Abstract
Purpose
miRNAs have been linked to chemosensitivity of breast cancer cells in-vitro. In patients, however, there is no clinically validated method for predicting chemotherapy response. The aim of this study was to assess whether I) a specific pattern of miRNA expression in pretherapeutic biopsies can predict response to neoadjuvant chemotherapy, and II) differential miRNA expression in residual tumor after completion of chemotherapy allows further prognostic stratification of non-responding patients.
Methods
Sixty-four patients with newly diagnosed large (≥3cm) or locally advanced primary breast cancers who underwent neoadjuvant anthracycline/taxane-based chemotherapy were included. Relative expression of 10 miRNAs likely to be associated with chemotherapy response (miR-7,-21,-29a,-29b,-34a,-125b,-155,-200c,-340,-451) was determined by quantitative RT-PCR from pretherapeutic biopsies (n=64) and residual invasive tumor after chemotherapy (n=42). Pathologic complete response (pCR) defined by absence of invasive tumor served as reference standard. In addition, miRNA expression was compared with disease-free and overall-survival.
Results
Nine (14%) of 64 patients achieved pCR. High expression of miR-7 and low expression of miR-340 in pretherapeutic biopsies predicted pCR with a negative-predictive-value of 96% and 97%, respectively (specificity 54% and 57%). The combined profile of miR-7high/miR-340low demonstrated improved specificity of 86% while maintaining a high negative-predictive-value (96%) to identify non-responders. Pretherapeutic expression of miR-200c and miR-155 showed prognostic information and low expression was associated with increased overall survival (115 vs. 90 months, p≤0.03). After chemotherapy, the overall survival of patients with residual invasive tumor was better for those demonstrating low miR-7 or high miR-125b (p=0.01).
Conclusions
Intratumoral expression of miR-7 and miR-340 prior to neoadjuvant chemotherapy could be used to predict pCR and a profile of miR-7low or miR-340high identified patients unlikely to achieve pCR who might benefit from alternative treatment options including earlier surgery. Our study identifies miRNAs as promising predictive biomarkers, which could aid in optimization of breast cancer management and treatment stratification.
Keywords: breast cancer, treatment response, prediction of response, neoadjuvant chemotherapy, miRNA, miR-7, miR-340
BACKGROUND
MicroRNAs (miRNAs) are involved in the development and progression of malignant tumors, mainly through post-transcriptional modulation of oncogenic and tumor suppressor pathways and provide prognostic information [2, 9, 16, 18, 31, 40, 48]. In breast cancer, differential miRNA expression has been demonstrated across breast cancer subtypes, with both tumor-promoting and tumor-suppressive functions for individual miRNAs [5, 8, 15, 17, 23, 44]. A recent study describing the global miRNA expression architecture in more than 1,000 human breast cancers confirmed these roles of individual miRNAs and revealed that miRNAs predominantly act as modulaters and fine-tuners of gene expression in these neoplasms [13].
Few studies have investigated the effect of chemotherapy on miRNA expression patterns or the predictive value of tissue-based miRNA expression profiles. Neoadjuvant systemic chemotherapy is increasingly used in women with newly diagnosed large or locally advanced breast cancer and is considered a valid therapeutic option in patients requiring chemotherapy [25, 47]. Neoadjuvant chemotherapy allows for assessment of treatment response in the resection specimen in addition to potentially reducing tumor volume and enabling more patients to be treated with breast-conserving surgery. Pathologic complete response (pCR) defined by the absence of invasive cancer is an important prognostic parameter associated with improved long-term outcome [11, 36, 43]. While clinical response is observed in up to 70% of patients, only about 15–30% achieve pCR [11, 25, 47].
Novel predictive biomarkers that can be assessed in the biopsy specimen before chemotherapy could help to individualize treatment decisions and to potentially avoid ineffective chemotherapies. The reliable assessment of miRNA expression profiles is feasible from routine formalin-fixed and paraffin-embedded archival tissues [12, 21, 42].
In the present study we explored further the potential of specific miRNAs to serve as predictive markers for response to neoadjuvant treatment in breast cancer. Ten candidate miRNAs were chosen, including four miRNAs (miR-7, -21, -34a and miR-451) previously associated with chemotherapy resistance of human breast cancer cells in-vitro [10, 24, 28, 33], and six miRNAs (miR-29a, -29b, -125b, -155, -200c, and miR-340) that we identified through in-silico analysis (described in materials and methods) of published gene expression changes in breast cancers after neoadjuvant chemotherapy [27, 34].
MATERIAL AND METHODS
Patients
A total of 64 patients with large (≥3cm) or locally advanced (T2 - 4) primary invasive breast cancer, with or without positive lymph node metastases at the time of presentation were included. All patients had been enrolled in a larger multi-center chemotherapy trial between 1998 and 2002 [41, 46]. Exclusion criteria were known distant metastases and prior chemo-, hormone-, or radiotherapy. Patients received epirubicin and paclitaxel in combination (90mg/m2 and 175mg/m2) or sequentially (150mg/m2 and 250mg/m2), or epirubicin plus cyclophosphamide (90mg/m2 and 600mg/m2) followed by docetaxel (100mg/m2). After completion of chemotherapy, all patients underwent breast-conserving surgery or mastectomy, followed by three cycles of adjuvant cyclophosphamide, methotrexate, and fluorouracil. In addition, patients with positive hormone receptor status received adjuvant tamoxifen for 5 years after completion of chemotherapy, and patients who underwent lumpectomy received radiotherapy of the breast.
For this miRNA analysis, consecutive patients from a single center, University Hospital Klinikum rechts der Isar of the Technische Universität München, were included if tumor tissue from pretherapeutic biopsies was available. Written informed consent for subsequent tissue analysis had been obtained from all patients and the study protocol for this analysis was approved by the institutional review board.
For all patients, the following histopathological parameters were assessed: invasive ductal vs. lobular subtype, grade (according to Bloom-Richardson modified by Elston and Ellis [14]), estrogen- and progesterone receptor status (positivity defined as >1% positive tumor cells), HER2-status (positivity defined as either IHC-score 3+ or amplification by FISH), tumor and nodal stage (ypT, ypN) and histopathologic response following chemotherapy. Last follow-up date was June 2012 and median follow-up time was 112 months. Disease-free and overall survival was calculated from the date of initial diagnosis. The REMARK reporting guidelines have been followed as far as applicable to this study [3]. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| total n=64 | [n] | % |
|---|---|---|
|
| ||
| Age [years] | ||
| Median (Range) | 52 (31; 68) | |
|
| ||
| Subtype | ||
| Invasive ductal | 55 | 86 |
| Invasive lobular | 8 | 13 |
| Mixed ductulo-lobular | 1 | 1 |
|
| ||
| Grade | ||
| G2 | 17 | 27 |
| G3 | 47 | 73 |
|
| ||
| Estrogen receptor status | ||
| Positive (≥1% positive tumor cells) | 41 | 64 |
| Negative | 23 | 36 |
|
| ||
| Progesterone receptor status | ||
| Positive (≥1% positive tumor cells) | 24 | 37 |
| Negative | 40 | 63 |
|
| ||
| HER2 status | ||
| Positive (IHC 3+ or FISH ratio ≥2.2) | 23 | 36 |
| Negative (IHC 0, 1+ or FISH ratio <2.2) | 37 | 58 |
| n/a | 4 | 6 |
|
| ||
| Surgical procedure | ||
| Breast conserving therapy | 33 | 52 |
| Mastectomy | 31 | 48 |
|
| ||
| ypT | ||
| 0 | 9 | 14 |
| 1 | 26 | 41 |
| 2 | 17 | 27 |
| 3 | 10 | 15 |
| 4 | 2 | 3 |
|
| ||
| ypN | ||
| 0 | 26 | 41 |
| 1 | 13 | 20 |
| 2 | 16 | 25 |
| 3 | 6 | 9 |
| n/a | 3 | 5 |
|
| ||
| Pathologic response after Chemotherapy | ||
| pCR | 9 | 14 |
| non-pCR | 55 | 86 |
|
| ||
| Follow-up [months] | ||
| Mean (95% confidence interval) | 116 (100; 131) | |
| Median (Range) | 112 (104; 120) | |
|
| ||
| Disease-free survival [months] | ||
| Mean (95% confidence interval) | 82 (68; 95) | |
| Median (Range) | 125 (52; 197) | |
|
| ||
| Overall survival [months] | ||
| Mean (95% confidence interval) | 103 (91; 115) | |
| Median (Range) | 115 (94; 135) | |
ypT, tumor stage assessed in the surgical specimen after neoadjuvant chemotherapy
ypN, nodal stage assessed in the surgical specimen after neoadjuvant chemotherapy
pCR, no residual invasive tumor in the breast, with or without residual non-invasive intraductal carcinoma, and irrespective of lymph node status (ypT0/is, ypNx)
Histopathologic Response
Pathologic complete response (pCR) was assessed in the surgical specimens post-chemotherapy, and defined by the complete absence of residual invasive tumor in the breast, with or without residual non-invasive intraductal carcinoma, and irrespective of lymph node status (ypT0/is, ypNX). All cases with residual invasive tumor of any size were classified as non-responding (non-pCR). Nine (13.6%) patients achieved pCR and 57 (86.4%) patients were histopathologic non-responders.
For assessment of pCR, surgical specimens were cut in 0.5 cm thick slices and evaluated for the presence of macroscopic tumor. Representative samples were taken from all macroscopically visible putative tumor as well as areas with marked fibrosis or scarring, and microscopically analyzed for the presence of residual tumor. The tumor bed was histologically identified by signs of tumor regression such as fibrosis, inflammation, and presence of macrophages or hemosiderin. Immunohistochemical staining with antibodies against cytokeratins was performed on selected sections to identify or verify tumor residues. The categorization of a case as pCR required extensive sampling from macroscopically suspicious as well as uninvolved areas of the surgical specimens.
Tissue samples
All biopsies and surgical specimens were fixed in 10% formaldehyde for 10 – 24 hours and subsequently paraffin-embedded. H&E stained sections of all cases were reviewed by an experienced pathologist (SA) to confirm subtype and grade and assess the percentage of viable invasive tumor cells, fibrosis or necrosis, and percentage of inflammatory cells. Breast cancer samples were further processed if at least one well circumscribed area with a tumor cellularity ≥70% was present to allow for manual microdissection, and if <10% inflammatory cells or <10% residual lymphocytes in lymph node metastases were present. For all biopsies and surgical specimens, tumor tissue from two or more different locations or biopsy cores was combined to account for intratumoral heterogeneity and avoid sampling bias.
A total of 123 samples from 64 patients showed sufficient tumor cell content, including 64 biopsies before chemotherapy, 42 post-chemotherapy specimens, and 17 lymph node metastases post-chemotherapy.
Selection of miRNA candidates potentially associated with chemotherapy resistance
Four miRNAs, including miR-7, -21, -34a and miR-451 were previously described to be associated with resistance of human breast cancer cells to anthracycline or taxane chemotherapy in-vitro [10, 24, 28, 33]. An additional six miRNA candidates were identified through in-silico analysis of published gene expression changes in breast cancers after chemotherapy. Perou’s seminal study [34] describing intrinsic molecular subtypes of breast cancer had also included gene expression profiles of 20 breast cancers before and after neoadjuvant anthracycline-based chemotherapy. A subsequent re-analysis identified 17 genes that showed significant changes in expression after neoadjuvant chemotherapy [27]. We utilized this set of 17 genes to identify potential regulatory miRNAs by in-silico analysis. Three different algorithms were used to predict putative regulatory miRNAs, including TargetScan [30] (Version 6.0), microRNA.org [4] (Release 10/2010) and PicTar [29]. miRNAs predicted to regulate two or more of the 17 genes or miRNAs predicted by 2 or more algorithms were chosen as candidates, including miRNAs miR-29a, -29b, -125b, -155, -200c, and miR-340.
RNA extraction
All tissue samples from the same patient (before and after chemotherapy) were processed simultaneously. Total RNA including small RNAs was extracted using the FFPE miRNeasy Kit (Qiagen, Hilden, Germany). We had previously studied the influence of various extraction parameters on quality and quantity of RNA, and a section thickness of 10μm and buffer volume of 150μl was determined as optimal [37]. For each sample, between 2 and 20 unstained 10μm sections were manually microdissected after deparaffinization, and RNA was extracted using 150μl proteinase K digestion buffer according to manufacturer’s instructions. Total RNA was measured with a NanoDrop photospectrometer (NanoDrop, Wilmingtom, DE).
miRNA expression analysis by quantitative PCR using TaqMan miRNA Assays
The expression of 10 miRNAs, including miR-7, -21, -29a, -29b, -34a, -125b, -155, -200c, -340, and miR-451, as well as small nucleolar RNA U48 for normalization, was determined by quantitative reverse-transcription polymerase chain reaction using TaqMan miRNA Assays (Applied Biosystems, Carlsbad, CA, USA). We had previously determined RNU48 as optimal reference for normalization, showing least intra- and inter-individual variation and high abundance in a range of breast cancer samples, and demonstrated high technical reproducibility of both RNA extraction procedure and quantitative PCR analysis for assessment of miRNA expression [37].
The assays were performed according to the manufacturer’s instructions, scaling the total reaction volume to 10μl each for the reverse transcription and the real-time PCR amplification reaction. A standard input of 6ng total RNA (manufacturer’s recommendation 1–10ng) was used, and 3ng and 9ng were used as control input to confirm that the RT reaction is performed within a linear dynamic range. Two negative controls were included in the RT, including one reaction without template and one without enzyme. During real-time PCR, all samples and serial dilutions were run in triplicates, and negative controls in duplicates.
For each individual assay, all 123 samples were analyzed at the same time on two 384-well plates, and corresponding pre- and post-therapeutic samples from one patient were analyzed on the same plate to avoid bias. A pool of 90 samples for which abundant quantity was available was used to prepare serial dilutions. For each assay, a standard curve including 6 serial dilutions was used to calculate and correct for amplification efficiency. Real-time PCR was carried out on a Light Cycler 480 instrument (Roche Diagnostics).
Analysis of quantitative PCR data was performed using the Light Cycler Software (Roche LC Software V1.5) and the arithmetic mean of each triplicate measurement was used for further analysis. Relative quantification of miRNA expression was performed using a non-linear algorithm (second derivative maximum, Roche LightCycler Software V1.5) with a standard curve for each individual assay to calculate and correct for amplification efficiency, and using RNU48 as reference for normalization.
Statistical analysis
The miRNA expression analysis was performed blinded to patients’ histopathological response status (pCR). The Mann-Whitney test was used to compare relative expression of a given miRNA between groups of patients. The Wilcoxon signed rank test was used to compare miRNA expression before and after chemotherapy. miRNAs showing a significant association with pCR were used to predict response by using the median expression value as a cutoff. The Chi-Square test was used to calculate the likelihood ratio for an association between low versus high miRNA expression (using median as cutoff) or clinico-pathologic parameters and histopathologic response. Box plots are utilized to visualize differences in relative miRNA expression, showing the median (line within the box), 25th and 75th percentiles, and whiskers showing 1.5 times the interquartile range. Spearman’s rank correlation was used to assess bivariate relationship of quantitative parameters. Kaplan Meier analysis and log-rank test were applied to compare survival probability between groups of patients. Multivariate analysis using Cox regression model was performed for various combinations of only two parameters, including miRNA expression and clinico-pathologic parameters, to account for the limited number of events. Quantitative parameters are expressed as median and range or mean ± one standard deviation. All statistical analyses were performed at a two-sided 5% level of significance using IBM SPSS Statistics (Version 21).
RESULTS
miR-7 and miR-340 expression at baseline is different in patients achieving pCR vs. non-PCR and predicts treatment response
For all 64 patients, tumor tissue from pretherapeutic core needle biopsies was available to assess miRNA expression at baseline prior to the start of chemotherapy. First, we assessed differences in miRNA expression between patients who achieved pCR (n=9) versus non-responding patients (non-PCR; n=55). Two miRNAs demonstrated significant differences in expression, with higher expression of miR-7 (mean 1.6 versus 0.9) and lower expression of miR-340 (mean 2.5 versus 5.7) associated with pCR vs. non-pCR (p=0.02; Figure 1). The expression of miR-7 and miR-340 were not statistically correlated with each other. All other miRNAs did not show an association with pCR.
Figure 1. Differential expression of miR-7 and miR-340 in patients achieving pCR versus non-pCR.
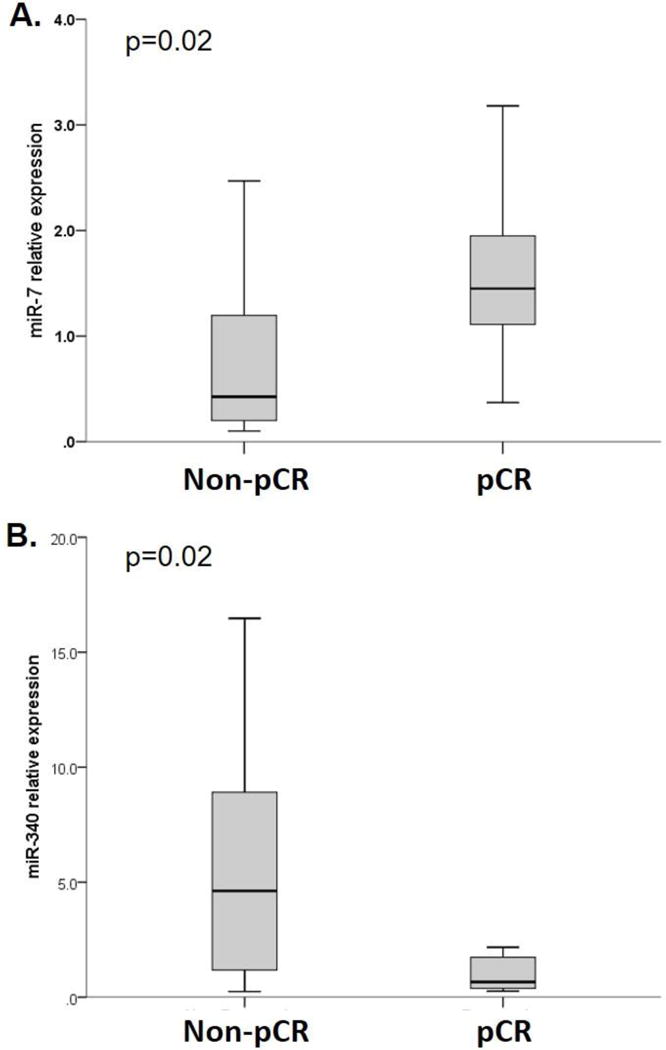
(A) miR-7 showed significantly higher expression at baseline in patients who achieved pCR versus non-pCR (p=0.02), and (B) miR-340 showed significantly lower expression in patients who achieved pCR versus non-pCR (p=0.02). Box plots are showing the median (line within the box), 25th and 75th percentiles, and whiskers are showing 1.5 times the interquartile range.
We next assessed the predictive value of miR-7 and miR-340 to identify treatment response versus non-response (pCR vs. non-pCR). Using the median value as cutoff, high expression of miR-7 and low expression of miR-340 in pretherapeutic biopsies each predicted pCR with a high negative predictive value of 96% and 97%, respectively (corresponding specificity 54% and 57%). The combined expression pattern of miR-7high/miR-340low predicted pCR with an equally high negative predictive value of 96% and improved specificity of 86% (Table 2).
Table 2.
Prediction of response (pCR) by expression of miR-7 and miR-340 at baseline.
| [%] | NPV | Sensitivity | Specificity | PPV | Likelihood Ratio (p) |
|---|---|---|---|---|---|
|
miR-7 cutoff >0.55 |
96 | 86 | 54 | 21 | 4.3 (0.03) |
|
miR-340 cutoff <4.0 |
97 | 89 | 57 | 26 | 7.1 (<0.01) |
|
Combined profile* miR-7high/miR-340low |
96 | 71 | 86 | 42 | 9.8 (<0.01) |
p, two-sided level of significance for likelihood ratio assessed by Chi-Square test
NPV, negative predictive value; PPV, positive predictive value
prediction of pCR by a combination of high expression of miR-7 (>0.55) and low miR-340 (<4.0); patients showing expression outside the cutoff for either miR-7 or miR-340 were considered non-responders
Associations between baseline miRNA expression and clinico-pathologic parameters
We next assessed associations between miRNA expression and common clinico-pathological tumor characteristics to identify potential confounding factors.
A higher expression of miR-7 at baseline was associated with invasive ductal versus lobular subtype (mean 1.0 vs. 0.5; p<0.01), grade 3 versus grade 2 (1.1 vs. 0.7; p=0.04) and HER2-positive versus -negative status (1.1 vs. 0.5; p=0.04). Lower expression of miR-340 was associated with HER2-negativity (mean 4.0 vs. 7.7; p<0.01) and estrogen receptor-positive/HER2-negative subtype (mean 4.0 vs. 6.5; p=0.02). All statistically significant associations between miRNA expression and clinico-pathologic parameters are summarized in Table 3.
Table 3.
Associations between baseline miRNA expression and clinico-pathologic parameters
| miR-7 | miR-200c | miR-340 | miR-125b | miR-155 | ||
|---|---|---|---|---|---|---|
| [p] | ||||||
| ductal type | ↓ <0.01 | ↓ 0.04 | ||||
| Grade 3 | ↓ 0.03 | ↓ 0.01 | ||||
| Estrogen receptor positivity | ↓ 0.04 | ↓ 0.03 | ||||
| Progesterone receptor positivity | ↓ <0.01 | |||||
| HER2 positivity | ↓ 0.03 | ↓ <0.01 | ||||
| Estrogen receptor-positive/HER2 negative status | ↓ <0.01 | ↓ 0.02 | ↓ 0.02 | |||
p, two-sided level of significance assessed by Mann-Whitney test
↑, higher expression associated with the respective clinico-pathologic parameter
↓, lower expression associated with the respective clinico-pathologic parameter
miRNAs not demonstrating significant associations with any clinico-pathologic parameter (miR-21, -29a, -29b, -451, -34a) are not shown
Predictive value of clinico-pathologic parameters for pathologic response (pCR)
To further assess potential confounding factors, we assessed possible associations between clinico-pathologic parameters and pCR. Patients with invasive ductal carcinoma showed a tendency for better response to chemotherapy compared to lobular carcinomas, although this was not statistically significant. We did not observe statistically significant associations between tumor grade, hormone receptor- or HER2-status and subsequent pCR in this patient cohort.
Expression of miR-200c and miR-155 at baseline provides prognostic information
We next assessed the prognostic value of miRNA expression at baseline prior to the start of neoadjuvant chemotherapy using disease free and overall survival as reference endpoint. Using the median expression values as cutoff, low-expression of miR-200c was significantly associated with longer disease free (101 vs. 67 months; p=0.04) and overall survival (115 vs. 90 months; p=0.02) compared to high expression of miR-200c. Similarly, low expression of miR-155 was associated with longer disease free (104 vs. 60 months; p<0.01) and overall survival (117 vs. 89 months; p=0.03; Fig 2). Despite being associated with response, miR-7 and miR-340 demonstrated no association with survival. The pretherapeutic expression levels of other miRNAs were not associated with survival.
Figure 2. Low expression of miR-200c and miR-155 in pretherapeutic biopsies is associated with longer overall survival.
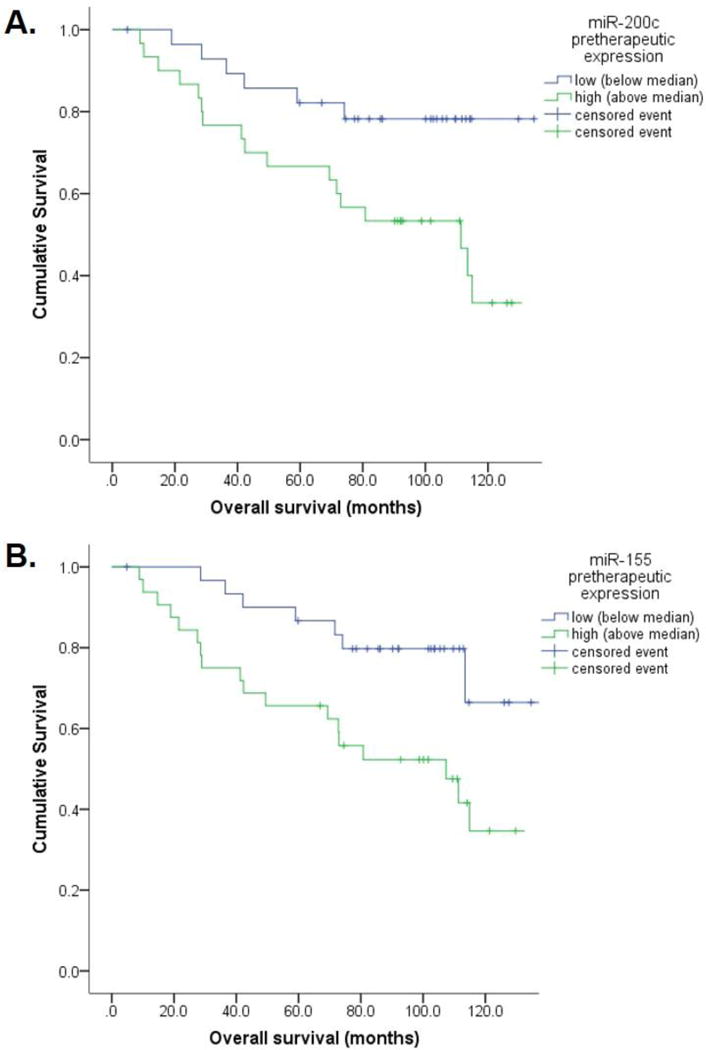
(A) Low expression of miR-200c in pretherapeutic biopsies (below median, <0.85) was associated with longer overall survival of 115 vs. 90 months compared to high expression (p=0.02); (B) Similarly, low expression of miR-155 (below median, <1.0) was associated with longer overall survival of 117 vs. 89 months (p=0.03).
Amongst clinico-pathologic parameters, estrogen receptor positivity, grade 2 (versus 3), and negative nodal status after chemotherapy were significantly associated with longer overall survival, and patients achieving pCR showed a tendency for longer disease free and overall survival although this was not statistically significant (Table 4).
Table 4.
Associations of baseline miRNA expression and clinico-pathologic parameters with disease free and overall survival
| Disease free survival [months] | p | Overall survival [months] | p | |
|---|---|---|---|---|
|
| ||||
| miR-200c High (≥0.85; n=32) | 67 | 0.03 | 99 | 0.02 |
| Low (<0.85; n=32) | 101 | 115 | ||
|
| ||||
| miR-155 High (>1.0; n=32) | 60 | <0.01 | 89 | 0.03 |
| Low (<1.0; n=32) | 104 | 117 | ||
|
| ||||
| Estrogen Receptor Positive (n=41) | 94 | <0.01 | 115 | 0.03 |
| Negative (n=23) | 60 | 81 | ||
|
| ||||
| Grade 2 (n=17) | >0.1 | 124 | 0.04 | |
| 3 (n=47) | 95 | |||
|
| ||||
| ypN 0 (n=26) | >0.1 | 125 | 0.01 | |
| ≥1 (n=38) | 90 | |||
|
| ||||
| pCR (n=9) | 99 | >0.1 | 124 | >0.1 |
| non-pCR (n=55) | 77 | 0.07 | 99 | 0.09 |
High, relative miRNA expression above the median value
Low, relative miRNA expression below the median value
p, two-sided level of significance assessed by log-rank test
Multivariate analysis using Cox regression was performed for various combinations of miR-200c or miR-155 with one additional clinico-pathologic parameter. Low expression of miR-200c remained significantly associated with longer overall survival in multivariate combinations with tumor grade (p=0.03), HER2 status (p=0.02), and negative nodal status after chemotherapy (p=0.02) and remained borderline significant in combinations with ER status (p=0.06) and pCR (p=0.07).
Similarly, low miR-155 remained significantly associated with longer overall survival in multivariate combinations with tumor grade (p=0.01) and HER2 status (p=0.04), and remained borderline significant in combinations with ER status (p=0.07), negative nodal status after chemotherapy (p=0.06), and pCR (p=0.05).
Expression of miR-7 and miR-125 in residual invasive tumor provides prognostic stratification of non-responding patients
Out of 55 non-responding patients (non-pCR) sufficient residual tumor to allow for microdissection and miRNA analysis was obtained from 42 patients. miRNA expression in residual invasive tumor after chemotherapy provided additional prognostic stratification of histopathologic non-responders. Using the median expression value as cutoff, low expression of miR-7 was associated with longer disease free (100 vs. 52 months, p=0.01) and overall survival (119 vs. 83 months; p=0.03). Similarly, high expression of miR-125b was associated with longer disease free (92 vs. 57 months, p=0.02) and overall survival (116 vs. 77 months, p<0.01) (Figure 3). All other miRNAs showed no association with survival parameters.
Figure 3. Prognostic stratification of non-responding patients (non-pCR) by high expression of miR-125b and low expression of miR-7 in residual invasive tumor after chemotherapy.
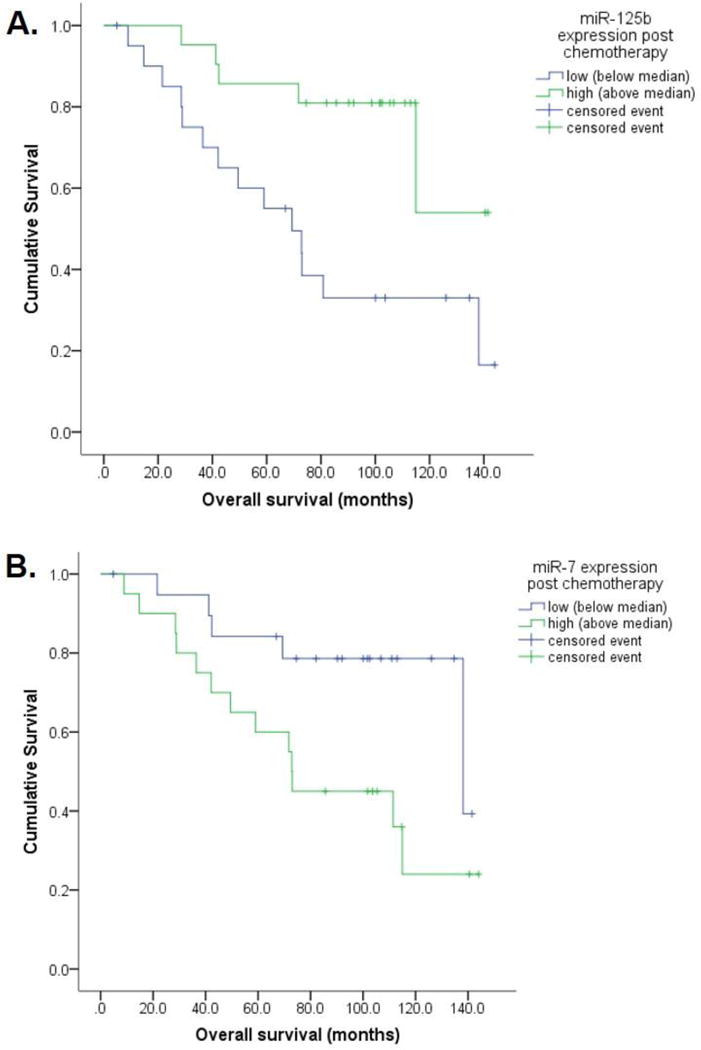
(A) High expression of miR-125b (above median, >1.2) in residual invasive tumor was associated with longer overall survival of 116 vs. 77 months compared to low miR-125b (p<0.01); (B) Similarly, low expression of miR-7 (below median, <0.2) in residual invasive tumor was associated with longer overall survival of 119 vs. 83 months (p=0.03).
Overall, the majority of miRNAs (miR-7, -21, -155, -200c, -340) showed a significant decrease in expression in residual tumor after chemotherapy compared to baseline, while two miRNAs, miR-125b and miR-34a showed a significant increase (Supplementary Table 1).
Axillary lymph node metastases were obtained from 17 out of 55 non-responding patients after chemotherapy. There was no consistent association between miRNA expression in the primary tumor and corresponding lymph node metastases.
DISCUSSION
We demonstrated that high intratumoral expression of miR-7 and low expression of miR-340 predicted pathologic complete response (pCR) of breast cancer prior to anthracycline/taxane-based neoadjuvant chemotherapy. In the neoadjuvant setting the early identification of non-responding breast carcinomas (non-pCR) would be most important to avoid ineffective treatments and potentially switch to alternative therapies earlier such as surgery or using a non-cross resistant chemotherapy regimen. High intratumoral expression of miR-7 and low expression of miR-340 prior to neoadjuvant chemotherapy identified histopathologic non-response (non-pCR) with a high negative predictive value (96–97%) identifying more than about half of patients that would have non-pCR at completion of neoadjuvant chemotherapy (specificity 54–57%). Using a combined profile of miR-7high/miR-340low identified non-responding patients with an improved specificity of 86% maintaining a high negative predictive value of 96%. A high negative predictive value is desirable to allow possible changes to an alternative non-cross resistant chemotherapy regimen in non-responders, while assuring that therapy is not withheld from potentially responding patients. Of note, the predictive value of miR-7 and miR-340 expression for histopathologic response was independent of previously established clinic-pathologic parameters such as tumor grade or tumor subtype.
Little is known so far about the predictive value of tissue-derived miRNAs but few studies assessed changes in circulating miRNAs from plasma samples during neoadjuvant chemotherapy. However, changes in plasma and tumor-based miRNA were not associated with each other as non-tumoral sources such as endothelial-derived miRNAs may have contributed to the pattern of circulating miRNAs in plasma [7, 20]. Zheng et al. reported increased miR-125b and miR-141 associated with non-pCR after anthracycline-taxane based chemotherapy in 21 breast cancers [50]. In 246 patients with estrogen-receptor positive breast cancers, higher levels of miR-30 and miR-182 were associated with benefit from adjuvant tamoxifen treatment and longer progression-free survival [38].
miR-7 was chosen in our panel because this microRNA has been previously associated with chemotherapy resistance [24, 35] and was linked with tumor-suppressor properties [49] and immunosurveillance of breast cancer cells in-vitro [1]. miR-7 was downregulated in taxane- and platinum resistant MCF-7 breast cancer cells [24, 35] and was suggested to regulate a drug efflux pump (multidrug resistance protein 1) [35] which is often upregulated in residual breast cancer after anthracycline- and/or taxane-based chemotherapy [26, 39]. Furthermore, breast cancer cells with upregulated miR-7 expression have been found to be more susceptible to cytotoxic T lymphocytes and may therefore contribute to a favorable immune-response [1].
miR-340 has not been previously linked with chemoresistance. Using in-silico analysis we identified that miR-340 is predicted to target several genes that were previously reported to be upregulated in breast cancer specimens after anthracycline-based chemotherapy [27]. miR-340 is also predicted to regulate tumor growth and proliferation, including Jun- and Fos-oncogenes, Cyclin Dependent Kinase 5 (Cdk5) and Connective Tissue Growth Factor (CTGF).
In our study, low intratumoral expression of miR-200c and miR-155 at baseline were both significantly associated with longer disease free and overall survival (115 vs. 90 and 117 vs. 89 months; p≤0.03). The prognostic value of both miRNAs was independent of other clinico-pathologic parameters, and low expression of miR-200c and miR-155 in baseline biopsies was better in predicting overall survival than pCR, estrogen receptor and HER2 status or tumor grade. The best prediction of overall survival was achieved by negative nodal status after chemotherapy. While miR-155 has a well-known oncogenic role in solid cancers including breast cancer (reviewed in [22, 32]) and its downregulation could conceivably be associated with good prognosis, the prognostic value of miR-200c in breast cancer is less well established. A recent study found opposing prognostic effects of miR-200c in progesterone receptor-positive versus -negative breast cancers [45].
Achievement of histopathologic complete response (pCR) correlates with improved long-term outcome, in particular for aggressive triple-negative or HER2-positive breast cancer subtypes [6, 11, 19, 25, 47]. However, the majority of patients do not achieve pCR. Further prognostic stratification of this large and heterogeneous group of non-responders would be clinically desirable but reliable markers are currently lacking.
We observed that tumor tissue levels of most miRNAs showed a treatment induced decrease in expression; however, miR-125b and miR-34a were significantly increased after chemotherapy. This is in line with a prior report of increased miR-34a in residual tumor after neoadjuvant chemotherapy [20].
Our results demonstrate that patients with residual tumor with high expression of miR-125b and low expression of miR-7 showed longer overall survival of 116 vs. 77 months (p<0.01) and 119 vs. 83 months (p=0.03), respectively. This information might be not translatable to different treatment stratification at this time; nevertheless, patients with a poorer prognosis might benefit from closer surveillance for disease recurrence.
Our study has some limitations related to the retrospective nature and limited size of the patient cohort (n=64 patients; n=123 samples). An important strength is the homogenous cohort consisting of consecutive patients from a previously reported clinical trial, which included large and locally advanced, higher grade (G2–3) breast cancers who received the same treatment regimen of epirubicin plus taxane chemotherapy [41, 46]. Furthermore, tumor tissue from two or more individual locations or biopsy cores was combined in each case to account for intratumoral heterogeneity and to avoid sampling bias. All samples were microdissected to ensure high tumor cell content (>70%). Our selection of miRNA candidates was performed prospectively based on mechanistic associations with chemo-sensitivity/-resistance in-vitro or based on predicted regulation of target genes that are altered in breast cancer after chemotherapy.
Based on our findings further prospective studies are necessary to confirm the predictive value of these miRNAs in defined breast cancer subtypes and for different therapeutic regimens such as those containing anti-HER2 treatment in addition to chemotherapy.
CONCLUSIONS
In conclusion, our results establish a potential role for using aberrant miRNA expression in predicting response or resistance to chemotherapy as well as disease free and overall survival in breast cancer. This information could be used for treatment stratification considering alternative treatment options. In addition, miRNA-targeted therapies are under investigation, and our results may provide a rationale for further assessment of miR-7 or miR-340 as targets to enhance chemosensitivity or manage drug-resistant breast cancers in the future.
Supplementary Material
Acknowledgments
This study was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation, DFG) Grant No SA1698/1-2 awarded to SA. MR was supported by a fellowship of the Technische Universität München. SA is supported by the Clinical and Translational Science Collaborative of Cleveland (KL2TR000440) from the National Center for Advancing Translational Sciences (NCATS) component of the NIH.
Footnotes
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
SA conceived of the study. SA, MR and HB analyzed and interpreted data. SA, MR and TB carried out experiments. HB provided study material and clinical follow-up data. WW and MK participated in data interpretation. All authors were involved in writing the paper and had final approval of the submitted and published versions.
ETHICAL APPROVAL
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Akalay I, Tan TZ, Kumar P, et al. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene. 2015;34:2261–2271. doi: 10.1038/onc.2014.151. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khanbashi M, Al-Moundhri M. Micro-Ribonucleic Acid and Carcinogenesis: Breast Cancer as an Example. Oncol Rev. 2015;9:279. doi: 10.4081/oncol.2015.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman DG, Mcshane LM, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC medicine. 2012;10:51. doi: 10.1186/1741-7015-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betel D, Wilson M, Gabow A, et al. The microRNA.org resource: targets and expression. Nucleic acids research. 2008;36:D149–153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blenkiron C, Goldstein LD, Thorne NP, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumour subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:93–100. doi: 10.1200/JCO.1998.16.1.93. [DOI] [PubMed] [Google Scholar]
- 7.Bovy N, Blomme B, Freres P, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6:10253–10266. doi: 10.18632/oncotarget.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buffa FM, Camps C, Winchester L, et al. microRNA-associated progression pathways and potential therapeutic targets identified by integrated mRNA and microRNA expression profiling in breast cancer. Cancer Res. 2011;71:5635–5645. doi: 10.1158/0008-5472.CAN-11-0489. [DOI] [PubMed] [Google Scholar]
- 9.Cekaite L, Eide PW, Lind GE, et al. MicroRNAs as growth regulators, their function and biomarker status in colorectal cancer. Oncotarget. 2016;7:6476–6505. doi: 10.18632/oncotarget.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen GQ, Zhao ZW, Zhou HY, et al. Systematic analysis of microRNA involved in resistance of the MCF-7 human breast cancer cell to doxorubicin. Med Oncol. 2010;27:406–415. doi: 10.1007/s12032-009-9225-9. [DOI] [PubMed] [Google Scholar]
- 11.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 12.Doleshal M, Magotra AA, Choudhury B, et al. Evaluation and validation of total RNA extraction methods for microRNA expression analyses in formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2008;10:203–211. doi: 10.2353/jmoldx.2008.070153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvinge H, Git A, Graf S, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature. 2013;497:378–382. doi: 10.1038/nature12108. [DOI] [PubMed] [Google Scholar]
- 14.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 15.Enerly E, Steinfeld I, Kleivi K, et al. miRNA-mRNA integrated analysis reveals roles for miRNAs in primary breast tumors. PLoS One. 2011;6:e16915. doi: 10.1371/journal.pone.0016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabris L, Ceder Y, Chinnaiyan AM, et al. The Potential of MicroRNAs as Prostate Cancer Biomarkers. Eur Urol. 2016;70:312–322. doi: 10.1016/j.eururo.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farazi TA, Horlings HM, Ten Hoeve JJ, et al. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443–4453. doi: 10.1158/0008-5472.CAN-11-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farazi TA, Spitzer JI, Morozov P, et al. miRNAs in human cancer. The Journal of pathology. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher ER, Wang J, Bryant J, et al. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 20.Freres P, Josse C, Bovy N, et al. Neoadjuvant Chemotherapy in Breast Cancer Patients Induces miR-34a and miR-122 Expression. J Cell Physiol. 2015;230:473–481. doi: 10.1002/jcp.24730. [DOI] [PubMed] [Google Scholar]
- 21.Hasemeier B, Christgen M, Kreipe H, et al. Reliable microRNA profiling in routinely processed formalin-fixed paraffin-embedded breast cancer specimens using fluorescence labelled bead technology. BMC Biotechnol. 2008;8:90. doi: 10.1186/1472-6750-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgs G, Slack F. The multiple roles of microRNA-155 in oncogenesis. Journal of clinical bioinformatics. 2013;3:17. doi: 10.1186/2043-9113-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 24.Kastl L, Brown I, Schofield AC. miRNA-34a is associated with docetaxel resistance in human breast cancer cells. Breast Cancer Res Treat. 2012;131:445–454. doi: 10.1007/s10549-011-1424-3. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann M, Von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Annals of surgical oncology. 2012;19:1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 26.Kim B, Fatayer H, Hanby AM, et al. Neoadjuvant chemotherapy induces expression levels of breast cancer resistance protein that predict disease-free survival in breast cancer. PLoS One. 2013;8:e62766. doi: 10.1371/journal.pone.0062766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korn EL, Mcshane LM, Troendle JF, et al. Identifying pre-post chemotherapy differences in gene expression in breast tumours: a statistical method appropriate for this aim. Br J Cancer. 2002;86:1093–1096. doi: 10.1038/sj.bjc.6600216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovalchuk O, Filkowski J, Meservy J, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 29.Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 30.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 32.Mattiske S, Suetani RJ, Neilsen PM, et al. The oncogenic role of miR-155 in breast cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 33.Mei M, Ren Y, Zhou X, et al. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol Cancer Res Treat. 2010;9:77–86. doi: 10.1177/153303461000900109. [DOI] [PubMed] [Google Scholar]
- 34.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 35.Pogribny IP, Filkowski JN, Tryndyak VP, et al. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127:1785–1794. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- 36.Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2015 doi: 10.1038/modpathol.2015.74. [DOI] [PubMed] [Google Scholar]
- 37.Raychaudhuri M, Schuster T, Buchner T, et al. Intratumoral Heterogeneity of MicroRNA Expression in Breast Cancer. J Mol Diagn. 2012;14:376–384. doi: 10.1016/j.jmoldx.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Gonzalez FG, Sieuwerts AM, Smid M, et al. MicroRNA-30c expression level is an independent predictor of clinical benefit of endocrine therapy in advanced estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2011;127:43–51. doi: 10.1007/s10549-010-0940-x. [DOI] [PubMed] [Google Scholar]
- 39.Rudas M, Filipits M, Taucher S, et al. Expression of MRP1, LRP and Pgp in breast carcinoma patients treated with preoperative chemotherapy. Breast Cancer Res Treat. 2003;81:149–157. doi: 10.1023/A:1025751631115. [DOI] [PubMed] [Google Scholar]
- 40.Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Archiv: an international journal of pathology. 2008;452:1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarz-Dose J, Untch M, Tiling R, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–541. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 42.Siebolts U, Varnholt H, Drebber U, et al. Tissues from routine pathology archives are suitable for microRNA analyses by quantitative PCR. J Clin Pathol. 2009;62:84–88. doi: 10.1136/jcp.2008.058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 44.Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuomarila M, Luostari K, Soini Y, et al. Overexpression of microRNA-200c predicts poor outcome in patients with PR-negative breast cancer. PLoS One. 2014;9:e109508. doi: 10.1371/journal.pone.0109508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Untch M, Mobus V, Kuhn W, et al. Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol. 2009;27:2938–2945. doi: 10.1200/JCO.2008.20.3133. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Hage JA, Van De Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 48.Varamo C, Occelli M, Vivenza D, et al. MicroRNAs Role as Potential Biomarkers and Key Regulators in Melanoma. Genes Chromosomes Cancer. 2016 doi: 10.1002/gcc.22402. [DOI] [PubMed] [Google Scholar]
- 49.Webster RJ, Giles KM, Price KJ, et al. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284:5731–5741. doi: 10.1074/jbc.M804280200. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Li S, Boohaker RJ, et al. A MicroRNA Expression Signature In Taxane-anthracycline-Based Neoadjuvant Chemotherapy Response. Journal of Cancer. 2015;6:671–677. doi: 10.7150/jca.11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


