Abstract
Rationale
Global tobacco-related mortality dwarfs that of all other drugs. Nicotine is believed to be the primary agent responsible for tobacco use and addiction. However, nicotine is a relatively weak and inconsistent reinforcer in nonhumans and nicotine reinforcement has not been demonstrated in never-smokers.
Objectives
This study investigated the discriminative, subjective, and reinforcing effects of nicotine in never-smokers.
Methods
Eighteen never-smokers (<50 lifetime nicotine exposures) participated in a double-blind study. During a drug discrimination phase, volunteers ingested oral nicotine and placebo capsules (quasi-random order) at least 2 hours apart and rated subjective effects repeatedly for 2 hours after ingestion in daily sessions. Blocks of 10 sessions were continued until significant discrimination was achieved (p≤.05, binomial test; ≥8 of 10). Following discrimination, nicotine choice was tested by having volunteers choose which capsule set to ingest on each daily session. Successive blocks of 10 sessions were conducted until choice for nicotine or placebo met significance within each volunteer (≥8 of 10 sessions).
Results
All 18 volunteers significantly discriminated nicotine from placebo; the lowest dose discriminated ranged from 1.0–4.0 mg/70kg. Nine volunteers significantly chose nicotine (choosers) and nine significantly chose placebo (nicotine avoiders). The choosers reported predominately positive nicotine subjective effects (e.g., alert/attentive, good effects, liking), while avoiders tended to report negative effects (e.g., dizzy, upset stomach, disliking). Both choosers and avoiders attributed their choice to the qualitative nature of drug effects.
Conclusions
These results provide the first evidence that nicotine can function as a reinforcer in some never-smokers.
Keywords: nicotine, oral, never-smoker, drug discrimination, reinforcement, self-administration, subjective effects
Introduction
Tobacco is one of the most widely used mood altering drugs in the world. It is widely believed that nicotine is the principal constituent of tobacco that functions as a behavioral reinforcer and leads to tobacco use and addiction (Benowitz 1996; Stolerman and Shoaib 1991). Despite the widespread use of nicotine, both human and nonhuman animal studies have shown inconsistent or less robust reinforcing effects of nicotine relative to other drugs of abuse (Griffiths et al. 1979; Henningfield and Goldberg 1983; Goodwin et al. 2015). Some researchers have expressed doubts that regular smoking and tobacco use are evidence of addiction to nicotine per se (Dar and Frenk 2004; 2007; Robinson and Pritchard 1992). These reservations arise from a number of observations including: the difficulty or inconsistency of establishing nicotine self-administration in laboratory animals (Dar and Frenk 2002; Griffiths et al. 1979; Le Foll and Goldberg 2009; Koffarnus and Winger 2015; Goodwin et al. 2015), the relatively limited success of nicotine replacement therapies for smoking cessation (Etter and Stapleton 2006; Hughes et al. 2003; McClure and Swan 2006), the lack of consistent mood-elevating or “euphoric” effects of nicotine (Dar et al. 2007; Hughes et al. 2000; Kalman and Smith 2005), and the observation that denicotinized cigarettes are able to reduce cigarette craving and withdrawal (Rose et al. 2000; Shahan et al. 1999). Although the reinforcing effects of nicotine in nicotine users have been demonstrated (e.g. Harvey et al. 2004; Le Foll and Goldberg 2009; Perkins 2004), these effects appear relatively inconsistent. To our knowledge, nicotine reinforcement has never been unequivocally demonstrated in humans without histories of nicotine use (e.g., tobacco users; but see Perkins et al. 2001). In fact, in nicotine non-users, nicotine delivery via gum or nasal spray has sometimes been shown to be punishing in nicotine versus placebo choice procedures (Hughes et al. 2000; Perkins et al. 1997). Collectively, these observations underscore our limited understanding of the conditions under which nicotine can function as a reinforcer. This study was undertaken to attempt to reconcile the widespread use of nicotine with its apparent lack of reinforcing effects in volunteers with neither current nor significant past use of nicotine.
The discriminative and reinforcing effects of drugs are often considered to be separate yet overlapping domains of drug action that contribute to the onset and maintenance of nicotine dependence (Perkins 1999a; 1999b). The present study investigated the discriminative and reinforcing effects of oral nicotine in healthy volunteers who did not use nicotine. The oral route (via capsules) was chosen for several reasons: the double-blind design of the study could be maintained, precise nicotine doses could be administered, and oral administration minimizes tissue irritation in contrast to delivery via nasal sprays or by inhalation. The study used a nicotine versus placebo drug discrimination procedure to establish discriminative control by a low threshold dose of nicotine. Following acquisition of nicotine discrimination, a choice procedure was implemented in which volunteers chose to self-administer either nicotine or placebo. This study was designed to explore whether low nicotine doses that have discriminative stimulus effects also have reinforcing effects in volunteers with neither current nor significant past use of nicotine. We hypothesized that volunteers who rated subjectively positive effects following nicotine administration would be more likely to choose to self-administer nicotine (i.e., show reinforcing effects of nicotine).
Methods
Participants
Eighteen healthy volunteers (3 men) who ranged in age from 23 to 47 years old completed both the discrimination phase and choice phase of the study. Volunteers had less than 50 lifetime exposures to nicotine from any route of administration. Previous research and the Centers for Disease Control and Prevention have defined “never-smoker” as those who report smoking zero or less than 100 cigarettes in their lifetime (Hughes 1996; Schoenborn and Adams 2010). The present study used the smoking status designation of “never-smoker,” but employed a more rigorous definition of less than 50 lifetime exposures to nicotine by any route of administration. Volunteers underwent medical screening, including assessment of medical history, drug use history, and mood and personality measures. All 18 volunteers had at least some college education (with 11 holding advanced degrees) and all held regular full-time jobs at the Johns Hopkins Bayview Medical Center. No volunteer reported psychoactive drug use (other than alcohol or caffeine) in the past 30 days or past or current drug dependence (excluding caffeine). Drug free status was confirmed with a urinalysis for common drugs of abuse (EMIT, Syva Co.). Pregnancy and significant medical or psychiatric illness (e.g., insulin-dependent diabetes, schizophrenia) were exclusionary. The Johns Hopkins University Institutional Review Board approved the study and all volunteers provided written informed consent.
To minimize the confounding effects of expectancy bias, the instructions to volunteers and the consent form obscured that the purpose of the study was to test the discriminative and reinforcing effects of nicotine versus placebo. Rather, volunteers were informed that they would receive two sets of capsules daily and the compounds that they could potentially receive were: inactive placebo, ginseng, ginkgo biloba, guarana, chamomile, peppermint, kava, chlorogenic acids, aspartame, diterpenes, caffeine, rhodiola rosea, absinthin, nicotine, poppy extracts, valerian, tannin, sugar, yerba mate, and theobromine. Volunteers were told they would receive two of the listed compounds. Volunteers were paid for their participation, which partly depended on their performance during drug discrimination session days (described below).
General Procedures
This protocol included three phases: 1) drug discrimination acquisition and dose determination phase, 2) drug discrimination phase, and 3) choice phase. Volunteers were not told how their performance in the discrimination or choice phases would affect their duration of participation. Volunteers reported to the laboratory on weekdays between 8:00 AM and 5:00 PM.
Questionnaires
In all phases, on the morning of each session day, volunteers were given a subjective effects questionnaire packet to be completed that day. For the subjective effects questionnaire, volunteers first rated “the overall strength of the drug effect you experienced from this set of capsules” using a 5-point scale ranging from: “No drug effect at all” to “Very strong drug effect.” For the next questionnaire item, volunteers rated drug liking using a 9-point scale ranging from: “Dislike very much”(−4), “Neutral, or no effect” (0), to “Like very much”(+4). Both liking and disliking scores were derived from this scale. For ratings of liking, ratings were assigned a score of 0 if the volunteer indicated disliking. For ratings of disliking, ratings were assigned a score of 0 if the volunteer rated liking, and negative scores were converted to positive scores. Volunteers rated 20 additional subjective effects items on a 5-point scale ranging from “Not at all” (0) to “Extremely” (4). The items are shown in the left column of Table 1. Each of these items was rated immediately before (time “0”), and at 15, 30, 45, 60, 75, 90, and 120 minutes after swallowing two identically appearing capsules. At 120 minutes, volunteers provided a brief written description of the subjective effects of the capsules.
Table 1.
Significant peak effects for subjectively positive and negative ratings during drug discrimination for the 9 nicotine choosers and 9 nicotine avoiders1
| Nicotine Choosers | Nicotine Avoiders | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volunteer Number | S-3 | S-7 | S-8 | S-14 | S-15 | S-24 | S-25 | S-27 | S-34 | S-12 | S-17 | S-19 | S-21 | S-22 | S-23 | S-26 | S-28 | S-32 |
| Dose (mg/70kg) | 4.0 | 3.0 | 3.0 | 2.5 | 2.0 | 1.5 | 1.0 | 4.0 | 1.0 | 2.5 | 1.0 | 1.5 | 3.5 | 3.5 | 3.0 | 2.5 | 3.5 | 3.0 |
| Significant Choice of: | Nic | Nic | Nic | Nic | Nic | Nic | Nic | Nic | Nic | Plac | Plac | Plac | Plac | Plac | Plac | Plac | Plac | Plac |
| Subjectively Positive Effects | ||||||||||||||||||
| Liking | ■ | ■ | ■ | ○ | ■ | ○ | ||||||||||||
| Good Effects | ■ | ■ | ■ | ■ | ○ | |||||||||||||
| Alert/Attentive | ■ | ■ | ■ | ■ | ■ | ○ | ○ | |||||||||||
| Stimulated | ■ | ■ | ■ | ■ | ○ | ○ | ||||||||||||
| Social/Talkative | ■ | ■ | ■ | ○ | ○ | ○ | ||||||||||||
| Urge to do Tasks | ■ | ■ | ■ | ○ | ○ | |||||||||||||
| Energetic | ■ | ■ | ■ | ○ | ○ | |||||||||||||
| Mellow | ■ | ○ | ■ | ■ | ■ | |||||||||||||
| Increased Concentration | ■ | ■ | ■ | ○ | ○ | |||||||||||||
| Content/Well-being | ■ | ■ | ○ | |||||||||||||||
| Relaxed | ○ | ■ | ■ | |||||||||||||||
| Subjectively Negative Effects | ||||||||||||||||||
| Disliking | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ||||||||||
| Bad Effects | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |||||||||||
| Lightheaded/Dizzy | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |||||||||
| Foggy/Not Clear-Headed | ■ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ||||||||||
| Upset Stomach/Nauseated | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |||||||||||
| Lethargy | ■ | ○ | ■ | ■ | ○ | |||||||||||||
| Drowsy/Sleep | ■ | ■ | ○ | ○ | ○ | |||||||||||||
| Headache | ■ | ○ | ○ | |||||||||||||||
| Jittery/Shaky | ○ | ○ | ||||||||||||||||
| Irritable/Cross/Grumpy | ○ | |||||||||||||||||
| Anxious/Nervous | ||||||||||||||||||
Presence of squares or circles in the table indicate a statistically significant difference between nicotine and placebo in the indicated volunteer during drug discrimination sessions at the lowest dose of nicotine discriminated immediately preceding the choice phase. The 22 subjective effects domains were divided a priori into two categories: “positive” effects (e.g., “Liking” and “Good Effects”) and “negative” effects (e.g., “Disliking” and “Bad Effects”). Filled squares indicate a statistically significant increase in subjectively positive effects or a decrease in subjectively negative effects; such effects would be hypothesized to occur in individuals in whom nicotine would function as a reinforcer. Unfilled circles indicate a decrease in subjectively positive effects or an increase in subjectively negative effects; such effects would be hypothesized to occur in individuals in whom nicotine would be avoided. The distribution of closed squares and open circles shows that subjective effects differences during discrimination corresponded to subsequent choice or avoidance of nicotine during the choice phase. For example, among the 9 nicotine choosers, 8 have one or more closed squares and, across items, there are 36 closed squares total for nicotine choosers. In contrast, among the 9 nicotine avoiders, only 5 has a closed square and there are 8 closed squares total. An opposite distribution of open circles among nicotine choosers and avoiders is apparent. Nic indicates choice of nicotine; Plac indicates choice of placebo.
There were two additional questionnaires. On drug discrimination session days, volunteers guessed the identity of the two sets of capsules (using the letter codes assigned for that volunteer, see below), provided a written description of why they made that guess, and rated their confidence level in their guess on a 4-point scale ranging from “not at all” (0) to “Very much” (3). At the completion of the choice block (described below) for a dose of nicotine, volunteers completed the End of Choice Questionnaire in which they rated the relative importance (“Not Important,” “A little important,” “Moderately important,” or “Very important”) of several subjective dimensions in differentiating between the two capsule sets. The subjective dimensions were the same as shown in the left column of Table 1.
Phase 1
During the drug discrimination acquisition and dose determination phase, volunteers acquired a placebo versus nicotine discrimination. Volunteers swallowed two pairs of capsules (labeled “Set 1” and “Set 2”) each day and answered the subjective effects questionnaire after each capsule set. All capsules were identical in appearance. One capsule set was always placebo and one capsule set was always nicotine. To maintain double-blind procedures throughout the study, placebo and nicotine were assigned a random letter for each volunteer (e.g., Drug X or Drug Y). On Session Day 1 of the study, volunteers reported to the laboratory and completed the initial time point (time “0”) on the subjective effects questionnaire. Volunteers were then administered the first capsule set and were told the letter corresponding to that capsule set. They were instructed to attend to the subjective effects and to answer the questionnaire items at the specified time points. Volunteers were given a digital timer with preset alarms to prompt completion of the questionnaire. Volunteers left the laboratory to return to their usual daily activities (e.g., work). After at least two hours, volunteers returned to the laboratory. The subjective effects questionnaire for the first capsule set was returned and volunteers completed the initial time point (time “0”) on the second subjective effects questionnaire (i.e., the questionnaire corresponding to the second capsule set). Volunteers were administered the second capsule set and told the letter that corresponded to that capsule set. Volunteers were again instructed to pay attention to the subjective effects of that capsule set and to answer each item at the specified time point. Volunteers left the laboratory to return to work while completing the questionnaire. The sequence of initial exposure to placebo and nicotine was randomized across volunteers.
On Session Day 2 and subsequent discrimination session days including Phase 2, the above routine was followed except that the volunteers were not told the letter corresponding to each capsule set. Instead, capsule sets were labeled “Set 1” and “Set 2.” The order of exposure to placebo and nicotine was quasi-random across session days, and the subjective effects questionnaire was completed after each set of capsules. When the last time point of the second subjective effects questionnaire was completed, volunteers called the laboratory by telephone to verbally guess which letter corresponded to which capsule set. Also, they rated their confidence in the accuracy of their guess (described previously) and were asked to verbally report what effects they noticed after taking each capsule set. The guess and reported effects were recorded by research staff blinded to the drug and capsule conditions. After the guess was entered and recorded, the research staff member opened a sealed envelope containing the correct letter codes for each capsule set and told the volunteer whether his/her answer was correct or incorrect.
All volunteers began the protocol at a dose of 1.5 mg/70 kg nicotine. Two to three discrimination session days were conducted at this dose. If the volunteer reported no discernible effects of either the nicotine or placebo capsules, or reported effects but were not confident in their guesses, the dose was increased by 0.5 mg/70 kg. This procedure for incrementally increasing the nicotine dose was repeated until volunteers reported effects with confidence and correctly identified the capsule sets. Each time the dose of nicotine was increased, volunteers were instructed that either the dose of one compound would increase or the dose of the other compound would decrease to facilitate discrimination between the capsule sets. “Day 1” procedures, in which the capsule set letters were revealed to the volunteer at the time of consumption, were not repeated.
Phase 2
During discrimination testing, session days at a given dose of nicotine were repeated in blocks of 10 until volunteers could reliably discriminate between placebo and nicotine for ≥8 out of 10 session days within the block (i.e., p≤.05 according to the binomial probability distribution). If a volunteer failed to discriminate at a dose associated with effects (or had no discernible effects or no confidence in their guess), the dose was increased by 0.5 mg/70 kg and a new block of 10 session days was begun.
Phase 3
The choice phase of the protocol followed the discrimination phase. During a daily choice session, the volunteer reported to the laboratory and was asked to choose which one of the two letter-coded drug capsules they wanted to take that day (e.g., Drug X). In addition to making a choice between capsule sets, the volunteer was asked to write an explanation for why he/she chose the capsule set. The choice and reason for making that choice were recorded and the volunteer was administered the pair of capsules corresponding to the letter code. Volunteers then completed a subjective effects questionnaire packet as described above. Volunteers completed daily choice sessions in blocks of 10 sessions during the choice phase. Blocks of 10 daily choice sessions were repeated until volunteers significantly chose one letter-coded pair of capsules over the other for ≥8 out of 10 sessions within the block (i.e., p≤.05). To ensure that the discrimination was maintained during the choice phase, discrimination session days were interspersed throughout choice sessions (e.g., a discrimination session day was conducted after three to four consecutive choice sessions). If the volunteer incorrectly discriminated between nicotine and placebo twice during the choice phase, volunteers returned to the discrimination phase until they correctly discriminated between the capsules for at least three consecutive discrimination session days. The choice phase was then resumed. At the completion of a choice block (i.e., 10 daily choice sessions and interspersed discrimination session days) with a given dose of nicotine, volunteers completed the End of Choice Questionnaire, as described above. Volunteers who showed a significant choice of nicotine were discontinued from further participation.
Testing lower doses of nicotine
Volunteers who showed significant choice of placebo during the choice phase or who did not show a signficant choice for either nicotine or placebo restarted Phase 2 at a lower dose of nicotine, and subsequently proceeded to Phase 3 again. The rationale for testing lower doses of nicotine in participants who did not initially demonstrate significant nicotine choice was based on the knowledge that unpleasant effects of nicotine increase as a function of dose. It was reasoned that the dose of nicotine necessary to initially establish the discrimination might be too high to maintain nicotine choice; after the volunteer had acquired experience discriminating nicotine, it was reasoned, the dose might be able to be lowered while maintaining the discriminative effects. Related to this, previous research showed decreases in some negative subjective effects of nicotine after repeated exposure (Heishman and Henningfield 2000). Thus, it seemed possible that decreasing the dose of nicotine after acquisition of the discrimination would unmask positive subjective effects of nicotine. In these volunteers, nicotine was decreased by 0.5 mg/70 kg and the drug discrimination conditions were reinstated. Volunteers were assigned new letters for each capsule pair to avoid biases based on their previous experience. The discrimination and choice data presented are from the lowest dose of nicotine each volunteer significantly discriminated.
Compensation
During discrimination, volunteers received $10 per session day for taking the capsules and completing subjective effects questionnaires, and they received an additional $10 per session day for a correct guess. During choice, volunteers received $20 per session day for choosing and taking capsules and for completing the subjective effects questionnaire. In addition, volunteers completing the entire protocol received an additional $2 per session day as a completion bonus.
Drug
Identically appearing nicotine and placebo capsules were prepared in opaque size 0 gelatin capsules. Each administration of nicotine and placebo consisted of two capsules to accommodate the maximum possible dose of nicotine that could be administered in the study. Nicotine capsules were filled with (−) nicotine hydrogen tartrate (Sigma-Aldrich Inc, St. Louis, MO) and lactose. Placebo capsules were filled with lactose. Nicotine doses are expressed as the free base. All volunteers began with a dose of 1.5 mg/70 kg nicotine in Phase 1 of discrimination. The dose was increased as necessary in 0.5 mg/70 kg increments to a maximum dose of 4.0 mg/70 kg. All capsules were swallowed with water. Initial starting and maximum nicotine doses were chosen based on the bioavailability of oral nicotine, previous research using nicotine non-users, and the doses available in over-the-counter nicotine replacement therapies.
Statistics
Significant discrimination between nicotine and placebo was defined as 8, 9, or 10 correct discriminations out of a block of 10 discrimination session days (p≤0.05, binomial probability distribution). Significant nicotine choice was defined as choice of nicotine capsules on 8, 9, or 10 of the 10 choice sessions; significant nicotine avoidance was defined as 0, 1, or 2 nicotine choices. For purposes of making decisions within the study, drug discrimination and drug choice were determined based on the first block of 10 session days in which the volunteer reliably discriminated between nicotine and placebo or reliably chose nicotine or placebo. Ratings from the subjective effects questionnaire during drug discrimination were expressed as peak change scores from the pre-capsule time point for each capsule set within each session day. For these data, differences between nicotine and placebo were analyzed within each volunteer using two-tailed paired t-tests to determine significance (p≤0.05). Drug discrimination performance and subjective effects data presented in Figure 1 and Table 1 are from the first block of 10 session days at the lowest dose of nicotine that each volunteer significantly discriminated and any subsequent sessions at that dose of nicotine.
Figure 1.
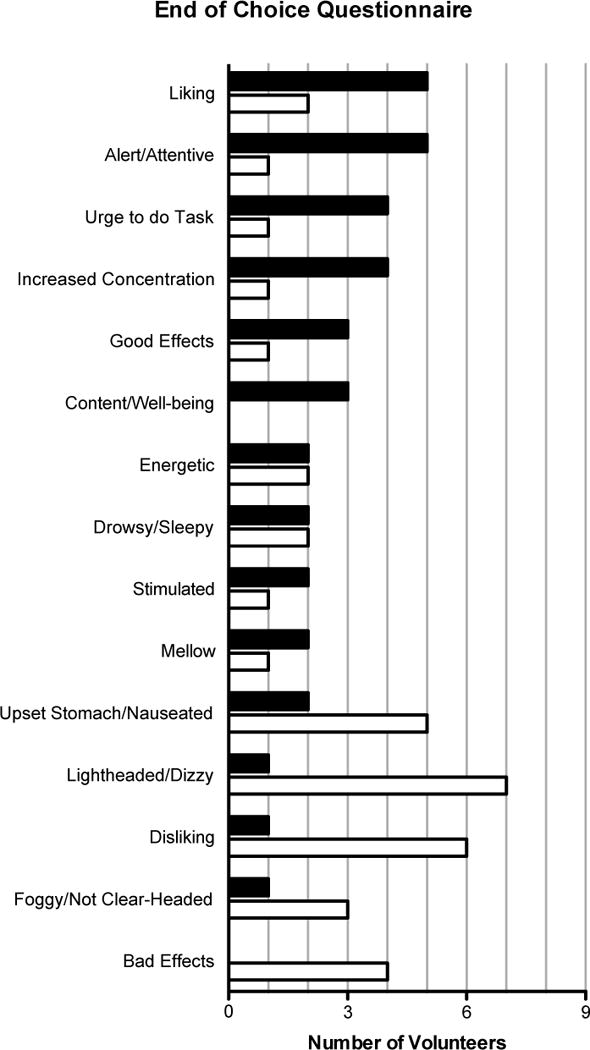
Data from the End of Choice questionnaire in which volunteers rated how important each subjective effect dimension was in making the discrimination. The number of volunteers who reported each subjective effect item as being “moderately” or “very” important in making the discrimination between nicotine and placebo is shown; items not rated as moderately or very important by two or more choosers or avoiders are not shown. Data are presented separately for those who chose nicotine (black bars, “choosers”) and those who avoided nicotine (white bars, “avoiders”) during the choice procedure.
Results
Participant Retention
Thirty-five participants signed informed consent. Of these, eight dropped out of the study during drug discrimination (Phase 1 or Phase 2) due to time commitment problems. Four volunteers were discharged by the investigators due to noncompliance (e.g., failing to come to laboratory sessions). Three volunteers were discharged due to medical problems not related to the study. One volunteer reached the maximum number of sessions approved for this protocol before acquiring the discrimination and was therefore discharged from the study. Nineteen volunteers acquired the nicotine versus placebo discrimination. One of these volunteers, who acquired the discrimination at a nicotine dose of 1.5 mg/70kg, was dropped from the study due to noncompliance (i.e., failing to report for sessions); this dropout did not appear to be related to specific study procedures or negative side effects from nicotine.
Nicotine Discrimination and Choice
Eighteen volunteers completed both the discrimination and choice phases of the study. Across participants, the mean (SEM) number of total discrimination sessions at all nicotine doses was 42.2 (5.5) and ranged from 10 to 85. The mean (SEM) number of discrimination sessions at the lowest dose of nicotine discriminated was 12.2 (1.0) and ranged from 10 to 20. The mean (SEM) number of choice sessions at the lowest nicotine dose discriminated was 11.1 (0.7) and ranged from 10 to 20. The total number of discrimination sessions as well as the number of discrimination and choice sessions at the lowest nicotine dose discriminated were not significantly different between the nicotine choosers and the nicotine avoiders (two-tailed t-tests, n=9 in each group).
As shown in Table 1, the lowest nicotine dose that was significantly discriminated (which is also the nicotine dose used in the choice phase) ranged from 1.0 to 4.0 mg/70 kg. Table 1 also shows significant nicotine versus placebo differences on subjective effects ratings for each volunteer during discrimination sessions. Of the 18 volunteers, nine significantly chose nicotine (≥8 of 10 choices, p≤0.05, binomial probability distribution), and nine significantly avoided nicotine (≤2 of 10 choices). The mean (± SEM) dose of nicotine that was discriminated was not significantly different between nicotine choosers (2.4 mg/70 kg ± 0.38) and nicotine avoiders (2.7 mg/70 kg ± 0.30; t-test, p>.05).
Of the 18 volunteers who completed the first choice phase, five (S-3, S-7, S-8, S-24, S-27) significantly chose nicotine and were discontinued from further participation. Of the remaining 13 (who met criteria for testing lower nicotine doses), 10 showed significant choice of placebo and 3 did not show significant choice of either placebo or nicotine. Of the latter group, all three (S-15, S-25, S-34) significantly discriminated and chose a lower dose of nicotine when lower doses of nicotine were tested. Of the 10 volunteers who initially chose placebo, only one (S-14) subsequently chose nicotine at the lower dose. The remaining 9 volunteers either continued to choose placebo (n=1, S-17) or failed to discriminate between nicotine and placebo at the lower nicotine dose (S-12, S-19, S-21, S-22, S-23, S-26, S-28, S-32). Thus, all 9 of these volunteer were designated as placebo choosers (i.e. nicotine avoiders).
As shown in Table 1 and described in the table footnote, the distribution of significant positive and negative subjective effects during the drug discrimination phase corresponded to subsequent choice or avoidance of nicotine during the choice phase. For example, the closed squares (which indicate a significant increase in subjectively positive effects or a decrease in subjectively negative effects) would be hypothesized to occur more frequently in nicotine choosers than in nicotine avoiders. In fact, among the 9 nicotine choosers, 8 have one or more closed squares and, across items, there are 36 closed squares total. In contrast, among the 9 nicotine avoiders, only 5 have a closed square and there are 8 closed squares total. The mean number of closed squares (± SEM) was significantly higher in choosers (4 ± 1.1) than avoiders (0.8 ± 0.3; t-test p≤ 0.05). Open circles (which indicate a decrease in subjectively positive effects or an increase in subjectively negative effects) show an opposite distribution. The mean number of open circles (± SEM) was significantly lower in the nicotine choosers (1.4 ± 0.6) compared to the nicotine avoiders (6 ± 1.2; t-test p ≤.05).
Table 2 shows representative verbatim written comments summarizing the most frequently reported reason that each volunteer chose or avoided nicotine during the choice phase. In general, the volunteers who signficantly chose nicotine indicated that they did so based on subjectively positive effects following nicotine administration. In contrast, volunteers who signficantly avoided nicotine (i.e., chose placebo) indicated that they did so to avoid subjectively negative effects experienced following nicotine administration.
Table 2.
Representative verbatim comments summarizing the most frequently reported reason that each volunteer chose or avoided nicotine1
| Subject | Volunteers
that significantly chose nicotine Reason for Choice |
Subject | Volunteers that significantly chose
placebo Reason for Choice |
|---|---|---|---|
| S-3 | “Nicotine” improves my mood, increases concentration, desire to work, focus, and clear-headedness. | S-12 | To avoid side effects of “nicotine” like lightheadedness. |
| S-7 | “Nicotine” increases alertness; I was more focused and had high levels of concentration after “nicotine” compared to “placebo.” “Nicotine” helped me towards work-related tasks and had pleasant effects. | S-17 | Because “placebo” has no effects. |
| S-8 | “Nicotine” improves my mood, concentration, and productivity at work. | S-19 | “Nicotine” makes me feel lightheaded, so I choose “placebo” to avoid negative effects. |
| S-14 | I like the pleasant effects of “nicotine” - I feel upbeat, social, and positive. | S-21 | “Nicotine” gives me negative side effects, so I choose “placebo.” |
| S-15 | I like the effects of “nicotine.” | S-22 | “Placebo” makes me alert and attentive and doesn’t make me sick like “nicotine.” |
| S-24 | “Nicotine” makes me feel more alert, focused, and energized. I have some negative effects at the beginning (dizziness and lightheadedness), but the good effects outweigh the bad. | S-23 | To avoid side effects of “nicotine”: dizziness and upset stomach. |
| S-25 | “Nicotine” produces good alertness and stimulation that I like, and there aren’t usually negative effects. | S-26 | “Nicotine” increases lightheadedness and dizziness that interfere with normal activities. |
| S-27 | “Nicotine” gives me a boost in the afternoon, increases wakefulness, improves energy and concentration. | S-28 | I choose “placebo” because I do not like the headache and nausea associated with “nicotine.” |
| S-34 | “Nicotine” makes me feel content, pleasant and mellow. “Placebo” just makes me tired. | S-32 | “Placebo” gives me more energy for my job. |
Representative verbatim written comments were selected for each volunteer to reflect the most frequently reported reason provided for making his/her choice. Written comments were provided at the time the capsule set was chosen on each choice session. Left and right columns show comments from nicotine choosers and nicotine avoiders, respectively. In these comments, capsule drug letter codes (e.g. “Drug A” or “Drug B”) were replaced by the corresponding compound: “nicotine” or “placebo.” 1
The End of Choice questionnaire administered following the completion of the choice phase of the study provides additional evidence that nicotine produced qualitative differences between choosers and avoiders. Figure 1 shows the number of nicotine choosers and avoiders who rated each subjective effect item as either “moderately” or “very” important in differentiating the two capsule conditions. Volunteers who chose to self-administer nicotine tended to rate subjectively positive effects as important for making the discrimination, while those who avoided nicotine tended to rate negative subjective effects as important. More specifically, the figure shows that ratings of liking, alert/attentive, urge to do tasks, increased concentration, good effects, and content/well-being were all endorsed by 3 or more of the nicotine choosers. In contrast, 3 or more volunteers who avoided nicotine endorsed upset stomach/nauseated, lightheaded/dizzy, disliking, foggy/not clear-headed, and bad effects as important.
Discussion
In the present study, orally administered nicotine functioned as a discriminative stimulus in 19 out of 20 healthy volunteers with less than 50 lifetime exposures to nicotine. Discrimination between nicotine and placebo was acquired and maintained at a range of doses (1.0–4.0 mg/70 kg) with at least 80% accuracy. Of the 18 volunteers who completed the study, nine significantly chose to subsequently self-administer nicotine (nicotine “choosers”) while nine significantly chose placebo (nicotine “avoiders”). The choosers reported predominately positive subjective effects of nicotine (e.g., significant increases in alert/attentive, good effects, liking), while avoiders tended to report negative effects (e.g., significant increases in dizzy, upset stomach, disliking). Both choosers and avoiders attributed their choice to the qualitative nature of the nicotine effects. Furthermore, in both ratings and written comments summarizing the reasons for their choices, nicotine choosers indicated their choice was based on subjectively positive effects of nicotine administration in contrast to nicotine avoiders who indicated their choices were based on avoiding subjectively negative effects of nicotine administration. The concordance of qualitative subjective reports with choice or avoidance of nicotine, along with the procedure of requiring volunteers to make a minimum of ten repeated choices between nicotine and placebo, suggests that the designation given to volunteers as a chooser or avoider was not based on chance responding. We believe this is the first demonstration that nicotine can function as a reinforcer in humans who are neither current nicotine users nor have a significant past history of nicotine use from any route of administration.
In contrast to prior studies that showed that nicotine did not function as a reinforcer in never-smokers (Perkins et al. 1997; 2001; Hughes et al. 2000), the present study showed that 50% of never-smokers demonstrated nicotine choice after acquiring a low dose nicotine versus placebo discrimination over an average of 42 sessions (range, 10 to 85). In contrast, laboratory studies using similar choice procedures comparing d-amphetamine and placebo in non-drug using participants have shown that d- amphetamine is preferred to placebo in the majority of participants after as few as two exposures each to drug and placebo and in absence of explicit discrimination training (de Wit et al. 1986; 1987; Foltin and Fischman 1991). Thus, although the present study does demonstrate nicotine reinforcement in never-smokers, oral nicotine appears to have substantially less efficacy as a reinforcer than oral d-amphetamine when tested under reasonably similar laboratory conditions.
Although the prevalence of nicotine use disorder (i.e. addiction) is high in the general population, nicotine is an atypical drug of abuse in that its initial subjective effects are often not pleasant and, furthermore, positive subjective effects are not a robust predictor of the development of subsequent addiction (de Wit and Phillips 2012; Haertzen et al. 1983). In the present study, nicotine choosers reported choosing nicotine because of positive subjective effects while nicotine avoiders reported choosing placebo to avoid negative subjective effects. However, it is interesting to note that five of the nine choosers showed a significant increase in at least one negative subjective effect (Table 1, open circles for nicotine choosers), while four of the nine avoiders showed a significant increase in at least one of the positive subjective effects (Table 1, filled squares for nicotine avoiders). These findings underscore the complex role between subjective effects and nicotine reinforcement in never-smokers. Although researchers have described a progression of stages from initial nicotine exposure to nicotine addiction, the empirical evidence for such stage-specific predictors is weak (USDHHS 2010). The present study does not provide any information about the relationship of the initial subjective and reinforcing effects to the possible development of subsequent nicotine use disorder.
Although the sample size was small, there was no compelling evidence that past use of nicotine was a significant determinant of the reinforcing effects of nicotine in this study, as has been suggested by previous studies (Neugebauer et al. 2014). The volunteers in this study were current nicotine non-users with less than 50 lifetime exposures to nicotine. Of the nine who reported any past exposure, six and three were nicotine choosers and avoiders, respectively.
The study was conducted with oral nicotine administration although inhaled nicotine is the most common route of administration. The present study used the oral route of administration to facilitate double-blind administration procedures and to allow for the administration of exact nicotine doses, which is problematic with inhaled delivery. Oral bioavailability of nicotine is 20 to 45%, likely because of first-pass metabolism (Hukkanen et al., 2005). Administration of 4 mg of oral nicotine (approximately equivalent to the highest dose administered in the present study) had a Cmax of 6.4 to 7.5 ng/ml and a Tmax of 1.3 to 1.5 hours (Benowitz et al., 1991; D’Orlando and Fox, 2004). In contrast, the bioavailability of inhaled nicotine from cigarette smoke is 80 to 90%, with a Cmax of 15 to 30 ng/ml and a Tmax of 5 to 8 minutes (Hukkanen et al. 2005).
Several limitations of the study should be noted. Female participants were over-represented. Also, how representative the small population of study participants is of the general population of never-smokers is unknown. Although volunteers were instructed to complete questionnaires in response to timer alarms, real time assessment of subjective effects responses using Ecological Momentary Assessment methods would have been preferable (Stone and Shiffman 2002). A potential concern about the study design is that the procedure of using successive blocks of 10 sessions to establish significant discrimination accuracy or significant choice behavior would have theoretically resulted in significance eventually by chance alone. However, the procedure is very unlikely to account for the present results because significant discrimination and significant choice occurred after a mean of only 12.2 and 11.1 sessions, respectively, in the relevant dose conditions. Therefore, there were too few sessions for significant discrimination or choice to have occured by chance.
In conducting this study of the potential reinforcing effects of nicotine in volunteers with neither current nicotine use nor significant previous use of nicotine, careful consideration was given to the theoretical risk that, after the study, participants might seek out nicotine and become habitual users. In a discussion of human participant issues in drug abuse research, the College on Problems of Drug Dependence concluded that exposure of drug naïve individuals to abused drugs in a medically monitored setting is unlikely to create addiction or exacerbate pre-existing risk factors for addiction (College on Problems of Drug Dependence, 1995). Furthermore, oral nicotine delivery and other forms non-inhaled nicotine delivery are considered to have a very low abuse potential in never-smokers (Henningfield and Keenan, 1993; Houtsmuller et al., 2002; Gerlach et al. 2008, but see Etter 2007). In addition, to futher reduce the possibility that volunteers would start using nicotine after the study, volunteers were informed that they could receive a wide range of different substances and they were never debriefed about the study objectives or use of nicotine. To the authors’ knowledge, no participant engaged in tobacco smoking behavior or use of nicotine-containing products during the study or in the two weeks immediately following completion of the study.
Future research would benefit from the assessment of plasma and saliva nicotine levels to determine if rate of onset, peak plasma levels, or other metabolic differences are important determinants of nicotine reinforcement in human nicotine non-users as is suggested by studies in animals (e.g., Pastor et al. 2013; Wing and Shoaib 2013). Likewise, an examination of the possible role of genetic polymorphisms, age, gender, and ethnicity as determinants of individual differences in the reinforcing effects of nicotine would be of value (e.g., Morel et al. 2014; Schuck et al. 2014).
Nicotine addiction in the form of cigarette smoking is a leading cause of mortality world-wide (USDHHS 2014; WHO 2013). Improved understanding of vulnerability to nicotine reinforcement in nicotine naïve individuals may be vital for understanding the development of tobacco addiction, and improving smoking prevention interventions. The rapidly developing technology and expanding marketing of electronic nicotine delivery devices (King et al. 2014), especially to youthful nicotine non-users (McMillen et al. 2014; Vakkalanka et al. 2014) underscores the importance of further research of nicotine reinforcement in nicotine naïve populations.
Acknowledgments
Conduct of this research was supported by NIH R01DA03890 and T32 DA07209. We thank Eric Richter, Crystal Barnhouser, Samantha Gebhart, Samuel King, Kevin Strouse, Jessica Vanderhoff, Elana Schwartz, and Julia Kane for serving as session monitors, Lisa Shade for technical assistance, Linda Felch for statistical assistance, and the pharmacy and medical staff.
Footnotes
Disclosures: The study was conducted in compliance with United States laws. Dr. Reissig is an employee of the U.S. Food and Drug Administration (FDA), however, the views presented in this article do not necessarily reflect those of the FDA and no official support or endorsement of this article by the FDA is intended or should be inferred.
References
- Benowitz NL. Pharmacology of nicotine: Addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Denaro C, Jenkins R. Stable isotope studies of nicotine kinetics and bioavailability. Clinical Pharmacology and Therapeutics. 1991;49:270–277. doi: 10.1038/clpt.1991.28. [DOI] [PubMed] [Google Scholar]
- College on Problems of Drug Dependence. Human subject issues in drug abuse research. College on Problems of Drug Dependence. Drug Alcohol Depend. 2005;37:167–175. [PubMed] [Google Scholar]
- Dar R, Frenk H. Nicotine self-administration in animals: a reevaluation. Addiction Research & Theory. 2002;10:545–579. [Google Scholar]
- Dar R, Frenk H. Do smokers self-administer pure nicotine? A review of the evidence. Psychopharmacology. 2004;173:18–26. doi: 10.1007/s00213-004-1781-2. [DOI] [PubMed] [Google Scholar]
- Dar R, Frenk H. Reevaluating the nicotine delivery kinetics hypothesis. Psychopharmacology. 2007;192:1–7. doi: 10.1007/s00213-007-0768-1. [DOI] [PubMed] [Google Scholar]
- Dar R, Kaplan R, Shaham L, Frenk H. Euphoriant effects of nicotine in smokers: fact or artifact? Psychopharmacology. 2007;191:203–210. doi: 10.1007/s00213-006-0662-2. [DOI] [PubMed] [Google Scholar]
- D’Orlando KJ, Fox BS. Tolerability and pharmacokinetics of single and repeated doses of nicotine with The Straw, a novel nicotine replacement product. Nicotine and Tobacco Research. 2004;6:63–70. doi: 10.1080/14622200310001656876. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neuroscience and Biobehavioral Reviews. 2012;36:1565–1576. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. The reinforcing properties of amphetamine in overweight subjects and subjects with depression. Clinical Pharmacology and Therapeutics. 1987;42:127–136. doi: 10.1038/clpt.1987.122. [DOI] [PubMed] [Google Scholar]
- Etter JF. Addiction to the nicotine gum in never smokers. BMC Public Health. 2007;7:159–164. doi: 10.1186/1471-2458-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tobacco Control. 2006;15:280–285. doi: 10.1136/tc.2005.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Methods for the assessment of abuse liability of psychomotor stimulants and anorectic agents in humans. Br J Addict. 1991;86:1633–1640. doi: 10.1111/j.1360-0443.1991.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Gerlach KK, Rohay JM, Gitchell JG, Shiffman S. Use of nicotine replacement therapy among never smokers in the 1999–2006 National Health and Nutrition Surveys. Drug and Alcohol Dependence. 2008;98:154–158. doi: 10.1016/j.drugalcdep.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Hiranita T, Paule MG. The reinforcing effects of nicotine in humans and nonhuman primates: A review of intravenous self-administration evidence and future directions for research. Nicotine Tobacco Research. 2015 doi: 10.1093/ntr/ntv002. in press. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bradford LD. Advances in Behavioral Pharmacology. Vol. 2. New York: Academic Press, Inc; 1979. Predicting the abuse liability of drugs with animal drug self-administration procedures: Psychomotor stimulants and hallucinogens; pp. 163–208. [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Dependence. 1983;11:147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as a reinforce of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology. 2004;175:134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology. 2000;152:321–333. doi: 10.1007/s002130000541. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacology Biochemistry Behavior. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002;72:559–568. doi: 10.1016/s0091-3057(02)00723-2. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Who are these “nonsmokers”? Am J Public Health. 1996;86(5):745–746. doi: 10.2105/ajph.86.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Rose GL, Callas PW. Do former smokers respond to nicotine differently from never smokers? A pilot study. Nicotine Tob Res. 2000;2:225–262. doi: 10.1080/14622200050147529. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of the over-the-counter nicotine replacement. Tobacco Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacological Reviews. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of subjective effects of nicotine nasal spray and intravenous studies with smokers and nonsmokers. Nicotine Tobacco Research. 2005;7:317–333. doi: 10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among U.S. Adults 2010–2013. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Winger G. Individual differences in the reinforcing and punishing effects of nicotine in rhesus monkeys. Psychopharmacology. 2015;232:2393–2403. doi: 10.1007/s00213-015-3871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009;192:335–367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Swan GE. Tailoring nicotine replacement therapy: rationale and potential approaches. CNS Drugs. 2006;20:281–291. doi: 10.2165/00023210-200620040-00002. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: Use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B, De Biasi M, Lathrop M, Fratta W, Maskos U, Faure P. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol Psychiatry. 2014;19(8):930–936. doi: 10.1038/mp.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugenbauer NM, Cortright JJ, Sampedro GR, Vezina P. Exposure to nicotine enhances its subsequent self-administration: Contribution of nicotine-associated contextual stimuli. Behavioural Brain Research. 2014;260:155–161. doi: 10.1016/j.bbr.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor V, Andres ME, Bernabeu RO. The effect of previous exposure to nicotine on nicotine place preference. Psychopharmacology. 2013;226(3):551–560. doi: 10.1007/s00213-012-2928-1. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Nicotine discrimination in men and women. Pharmacology Biochemistry Behavior. 1999a;64(2):295–299. doi: 10.1016/s0091-3057(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Nicotine self-administration. Nicotine Tobacco Research. 1999b;1:S133–S137. doi: 10.1080/14622299050011951. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Response to Dar and Frenk (2004) “Do smokers self-administer pure nicotine? A review of the evidence”. Psychopharmacology. 2004;175:256–258. doi: 10.1007/s00213-004-1930-7. author reply 259–261. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Fonte C, Wilson A. Reinforcing effects of nicotine as a function of smoking status. Exp Clin Psychopharmacol. 2001;9:243–250. doi: 10.1037//1064-1297.9.3.243. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sanders M, D’Amico D, Wilson A. Nicotine discrimination and self-administration in humans as a function of smoking status. Psychopharmacology. 1997;131:361–37. doi: 10.1007/s002130050304. [DOI] [PubMed] [Google Scholar]
- Robinson JH, Pritchard WS. The role of nicotine in tobacco use. Psychopharmacology. 1992;108(4):397–407. doi: 10.1007/BF02247412. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology Biochemistry Behavior. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA, Adams PF. Health behaviors of adults: United States 2005–2007. National Center for Health Statistics. Vital Health Stat. 2010;10(245) [PubMed] [Google Scholar]
- Schuck K, Otten R, Engels RC, Kleinjan M. Initial responses to the first dose of nicotine in novel smokers: the role of exposure to environmental smoking and genetic predisposition. Psychol Health. 2014;29(6):698–716. doi: 10.1080/08870446.2014.884222. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology. 1999;147:210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12(12):467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Capturing momentary, self-report data: a proposal for reporting guidelines. Ann Behav Med. 2002;24(3):236–43. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Chapter 4: Nicotine Addiction: Past and Present. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [Google Scholar]
- U.S. Department of Health and Human Services. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking—50 Years of Progress. Accessed 18 Dec 2014. [Google Scholar]
- Vakkalanka JP, Hardison LS, Jr, Hostege CP. Epidemiological trends in electronic cigarette exposures reported to U.S. Poison Centers. Clin Toxicol. 2014;52(5):542–548. doi: 10.3109/15563650.2014.913176. [DOI] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Effect of infusion rate on intravenous nicotine self-administration in rats. Behav Pharmacol. 2013;24(5–6):517–522. doi: 10.1097/FBP.0b013e3283644d58. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic. Enforcing bans on tobacco advertising, promotion, and sponsorship. 2013 http://www.who.int/tobacco/global_report/2013/en. Accessed on 18 Dec 2014.


