Abstract
Introduction
Oral contraceptive pills have been implicated in the pathophysiology of breast cancer. Although many studies have examined the relationship between combined oral contraceptives (COCs) and breast cancer, there is a paucity of literature that discusses progestin-only oral contraceptives (POCs) and breast cancer. The purpose of this investigation is to examine potential associations between different types of oral contraceptives and breast cancer mortality in the South Carolina Medicaid population among different racial/ethnic groups.
Methods
Subjects included 4,816 women diagnosed with breast cancer between 2000 and 2013. Kaplan-Meier curves were calculated to determine time-to-mortality rates among users of oral contraceptives. Competing-risks models and Cox multivariate survival models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of breast cancer and other-cause mortality, as well as all-cause mortality.
Results
POCs were associated with a significantly decreased risk of breast cancer mortality (HR: 0.07; 95% CI: 0.01, 0.52) and a non-significant increased risk of all-cause mortality (HR: 1.04; 95% CI: 0.52, 2.07). COCs increased the risk of breast cancer mortality (HR: 1.61; 95% CI: 1.14, 2.28) and all-cause mortality (HR: 1.83; 95% CI: 1.30, 2.57).
Conclusion
Use of POCs may be associated with a decreased risk of breast cancer mortality. Due to the small sample size of POC users in the current study, additional research is needed to confirm these findings.
Keywords: breast cancer, oral contraceptives, progestin, health disparities, competing risk, survival analysis
Introduction
Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer-related deaths among women [1]. In 2015, approximately 30% of new female cancer cases were located in the breast. Reproductive factors, such as oral contraceptive (OC) use, late pregnancy, and nulliparity, as well as genetic factors, family history, and age, increase breast cancer risk [1–5]. There are currently approximately sixty-two million women in their childbearing years (15–44 years of age) and it is reported that more than 99% of those who are sexually active use at least one contraception method [6]. Contraceptive pills, specifically combined oral contraceptives (COCs) that combine estrogen + progestin, and sterilization are the leading methods of choice [7]. Since the introduction of the birth control pill in the 1960’s, there has been a lot of debate about COCs potential health effects [4,8]. With the increasing popularity of OCs, it is important to focus on the potential health risks they may have on society and to consider ways of reducing these risks.
In the National Survey of Family Growth (NFSG), the majority of American women who reported ever having used a contraceptive method identified themselves as using OCs, specifically combined OCs. Of those currently using an OC method, 98% used an estrogen+ progestin method or COC method, and 2% reported using progestin-only contraceptive pills (POCs) [8]. Within one year of use, similar rates of unintended pregnancies are observed among COC and POC users with typical (i.e., occasional incorrect use) and perfect use (9 cases/100 women and 3 cases/1000 women, respectively) [5,9]. Typical use refers to pregnancy rates that include inconsistent or incorrect use and perfect use applies to pregnancy rates that reflect women following the directions (e.g., no missed pill) [10]. In fact, both COCs and POCs are more than 99% effective [3,11–12]. However, COCs are associated with an increased risk of venous thromboembolic events (VTEs), high blood pressure, and breast cancer [7,11–14]. Compared to women who had exclusively used POCs, exclusive users of COCs had a 30% increased risk of breast cancer [3]. Despite similarities in efficacy and increased risks of adverse effects, COCs remain more popular than POCs. Plausible reasons for the existing disparities in OC use include physician prescription patterns, knowledge and attitudes of OC types, longer biological adjustment periods and lack of communication between provider and patient [15].
Many studies have investigated whether COCs increase the risk of breast cancer [16–18]; however, there is a paucity of literature assessing different types of OCs (non-estrogen-containing formulations) and breast cancer risk. Due to significant health disparities in breast cancer mortality among African American (AA) and European American (EA) women, and potential differences in types of OCs used between races, we will focus on the differences in breast cancer mortality by race [19–21]. South Carolina can provide insight into potential reasons for racial/ethnic disparities in cancer mortality, with EA women having a higher incidence of breast cancer yet higher survival than their AA counterpart [22]. The purpose of this study is to determine the association between the type of OC use and breast cancer mortality in the South Carolina Medicaid population using a competing risk model [13]. Secondary goals include assessing type of OC use and all-cause mortality as well as mortality from other (or “non-breast cancer”) causes.
Methods
Data Sources and Study Design
All data used for this analysis were collected through the Office of Research and Statistics (ORS)/SC Revenue and Fiscal Affairs Office (RFA) Medicaid administrative enrollment and claims data and linked to the South Carolina Central Cancer Registry (SCCCR) using probabilistic matching techniques. The SCCCR maintains a gold-certified rating through the National Association of American Cancer Registries (NAACR), indicating data of exceptionally high quality, validity, and completeness. Data was linked by matching on name, social security number (SSN), and other identifying variables. This study was granted an exemption from the institutional review board of the University of South Carolina.
We used a retrospective cohort to examine the relationship between OCs and breast cancer mortality among low-income populations by race/ethnicity. Women exposed to POCs, COCs, or POC+COC are compared to individuals who have never been exposed to OCs. Our study is composed of an open cohort, where individuals can leave and enter the population at different time points, from 2000 to 2012.
Study Population
Medicaid data consists of an open population limited to individuals with ≥12 months of eligibility. The study population included women enrolled in Medicaid in South Carolina between 2000 and 2012. Women were excluded if they did not have a race designation of EA or AA. Medicaid pharmacy files, classified by the National Drug Code (NDC), included information regarding the OC pill type, date dispensed, quantity, and the number of refills. Women with NDC codes with therapeutic class 681200 were flagged and women with a prescription for a POC or a COC were included in our study. Women using both POC and COC were included in the POC+COC group.
Breast cancer occurrence was determined according to the International Classification of Diseases (ICD-9) codes from the ORS/RFA and SCCCR. Individuals with ICD-9 codes for malignant neoplasms of female breast: 174.X; carcinoma in situ of breast: 233.0; and neoplasms of uncertain or unspecified behavior (excluding skin of breast): 238.3 and 239.3. The SEER staging manual (2000) was used to classify breast cancer stages.
Covariates
To evaluate the association between OC use and breast cancer mortality risk, individual baseline and demographic variables were considered in the analysis: year of diagnosis (continuous), education (categorical), marital status (categorical), race (categorical), tobacco (categorical), duration of pill use (continuous), follow-up (continuous), stage of disease (categorical), mean age (continuous), and time-to-mortality (continuous). Education was categorized as < high school/some high school/high school graduate/≥ high school/missing; marital status: married/not married; race: EA/AA; tobacco: yes/no; duration of pill use (months); follow-up after OC use (months); stage of disease: in situ or noninvasive, local, regional/distant; ; age (years); and time-to-mortality (days). Based on the directed acyclic graph (DAG, Figure 1), race and age are confounders, or covariates that create a biasing path [23]. Adjusting for age and race is a minimally sufficient set for estimating the direct effect of OC use on breast cancer [24, 25].
Figure 1.
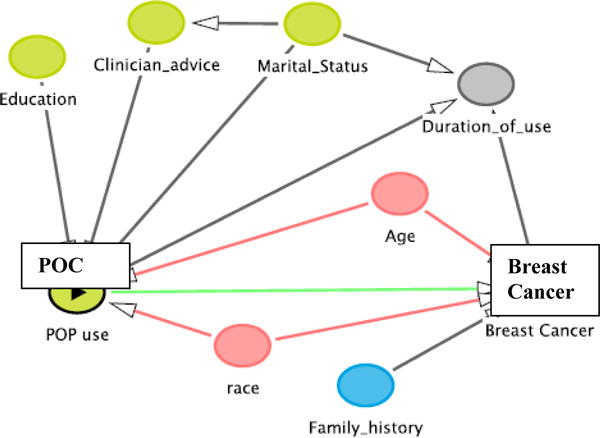
Directed Acyclic Graph illustrating the association of POC use and breast cancer risk
Statistical Methods
Descriptive statistics, stratified by OC use, were calculated for all baseline demographic variables. All categorical and continuous variables were assessed using chi-square test and two-tailed t-tests, respectively. The continuous variables are presented by mean (standard deviation (SD)) and categorical variables by frequencies (percentages (%)). All P values were 2-tailed, and significance was assessed as a Type I error rate of alpha 0.05. Kaplan-Meier survival curves were calculated, and the log-rank test statistic was used to assess statistical differences between OC groups for breast cancer mortality. Competing risk models were performed and models fitted using the Lunn McNeil approach to estimate cause-specific mortality (breast cancer and other causes)[26]. Associations among all-cause mortality rates, race, and other important covariates were estimated using Cox proportional hazards (PH) model. In the competing risk model, events were classified as breast cancer or other cause mortality and in the Cox PH model, events were classified as all-cause mortality. Total survival time was calculated for all subjects as the time from OC use to the time of death or censoring. In the Cox PH model, women were followed from OC use until any event occurred (breast cancer or other cause) to determine all-cause mortality. In the competing risk models, survival time was calculated from OC use to breast cancer mortality and in a separate model, from OC use to non-breast cancer deaths, or other causes of deaths. Women were censored at either the date of death for their respective model or at the end of the study period, whichever occurred first.
Separate competing risk models were performed by race (EA/AA), adjusting for other baseline and demographic variables. Cox PH models were used to assess the association between baseline and demographic variables with hazard risk from overall mortality by race. We evaluated PH assumption and the time-dependent relationship between OC exposure and all-cause mortality using Cox PH models. In our final competing risk model, we adjusted for OC use, marital status, year of diagnosis and age for EA women and OC use, stage, and age for AA women, when assessing breast cancer mortality. Adjustments varied slightly when assessing other cause mortality by race (Table 2). Covariates were determined based on backward elimination with an entry level of 0.10. However, OC use, our main variable, was kept in all models based on the a priori research question. In the reduced model, the p-value < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (Cary, NC).
Table 2.
Competing Risk Regression Analysis for Death from Breast Cancer and Other Causes in Medicaid Patients by Race
| Breast Cancer Mortalityα | Other cause Mortalityβ | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | No. of Patients | No. of Events | Estimate | HR | P-Value | Estimate | HR | P-Value |
| European American | ||||||||
|
| ||||||||
| Oral Contraceptive | ||||||||
| Never use | 427 | 320 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| POC | 118 | 82 | −12.88 | <0.01 | 0.95 | 0.07 | 1.08 | 0.99 |
| COC | 3773 | 2575 | 0.65 | 1.91* | <0.01 | 0.08 | 1.08 | 0.99 |
| POC+COC | 259 | 225 | 1.11 | 3.02* | <0.01 | 0.08 | 1.09 | 0.99 |
| Married | ||||||||
| No | 3196 | 2391 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Yes | 1327 | 795 | 0.13 | 1.13 | 0.21 | −3.95 | 0.02* | <0.01 |
| Follow-up time | 4577 | 3202 | £ | £ | £ | 0.01 | 1.01* | <0.01 |
| Year of Diagnosis | 4577 | 3202 | −0.34 | 0.71* | <0.01 | 0.69 | 0.71* | <0.01 |
| Age | 4577 | 3202 | −0.04 | 0.96* | <0.01 | £ | £ | £ |
| African American | ||||||||
|
| ||||||||
| Oral Contraceptive | ||||||||
| Never use | 31 | 22 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| POC | 17 | 14 | −1.49 | 0.23 | 0.17 | −0.24 | 0.58 | 0.82 |
| COC | 185 | 123 | −0.72 | 0.49 | 0.10 | −0.55 | 0.50 | 0.71 |
| POC+COC | 06 | 03 | −0.24 | 0.78 | 0.82 | N/A± | N/A± | N/A± |
| Stage | ||||||||
| In situ | 36 | 17 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Local | 94 | 62 | 1.47 | 4.34* | 0.04 | £ | £ | £ |
| Regional | 93 | 68 | 2.08 | 8.04* | <0.01 | £ | £ | £ |
| Distant | 12 | 11 | 3.65 | 38.50* | <0.01 | £ | £ | £ |
| Year of Diagnosis | 239 | 162 | £ | £ | £ | −0.51 | 0.60* | <0.01 |
| Age | 239 | 162 | 0.04 | 1.04* | 0.05 | £ | £ | £ |
EA: adjusted for marital status, year of diagnosis, and age; AA: adjusted for stage, and age;
EA adjusted for marital status, follow-up, and year of diagnosis; AA: adjusted for year of diagnosis;
Not included in reduced model (Overall model >0.05); HR: Hazard Ratio; CI: Confidence Interval;
Sample size/count < 5;
Significant (p< 0.05)
Results
Participants
Baseline characteristics according to OC use are presented in Table 1. Of the 4,816 women included in the analysis, approximately 11% did not survive. The OC distribution was POC (n= 135; 2.8%), COC (n= 3,958; 82.2%), and POC + COC (n= 265; 5.5%). Never users consisted of 9.5% of the study population. The highest mean age (41.5 years (SD: 8.2)) of use was seen among POC+COC users, a group which primarily consisted of married women (80.4%) and women who had more than a high school education. The shortest mean follow up was seen among POC users (99.3 months (SD: 43.7)).
Table 1.
Baseline demographic characteristics of Medicaid participants by OC use, 2000–2012
| Oral Contraceptive Types | |||||
|---|---|---|---|---|---|
| Characteristics (N, %) | Never Use (n=458) | POC (n=135) | COC (n=3958) | POC+ COC (n=265) | P Value |
| Vital Status | <0.01 | ||||
| Alive | 421 (91.9) | 124 (91.9) | 3307 (83.6) | 239 (90.2) | |
| Not Alive | 37 (8.1) | 11 (8.2) | 496 (16.4) | 26 (9.8) | |
| Race | <0.01 | ||||
| White | 427 (93.2) | 118 (87.4) | 3773 (95.3) | 259 (97.7) | |
| Black | 31 (6.8) | 17 (12.6) | 185 (4.7) | 6 (2.3) | |
| Education | 0.31 | ||||
| < High School | 26 (5.7) | 14 (10.4) | 532 (13.4) | 0 (0.0) | |
| Some high school | 5 (1.1) | 0 (0.0) | 32 (0.8) | 0 (0.0) | |
| High school graduate | 12 (2.6) | 2 (1.5) | 121 (3.1) | 0 (0.0) | |
| > High school | 171 (37.3) | 10 (7.4) | 1215 (30.7) | 111 (41.9) | |
| Missing | 244 (53.3) | 109 (80.7) | 2058 (51.9) | 135 (58.1) | |
| Stage | <0.01 | ||||
| In situ | 17 (3.7) | 3 (3.2) | 343 (12.8) | 22 (9.7) | |
| Local | 156 (34.1) | 48 (51.1) | 1175 (44.0) | 12 (5.3) | |
| Regional/Distant | 168 (36.7) | 43 (45.7) | 1156 (43.3) | 194 (85.1) | |
| Missing | 117 (25.5) | 41 (30.4) | 1284 (32.4) | 37 (14.0) | |
| Tobacco | 0.31 | ||||
| Yes | 17 (10.9) | 28 (82.4) | 352 (28.7) | 0 (0.0) | |
| No | 139 (89.1) | 6 (17.7) | 876 (71.3) | 16 (100.0) | |
| Married | <0.01 | ||||
| Yes | 128 (30.1) | 45 (37.2) | 992 (25.1) | 213 (80.4) | |
| No | 298 (70.0) | 76 (62.8) | 2954 (74.9) | 52 (19.6) | |
| Mean (SD) | |||||
| Age of OC use | 34.4 (5.3) | 35.0 (6.7) | 36.2 (7.3) | 41.5 (8.2) | <0.01 |
| Follow-up after OC use | 121.9 (15.7) | 99.3 (43.7) | 139.4 (29.2) | 135.7 (21.9) | <0.01 |
| Duration of use | N/A | 5.0 (4.4) | 13.3 (17.5) | 22.1 (30.4) | 0.03 |
Figure 2 presents the univariable hazard ratios for risk of breast cancer and all-cause mortality by race and OC type. The crude analysis suggests an increased risk of breast cancer mortality among COC users of all races (HR: 1.80; 95% CI: 1.29, 2.55) and among EA women (HR: 2.10; 95% CI: 1.44, 3.06), specifically. The decreased risk seen in AAs using COC is non-significant (p-value: 0.21). Similar findings were observed among COC users and all-cause mortality (all races-HR: 2.38; 95% CI: 1.71, 3.32, EA-HR: 2.85; 95% CI: 1.96, 4.14, AA-p-value: 0.26). POC was inversely associated with breast cancer-specific mortality among all races (HR: 0.04; 95% CI: 0.01, 0.33, p-value: < 0.01) but was not associated with all-cause mortality (HR: 0.60; 95% CI: 0.30, 1.20, p-value: 0.28). Competing risks regression analyses that controlled for marital status, follow up, year of diagnosis, age, and stage of breast cancer diagnosis were performed to assess whether the use of OC was associated with longer survival times compared to no OC use. Tobacco and duration were removed in the final model due to missing information and nonsignificant findings.
Figure 2.
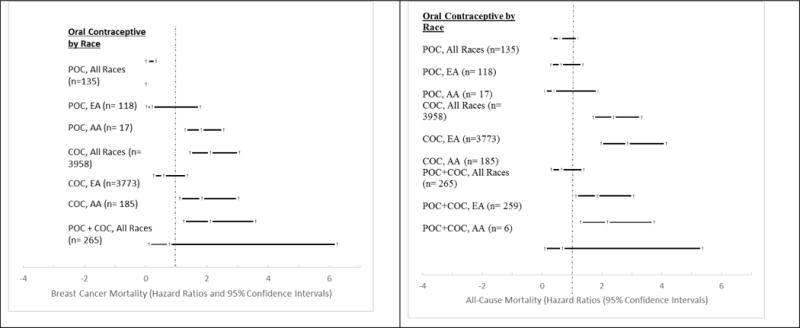
Univariable hazard ratios for risk of breast cancer mortality and all-cause mortality by race and oral contraceptive type
Kaplan-Meier survival curves of Medicaid cohort are stratified by race and OC exposures (never users, POC, COC, or POC+COC) among women in the total population and among women who previously had breast cancer (Figure 3). There are significant differences in survival by OC type, especially among AA women. POC use was associated with better long term survival. As shown in the reduced models (Table 2), EA women had a significantly higher likelihood of breast cancer death when using either COC (HR: 1.91, p-value: <0.01) or POC+COC (HR: 3.02, p-value: <0.01) compared to never use. EA also had a reduction in risk of breast cancer mortality with each additional year of diagnosis and age. OC use was not significantly associated with an increased risk of other-cause mortality among EA and AA women and did not significantly decrease the risk of breast cancer mortality among AA women. Overall, POC was associated with a reduced risk of breast cancer mortality (HR: 0.07, p-value: <0.01) and both COC and POC+COC were associated with increased risks of breast cancer mortality (HR: 1.61 and 2.09, p-value: <0.01) (Table 3).
Figure 3.
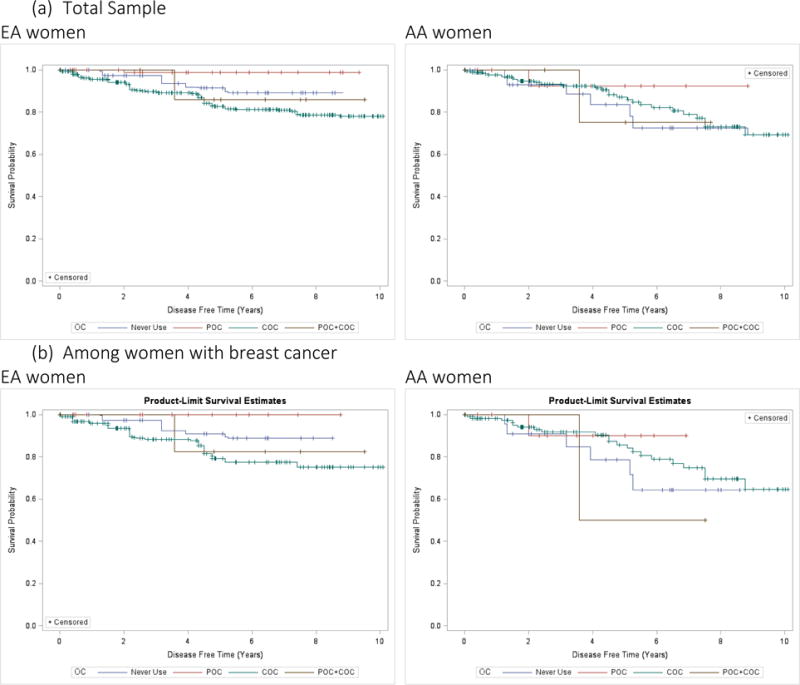
Kaplan-Meier (KM) Survival Curves (“Time-to-mortality”) for different Oral Contraceptive Users (Never Users/POC/COC/POC+COC)
Table 3.
Competing Risk for Breast Cancer Mortality Using the Total Population (all races)
| No. of Patients | No. of Events | Estimate | HR±* | |
|---|---|---|---|---|
| Oral Contraceptive | ||||
| Never use | 916 | 458 | (ref) | (ref) |
| POC | 270 | 135 | −2.65 | 0.07* |
| COC | 7916 | 3958 | 0.48 | 1.61* |
| POC+COC | 530 | 265 | 0.74 | 2.09* |
Adjusted for marital status, follow-up, year of diagnosis, and age;
Significant (p< 0.05)
Table 4 shows the results of all-cause survival using the Cox PH model for AA, EA, and total (AA+EA) women. Overall, OC use was not significantly associated with all-cause mortality among AA women. However, without stratification, the total population had an increased mortality when using either COCs or POC+COC (HR: 1.83; 95% CI: 1.30, 2.57 and HR: 2.85, 95% CI: 1.72, 4.72, respectively). The increased all-cause mortality risk was also noted among EA women using COCs and POC+COC. EA women and the total population saw 2–3 fold increased risk of all-cause deaths among COC and POC+COC users.
Table 4.
Cox regression analysis for all-cause mortality in patients in the Medicaid cohort
| Race | N | All-cause Mortalityβ | |
|---|---|---|---|
| Oral Contraceptives | |||
| Never Use | EA£ | 427 | 1.00 |
| AA‡ | 31 | 1.00 | |
| Total¥ | 458 | 1.00 | |
| POC | EA | 118 | 1.00 (0.47, 2.13) |
| AA | 17 | 0.26 (0.05, 1.26) | |
| Total | 135 | 1.04 (0.52, 2.07) | |
| COC | EA | 3773 | 2.35 (1.61, 3.42)* |
| AA | 185 | 0.44 (0.20, 0.96) | |
| Total | 3958 | 1.83 (1.30, 2.57)* | |
| POC+ COC | EA | 259 | 3.38 (1.97, 5.79)* |
| AA | 6 | 0.30 (0.04, 2.42) | |
| Total | 265 | 2.85 (1.72, 4.72)* |
all models did not satisfy PH assumption, hence time-dependent model fitted;
Significant (p< 0.05)
Model adjusted for year of diagnosis, and (year of diagnosis as a function of time)
Model adjusted for marital status, year of diagnosis, and (year of diagnosis as a function of time)
Model adjusted for marital status, follow-up time, year of diagnosis, and (follow-up time and year of diagnosis as a function of time)
Discussion
In this large study of 4,816 Medicaid women, we found that COC and POC+COC use were positively associated with breast cancer mortality. After adjustment for relevant covariates, EA women using COCs and POC+COCs had 1.91 to 3.02 times the risk of breast cancer death and 2.35 to 3.38 times the risk of all-cause mortality compared with never users. The association between OC use and breast cancer mortality among AAs was not significant. Mortality in the AA population was primarily determined by stage of breast cancer diagnosis, which was associated with a twenty-fold increased risk.
Recently, a meta-analysis of contraceptive use and breast cancer risk showed POCs were not associated with increased breast cancer risk [27]. In this current study, we found a significantly reduced risk between POC use and breast cancer mortality in the total population (HR: 0.07; p-value: <0.01). Yet, POCs were associated with non-significantly increased likelihood of death from other causes (non-breast cancer mortality) and non-significantly reduced risk of all-cause mortality. Other studies have hypothesized that medical surveillance may bias the relationship between COCs and breast cancer [3, 28] and similarly, access to health care may bias the relationship between COCs and breast cancer mortality. However, this biasing relationship is reduced in our study because POC and POC+COC users have undergone similar medical surveillance procedures and have similar access to health care.
This study suggests that OCs play a differential role in breast cancer, other cause and all-cause mortality by race. However, the lack of a clear association among AA women using OCs and our outcomes of interests requires more attention. Larger sample sizes of AA women may be necessary in the future to examine this relationship. There were only six AA women using POC+COCs, which reduces our power in interpreting POC+COC utilization in this population. Understanding the role of OC types on women’s health could help minimize the burden of cancer and more epidemiologic studies need to be done to explore the effect of POCs. The only marketed POC in the United States is norethindrone .35 mg tablet, which includes Camila, Errin Nor-QD, Ovrette, Jolivette, OrthoMicronor and generic medications. Typically, POCs are recommended for women who have certain contraindications to estrogen-containing formulations. For example, in our population, smoking was more common among POC users (82.4%). Despite 28.7% of COC users being smokers, smoking is a known contraindication of COC use and may result in serious adverse events (e.g. VTE, stroke) [14, 29, 30]. Most of the current studies available are case-control studies and focus only on COC users. Comparing various types of contraceptive preparations may provide insight to safer alternatives to pregnancy prevention, especially among high-risk groups. We performed sensitivity analyses on a crude proxy of menopausal status (age > 50) and excluded women diagnosed with breast cancer in the first year of the cohort and found similar results.
This study should be replicated in more generalizable populations but still provides insight into a potentially less risky alternative to COCs. Another study also suggests raising awareness of POCs in at-risk populations such as smokers, those with family history of breast cancer and cardiovascular disease, and who may be more susceptible to the estrogen component in the OC pill if these results are confirmed in other studies [31]. The use of OCs can vary drastically by region, and studies have shown that there is a considerable amount of misinformation among health care providers about contraception health, with older providers and primary care physicians tending to demonstrate a larger gap [20]. In the US, approximately 17% of women aged 15–45 years use COCs, which is only half the number of women using OCs in Europe and twice the number of women in Africa [32]. Future research should examine this relationship by region and using different racial and ethnic cohorts.
Strengths and limitations
Strengths of our study include the large overall sample size and detailed information on medication use from enrollment and administrative claims data. Our study did not exclude POC formulations when considering types of OCs, which makes it unique. Furthermore, our sample population is restricted to Medicaid beneficiaries, which provides us with unique insight to underrepresented groups. However, this data registry did not provide information on potential confounders such as diet, physical activity, serum lipids, blood pressure, family history, and other reproductive factors (e.g. menstrual history), estrogen/progesterone receptor status, and post-operative treatment. Limited information was provided on tobacco use, and stratification of events by type of OC use and race led to small numbers and reduced the strength of the association. It would also be interesting to study the long-term use of OCs (including POCs) and breast cancer mortality.
Conclusion
Among premenopausal women using OCs, COCs were the strongest predictor of breast cancer mortality and POC+COCs were the strongest predictor of overall mortality. The type of OC used should be taken into account when assessing breast cancer mortality risk. POCs may be associated with less short- and long-term adverse events related to the estrogen component of COCs. However, due to the small sample size of POC users in the current study, additional research is necessary to confirm whether the association with breast cancer mortality differs by OC type.
Highlights.
There were significant differences in the rates of survival of a sample of women previously diagnosed with breast cancer who had taken different types of oral contraceptive, especially among African American women.
Progesterone-only contraceptives were associated with a significantly decreased risk of breast cancer mortality and may be a better alternative to combined oral contraceptives among high-risk populations.
Exogenous estrogen and progesterone should be investigated further for oral contraceptive use as well as for prevention of breast cancer.
Acknowledgments
Funding
Marsha Samson received partial funding from NIH-NIGMS (Grant Number T32-GM081740).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MES was responsible for data collection and analysis, and drafted the paper.
SAA was responsible for the conceptual layout and reviewed the manuscript.
CMM helped with the data analysis.
JZ reviewed the data analysis.
CLB reviewed the manuscript and helped with pharmaceutical concepts.
JH reviewed the manuscript and helped with epidemiologic concepts.
SES oversaw the drafting of the manuscript and contributed to its revision.
All authors participated in the work and saw and approved the paper prior to submission.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH. This work has not been presented previously.
Conflict of interest
The authors declare that they have no financial or non-financial conflict of interest.
Ethical approval
There was no human experimentation and no animals were used in this study. Analysis of deidentified, publicly available data does not constitute human subjects research as defined in federal regulations, and therefore the present study did not require IRB approval. IRB approval ‘review type exempt’ was received from the University of South Carolina IRB Committee (Pro00039778).
Provenance and peer review
This article has undergone peer review.
References
- 1.ACS. Cancer Facts & Figures 2015 [Internet] 2015 [cited 2015 Oct 12]. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
- 2.DHEC. DHEC: Breast Cancer [Internet] 2015 [cited 2015 Jan 25]. Available from: http://www.scdhec.gov/Health/DiseasesandConditions/Cancer/BreastCancer/mindex.htm.
- 3.Kumle M, Weiderpass E, Braaten T, Persson I, Adami H-O, Lund E. Use of oral contraceptives and breast cancer risk: The Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2002 Nov;11(11):1375–81. [PubMed] [Google Scholar]
- 4.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast Cancer Before Age 40 Years. Semin Oncol. 2009 Jun;36(3):237–49. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006 Jun 7;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 6.Dye J. Fertility of American Women: 2008 [Internet] U.S. Department of Commerce Economics and Statistics Administration; 2008. Available from: http://www.census.gov/prod/2010pubs/p20-563.pdf. [Google Scholar]
- 7.Daniels K, Mosher W, Jones J. Contraceptive Methods Women Have Ever Used: United States, 1982–2010 [Internet] 2013 Feb; Report No.: 62. Available from: http://www.cdc.gov/nchs/data/nhsr/nhsr062.pdf. [PubMed]
- 8.Hall KS, Trussell J, Schwarz EB. Progestin-only contraceptive pill use among women in the United States. Contraception. 2012 Dec;86(6):653–8. doi: 10.1016/j.contraception.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCI. Breast Cancer Risk in American Women [Internet] National Cancer Institute; 2015. [cited 2015 Sep 17]. Available from: http://www.cancer.gov/types/breast/risk-fact-sheet. [Google Scholar]
- 10.Trussell J. Contraceptive failure in the United States. Contraception. 2011 May;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain SF. Progestogen-only pills and high blood pressure: is there an association? A literature review. Contraception. 2004 Feb;69(2):89–97. doi: 10.1016/j.contraception.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Trussell Effectiveness of Family Planning Contraceptive Methods [Internet] [cited 2015 Oct 12]. Available from: http://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/contraceptive_methods_508.pdf.
- 13.Kubba AA. Breast cancer and the pill. J R Soc Med. 2003 Jun;96(6):280–3. doi: 10.1258/jrsm.96.6.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FSRH. Faculty of Sexual & Reproductive Healthcare Clinical Guidance: Progestogen-only Pills Clinical Effectiveness Unit [Internet] England: Faculty of Sexual and Reproductive Healthcare; 2009. pp. 1–19. Available from: http://www.fsrh.org/pdfs/CEUGuidanceProgestogenOnlyPill09.pdf. [Google Scholar]
- 15.Kovacs G. Progestogen-only pills and bleeding disturbances. Hum Reprod Oxf Engl. 1996 Oct;11(Suppl 2):20–3. doi: 10.1093/humrep/11.suppl_2.20. [DOI] [PubMed] [Google Scholar]
- 16.Hunter DJ, Colditz GA, Hankinson SE, Malspeis S, Spiegelman D, Chen W, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2010 Oct;19(10):2496–502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longman SM, Buehring GC. Oral contraceptives and breast cancer. In vitro effect of contraceptive steroids on human mammary cell growth. Cancer. 1987 Jan 15;59(2):281–7. doi: 10.1002/1097-0142(19870115)59:2<281::aid-cncr2820590218>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Beaber EF, Malone KE, Tang M-TC, Barlow WE, Porter PL, Daling JR, et al. Oral contraceptives and breast cancer risk overall and by molecular subtype among young women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2014 May;23(5):755–64. doi: 10.1158/1055-9965.EPI-13-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehlendorf C, Foster DG, de Bocanegra HT, Brindis C, Bradsberry M, Darney P. Race, Ethnicity and Differences in Contraception Among Low-Income Women: Methods Received by Family PACT Clients, California, 2001–2007. Perspect Sex Reprod Health. 2011 Sep;43(3):181–7. doi: 10.1363/4318111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehlendorf C, Levy K, Ruskin R, Steinauer J. Health care providers’ knowledge about contraceptive evidence: a barrier to quality family planning care? Contraception. 2010 Apr;81(4):292–8. doi: 10.1016/j.contraception.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littlejohn KE. Hormonal contraceptive use and discontinuation because of dissatisfaction: differences by race and education. Demography. 2012 Nov;49(4):1433–52. doi: 10.1007/s13524-012-0127-7. [DOI] [PubMed] [Google Scholar]
- 22.Samson M,MSPH,MHSA, Porter N MSc, Hurley D,MSPH, Adams S, PhD, Eberth J, PhD. Disparities in breast cancer incidence, mortality, and quality of care among African American and European American women in South Carolina. Southern Medical Journal. 2016;109(1):24–30. doi: 10.14423/SMJ.0000000000000396. 2015 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiol Camb Mass. 2011 Sep;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 24.Samson ME, Adams SA, Orekoya O, Hebert JR. Understanding the Association of Type 2 Diabetes Mellitus in Breast Cancer Among African American and European American Populations in South Carolina. J Racial Ethn Health Disparities. 2015 Oct;20:1–9. doi: 10.1007/s40615-015-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleischer NL, Diez Roux AV. Using directed acyclic graphs to guide analyses of neighbourhood health effects: an introduction. J Epidemiol Community Health. 2008 Sep;62(9):842–6. doi: 10.1136/jech.2007.067371. [DOI] [PubMed] [Google Scholar]
- 26.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995 Jun;51(2):524–32. [PubMed] [Google Scholar]
- 27.Samson M, Adams Swann, Porter N, Orekoya O, Hebert J, Steck S. Progestin and Breast Cancer Risk: A Systematic Review. Breast Cancer Research and Treatment. 2016 doi: 10.1007/s10549-015-3663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro S. Bias in the Evaluation of Low-Magnitude Associations: An Empirical Perspective. Am J Epidemiol. 2000 May 15;151(10):939–45. doi: 10.1093/oxfordjournals.aje.a010135. [DOI] [PubMed] [Google Scholar]
- 29.FSRH. Faculty of Sexual & Reproductive Healthcare Clinical Guidance: Combined Hormonal Contraception Clinical Effectiveness Unit [Internet] England: Faculty of Sexual and Reproductive Healthcare; 2011. pp. 1–19. Available from: http://www.fsrh.org/pdfs/CEUGuidanceProgestogenOnlyPill09.pdf. [Google Scholar]
- 30.Curtis Kathryn. US Medical Eligibility Criteria for Contraceptive Use, 2010 [Internet] 2010 [cited 2015 Sep 17]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a1.htm?s_cid=rr5904a1_e.
- 31.Samson M, Adams S, Merchant A, Zhang J, Bennett C, Hebert J. Cardiovascular Disease Incidence among Females in South Carolina by Type of Oral Contraceptives, 2000–2013: a retrospective cohort study. Archives of Gynecology and Obstetrics. 2016 doi: 10.1007/s00404-016-4143-5.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brynhildsen J. Combined hormonal contraceptives: prescribing patterns, compliance, and benefits versus risks. Ther Adv Drug Saf. 2014 Oct;5(5):201–13. doi: 10.1177/2042098614548857. [DOI] [PMC free article] [PubMed] [Google Scholar]


