology different to those from the main island (Mainland) and the South Isles (Corbet 1964). Divergence within Orkney vole populations has been attributed to either founding events (Berry 1996) or fast adaptive radiation (Corbet 1986). A recent study has also revealed higher genetic diversity in Orkney voles from Mainland (compared to those of the outer isles). These data suggest an initial introduction of voles to Mainland, from where successive founding events occurred in other islands of the group through further human‐mediated dispersal (Martínková et al. 2013).
To investigate microevolution in Orkney voles through time, we used molar morphology as a phenotypic marker. Vole teeth are the most abundant and diagnostic element in the fossil and subfossil record, and their complex form is evolutionary tractable (Guthrie 1965; Renvoisé et al. 2012). First we examined the extent of molar size and shape divergence of Orkney voles from their continental counterparts and from other insular populations. Increase in molar size (as a marker for body size increase) is expected in insular populations of rodents according to the island rule (Foster 1964; van Valen 1973), whereas niche widening and reduction of predation and interspecific competition (together with founder events and drift in small, isolated populations) may induce rapid molar shape change (Renaud et al. 2011, 2013). To test the influence of climatic gradients (Piras et al. 2009; McGuire 2010) on the phenotype of M. arvalis, we assessed how much molar size and shape covaries with climate among populations from Western Europe—‐including Orkney (Fig. 1).
We then investigated morphological divergence among Orkney populations since their introduction. To test the expectation of greater divergence on more remote and smaller islands (Renaud and Millien 2001; Millien 2011), we examined phenotypic variation in the context of interisland distances, as well as island areas. The suggestion of dispersal throughout the archipelago from a source population on Mainland (Martínková et al. 2013) was tested using the correlation between molar shape and geographic distances calculated from Mainland. Further, the hypothesis of stochastic processes of divergence by, for example, founding events and genetic drift (Berry 1996) was assessed by testing (i) the influence of the time elapsed since introduction using direct radiocarbon age of some of the samples and (ii) the covariance between dental morphology and neutral molecular markers from the same modern and ancient individuals from Orkney. A significant correlation between morphological and neutral genetic variation is expected if random processes underlie phenotypic diversification in Orkney voles since their introduction (Clegg et al. 2002). Finally, because of the detailed archaeological record on Mainland Orkney, we were able to examine the pace of evolutionary change in molar shape for a single island, along a time series spanning the 5000 years since the introduction of M. arvalis to the Orkney archipelago.
Material and Methods
MODERN AND ANCIENT SAMPLES
A total of 853 M. arvalis first lower molars (M1) were measured: 582 modern (Table 1) and 271 ancient (Table 2). The modern dataset includes 378 specimens from across mainland continental Europe, 73 specimens from three islands off the coast of France (Guernsey, Yeu, and Noirmoutier), and 131 M. arvalis orcadensis from seven Orkney Islands (Fig. 1). The modern continental dataset covers several (previously identified) mitochondrial DNA (mtDNA) haplogroups of M. arvalis (Table 1): the Western‐North (WN), Western‐South (WS), Italian (IT), Eastern (EA), and Central (CE) clades (and the Orkney [OR] clade within the WN clade), based either on direct genotyping or on the geographic distribution of evolutionary lineages (Haynes et al. 2003; Heckel et al. 2005; Tougard et al. 2008; Braaker and Heckel 2009; Martínková et al. 2013). The three coastal island populations of Guernsey, Yeu, and Noirmoutier are considered here as subspecies and potential relic populations from the Last Glacial Maximum (LGM, Berry and Rose 1975), although no fossil evidence exists to support this assumption. The Guernsey voles belong to the same WN haplogroup as the Orkney vole but with very distinctive mtDNA haplotypes (Martínková et al. 2013), whereas Noirmoutier and Yeu voles belong to the WS haplotype lineage.
Table 1.
Modern samples of Microtus arvalis
| Country | Localities | MtDNA Lineages | Code | N |
|---|---|---|---|---|
| France | Various (M) | M. agrestis | 29 | |
| United Kingdom (I) | Orkney, Burray (T) | Orkney | ORbur | 2 (2) |
| Orkney, Mainland (T) | Orkney | ORmai | 46 (19) | |
| Orkney, Rousay (T) | Orkney | ORrou | 10 (1) | |
| Orkney, Sanday (T) | Orkney | ORsan | 20 (3) | |
| Orkney, Shapinsay (T) | Orkney | ORsha | 3 | |
| Orkney, South Ronaldsay (T) | Orkney | ORsro | 17 (15) | |
| Orkney, Westray (T) | Orkney | ORwes | 33 (10) | |
| Guernsey (M) | Western‐North | WNgue | 35 | |
| France (I) | Noirmoutier (T) | Western‐South | WSnoi | 30 |
| Yeu (T) | Western‐South | WSyeu | 8 | |
| France (C) | Morbihan (T) | Western‐South | WSmor | 13 |
| Vendée (T) | Western‐South | WSven | 19 | |
| Loire Atlantique (M) | Western‐South | WSlat | 12 | |
| Mont Saint‐Michel (T) | Western‐North | WNmsm | 8 | |
| Caen (T) | Western‐North | WNcae | 29 | |
| Calais (T) | Western‐North | WNcal | 23 | |
| Île‐de‐France (T) | Western‐North | WNidf | 17 | |
| Vienne (T) | Western‐North | WNvie | 13 | |
| Cantal (M) | Western‐North | WNcan | 27 | |
| Côte‐D'or (M) | Western‐North | WNcdo | 24 | |
| Aude (T) | Western‐South | WSaud | 30 | |
| Haute‐Savoie (M) | Western‐North | WNhsa | 19 | |
| Spain (C) | Various (M) | Western‐South | WSspa | 15 |
| Germany (C) | Wolfach (T) | Western‐North | WNwol | 17 |
| Lower Saxony (M) | Central | CElsa | 23 | |
| Italy (C) | Various (M) | Italian | IT | 6 |
| Balkan countries (C) | Various (M) | Eastern | EAblk | 30 |
| Hungary (C) | Various (M) | Eastern | EAhun | 24 |
I, insular samples; C, continental samples; M, material from museum collections; T, trapped animals (genotype available); N, number of molars included in the morphometric study with, in parentheses, the number of specimens with mt‐cytb sequence (see Supporting Information 4) .
Table 2.
Ancient samples of Microtus arvalis
| Location | Site | Context | Period | Chronology | Group | N |
|---|---|---|---|---|---|---|
| Orkney Mainland | Quanterness | Cairn | Neolithic | Late 4th mill. BC | Qu | 29 |
| Skara Brae | Village | Neolithic phase 0 Neolithic phase 1 | 3360–3160 BC 2910–2820 BC | SB1 | 17 | |
| Abandonment | 2850 BC | SBt | 12 | |||
| Neolithic phase 2 | 2850–2400 BC | SB2 | 10 | |||
| Howe | Broch | Iron Age | 4th–7th c. AD | Ho | 25 (3) | |
| Earl's Bu | Viking building | Norse | 11th–12th c. AD | EB | 15 | |
| Orkney Westray | Point of Cott | Cairn | Neolithic | c3500–2800 BC | PC | 27 (3) |
| Holm of Papa Westray North | Cairn | Neolithic | c3500, c3000, c2600 BC | HW | 20 (3) | |
| Pierowall Quarry | Cairn | Neolithic | c2900–2600 BC | PQ1 | 10 (2) | |
| PQ2 | 15 | |||||
| PQ4 | 13 | |||||
| The Links of Noltland | Village | Neolithic | c2900–2600 BC | LN | 22 | |
| Orkney Sanday | Tofts Ness | Village | Late Bronze Age | c1000 BC | TN | 22 |
| Spain | L'Abreda | Cave | Solutrean | 22,000–17,000 BP | Abr1 | 8 |
| France | L'Abeurador | Rock shelter | Mesolithic | 8000–6000 BC | Abe | 11 |
| Melun Grüber | Village | Iron Age | 1st–4th c. AD | MG | 7 | |
| Belgium | Abbaye Ninove | Building | Medieval | 12th c. AD | AN | 8 |
Period: Chronocultural context of the vole samples. Chronology: Time frame of the context in which samples have been collected (BP, Before Present; BC, Before Christ; AD, Anno Domini; c., century). Group: Sample codes used throughout this study. N: number of molars considered in the study with, in parentheses, the number of specimens with mt‐cytb sequence (see Supporting Information 4).
The archaeological samples included 237 M. a. orcadensis from nine sites in Orkney and 34 M. arvalis from four continental European sites (Fig. 1, Table 2). The M. arvalis subfossils from continental Europe required preliminary identification (see Statistical analyses), which was unnecessary for Orkney voles. The subfossil Orkney voles were sampled from archaeological sites on Mainland, Westray, Papa Westray, and Sanday (Fig. 1). Mainland samples span the longest temporal transect; from Neolithic Skara Brae (SB, Clarke 1976) and Quanterness (Qu, Renfrew 1979)—dating between 3500 and 2500 BC, the Iron Age broch at Howe (Ho, Smith and Carter 1994)—dating between 4th and 7th centuries AD, and Medieval Earl's Bu (EB, Batey et al. 1993)—dating between the 11th and 12th centuries AD. Northern isles samples include those from Westray, Papa Westray, and Sanday. From the former two islands, specimens have been recovered from four Neolithic contexts; the chambered tombs at Point of Cott (Barber 1997), Pierowall Quarry (Sharples 1984), Holm of Papa Westray North (Ritchie 2009), and the settlement at the Links of Noltland (Clarke and Sharples 1985), whereas on Sanday, samples have been collected from the Late Bronze Age phase of occupation at Tofts Ness (Dockrill et al. 1994; Simpson and Dockrill 1996).
Ancient samples from continental Europe (Table 2) include those from the Upper Pleistocene cave deposits from L'Abreda (M. S. Segui, pers. comm.) in Spain, the Mesolithic deposits of L'Abeurador cave (Marquet 1993) in France, the Iron Age settlement of Melun Grüber (Mistrot 2000) in France, and the Late Medieval Abbaye Ninove (Wouters and Peersman 1994; A. Ervynck, pers. comm.) in Belgium.
Geometric morphometrics of the first lower molar
To quantify molar size and shape, we used a two‐dimensional geometric morphometric approach (Zelditch et al. 2012; Adams et al. 2013). The form of the occlusal surface of the M1 in M. arvalis (Fig. 2A) was quantified using 18 landmarks positioned at the maximum curvature of the salient and reentrant angles of the posterior loop and the triangle cusps on the buccal and lingual sides of the M1 (Fig. 2B). The smooth curve of the anterior loop (Fig. 2A), which is lacking landmarks, was further quantified using 12 equidistant sliding semilandmarks (Fig. 2B) to extend the landmark‐based statistics to curves (Mitteroecker and Gunz 2009). The Cartesian coordinates of the landmarks and semilandmarks were captured using TPSdig2 v.2.16 (Rohlf 2010a).
Figure 2.
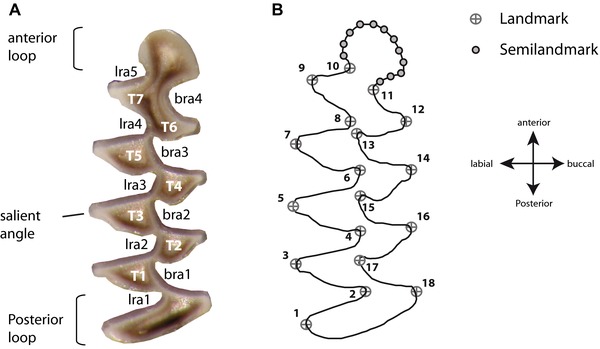
(A) Occlusal surface of Microtus arvalis right first lower molar (M1): T, triangle; bra, buccal reentrant angle; lra, lingual reentrant angle. (B) Position of the 18 landmarks and 12 semilandmarks.
This molar shape dataset (Supporting Information 1) was analyzed using a generalized Procrustes analysis (GPA, (Rohlf and Slice 1990). Using this procedure, information on position, scale, and orientation are removed from the Cartesian coordinates configuration and the semilandmarks are forced to slide on a tangent according to the Bending Energy algorithm (Bookstein 1997). The resulting Procrustes shape coordinates (Supporting Information 1) from this superimposition were used as shape variables for subsequent statistical analyses. Overall M1 size was measured by the centroid size (CS), that is, the square root of the sum of the squared distances between each point and the centroid of the configuration (Supporting Information 1). GPA was performed with TPSrelw v. 1.49 (Rohlf 2010b).
Acquisition of climatic, geographic, temporal and genetic data
Geoclimatic data—i.e., longitude, latitude, altitude and annual mean monthly, maximal monthly, minimal monthly values of precipitation and temperature (Supporting Information 2)—were collected throughout the distribution of the modern vole dataset (Fig. 1) from weather stations between 1960 and 1995 (World Meteorological Organization [WMO] stations; sources: National Oceanic and Atmospheric Administration [NOAA], National Climatic Data Center [NCDC], and Global Climate Perspectives System [GCPS]). Geographic characteristics (Supporting Information 3) of the different Orkney Islands, such as their size (km2), and their shortest interisland linear distances (km), were acquired from the EDINA Digimap collections software (Edinburgh University).
Dating of Orkney vole specimens collected from the different archaeological sites was ascertained through direct 14C assays using Accelerator Mass Spectrometry (AMS) on hemimandibles (Martínková et al. 2013). The mean of the calibrated 14C age values in years before present (BP) for each archaeological site was used as the radiocarbon age of the different Orkney vole samples in our dataset. Vole samples without direct 14C dating (such as those from the Links of Noltland and Tofts Ness) have been assigned ages in calendar years BP estimated from 14C dating of the archaeological context from which they have been collected.
We could match 61 modern and ancient specimens from Orkney for combined geometric morphometric and genetic analyses (Supporting Information 4), using the molar and mtDNA sequence data (mt‐cytb, Martínková et al. 2013). Modern samples that were morphologically and genetically analyzed were from locations widely distributed over the Orkney archipelago (Table 1), whereas ancient specimens similarly analyzed were from Neolithic Westray (Orkney) and Iron Age Mainland (Orkney, see Table 2). The molecular distance between individuals (Supporting Information 5) was generated using the Kimura 2‐parameter (K2P) model in PAUP* (Swofford 2002).
STATISTICAL METHODS
Taxonomic identification of archaeological specimens
Two sympatric species of Microtus (arvalis and agrestis) occur in continental Europe and display very similar tooth morphology (Chaline and Mein 1979) that can result in misidentification (Hall and Yalden 1978). Therefore, species identification of archaeological voles from continental Europe was performed with a predictive approach based on a linear discriminant analysis (LDA) using a modern comparative sample of 31 M. arvalis and 29 M. agrestis from France (Table 1). Of the available 26 principal components (PC) of shape, the first nine were used to classify specimens following dimensionality reduction based on cross‐validation percentages (leave‐one‐out procedure) of correct reassignments (Baylac and Friess 2005; Sheets et al. 2006). This classification was based on the generalized distance (D 2) and the associated probability of group membership between the archaeological specimen and the centroid of both reference taxonomic groups. Only ancient specimens associated to M. arvalis with a predictive probability above 0.9 have been included in the study.
Differentiation of molar size and shape in M. arvalis
Molar size variation was assessed among samples by an analysis of variance (ANOVA) on the log‐transformed CS, with subsequent pairwise t‐tests between samples using a Bonferroni correction. The molar shape variation among samples was tested using multivariate analysis of variance (MANOVA) and measured by Procrustes distances (Euclidian distances between two configurations of Procrustes coordinates) using permutation tests (10,000 runs). The main axes of variation are displayed with a principal components analysis (PCA). The visualization of molar shape‐change along the principal axes is depicted by the magnitude of change in Procrustes distances.
Covariates of molar size and shape differentiation
To assess the influence of climatic factors (latitude, longitude, altitude, precipitation, temperature), island size (area), and time elapsed since introduction on molar size and shape among Orkney vole populations, we used univariate (CS) and multivariate (Procrustes coordinates) linear regressions with permutation tests (10,000 runs). The influence of geographic distance from Mainland Orkney on shape divergence among other Orkney populations was tested with a linear regression model and Pearson's R correlation test between the paired geographic and shape distances of each modern sample from Mainland, respectively measured in kilometers and Procrustes distances.
The size‐related shape changes (allometry) among samples were tested with a multivariate analysis of covariance (MANCOVA) with Procrustes coordinates as dependent variables and log CS as a covariate. The lack of a significant interaction between the shape and size differences among samples would signal a lack of influence of size over the patterns of shape divergence, interpreted as common allometric trajectories among samples. The amount of shape change explained by size difference was assessed by multivariate regression with Procrustes coordinates as dependent variables and the log‐transformed CS as the independent variable. To test the null hypothesis of independence, permutation tests (1000 runs) were carried out.
The influence of the interisland and genetic distances over molar shape differentiation among the Orkney vole populations was tested using PROTEST, since it has proven to perform better than the Mantel test (Jackson 1995; Peres‐Neto and Jackson 2001). We used a principal coordinate analysis (PCoA) to convert distances matrices into a Cartesian coordinate system (Gower 1966) and 1000 random permutations to test for the significance of the correlation index (Monte‐Carlo, Jackson 1995).
Allochronic changes along the Orkney Mainland time series
The evolution of molar shape changes in Mainland Orkney voles, from their Neolithic introduction until the present time, is an allochronic design (Hendry and Kinisson 1999), involving Mainland Orkney populations composed of six assemblages representing a time series of four broad chronological periods: Neolithic (Qu, SB1 and SB2), Iron Age (Ho), Medieval (EB), and present‐day (Modern Mainland), with varying time intervals between the successive samples. Evolutionary rates are dependent on the time interval over which they are measured, with shorter time intervals tending to lead to higher observed evolutionary rates, because short‐term fluctuations are buffered over long time periods (Gingerich 1983). The limited number of successive samples and the uneven time intervals separating them, renders it difficult to calculate evolutionary rates accurately. Instead, morphological distances among successive samples were calculated. Mahalanobis's distances (D, Mahalanobis 1936) were chosen, because expressing among‐sample difference relative to within‐sample variance provides a multivariate analog to the morphological distances used to estimate evolutionary rate of complex traits in haldanes (Lerman 1965; Cherry et al. 1982), appropriate for geometric morphometric analyses (Arnegard et al. 2010; Carlson et al. 2011; Adams 2014). The calculation of D was performed based on 32 PCs, according to a procedure of dimensionality reduction described previously (Taxonomic identification of archaeological specimens).
Size (log CS), shape (estimated by the second axis of the analysis focused on Orkney) and morphological distances (D) were expressed against time to provide a comprehensive picture of phenotypic evolutionary change in Orkney following initial divergence.
PCA, multivariate regressions, and visualizations of shape change were performed with MorphoJ (Klingenberg 2010). Linear regressions, PCA, LDA, ANOVA, MANOVA/MANCOVA, and PROTEST were carried out with R version 2.13.0 (R development Core Team) with the ade4 package (Dray and Dufour 2007) and the library Rmorph (Baylac 2012).
Results
DIVERGENCE OF ORKNEY VOLES FROM THEIR CONTINENTAL AND OTHER INSULAR RELATIVES: MOLAR SIZE, SHAPE, AND ALLOMETRY
Ancient and modern Orkney voles have larger molars than ancient and modern continental and other insular populations of M. arvalis (F = 79.77, P < 0.0001), a tooth size only matched by the insular population from Guernsey (Fig. 3). Molar sizes in ancient voles are 5% larger than present‐day ones, both in continental Europe (F = 40.03, P < 0.0001) and Orkney (F = 10.11, P < 0.0001)—except in Spain where M. arvalis shows no reduction in molar size since the LGM. Despite significant differences (F = 49.06, P < 0.0001), the size range of M. arvalis’ molars remains stable, from the LGM (Spanish Abr1) till Medieval times (Belgium AN), and shows no evidence for the drastic size reduction manifested in current populations (Fig. 3). Modern Orkney voles display a molar size reduction of 5% compared to archaeological samples (Fig. 3, Supporting Information 6). However, only the medieval specimens from EB are significantly smaller than all the other archaeological samples from Orkney (Supporting Information 6), suggesting that in Orkney, a decrease in molar size occurred earlier than on the continent—i.e., between the 4th and 12th centuries AD.
Figure 3.
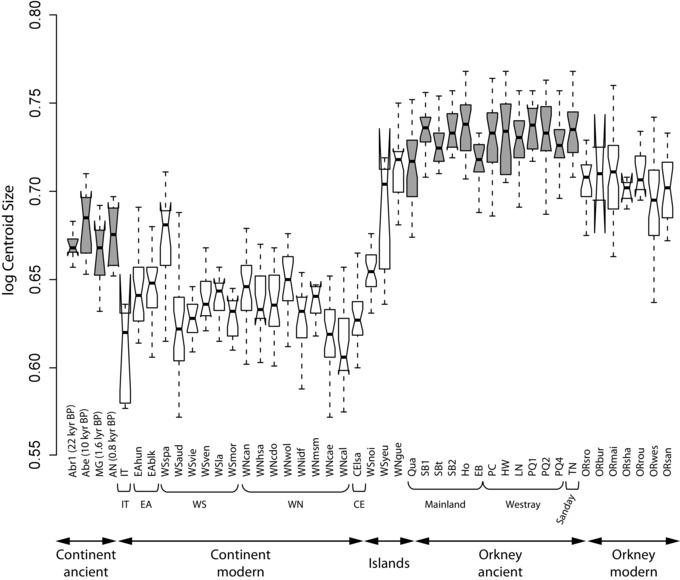
Variation in molar size (log‐transformed centroid size) in ancient (gray boxes) and modern (open boxes) Microtus arvalis samples (see Fig. 1 for localization and Tables 1 and 2 for sample details). Left, modern continental European samples include specimens grouped according to their genetic lineage (IT, Italian; EA, Eastern; WS, Western‐South; WN, Western‐North; CE, Central) and insular origin (Yeu, WSyeu; Noirmoutier, WSnoi; Guernsey, WNgue). Right, ancient Orkney specimens are grouped according to their geographic location in the archipelago.
Despite great variability of molar size across recent continental European samples (ANOVA, F = 30.83, P < 0.0001), tooth size does not correlate with genetic lineage (Fig. 3). Insular samples tend to have a larger M1 compared to their continental European counterparts (Supporting Information 6), with only Orkney and Guernsey (WNgue) displaying “gigantism” (Fig. 3). No significant size difference was found amongst modern Orkney populations according to pairwise t‐tests (Supporting Information 6).
As with size, molar shape distinguishes Orkney and Guernsey voles from other M. arvalis (Fig. 4A). The main variation in M. arvalis molar shape corresponds to a broadening/narrowing of the M1 anterior loop associated with the closing/narrowing of reentrant angle (lra5) between triangle 7 and the anterior loop (Fig. 4B). The shape disparity among Orkney voles contrasts with the conservatism observed in continental M. arvalis over time, where (despite significant variation among samples [Pillai's = 6.434; F = 2.405; P < 0.0001]) no clear patterning emerges among genetic lineages or between ancient and modern samples (Fig. 4A). No intermediate phenotype can be observed between continental and Orkney voles, not even in the samples from northern coastal France and medieval Belgium (AN)—areas considered likely geographic sources of Orkney voles (Martínková et al. 2013).
Figure 4.
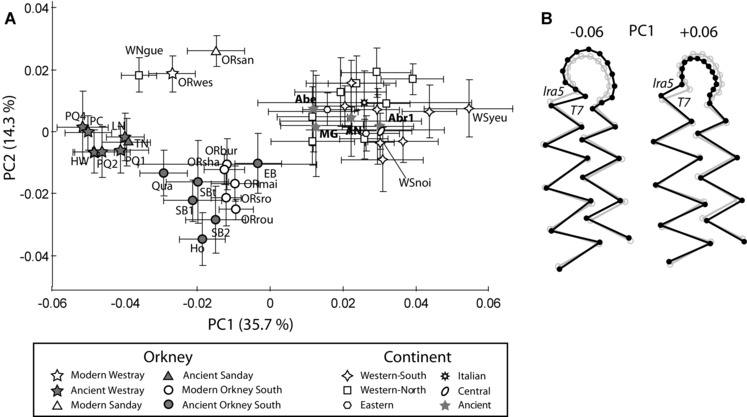
Molar shape differentiation of modern and ancient Microtus arvalis samples from continental Europe (islands included) and Orkney. (A) Scatter plot of the two first principal components of the morphometric analysis. Each symbol with its group code (Tables 1 and 2) corresponds to the mean value of a modern or ancient group, bracketed by the 95% confidence interval. (B) Molar shape change associated with PC1 is depicted with a wireframe graph connecting the landmarks and semilandmarks. The gray wireframe represents the mean shape and the black wireframe represents the shape changes along PC1 in negative (−0.06) and positive (+0.06) directions.
Overall, there is no common signature for island populations, since Noirmoutier (WSnoi) and Yeu (WSyeu) lie within the range of variation of mainland European samples (Fig. 4A). Guernsey voles show aspects of shape that are close to voles from the northern Orkney Isles (Westray and Sanday) along PC1 and PC2. However, the shape of Orkney and Guernsey voles is highly differentiated (Pillai's = 3.432; F = 6.976; P < 0.0001; Procrustes distance: 0.049; P < 0.001).
What does differentiate the Orkney and the Guernsey populations of M. arvalis from all their continental (mainland and island) European relatives is the hypertrophy of the anterior loop of their molars. This peculiar trait is partly size‐related as suggested (first) by the relationship between the PC1 (Fig. 4) and the CS (slope = −10.449; intercept = 4.813; r2 = 0.469; permut. P < 0.0001) and (second) by the significant allometric component explaining 20% of the shape variation (permut. P < 0.0001) mainly localized in the anterior part of the M1 (Fig. 5). However, a MANCOVA with tooth shape as a dependent factor showed significant effects of size (Pillai's = 0.4788; F = 10.4103, P < 0.0001), geographic groups (Pillai's = 8.3995; F = 2.6993, P < 0.0001), and size × group interaction (Pillai's = 4.1818; F = 1.2083, P < 0.0001). The latter indicates that the allometric pattern varies among samples.
Figure 5.
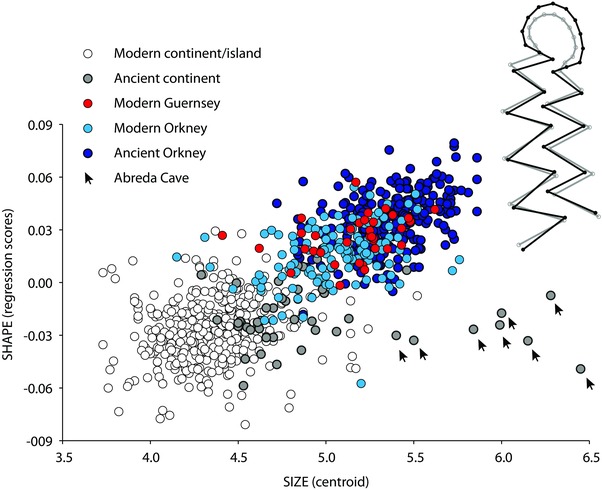
Allometric trend in modern and ancient Microtus arvalis estimated from the regression of Procrustes coordinates on centroid size. Insert: the gray lines and open circles represent the average shape and the black lines and circles represent the predicted shape for a centroid size increase of 2 mm.
LOCAL DIVERGENCE AMONG ORKNEY VOLES
The diversification in modern and ancient Orkney voles (Fig. 6) seems to have been driven first by the geography of the archipelago (PC1 separating southern from northern populations) and second by the time elapsed since their introduction (as shown by variation along PC2). Voles from Mainland (ORmai) and its satellite islets of Shapinsay (ORsha), Burray (ORbur), South Ronaldsay (ORsro), and Rousay (ORrou) are phenotypically close compared to the highly divergent (P < 0.001) Westray (ORwes) and Sanday (ORsan) specimens (Fig. 6A). The molar shape differences associated with this geographic divergence are related to modifications in the buccal (bra4) and labial (lra5) reentrant angles of the sixth and seventh cusps, with a relatively smaller and less rounded anterior loop present in the southern isles populations (Fig. 6B).
Figure 6.
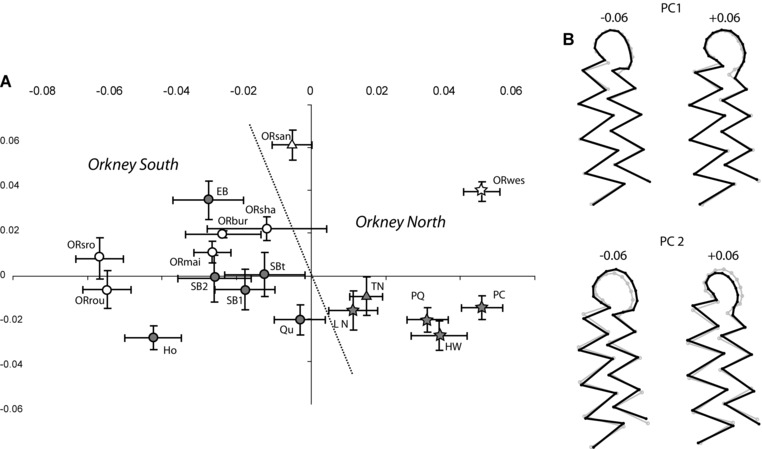
Molar shape variation in modern and ancient Microtus arvalis samples from Orkney. (A) Scatter plot of the two first principal components of the morphometric analysis. The modern samples are represented by empty symbols whereas the ancient samples are shown by gray‐filled circles. The dotted line separates northern from southern populations. (B) Molar shape change associated with PC1 and 2 is depicted by a wireframe graph connecting the landmarks and semilandmarks. The gray wireframe represents the mean shape and the black wireframes indicate the shape change of the PC score by 0.6 units in the positive and negative directions.
Time elapsed since introduction appears to have contributed to the pattern of diversification in Orkney voles, according to the significant association between radiocarbon age of the ancient samples and their molar shape (permut P: 0.038)—predicting 22% of variation. Shape divergence over time, which has been more pronounced in the northern isles (Fig. 6A), is associated with a reduction of the anterior loop (Fig. 6B). On the Orkney Mainland, the temporal divergence is less substantial and suggests periods of relative morphological stability, contrasting with intervals of marked changes.
Part of the divergence in shape of Orkney voles since introduction is associated with a small (7.2%) but significant (P < 0.0001) allometric adjustment attributable to molar size reduction between ancient and extant populations (Fig. 5).
CLIMATIC, GEOGRAPHIC AND GENETIC DETERMINANTS OF MORPHOLOGICAL DIVERGENCE
The climatic factors tested in relation to molar variation in the modern M. arvalis populations of our dataset (Tables 3 and 4) show that only size (not shape) is influenced within the geographic range studied. Thirty percent of the molar size variation is correlated with latitude, mean and maximum precipitation, and maximum temperature (Table 3) suggesting that M. arvalis molar size is influenced by the latitudinal gradient.
Table 3.
A. Linear regression model testing for influence of climatic factors on centroid size. B. Multivariate regression model testing for influence of climatic factors on shape (Procrustes coordinates)
| A | Slope | Error | Intercept | Error | r 2 | Permutation P |
|---|---|---|---|---|---|---|
| Latitude | 21.0740 | 8.0120 | 16.6440 | 12.0780 | 0.30 | 0.0302 * |
| Longitude | 0.0131 | 0.0272 | 4.5113 | 4.5113 | 0.01 | 0.6483 |
| T m | −0.56663 | 0.89727 | 13.759 | 4.0787 | 0.01 | 0.6483 |
| T max | −2.6312 | 1.0362 | 30.444 | 4.7101 | 0.29 | 0.0327 * |
| T min | 1.1388 | 1.0088 | −0.67746 | 4.5857 | 0.07 | 0.2681 |
| P m | 14.625 | 5.3215 | 2.2146 | 24.19 | 0.33 | 0.0128 * |
| P max | 124.5200 | 58.1860 | −86.9090 | 87.7180 | 0.22 | 0.0460 * |
| P min | 14.1480 | 19.4410 | 18.6460 | 21.8470 | 0.03 | 0.4767 |
| B | Predicted Sum of Squares | Residual Sum of Squares | Percentage predicted | Permutation P |
|---|---|---|---|---|
| Latitude | 0.001571 | 0.012390 | 11.25 | 0.0989 |
| Longitude | 0.001262 | 0.012699 | 9.04 | 0.1504 |
| Altitude | 0.000421 | 0.013540 | 3.01 | 0.0824 |
| T m | 0.001667 | 0.012294 | 11.94 | 0.0674 |
| T max | 0.001724 | 0.012237 | 12.35 | 0.0743 |
| T min | 0.007832 | 0.013178 | 5.61 | 0.4128 |
| P m | 0.001564 | 0.012396 | 11.21 | 0.0739 |
| P max | 0.001424 | 0.012537 | 10.20 | 0.1010 |
| P min | 0.000322 | 0.013639 | 2.31 | 0.9426 |
r2 and permutation P‐values in bold and followed by an asterisk (*) remain significant at 0.05 level.
Molar shape diversification among Orkney Island populations (Fig. 5) is neither related to island size (P = 0.992) nor to the distances between them (PROTEST, Monte‐Carlo = 0.569, P = 0.122). However, the distance from Mainland Orkney (probably the first island colonized and thus the source for subsequent dispersal: Martínková et al. 2013) appears to be a factor that has contributed significantly to this molar shape diversification according to Pearson's test (Pearson's R = 0.825, P = 0.042) and close to significant according to the linear regression (slope = 0.0016; intercept = 0.0531; r2 = 0.682; permut P < 0.0566). A significant correlation was found between pairwise genetic differentiation and shape differentiation measured by Procrustes distances among combined modern and ancient M. arvalis orcadensis populations (PROTEST, Monte‐Carlo = 0.404, P < 0.001), suggesting some concordance between molar shape and genetic divergence.
MORPHOLOGICAL CHANGES IN MAINLAND ORKNEY VOLES AFTER INITIAL DIVERSIFICATION
After the major divergence following their introduction, tooth morphology in Mainland Orkney voles did not follow the expected pattern of stasis (Fig. 7). During the earliest phase of the Neolithic, molar size experienced a further increase—seen between the sites of Quanterness (Qu) and Skara Brae (SB1), apparently followed by a stasis until the Late Iron Age—represented by specimens from Howe (Ho). Thereafter, specimens from Earl's Bu (EB) document a drastic size reduction between the 4th and the 12th century AD, which continued until the present time.
Figure 7.
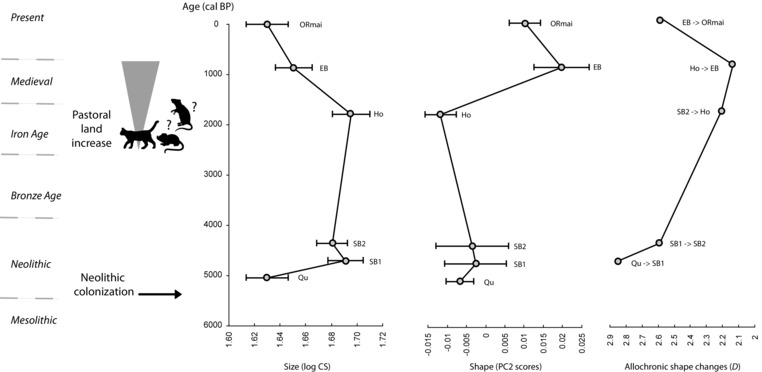
Morphological change over time in Mainland Orkney voles. (1) Molar size evolution depicted by mean size with confidence intervals. (2) Molar shape evolution depicted by mean scores along the second principal component axis detailed in Figure 6. (3) Allochronic shape changes expressed as Mahalanobis's distances (D) between each chronological step of the time series.
Far from displaying stasis after initial divergence, molar shape in Mainland Orkney voles also changed over time. During the early part of the record, limited divergence occurred more or less proportionally to the time elapsed. As was observed for size, a more substantial shift in molar shape occurred during the interval between Howe and Earl's Bu (i.e., between later prehistory and early Medieval times. Shape remained rather stable thereafter until modern times. This overall pattern is corroborated by considering morphological distance between successive samples, with the greatest morphological change occurring between Howe and Earl's Bu (Fig. 7).
Discussion
RAPID DIVERGENCE OF ORKNEY VOLES AFTER THEIR NEOLITHIC INTRODUCTION
The dental characteristics of Orkney voles—molar size gigantism and hypertrophied anterior loop—were acquired within less than a century (possibly within a few decades) after their Neolithic introduction and colonization of Orkney. No intermediate phenotype between continental European and Orkney M. arvalis was observed in the earliest (4th millennium BC) specimens from either Mainland Orkney or Westray, providing further evidence that newly colonizing island rodents exhibit extremely rapid initial divergence (Millien 2006; Nagorsen and Cardini 2009; Evans et al. 2012). Hence, phenotypic similarities that likely existed with a continental European source population were completely erased by this rapid morphological shift, rendering the identification of an ancestral phenotype among the continental populations all but impossible (Corbet 1986).
The molar size increase observed in M. arvalis on Orkney presumably reflects body size and may be a response to different levels of primary productivity (Rosenzweig 1968; Yom‐Tov and Geffen 2006, 2011; Medina et al. 2007; Blois et al. 2008) and/or a thermoregulatory response in accordance with Bergmann's rule. An alternative hypothesis would be that release of predation and competition pressure allowed the rodents to track their metabolic optimum by becoming larger (Damuth 1993.; Adler and Levins 1994; Michaux et al. 2002; Lomolino 2005; McNab 2010). This hypothesis is supported by a similar trend toward gigantism in Orkney and Guernsey voles, located on islands experiencing a different environmental regime but both characterized by the absence of the two major terrestrial predators of M. arvalis in continental Europe—i.e., stoats (Mustela erminea) and weasels (Mustela nivalis, Yalden 1999). Voles introduced to Orkney by Neolithic farmers faced only avian predation from hen harriers (Circus cyaneus) and short‐eared owls (Asio flammeus, Berry 1985)—and interspecific competition was reduced to only the wood mouse (Apodemus sylvaticus, Corbet 1979). In contrast, voles from Yeu and Noirmoutier—where weasels are present (Saint‐Girons and Nicolau‐Guillomet 1987)—do not display molar size gigantism as seen in the Guernsey and Orkney populations.
This molar gigantism apparently contributed to the hypertrophy seen in the anterior loop of the M1 through size allometry (Klingenberg 1996). In Microtus sp., the M1 anterior loop is the most variable and evolvable part of the tooth (Chaline et al. 1999; Jernvall et al. 2000; Renvoisé et al. 2009) due to its late development during morphogenesis (Jernvall et al. 2000). A similar mechanism has been evoked to explain parallel evolution in molar shape in insular house mice (Mus musculus domesticus, Renaud et al. 2011). The anterior elongation of the molar may correspond to a “line of least resistance to evolution” (Schluter 1996) related to developmental properties that are prone to being mobilized by size increase. Hence, drastic molar‐size increase driven by predation release and change in food resources (acting on overall body size) could have induced a broadening of the anterior loop of the molar.
The parallel evolution of a partly similar dental phenotype in Orkney and Guernsey voles—despite different phylogenetic signatures (Martínková et al. 2013)—supports the interpretation of a line of least evolutionary resistance, rather than a consequence of a common origin, as suggested by early 20th‐century naturalists (Miller 1909).
FOUNDING EVENTS AND ORKNEY VOLE DIVERSIFICATION
The molar shape divergence revealed among Orkney voles—first separating voles from the southern and northern isles, and then islands within the two parts of the archipelago—could fit a scenario of diversification related to progressive subdivision of a single landmass as a consequence of sea‐level rise. This scenario, however, is not supported by the geology of the archipelago. Although Orkney was a single island during the Younger Dryas 13,000 years ago (Bates et al. 2013), relative sea‐level around Orkney by the time of Neolithic colonization between 5300 and 5100 years ago, is thought to have been approximately only 2 m lower than today (Bates et al. 2013).
Given the environmental uniformity among the Orkney Islands, the most likely processes driving this local evolution are chance effects related to successive founder events and subsequent genetic drift in the progressive colonization of the archipelago, together with the impermeability of the local population (once installed) to later invaders (Granjon and Cheylan 1988; Hardouin et al. 2010). According to data from mitochondrial and nuclear DNA, colonization occurred first on Mainland Orkney, followed by human‐mediated transportation of a few founders to the northern islands (Martínková et al. 2013). This colonization scenario is supported by the influence of geographic distance from Mainland Orkney in molar shape divergence of current Orkney vole populations, the small but significant overall influence of time elapsed since introduction on molar shape divergence and the congruence between the divergence patterns provided by molar shape and neutral molecular markers.
ANTHROPOGENIC FORCES TRIGGERING POST‐ NEOLITHIC EVOLUTIONARY CHANGES
The archaeological time series on Mainland Orkney has also recorded phenotypic shifts in the morphology of Orkney voles millenia after their prehistoric introduction (Fig. 7). This finding contradicts the expectation that in an island setting, the voles should achieve an ecological optimum (after an initial divergence), and then change little after that. Instead, a major phenotypic shift is observed between the late Iron Age (4th to 7th centuries AD) and Medieval times (11th to 12th centuries AD). This phenotypic shift, long after their Neolithic introduction, suggests that evolution in the Orkney vole has been intimately linked with human influence on habitat and environment of the archipelago throughout its Holocene history (Hendry et al. 2008).
A later (post‐Medieval) molar size reduction in continental European M. arvalis also appears to be recorded in our data. This suggests that, for M. arvalis at least, body size reduction between fossil and modern specimens has not been a response to long‐term, natural global warming since the LGM (Millien et al. 2006), but rather a more recent anthropogenic phenomenon (Sheridan and Bickford 2011). A similar trend observed in mice species has been linked with pervasive anthropogenic perturbations—from habitat destruction to climate change (Cassaing et al. 2011; Stoetzel et al. 2013)—during very recent times. However, the archaeological record for Mainland Orkney shows size reduction in voles much earlier, between the 4th and 12th centuries AD, suggesting that anthropogenic impacts could have had greater/earlier effects on small mammals in confined/insular environments and/or in higher latitudinal locations.
The most dramatic shift in molar shape within Orkney voles is observed at the same time as size change, supporting the idea that major changes in the environment of the voles occurred at that period. Although human‐induced modification of the Orkney landscape was initiated as early as 5000 BP (Bunting 1994), the expansion of pastoral farming during the early and middle Iron Age (Bond 2002) led to an almost entirely open landscape with increasing numbers of livestock (Bunting 1994).
Around the same time, commensal mice (Mus musculus domesticus) and rats (Rattus rattus) were probably introduced to Orkney—they were present in mainland Britain during the Iron Age and Roman times, respectively (Yalden 1999). These commensal alien species might not have had a drastic effect on the Orkney vole populations since they did not compete for the same habitat. However, during the Iron Age, new terrestrial predators such as the domestic cat (Felis sylvestris) and fox (Vulpes vulpes)—both considered major predators of Orkney voles—were introduced to Orkney. Foxes are first recorded on Orkney in various Iron Age sites (Fairnell and Barrett 2007), whereas domestic cats are present as early as the 1st century AD at Ho (Ballin Smith 1994; O'Connor 2007.), Mine Howe (Mainland; unpublished data) and in later Iron Age deposits at Pool (Bond 2007) on Sanday. By the Viking and Norse periods, between 8th and 12th century AD, cats were clearly well established, occurring frequently in most archaeological sites of this period (Fairnell and Barrett 2007), while foxes disappear from the record.
The various changes associated with the increase in pasture land, and especially the introduction of foxes and cats, likely impacted Orkney vole populations (Whittaker 1998)—disturbing the local ecological equilibrium (Yom‐Tov et al. 1999; Sondaar 2000) of the species. Nevertheless, the impact of these changes on vole morphology seems to have become significant only when anthropogenic changes had become extensive.
Conclusion
Orkney voles have evolved their own particular dental phenotype, likely the result of human agency influencing its evolutionary trajectory in different ways over the last 5000 years. This human influence began with its Neolithic introduction to the Orkney Mainland at a time when there were no terrestrial predators and only one competing species (the wood mouse). The Orkney vole population rapidly diverged from continental European M. arvalis to reach a new ecological optimum, that included evolutionary changes in morphology of the molar teeth. Neolithic farmers then dispersed the species to other islands of the archipelago—from Mainland to Westray and during the Bronze Age to Sanday—generating several founding events contributing to idiosyncratic differences in dental characteristics. This initial divergence and diversification in Orkney voles was not followed by morphological stasis because the Orkney environment was subjected to continued human disturbance.
The case of the Orkney vole presented here demonstrates how, from Neolithic times, humans have played a major role in species evolution and suggests that anthropogenic modifications of the environment may have repeatedly disturbed the phenotypic evolutionary stasis of insular species. Given the continental‐scale and increasing intensity of human‐induced impact on ecosystems in the last centuries, such changes in the evolutionary trajectories of vertebrates are likely not restricted to insular systems.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1.
ACKNOWLEDGMENTS
We dedicate this article to the memory of our colleague Michel Pascal who passed away on the 5th January 2013 and to Anne Brundle who passed away on 18th January 2011. Michel was renowned in the field of biological invasions, particularly in relation to rodents, and he was known to everyone as the “Ratator.” Anne was museum curator at the Tankerness house Museum in Kirkwall and was instrumental in providing us with access to key archaeological collections of Orkney voles. We thank V. Bretille, N. Gould, J. S. Herman, E. P. Jones, S. Martínek, R. Marwick, J. Michaux, S. Montuire, J. Pauperio, C. Scott, B. Walther, and N. Wheale for field specimens. We are most grateful to A. Shepherd, A. Ritchie, M. S. Segui, A. Tresset, and V. Mistrot for the archaeological samples and archaeological advice. We thank A. Cardini and D. Polly for morphometric advice. We thank M. Fujita and L. Killick for their help in the acquisition of teeth images.
We thank the following for access to museum and archaeological samples: J. Barrett (MacDonald Institute, University of Cambridge), A. Brundle (Orkney Museum), C. David (Guernsey Museum), A. Ervynck (Flemish Heritage Institute), L. Gordon (Smithsonian Institute), J. S. Herman (National Museums of Scotland), D. Lee (Orkney College), R. Sabin (British Museum – Natural History, London), G. Veron (Muséum national d'Histoire naturelle, Paris). We thank A. R. Hoelzel for his contribution to the Orkney vole project. We acknowledge the receipt of major funding from the Arts and Humanities Research Council (project grant 119396) for KMD and JBS, a Marie Curie Intra European Fellowship (to NM), and support from a Wellcome Trust University award to KMD (GR071037). Finally, we are most grateful to Virginie Millien for all the useful comments of her review that have greatly improved the quality of the manuscript.
DATA ARCHIVING
The data are archived in the Supporting Information.
LITERATURE CITED
Associate Editor: Dean Adams
- Adams, D. C. 2014. Quantifying and comparing phylogenetic evolutionary rates for shape and other high‐dimensional phenotypic data. Syst. Biol. 63:166–177. [DOI] [PubMed] [Google Scholar]
- Adams, D. C. , Rohlf F. J., and Slice D. E.. 2013. A field comes of age: geometric morphometrics in the 21st century. Hystrix 24:7–14. [Google Scholar]
- Adler, G. H. , and Levins R.. 1994. The island syndrome in rodent populations. Q. Rev. Biol. 69:473–490. [DOI] [PubMed] [Google Scholar]
- Arnegard, M. E. , McIntyre P. B., Harmon L. J., Zelditch M. L., Crampton W. G. R., Davis J. K., Sullivan J. P., Lavoué S., and Hopkins C. D.. 2010. Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Am. Nat. 176:335–356. [DOI] [PubMed] [Google Scholar]
- Ballin Smith, B . 1994. Howe. Four millenia of Orkney prehistory. Society of Antiquaries of Scotland, Edinburgh. [Google Scholar]
- Barber, J. 1997. The excavation of a stalled cairn at the Point of Cott. Scottish Trust for Archaeological Research, Edinburgh, Westray, Orkney. [Google Scholar]
- Bates, M. R. , Nayling N., Bates R., Dawson S., Huws D., and Wickham‐Jones C.. 2013. A multi‐disciplinary approach to the archaeological investigation of a bedrock‐dominated shallow‐marine landscape: an example from the Bay of Firth, Orkney, U.K. Int. J. Naut. Archaeol. 42:24–43. [Google Scholar]
- Batey, C. E. , Harry R. C., and Morris C. D.. 1993. Excavations at the Earl's Bu. Glasgow University Archaeological Research Division, Glasgow, Orphir, Orkney. [Google Scholar]
- Baylac, M. 2012. Rmorph: A R geometric and multivariate morphometrics library.
- Baylac, M. , and Friess M.. 2005. Fourier descriptors, Procrustes superimposition, and data dimensionality: an example of cranial shape analysis in modern human populations Pp. 145–166 in Slice D. E., ed. Modern morphometrics in physical anthropology. University of Chicago, Chicago. [Google Scholar]
- Berry, R. J. 1985. The natural history of Orkney. Collins, Lond. [Google Scholar]
- Berry, R. J. . 1996. Small mammal differentiation on islands. Philos. Trans. R. Soc. Biol. Sci. 351:753–764. [DOI] [PubMed] [Google Scholar]
- Berry, R. J. , and Rose F. E. N.. 1975. Islands and the evolution of Microtus arvalis (Microtinae). J. Zool. 177:395–409. [Google Scholar]
- Blois, J. L. , Feranec R. S., and Hadly E. A.. 2008. Environmental influences on spatial and temporal patterns of body‐size variation in California ground squirrels (Spermophilus beecheyi). J. Biogeogr. 35:602–613. [Google Scholar]
- Blondel, J. , and Vigne J.‐D.. 1993. Space, time and man as determinants of diversity of birds and mammals in the Mediterranean region Pp. 135–146 in Ricklefs R. E. and Schluter D., eds. Species diversity in ecological communities. Ecological and geographical perspectives. Chicago Univ. Press, Chicago. [Google Scholar]
- Bond, J. 2002. Pictish pigs and Celtic cowboys: food and farming in the Atlantic Iron Age Pp. 177–184 in Ballin Smith B. and Banks I., eds. In the shadow of the Brochs. Tempus, Stroud, Gloucestershire. [Google Scholar]
- Bond, J. . 2007. The bioarchaeological evidence Pp. 169–286 in Hunter J., ed. Investigations in Sanday, Orkney. Vol 1: excavations at Pool, Sanday. The Orcadian Ltd, Kirkwall. [Google Scholar]
- Bookstein, F. L. 1997. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med. Image Anal. 1:225–243. [DOI] [PubMed] [Google Scholar]
- Braaker, S. , and Heckel G.. 2009. Transalpine colonisation and partial phylogeographic erosion by dispersal in the common vole (Microtus arvalis). Mol. Ecol. 18:2518–2531. [DOI] [PubMed] [Google Scholar]
- Bradshaw, W. E. , and Holzapfel C. M.. 2006. Evolutionary response to rapid climate change. Science 312:1477–1478. [DOI] [PubMed] [Google Scholar]
- Bunting, M. J. 1994. Vegetation history of Orkney, Scotland; pollen records from two small basins in West Mainland. New Phytol. 128:771–792. [Google Scholar]
- Bunting, M. J. . 1996. The development of heathland in Orkney, Scotland: pollen records from Loch of Knitchen (Rousay) and Loch of Torness (Hoy). Holocene 6:193–212. [Google Scholar]
- Carlson, B. A. , Hasan S. M., Hollmann M., Miller D. B., Harmon L. J., and Arnegard M. E.. 2011. Brain evolution triggers increased diversification of electric fishes. Science 332:583–586. [DOI] [PubMed] [Google Scholar]
- Carroll, S. P. , Hendry A. P., Reznick D. N., and Fox C. W.. 2007. Evolution on ecological time‐scales. Funct. Ecol. 21:387–393. [Google Scholar]
- Cassaing, J. , Sénégas F., Claude J., and de la Rivière B. L. P.. 2011. A spatio‐temporal decrease in molar size in the western European house mouse. Mammal. Biol. 76:51–57. [Google Scholar]
- Chaline, J. , and Mein P.. 1979. Les rongeurs et l'évolution. Doin, Paris. [Google Scholar]
- Chaline, J. , Brunet‐Lecomte P., Montuire S., Viriot L., and Courant F.. 1999. Anatomy of the arvicoline radiation (Rodentia): palaeogeographical, palaeoecological history and evolutionary data. Ann. Zool. Fennici 36:239–267. [Google Scholar]
- Cherry, J. F. 1990. The first colonization of Mediterranean islands: a review of recent research. J. Medit. Archaeol. 3:145–221. [Google Scholar]
- Cherry, L. M. , Case S. M., Kunkel J. G., Wyles J. S., and Wilson A. C.. 1982. Body shape metrics and organismal evolution. Evolution 36:914–933. [DOI] [PubMed] [Google Scholar]
- Clarke, D. V. 1976. The neolithic village at Skara Brae, Orkney 1972–1973 excavations. Pp. 4–27. National Museum of Antiquities of Scotland, Edinburgh. [Google Scholar]
- Clarke, D. V. , and Sharples N.. 1985. Settlement and subsistence in the third Millennium BC Pp. 54–82 in Renfrew C., ed. The prehistory of Orkney. Society of Antiquaries of Scotland, Edinburgh. [Google Scholar]
- Clegg, S. M. , Degnan S. M., Moritz C., Estoup A., Kikkawa J., and Owens I. P. F.. 2002. Microevolution in island forms: the role of drift and directional selection in morphological divergence of passerine birds. Evolution 56:2090–2099. [DOI] [PubMed] [Google Scholar]
- Clegg, S. M. , Frentiu F. D., Kikkawa J., Tavecchia G., and Owens I. P. F.. 2008. 4000 years of phenotypic change in an island bird: heterogeneity of selection over three microevolutionary timescales. Evolution 62:2393–2410. [DOI] [PubMed] [Google Scholar]
- Corbet, G. B. 1964. The identification of British mammals. British Museum, Lond. [Google Scholar]
- Corbet, G. B. . 1979. Report on rodent remains Pp. 135–137 in Renfrew C., ed. Investigations in Orkney. The Society of Antiquaries of London, Lond. [Google Scholar]
- Corbet, G. B. . 1986. Temporal and spatial variation of dental pattern in the voles, Microtus arvalis, of the Orkney Islands. J. Zool. 208:395–402. [Google Scholar]
- Damuth, J. 1993.. Cope's rule, the island rule and the scaling of mammalian population density. Nature 365:748–750. [DOI] [PubMed] [Google Scholar]
- Dockrill, S. J. , Bond J. M., Milles A., Simpson I., and Ambers J.. 1994. Tofts Ness, Sanday, Orkney. An integrated study of a buried Orcadian landscape Pp. 115–132 in Luff R. and Rowley‐Conwy P., eds. Whither Environmental Archaeology. Oxbow Monograph, Oxford, U.K. [Google Scholar]
- Dray, S. , and Dufour A. B.. 2007. The ade4 Package: implementing the duality diagram for ecologists. J. Stat. Softw. 22:1–20. [Google Scholar]
- Evans, A. R. , Jones D., Boyer A. G., Brown J. H., Costa D. P., Ernest S. K. M., Fitzgerald E. M. G., Fortelius M., Gittleman J. L., Hamilton M. J., et al. 2012. The maximum rate of mammal evolution. Proc. Natl. Acad. Sci. USA 109:4023–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairnell, E. H. , and Barrett J. H.. 2007. Fur‐bearing species and Scottish islands. J. Archaeol. Sci. 34:463–484. [Google Scholar]
- Foster, J. B. 1964. Evolution of mammals on islands. Nature 202:234–235. [Google Scholar]
- Ghalambor, C. K. , McKay J. K., Carroll S. P., and Reznick D. N.. 2007. Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21:394–407. [Google Scholar]
- Gingerich, P. D. 1983. Rates of evolution – effects of time and temporal scaling. Science 222:159–161. [DOI] [PubMed] [Google Scholar]
- Gower, J. C. 1966. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53:325–328 [Google Scholar]
- Granjon, L. , and Cheylan G.. 1988. Mécanismes de coexistence dans une guilde de muridés insulaires (Rattus rattus L., Apodemus sylvaticus L., et Mus musculus domesticus Rutty) en Corse: conséquences évolutives. Zeitschrift für Säugetierkunde 53:301–316. [Google Scholar]
- Guthrie, R. D. 1965. Variability in characters undergoing rapid evolution, an analysis of Microtus molars. Evolution 19:214–233. [Google Scholar]
- Hall, J. , and Yalden D. W.. 1978. A plea for caution over the identification of Late Pleistocene Microtus in Britain. J. Zool. 186: 556–560 [Google Scholar]
- Hardouin, E. , Chapuis J.‐L., Stevens M. I., van Vuuren J. B., Quillfeldt P., Scavetta R. J., Teschke M., and Tautz D.. 2010. House mouse colonization patterns on the sub‐Antarctic Kerguelen Archipelago suggest singular primary invasions and resilience agains re‐invasion. BMC Evol. Biol. 10:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, S. , Jaarola M., and Searle J. B.. 2003. Phylogeography of the common vole (Microtus arvalis) with particuliar emphasis on the colonization of the Orkney archipelago. Mol. Ecol. 12:951–956. [DOI] [PubMed] [Google Scholar]
- Heckel, G. , Burri R., Fink S., Desmet J.‐F., and Excoffier L.. 2005. Genetic structure and colonization processes in European populations of the common vole, Microtus arvalis . Evolution 59:2231–2242. [PubMed] [Google Scholar]
- Hendry, A. P. , and Kinnison M. T.. 1999. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution 53:1637–1653. [DOI] [PubMed] [Google Scholar]
- Hendry, A. P. , Farrugia T. J., and Kinnison M. T.. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17:20–29. [DOI] [PubMed] [Google Scholar]
- Jackson, D. A. 1995. PROTEST: a Procrustean randomization test of community environment concordance. Ecoscience 2:297–303. [Google Scholar]
- Jernvall, J. , Keränen S. V. E., and Thesleff I.. 2000. Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc. Natl. Acad. Sci. USA 97:14444–14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnison, M. T. , and Hairston N. G.. 2007. Eco‐evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 21:444–454. [Google Scholar]
- Klingenberg, C. P. 1996. Multivariate allometry Pp. 23–49 in Marcus L. F., Corti M., Loy A., Naylor G. J. P., and Slice D. E., eds. Advances in morphometrics. Plenum Press, New York. [Google Scholar]
- Klingenberg, C. P. . 2010. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11:353–357 [DOI] [PubMed] [Google Scholar]
- Lambrinos, J. G. 2004. How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology 85:2051–2070. [Google Scholar]
- Lerman, A. 1965. On rates of evolution of unit characters and character complexes. Evolution 19:16–25. [Google Scholar]
- Lomolino, M. V. 2005. Body size evolution in insular vertebrates: generality of the island rule. J. Biogeogr. 32:1683–1699. [Google Scholar]
- Losos, J. B. , Warheitt K. I., and Schoener T. W.. 1997. Adaptive differentiation following experimental island colonization in Anolis lizards. Nature 387:70–73. [Google Scholar]
- Mahalanobis, P. C. 1936. On the generalized distance in statistics. Proc. Natl. Inst. Sci. India 2:49–55. [Google Scholar]
- Major, C. I. 1905. The affinities of the Orkney vole (Microtus orcadensis Millais). Ann. Mag. Nat. Hist. 7:323–324. [Google Scholar]
- Marquet, J. ‐C. 1993. Paléoenvironnement et chronologie des sites du domaine atlantique français d'âge Pléistocène moyen et supérieur d'après l'étude des rongeurs. Les Cahiers de la Claise Supplément 2:1–346. [Google Scholar]
- Martínková, N. , Barnett R., Cucchi T., Struchen R., Pascal M., Pascal M., Fischer M. C., Higham T., Brace S., Ho S. Y. W., et al. 2013. Divergent evolutionary processes associated with colonization of offshore islands. Mol. Ecol. 22:5205–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, J. L. 2010. Geometric morphometrics of vole (Microtus californicus) dentition as a new paleoclimate proxy: shape change along geographic and climatic clines. Quat. Int. 212:198–205. [Google Scholar]
- McNab, B. K. 2010. Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164:13–23. [DOI] [PubMed] [Google Scholar]
- Medina, A. I. , Martí D. A., and Bidau C. J.. 2007. Subterranean rodents of the genus Ctenomys (Caviomorpha, Ctenomyidae) follow the converse to Bergmann's rule. J. Biogeogr. 34:1439–1454. [Google Scholar]
- Michaux, J. R. , Goüy de Bellocq J., Sarà M., and Morand S.. 2002. Body size increase in insular rodent populations: a role for predators. Global Ecol. Biogeogr. 11:427–436. [Google Scholar]
- Miller, G. S. 1909. Twelve new European mammals. Ann. Mag. Nat. Hist. 8:415–422. [Google Scholar]
- Millien, V. 2006. Morphological evolution is accelerated among island mammals. Pub. Lib. Sci. Biol. 4:1863–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millien, V. . 2011. Mammals evolve faster on smaller islands. Evolution 65:1935–1944. [DOI] [PubMed] [Google Scholar]
- Millien, V. , Kathleen Lyons S., Olson L., Smith F. A., Wilson A. B., and Yom‐Tov Y.. 2006. Ecotypic variation in the context of global climate change: revisiting the rules. Ecol. Lett. 9:853–869. [DOI] [PubMed] [Google Scholar]
- Mistrot, V. 2000. Les micromammifères, marqueurs de l'anthropisation du milieu. Études rurales 153‐154:195–206. [Google Scholar]
- Mitteroecker, P. , and Gunz P.. 2009. Advances in geometric morphometrics. Evol. Biol. 36:235–247. [Google Scholar]
- Nagorsen, D. W. , and Cardini A.. 2009. Tempo and mode of evolutionary divergence in modern and Holocene Vancouver Island marmots (Marmota vancouverensis) (Mammalia, Rodentia). J. Syst. Evol. Res. 47:258–267. [Google Scholar]
- O'Connor, T. P. 2007.. Wild or domestic? Biometric variation in the cat Felis silvestris Schreber. Int. J. Osteoarchaeol. 17 581–595. [Google Scholar]
- Palumbi, S. 2001. Humans as the world's greatest evolutionary force. Science 29:1786–1790. [DOI] [PubMed] [Google Scholar]
- Peres‐Neto, P. , and Jackson D.. 2001. How well do multivariate data sets match? The advantages of a Procrustean superimposition approach over the Mantel test. Oecologia 129:169–178. [DOI] [PubMed] [Google Scholar]
- Pigliucci, M. , Murren C. J., and Schlichting C. D.. 2006. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209:2362–2367. [DOI] [PubMed] [Google Scholar]
- Piras, P. , Marcolini F., Raia P., Curcio M. T., and Kotsakis T.. 2009. Testing evolutionary stasis and trends in first lower molar shape of extinct Italian populations of Terricola savii (Arvicolidae, Rodentia) by means of geometric morphometrics. J. Evol. Biol. 22:179–191. [DOI] [PubMed] [Google Scholar]
- Price, T. D. , Qvarnström A., and Irwin D. E.. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. Biol. Sci. 270:1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud, S. , and Millien V.. 2001. Intra‐ and interspecific morphological variation in the field mouse species Apodemus argenteus and A. speciosus in the Japanese archipelago: the role of insular isolation and biogeographic gradients. Biol. J. Linn. Soc. 74:557–569. [Google Scholar]
- Renaud, S. , Pantalacci S., and Auffray J. C.. 2011. Differential evolvability along lines of least resistance of upper and lower molars in island house mice. Pub. Lib. Sci. One 6:e18951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud, S. , Hardouin E. A., Pisanu B., and Chapuis J. L.. 2013. Invasive house mice facing a changing environment on the Sub‐Antarctic Guillou Island (Kerguelen Archipelago). J. Evol. Biol. 26:612–624. [DOI] [PubMed] [Google Scholar]
- Renfrew, C. 1979. Investigations in Orkney. Society of Antiquaries, Lond. [Google Scholar]
- Renvoisé, E. , Evans A. R., Jebrane A., Labruère C., Laffont R., and Montuire S.. 2009. Evolution of mammal tooth patterns: new insights from a developmental prediction model. Evolution 63:1327–1340. [DOI] [PubMed] [Google Scholar]
- Renvoisé, E. , Montuire S., Richard Y., Quéré J.‐P., Gerber S., Cucchi T., Chateau‐Smith C., and Tougard C.. 2012. Microevolutionary relationships between phylogeographical history, climate change and morphological variability in the common vole (Microtus arvalis) across France. J. Biogeogr. 39:698–712. [Google Scholar]
- Ritchie, A. 2009. On the fringe of neolithic Europe. Excavation of a chambered cairn on the Holm of papa westray. Society of Antiquaries of Scotland, Edinburgh, Orkney. [Google Scholar]
- Rohlf, F. J. 2010a. TpsDig 2‐thin plate spline digitizer. Ecology & Evolution, State University at Stony Brook, New York. [Google Scholar]
- Rohlf, F. J. . 2010b. TpsRelw 1.49‐thin plate spline relative warp. Ecology & Evolution, State University at Stony Brook, New York. [Google Scholar]
- Rohlf, F. J. , and Slice D. E.. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 39:40–59. [Google Scholar]
- Rosenzweig, M. L. 1968. The strategy of body size in mammalian carnivores. Am. Midl. Nat. 80:299–315. [Google Scholar]
- Saint Girons, M.‐C. , and Nicolau‐Guillomet P.. 1987. Les phénomènes d'insularité dans les îles du Ponant (France). Mammifères et oiseaux. Bulletin de la société zoologique de France 112:61–79. [Google Scholar]
- Schluter, D. 1996. Adaptive radiation along genetic lines of least resistance. Evolution 50:1766–1774. [DOI] [PubMed] [Google Scholar]
- Schüle, W. 1993. Mammals, vegetation and the initial human settlement of the Mediterranean islands: a palaeoecological approach. J. Biogeogr. 20:399–411. [Google Scholar]
- Sharples, N. M. 1984. Excavations at Pierowall Quarry, Westray, Orkney. Proc. Soc. Antiq. Scot. 114:75–126. [Google Scholar]
- Sheets, A. D. , Covino K. M., Panasiewicz J. M., and Morris S. R.. 2006. Comparison of geometric morphometric outline methods in discrimination of age‐related differences in feather shape. Front. Biol. 3:15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenbrot, G. I. , and Krasnov B. R.. 2005. An atlas of the geographic distribution of the arvicoline rodents of the world (Rodentia, Muridae: Arvicolinae). Pensoft Publishers. [Google Scholar]
- Sheridan, J. A. , and Bickford D.. 2011. Shrinking body size as an ecological response to climate change. Nature Clim. Change 1:401–406. [Google Scholar]
- Simpson, I. A. , and Dockrill S. J.. 1996. Early cultivated soils at Tofts Ness, Sanday, Orkney Pp. 130–144 in Hall A. M., ed. The quaternary of Orkney Field Guide. Quaternary Research Association, Cambridge. [Google Scholar]
- Smith, B. B. , and Carter S.. 1994. Howe: four millennia of Orkney prehistory excavations, 1978–1982. Society of Antiquaries of Scotland, Edinburgh. [Google Scholar]
- Sondaar, P. Y. 2000. Early human exploration and exploitation of islands. Tropics 10:203–230. [Google Scholar]
- Stoetzel, E. , Denys C., Michaux J., and Renaud S.. 2013. Mus in Morocco: a quaternary sequence of intraspecific evolution. Biol. J. Linn. Soc. 109:599–621. [Google Scholar]
- Swofford, D. L. 2002. Phylogenetic analysis using parsimony (*and other methods) v4.0b10. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Templeton, A. R. 1996. Experimental evidence for the genetic‐transilience model of speciation. Evolution 50:909–915. [DOI] [PubMed] [Google Scholar]
- Tougard, C. , Renvoisé E., Petitjean A., and Quéré J.‐P.. 2008. New insight into the colonization processes of common voles: inferences from molecular and fossil evidence. Pub. Lib. Sci. One 3:e3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Valen, L . 1973. Pattern and the balance of nature. Evol. Theory 1:31–49. [Google Scholar]
- Vigne, J. ‐D. 1999. The large "true" Mediterranean as a model for the Holocene human impact on the European vertebrate fauna? Recent data and new reflections Pp. 295–322 in Benecke N., ed. The Holocene history of the European vertebrate fauna. Modern aspects of research (Workshop, 6th–9th April 1998, Berlin). Deutsches Archaologisches Institut, Eurasian‐Abteilung, Berlin. [Google Scholar]
- Whittaker, R. J. 1998. Island biogeography: ecology, evolution and conservation. Oxford Univ. Press, Oxford, U.K. [Google Scholar]
- Williamson, M. 1981. Island populations. Oxford Univ. Press, Oxford, U.K. [Google Scholar]
- Wouters, W. , and Peersman J.. 1994. Een opgraving heropgegraven. Norbertijnen in Ninove (prov. Oost‐Vlaanderen). Interimverslag. Archeologie in Vlaanderen 3:339–357. [Google Scholar]
- Yalden, D. 1999. The history of British mammals. Poyser, Lond. [Google Scholar]
- Yom‐Tov, Y. , and Geffen E.. 2006. Geographic variation in body size: the effects of ambient temperature and precipitation. Oecologia 148:213–218. [DOI] [PubMed] [Google Scholar]
- Yom‐Tov, Y. , and Geffen E.. 2011. Recent spatial and temporal changes in body size of terrestrial vertebrates: probable causes and pitfalls. Biol. Rev. 86:531–541. [DOI] [PubMed] [Google Scholar]
- Yom‐Tov, Y. , Yom‐Tov S., and Moller H.. 1999. Competition, coexistence, and adaptation amongst rodent invaders to Pacific and New Zealand islands. J. Biogeogr. 26:947–958. [Google Scholar]
- Zelditch, M. L. , Swiderski D. L., Sheets A. D., and Fink W. L.. 2012. Geometric morphometrics for biologists. A primer. Elsevier, Berlin. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1.


