Abstract
Little is known about extensive nervous system growth after axons reach their targets. Indeed, postnatal animals continue to grow, suggesting that axons are stretched to accommodate the expanding body. We have previously shown that axons can sustain stretch-growth rates reaching 1 cm/day; however, it remained unknown whether the ability to transmit active signals was maintained. Here, stretch-growth did not alter sodium channel activation, inactivation, and recovery or potassium channel activation. In addition, neurons generated normal action potentials that propagated across stretch-grown axons. Surprisingly, Na and K channel density increased due to stretch-growth, which may represent a natural response to preserve the fidelity of neuronal signaling.
Keywords: Nervous system growth, Axon growth, Sodium channel, Potassium channel, Action potential
1. Introduction
Early in development, axons navigate via a growth cone over seemingly large distances to reach their target. However, well after axons integrate with their targets and establish synaptic connections, animals and their nervous systems continue to grow several orders of magnitude in size. For instance, axons of motor neurons initially extend a few hundred micrometers to innervate muscle. Yet, despite the absence of a growth cone, axons then elongate several centimeters to meters in length, keeping pace with the growth of the animal. It is conceivable that stretching forces, exerted on axons by the enlarging body, initiate and maintain stretch growth of the axon cylinder [1–4], thereby contributing to the formation of long nerves and white matter tracts.
Only a few studies have examined the effects of mechanical stretch on axons. In these reports, tension applied to the growth cones of single axons in culture was shown to induce transient lengthening [1,5–8]. To mimic the stretch growth of axons after target integration, we recently developed an in vitro system to investigate whether integrated axons could sustain rapid growth solely through the application of mechanical stretch. Using a microstepper motor system, two halves of a neuronal culture were separated to stretch the interconnecting axons. Far exceeding the approximately 1 mm/day rate of growth cone extension, integrated axons quickly adapted to stretch-growth rates reaching at least 1 cm/day, producing large axon fascicles 10 cm in length and potentially much longer. Remarkably, these extreme stretch-growth conditions stimulated expansion of the central portion of axon cylinders, while maintaining normal cytoskeletal ultrastructure [3,4,9].
While neurons can structurally accommodate rapid axon stretch growth, the important question of whether these neurons can still transmit active signals remained unknown. Naturally, the ability of the nervous system to retain function throughout animal development suggests that axons can transmit signals despite rapid periods of growth. The direct electrophysiological examination of axons under extreme stretch growth conditions, however, had not been explored. Therefore, we investigated whether neurons, following a period of rapid axon stretch growth in vitro, retain the ability to generate and convey action potentials. In addition, we examined whether stretch growth induces alterations in Na and K channel biophysics that underlie action potential generation and propagation.
2. Methods
2.1. Axon stretch growth
Dorsal root ganglia (DRG) were isolated from E15 rat embryos and maintained as described elsewhere [3,9]. For axon stretch growth, neurons were plated along the interface of two adjoining substrates in a custom designed bioreactor [3,9]. Within five days of plating, axons extended across the interface between the two adjoining substrates and into the population of neurons on either side. Axons were stretch-grown at a rate of 1 mm/day for 24 h, 2 mm/day for 12 h, 3 mm/day for 12 h, 4 mm/day for 12 h, and 6 mm/day for 48 h resulting in 1.75 cm long axon tracts; a total of 9 days in vitro (DIV) from day of plating. The result is two groups of neuronal somata at each end, interconnected by stretch-grown axons, Supplemental Fig. 1. The 6 mm/day rate was chosen as it is comparable to maximum body growth rates experienced by many animals such as rats.
Age matched control cultures were maintained in standard tissue culture dishes for 9 DIV, where axons were allowed to extend via growth cones without manipulation. Stretch-growth bioreactor substrates (Aclar 33C film, Structure Probe, West Chester, PA) and dishes for control cultures were prepared identically. Both surfaces were treated with 10 μg/mL poly-L-lysine, coated with type 1 rat-tail collagen (Becton Dickinson, Franklin Lakes, NJ), polymerized into a hydrogel (approximately 500 μm thick) by exposure to ammonia vapors for 2 min prior to cell plating.
2.2. Electrophysiology
Voltage gated Na and K channels were analyzed in neurons that had either undergone axon stretch-growth (stretch-grown cultures) or allowed to extend axons without manipulation (control cultures); both age-matched at 9 DIV. Somatic recordings were conducted with the whole cell patch clamp technique at room temperature using electrodes (4–8 MΩ, P-30, Sutter Instruments), Axopatch 1D amplifier, Digidata data acquisition system, and pClamp 8 software (Axon Instruments). To minimize instances of inadequate space clamp, neurons were only included in the analysis if 80% series resistance compensation was achieved and recordings where discarded if resistance increased >20% during the experiment.
All cultures were perfused continuously during the experiment. External solution consisted of (in mM) 115 NaCl, 5.6 KCl, 2 CaCl2, 1 MgCl2, 11 glucose, 1 NaH2PO4, and 25 NaHCO3 (equilibrated with 5% CO2, 95% O2). This formulation was chosen as the 115 mM concentration of NaCl, reduced from the more standard 130 mM, to avoid current saturation and inadequate space clamp. The pipette solution for recording Na currents consisted of (in mM) 135 CsCl, 1.21 CaCl2, 1.21 MgCl2, 6.04 Na HEPES, 3.96 HEPES, 3 EGTA, and 10 TEA Cl, pH 7.3 (CsOH). The pipette solution for recording K currents and action potentials consisted of (in mM) 5 NaCl, 135 KCl, 1 MgCl2, 3 EGTA, and 10 HEPES, pH 7.3 (NaOH). Since DRG neurons express tetrodotoxin resistant Na channels, K currents were isolated by blocking Na channels with 5 mM lidocane [10].
For channel activation, the membrane potential was stepped by 10 mV from a holding potential of −60 mV to 90 mV. Current–voltage (I–V) relationships were plotted from peak Na and steady-state K currents. Conductance was calculated using the equation G = I/(Vc−Vrev) where Vc was the command potential and Vrev the measured reversal potential. Na channel inactivation was analyzed by an h∞ protocol [11]. Pre-pulse command potentials were applied from −55 to +10 mV in steps of 5 mV, followed by a depolarizing pulse to +10 mV. Peak currents were normalized to maximum peak current. Na channel recovery from inactivation was assessed using a paired-pulse protocol that increased the time window between two depolarizing command potentials of +10 mV from 5 to 50 ms. Percent recovery was calculated by dividing the peak current from the second pulse by the peak of the first pulse.
Whole cell capacitance, read from the capacitance compensation potentiometer on the patch clamp amplifier, was recorded for each cell in the study. Current density was calculated by dividing the maximum channel current by whole cell capacitance. Since capacitance has the unit of farads per unit area, this provides a measurement of the amount of current flow per unit area of membrane.
For somatic action potentials, neurons held at −60 mV under current clamp conditions, were stimulated with 2 nA current over 1 ms. To detect action potential transmission, neuronal somata at one end of the stretch-grown culture were depolarized by a focal application of 100 mM KCl (BPS-4, ALA Scientific, Westbury, NY). Action potential propagation across the interconnecting axons was detected by simultaneously monitoring a current clamped neuron present in the opposite group at 10 kHz from a holding potential of −60 mV (Fig. 4).
Fig. 4.

Transmission of action potentials across stretch-grown axons. The right population of neurons was briefly depolarized with 100 mM KCl using a focal applicator while the membrane potential of a neuron in the left population was recorded. The preparation was superfused with external solution at a rate of >2 mL/min (in the direction of the arrow) washing KCl away from the population of neurons being recorded. The trace illustrates a recording of action potential transmission across stretch-grown axons. Initially, an application of external solution (first arrow) did not depolarize neurons or produce artificial signals. Upon application of the KCl, depolarization generated action potentials that propagated across stretch-grown axons and stimulated action potentials in an integrated neuron on the other side.
2.3. Immunocytochemical analysis
Samples of control cultures for Western blotting were rinsed, frozen on dry ice, and incubated in lysis buffer (RIPA with protease inhibitors, Roche, Nutley, NJ) for 5 min. Samples of axonal protein from control cultures were prepared by aspirating neuronal somata from the frozen dishes leaving isolated axons and collected as above. Lysates were pooled until a protein concentration of >0.5 mg/mL was achieved (BCA, Pierce, Rockford, IL). For stretch-grown samples (following the 6 mm/day axon stretch growth protocol), cultures were rinsed, frozen, and the substrates were cut away from the elongation device, Supplemental Fig. 1. Stretch-grown axons were isolated by cutting off the two populations of neuronal somata at each end. The axon and somata samples were then collected the same as control cultures. Thirty micrograms of protein from each sample was run on a 10% gel, immunoblotted (Pan-Nav, 1:1000, Kv1.2, 1:1000, Alomone Labs, Jerusalem, Israel), and detected by chemiluminescence (Pierce). Optical density of bands was analyzed using UN-SCAN-IT gel-scanning software (Silk Scientific, Orem, UT).
Fixed cultures were probed with antibodies against (Pan-Nav, 1:200) and (Kv1.2, 1:200). Confocal images were acquired at 60× with a Bio-Rad Radiance 2000-MP3 on a TE-300 microscope (Nikon, Melville, NY). Due to large changes in ion channel expression between control and stretch-grown samples, the photo multiplier tube (PMT) gain was adjusted, holding all other settings and constant, for visualization. An equivalent number of z-sections were acquired for each image and projected onto one image using a maximum pixel value method.
3. Results
The resting membrane potential was measured for each neuron and was not affected by axon stretch growth, −42.0 ± 2.19 mV for stretch-grown cultures and −37.4 ± 1.63 mV for control cultures (±SEM, P > 0.05, two-tailed unpaired t test). Whole cell capacitance was 35.5 ± 0.96 pF for stretch-grown cultures and 20.4 ± 1.40 pF for control cultures (P < 0.05). To examine whether stretch growth alters the biophysical properties of ionic channels underlying the action potential, voltage-dependent Na and K currents were analyzed. Interestingly, neurons from stretch-grown cultures (n = 15) produced larger peak Na currents compared to control cultures (n = 6, Fig. 1a). Peak I–V relationships, normalized by whole cell capacitance to eliminate the effect of cell size, demonstrated a 2-fold increase in peak Na current density in neurons from stretch-grown cultures compared to control cultures (P < 0.05). There were no obvious shifts in voltages for activation (−30 mV), peak current (−10 to 0 mV) or reversal potential (50 mV, Fig. 1b) suggesting that stretch did not alter the voltage sensitivity or Na channel selectivity. The 10–90% rise time of sodium currents measured at step depolarizations to 0 mV remained the same between static (0.488 ± 0.036 ms) and (0.486 ± 0.071 ms) stretch-grown neurons (P > 0.05).
Fig. 1.
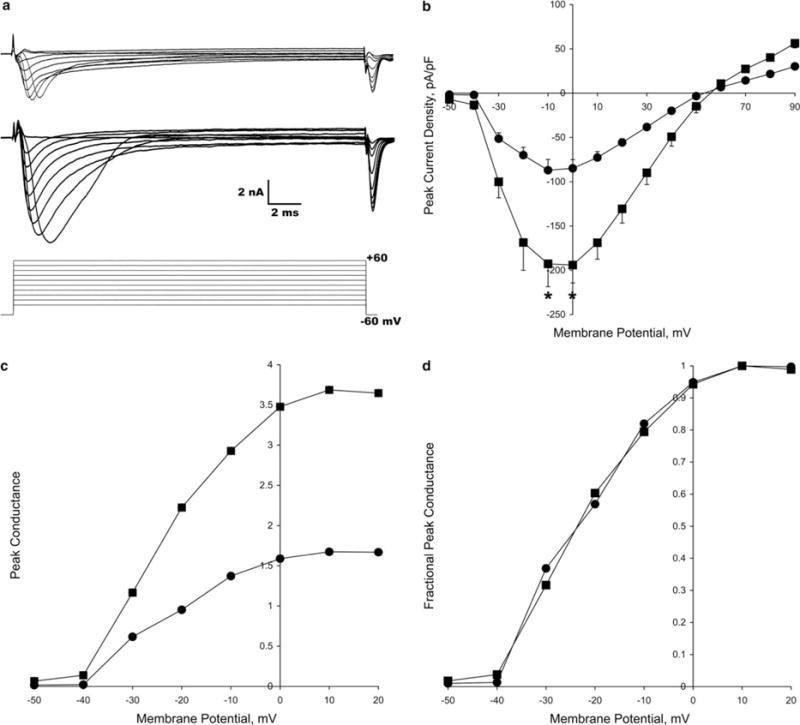
Analysis of Na channel currents in stretch-grown (squares) and control (circles) cultures. (a) Na currents measured from a neuron in a control (top) and stretch-grown (middle) culture using an activation protocol (bottom). (b) Peak Na channel I–V curves normalized to cell capacitance (bars = S.E.M.). Voltage dependent channel activation, reversal potential and maximum channel activation were not altered due to axon stretch growth (*P < 0.05). (c) Peak Na channel G–V curves, normalized to cell capacitance, showing that conductance increases >2-fold in stretch-grown neurons. (d) G–V curves, normalized to peak conductance, indicate that the voltage sensitivity of Na channels is not affected by axon stretch growth.
Conductance–voltage (G–V) curves, which remove the effects of variable driving forces at each command potential [12], were constructed to examine if axon stretch growth induced putative alterations in the voltage sensitivity of Na channels. Peak Na channel conductance, normalized to cell capacitance, reflected an increase in conductance per unit area of membrane (Fig. 1c). Current amplitude effects due to channel density, the number of channels present per unit area of membrane, were eliminated by normalizing to maximum conductance. The two curves overlaid each other (Fig. 1d), indicating that axon stretch growth does not induce shifts in the voltage sensitive kinetics of the Na channel.
Na channel inactivation was analyzed from neurons in control (n = 6) and stretch grown (n = 16) cultures. Consistent with previous reports, a wide variation in the kinetics of Na channel inactivation (data not shown) was found in both control and stretch-grown neurons [13]. However, no significant shifts in Na channel inactivation kinetics were present (P > 0.05). Na channel recovery from inactivation was also analyzed in neurons from control (n = 4) and stretch-grown (n = 11) cultures. Recovery curves from each neuron were fitted with a single exponential resulting in non-significantly different mean recovery time constants of 6.0 ± 1.2 ms for control and 4.7 ± 0.9 ms for stretch-grown cultures (P > 0.05, ±S.E.M.). This suggests that stretch does not alter Na channel recovery from inactivation.
Analogous to Na currents, steady-state K currents from neurons in stretch-grown cultures (n = 13) were observed to increase in amplitude compared to control cultures (n = 10, Fig. 2a). Peak steady-state K currents were normalized to cell capacitance and plotted as I–V relationships (Fig. 2b) revealing a 43% increase in K current due to stretch growth (P < 0.05) with no apparent shift in activation voltage (0 mV). G–V curves for peak K channel conductance (normalized to cell capacitance) show an increase in conductance per unit area of membrane (Fig. 2c). The G–V curves were normalized to maximum conductance revealing non-significantly different voltage sensitive kinetics of the K channel between control and stretch-grown cultures (Fig. 2d).
Fig. 2.

Analysis of K channel currents in stretch-grown (squares) and control (circles) cultures. (a) K currents measured from a neuron in a control (top) and a stretch-grown (middle) culture using an activation protocol (bottom). (b) Steady-state K channel I–V curves normalized to cell capacitance (bars = S.E.M.). Voltage dependent channel activation and maximum channel activation were not altered due to axon stretch-growth (*P < 0.05). (c) Peak K channel G–V curves, normalized to cell capacitance, showing that conductance increases 1.4× in stretch-grown neurons. (d) G–V curves, normalized to peak conductance, indicate that the voltage sensitivity of K channels is unaltered due to the axon stretch growth.
Since no alterations in basic biophysical properties of Na or K channels were identified, there are two possible explanations for the increase in Na and K current – an increase in channel density of the neuronal membranes or an increase in single channel conductance. In attempt to discriminate between these two possibilities, Western blots were used to examine Na and K channel expression. Na channel expression was increased by stretch growth in axons by 304% (Fig. 3a) and the neuronal soma by 187% (Fig. 3b) compared to control cultures. Interestingly, K channel expression increased in neuronal somata by 185% and not in axons (Fig. 3a and b). While this upregulation in channel expression can explain the increase in measured Na and K currents, it does not rule out alterations in single channel conductance.
Fig. 3.

Immunocytochemical analysis of Na and K channel expression in age-matched control (somata (C) and axon (C-Ax) fractions) and stretch-grown cultures (somata (SG-a/b) and axon (SG-Ax) fractions). (a) Western blots and densitometry measurements from isolated axons demonstrate an upregulation of Na channel, but not K channel, expression due to axon stretch growth. (b) Blots and densitometry measurements from neuronal somata indicate that axon stretch-growth induces an increase in both Na and K channel expression. (c, d) Fluorescent labeling of Nav Pan from control (c) and stretch-grown cultures (d). Despite the visual increase in fluorescent intensity in stretch-grown cultures, the PMT gain was increased from 10 to 80 for visualization of control cultures. Localization of Na channels appeared uniform over the length of stretch-grown axons and somata. (e, f) Fluorescent labeling of Kv 1.2 from control (e) and stretch-grown cultures (f). The PMT gain was increased from 25 for stretch-grown cultures to 70 in order to visualize the control cultures. Scale bar: 25 μm.
Augmenting the Western blot data, immunocytochemical labeling shows an upregulation of Na channels in both axons and somata as seen by the increase in fluorescence intensities (Fig. 3c and d). This increase in channel expression appeared uniform along axons and within the neuronal somata as indicated by the Western blot. Likewise, K channel expression appeared to increase in the somata with very little change in the axons (Fig. 3e and f).
While rapid axon stretch growth evidently induces changes in neuronal membrane properties, we further investigated whether action potential generation and propagation was effected. Action potentials were evoked in all neurons from both the control (n = 12) and stretch-grown cultures (n = 14) that were not significantly different with respect to rates of rise, action potential threshold, and half-width duration (P > 0.05). Stretch-grown cultures were then analyzed to determine if action potential propagation is maintained across the span of stretch-grown axons. Transmitted action potentials, initiated by focally applying KCl to the group of neuronal somata at one end of the culture, were recorded in patched neurons located in the group at the other end (n = 4, Fig. 4). To ensure that measured action potentials were not an artifact of the KCl application, a similar focal application of external recording solution was applied prior to the KCl. Incoming action potentials were only recorded when KCl, and not external recording solution, was applied.
4. Discussion
Between late embryogenesis and early adult life, integrated axons that initially span a very short distance continue to undergo enormous growth and can reach meters in length in large animals. The mechanical stretching forces resulting from the growth of an animal may be the key mechanism that initiates and maintains growth of the axon cylinder [1–4]. Here we found that Na and K channel density increased significantly following a period of sustained axon stretch growth, without altering channel kinetics or voltage sensitivity. Despite this active remodeling of membrane properties, neuronal somata retained the ability to generate normal action potentials that propagated across the stretch-grown axons.
One might imagine that as axons are forced to stretch-grow, the cytoskeletal structure and membrane composition would simply redistribute along the axon length. In striking contrast, axon stretch growth can induce a rapid expansion of the axon cylinder while maintaining a normal density of cytoskeletal proteins and organelles [3]. Nonetheless, if a redistribution of ion channels – a dilution of channel density – occurred as axons expand, the transmission of active signals would be impossible. Here we find that axon stretch growth also induces an increase in the density of Na and K channels and neurons retain the ability to transmit active signals. Accordingly, continuous mechanical stimulation may be a potential mechanism that induces modifications in membrane properties to preserve nervous system signaling as axons grow in length and caliber.
In addition to providing insight into developmental changes in axons as they grow, extreme axon stretch growth in vitro can be potentially exploited to repair the nervous system. We have recently used stretch-grown axons spanning two populations of neurons to bridge a 1 cm lesion in the rat spinal cord. It was found that transplanted stretch-grown cultures maintained their pre-transplant geometry, survived for at least 1 month and sent axons to integrate with viable host tissue at each end of the lesion [14]. Importantly, the findings of this study demonstrate that stretch-grown axon tracts have the potential to transmit active signals across a lesion.
Axon stretch growth is an understudied phase of extremely rapid growth that can provide significant insight into how the nervous system is remodeled after axons have integrated with their targets. Here we provide evidence that rapid axon stretch growth induces a unique change in the membrane properties of neuronal somata and axons. These data suggest that the mechanical stimulus that induces dramatic changes in the geometry of stretch-grown axons must also optimize ion channel density in order to maintain the fidelity of active signaling in nerves and white matter tracts that rapidly expand during development.
Supplementary Material
Acknowledgments
We thank Dr. L.S. Satin and Dr. R.G. Kalb for their insightful comments on this manuscript. This work was supported by NIH Grants AG21527, NS38104 and HD41699 (DHS) and NS45975 (ASC).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.febslet.2006.05.030.
References
- 1.Bray D. Axonal growth in response to experimentally applied mechanical tension. Dev Biol. 1984;102:379–389. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]
- 2.Weiss P. Nerve patterns: the mechanics of nerve growth. Growth, Third Growth Symp. 1941;5:163–203. [Google Scholar]
- 3.Pfister BJ, Iwata A, Meaney DF, Smith DH. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DH, Wolf JA, Meaney DF. A new strategy to produce sustained growth of central nervous system axons: continuous mechanical tension. Tissue Eng. 2001;7:131–139. doi: 10.1089/107632701300062714. [DOI] [PubMed] [Google Scholar]
- 5.Chada S, Lamoureux P, Buxbaum RE, Heidemann SR. Cytomechanics of neurite outgrowth from chick brain neurons. J Cell Sci. 1997;110:1179–1186. doi: 10.1242/jcs.110.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.Dennerll TJ, Lamoureux P, Buxbaum RE, Heidemann SR. The cytomechanics of axonal elongation and retraction. J Cell Biol. 1989;109:3073–3083. doi: 10.1083/jcb.109.6.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidemann SR, Lamoureux P, Buxbaum RE. Cytomechanics of axonal development. Cell Biochem Biophys. 1995;27:135–155. doi: 10.1007/BF02738107. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J, Lamoureux P, Santiago V, Dennerll T, Buxbaum RE, Heidemann SR. Tensile regulation of axonal elongation and initiation. J Neurosci. 1991;11:1117–1125. doi: 10.1523/JNEUROSCI.11-04-01117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfister BJ, Iwata A, Taylor AG, Wolf JA, Meaney DF, Smith DH. Development of transplantable nervous tissue constructs comprised of stretch-grown axons. J Neurosci Meth. 2006;153:95–103. doi: 10.1016/j.jneumeth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Scholz A, Vogel W. Tetrodotoxin-resistant action potentials in dorsal root ganglion neurons are blocked by local anesthetics. Pain. 2000;89:47–52. doi: 10.1016/S0304-3959(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 11.Hille B. Ionic Channels of Excitable Membranes. 2nd. Sinauer Associates Inc; Sunderland: 1992. [Google Scholar]
- 12.Dempster J. Computer analysis of electrophysiological signals. In: Sattelle D, editor. Biological Techniques. Academic Press; London: 1993. [Google Scholar]
- 13.Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. J Neurophysiol. 1994;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata A, Browne KD, Pfister BJ, Gruner JA, Smith DH. Long-term survival and outgrowth of mechanically engineered nervous tissue constructs implanted into spinal cord lesions. Tissue Eng. 2006;12:101–110. doi: 10.1089/ten.2006.12.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


