Abstract
Administration of cyclophosphamide following transplant (Post-transplant cyclophosphamide, PTC) has shown promise in the clinic as a prophylactic agent against graft vs. host disease. An important issue with regard to recipient immune function and reconstitution after PTC is the extent to which in addition to diminution of anti-host allo-reactive donor T cells, the remainder of the non-host allo-reactive donor T cell pool may be impacted. To investigate PTC’s effects on non-host reactive donor CD8 T cells, ova specific (OT-I) and gp100 specific Pmel-1 T cells were labeled with proliferation dyes and transplanted into syngeneic and allogeneic recipients. Notably, an intermediate dose (66mg/kg) of PTC which abrogated GVHD following allogeneic HSCT, did not significantly diminish these peptide specific donor T cell populations. Analysis of the rate of proliferation following transplant illustrated that lymphopenic driven donor non host reactive TCR Tg T cells in syngeneic recipients underwent slow division resulting in significant sparing of these donor populations. In contrast, following exposure to specific antigen at the time of transplant, these same T cells were significantly depleted by PTC demonstrating the global susceptibility of rapidly dividing T cells following encounter with cognate antigen. In total, our results employing both syngeneic and allogeneic minor antigen mismatched T cell replete models of transplantation, demonstrate a concentration of PTC that abrogates GVHD can preserve most cells that are dividing due to the accompanying lymphopenia following exposure. These findings have important implications with regard to immune function and reconstitution in recipients following allogeneic hematopoietic stem cell transplant.
Introduction
Allogeneic hematopoietic stem cell transplantation (AHSCT) is a curative therapy for some blood cancers and has the potential to be applied to many other malignancies, although such use is hindered by the complication of graft vs. host disease (GVHD) [1–5]. GVH responses are immediately initiated following transplant by rapidly cycling donor T cells that are not tolerant to host allogeneic transplantation antigens [6–10]. Efforts to remove anti-host alloantigen reactive T cells ex-vivo prior to transplant are ongoing, but practical as well as technical issues have thus far precluded development of an effective strategy [7, 11, 12]. Additionally, the low frequency of T cells reactive with non-HLA-encoded, i.e. minor transplantation antigens provides added challenges for successful ex-vivo deletion strategies,[13,14]. Alkylating compounds induce breaks in DNA which initiate the apoptosis of the affected cells upon entry into the replication cycle, or necrotic death dependent on the cell population and conditions present [15,16]. Regardless, these agents principally target dividing cells. Studies utilizing alkylating agents in attempts to impart immune tolerance were initiated in the late 1950’s in pre-clinical models [17–19]. Early studies demonstrated that cyclophosphamide, an alkylating agent, could diminish donor anti-host reactive T cells following an allogeneic tissue graft [20]. Subsequent work found that following low dose TBI conditioning and allogeneic bone marrow infusion, cyclophosphamide administration could prevent host T cells responding to donor antigens from rejecting the graft and enabled donor hematopoietic engraftment [21].
These findings, in part, re-kindled interest in cyclophosphamide as a transient immunosuppressive strategy for patients receiving AHSCT [22]. Recently, clinical trials have been performed at several centers to begin assessing the efficacy of post-transplant cyclophosphamide (PTC) administration to ameliorate GVHD [23–25]. http://clinicaltrials.gov/show/NCT01427881. Results are thus far promising for both safety and efficacy of high-dose PTC administration as well as GVHD occurrence after both non-myeloablative and myeloablative conditioning in HLA-mismatched and HLA-matched allogeneic HSCT recipients [26–28]. Dependent on the extent of conditioning and the status of the patient, T cell replete AHSCT is performed in the context of varying degrees of lympho-depletion in the recipient. This post-transplant environment therefore supports both lymphopenia induced proliferation (LIP) antigen as well as recipient allo-antigen antigen stimulated proliferation, the former driven by an excess of cytokines present that support T cell homeostasis and maintenance in lympho-replete immune compartments, e.g. IL-7, IL-15 [29–32].
Since a major challenge following HSCT is reconstituting immune function as quickly as possible [33–38]. A critical question following exposure to PTC concerns what populations of donor T cells are diminished or eliminated in recipients. Notably, pre-transplant conditioning was not employed in the historical allo-tissue graft experiments and in the pre-clinical studies examining engraftment, immune function was not examined. Questions therefore remain regarding the susceptibility of T cells undergoing LIP to deletion following PTC administration. The goal of the current study was to examine populations of T cells dividing due to lymphopenia alone or together with antigen driven activation in response to host alloantigen or specific peptide antigen in hematopoietic stem cell transplant models after exposure to PTC. The results demonstrated that PTC has a markedly different impact on host reactive compared to non-host reactive transplanted donor T cells – the latter were minimally affected by doses of PTC that ameliorated GVHD. These findings are discussed in the context of potential benefits of PTC to facilitate immune responsiveness and reconstitution following AHSCT.
Materials and Methods
Mice
Seven to Eight week-old female C57BL/6 (B6), BALB/c, C3H.SW, BALB.B (C.B10-H2b/LiMcdj), mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained in pathogen-free conditions in the Department of Microbiology and Immunology at the University of Miami Miller School of Medicine. B6 mice congenic for CD45 and expressing the CD45.1 allele (B6.SJL-Ptprc/BoAiTac), Pmel-1(B6.Cg-Thy1a/Cy Tg (TcrαTcrβ)8Rest/J)/RAG−/− mice (Pmel-1 a gift from Dr. Claudia Marcela Diaz) and OT-I(C57BL/6-Tg(TcrαTcrβ)1100Mjb/J)/Rag−/− mice were bred in facility.
Bone marrow transplantation
Donor C57BL/6 bone marrow, lymph nodes and spleen were aseptically removed. Single cell suspensions of marrow, spleen and lymph node cells were washed in PBS. Donor bone marrow cells were treated with anti-Thy-1.2 Miltenyi MACS magnetic beads and negatively selected to remove T cells and washed before transplant. Spleen and lymph node cells were incubated on anti-sIg- coated (Millipore) plastic dishes for 45 min at 4° C in order to remove B cells. Non-adherent cells were harvested and a small aliquot was stained with anti-CD4 and anti-CD8 mAb to determine percentage contributions. Recipient C3H.SW mice were irradiated with 10.5 Gy gamma-irradiation from an open beam Cobalt-60 source (Gamma beam 150). Within 4h after irradiation, experimental C3H.SW recipients were injected with C57BL/6 (45.1+) allogeneic bone marrow plus 2.3×106 sIg-depleted CD4 + and CD8 + allogeneic T cells (i.v. in 0.5 ml PBS). Transgenic donor CD8 T cell populations administered were enriched using Miltenyi MACS magnetic bead separation and infused at >90% purity. Recipient BALB.B mice were irradiated with 7.5 Gy gamma- irradiation from an open beam Cobalt-60 source (Gamma beam 150). Control TCD-BM transplant recipients allogeneic bone marrow (i.v. in 0.5 ml PBS). In recipients of post-transplant cyclophosphamide (Sigma), the indicated concentrations were administered intraperitoneally on Days 3, or 3 and 4 and adjusted for weight of recipients.
Immunofluorescence staining and analysis
Fluorescent antibodies were purchased from BD Biosciences, eBioscience, BioLegend, and Invitrogen/Life-Technologies – Molecular Probes and used for flow cytometric analysis: anti-vα2 (B20.1), anti-vβ5.1/5.2 (MR9-4), anti- CD90.1 (OX-7), anti-CD62L (MEL-14), anti-CD8α (53-6.7), anti-CD4 (RM4–5), anti-CD19 (6D5), anti-CD45.1 (A20), anti-CD44 (IM7), anti-IFN-γ (XMG1.2). For intracellular cytokine staining, single cell suspensions prepared from tissues were incubated (1×106 /ml) in 10% complete medium with 0.1 nM OVA257–284 (SIINFEKL) or 0.1 nM human gp10025–33, KVPRNQDWL (kindly provided by Dr. Marcela Diaz-Montero, Dept. of Medicine, University of Miami School of Medicine) and 0.7 µl BD GolgiStop protein transport inhibitor containing monensin for 4–18 hours at 37°C, surface stained, fixed and permeabilized with the FoxP3 staining kit (eBioscience) overnight, stained intra-cellularly with the appropriate antibodies, washed, and analyzed.
GVHD assessment
Recipients were monitored for changes in total body weight and overall survival. The clinical signs of GVHD were recorded for individual mice by means of a GVHD scoring system modified from Cooke et al. [39] Briefly, recipients were scored on a scale from 0 to 2 for 5 clinical parameters: weight loss (0, less than 10%; 1, 10% to 25%; 2, greater than 25%); diarrhea (0, not detectable; 1, mild; 2, severe); fur texture (0, unremarkable; 1, slight back ruffling; 2, entire body ruffling); posture (0, normal; 1, hunching noted only at rest; 2, severe hunching and impaired movement); alopecia (0, unremarkable; 1, mild to moderate; 2, severe with obvious areas of denuded skin). The clinical score for a BMT group represents the average of at least 5 mice per group.
Antigen Stimulation
Whole splenocytes from C57BL/6-Tg(CAG-OVA)916Jen/J, which express OVA in all tissues (kindly provided by Dr. Allison Bayer, Dept. of Microbiology and Immunology, DRI,UM School of Medicine), were infused i.p. and s.c. at 8 weeks post-transplant. Recipients were bled 3 days later to assess for expansion of OVA specific T lymphocytes (OT-I) by Vα2+ Vβ5+ CD8+.
Cell Proliferation
Cell proliferation was followed via the use of CFDA-SE (Invitrogen) or Cell Trace (Invitrogen). Cells were labeled with between 5–10mM proliferation dye and incubated in PBS at 10 million cells per ml for 15 minutes at 37 degrees Celsius. The reaction was quenched using RPMI with 10% FBS (Gibco) and washed twice in PBS. Cells were immediately assessed on a Becton-Dickson Fortessa Flow Cytometer.
Statistical Analysis
All cell population persistence post-transplant data are expressed as the mean % or number ± SEM. Weights and percentage in later divisions are expressed as mean values. Statistical comparisons were done using a nonparametric Mann-Whitney, two-tailed P value (95% confidence interval) test using the GraphPad Prism program. Statistical significance was defined as a P value <0.05.
Results
Identification of a PTC dose which ameliorated GVHD did not eliminate slowly dividing donor T cells
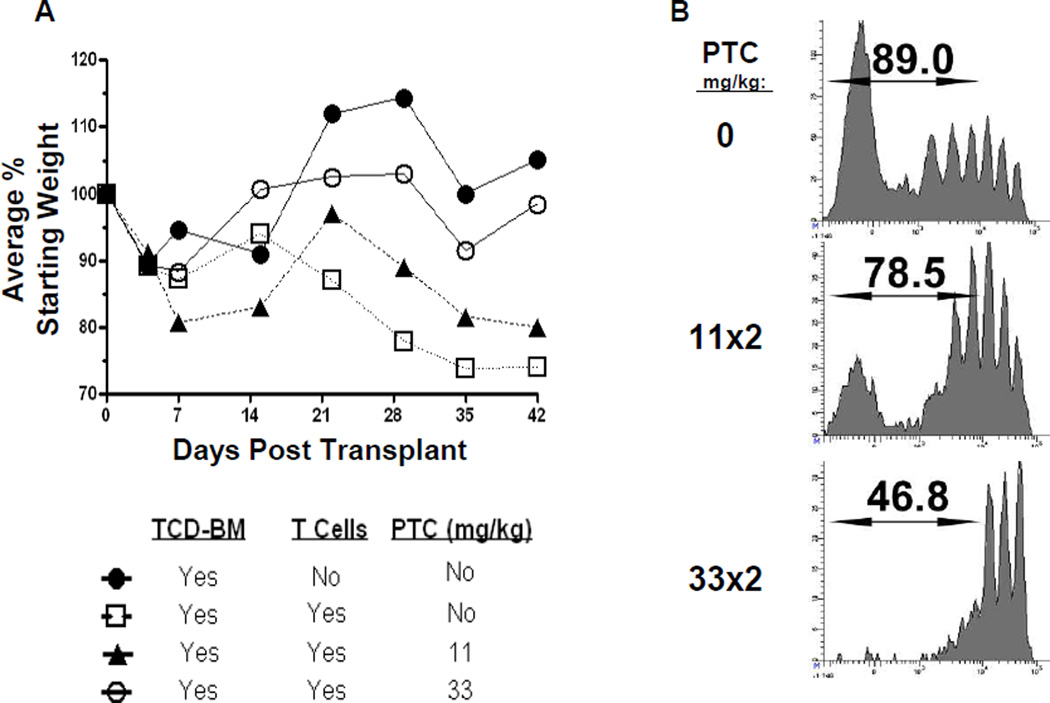
In order to identify an effective dose of PTC that would suppress donor anti-host allo-responses leading to GVHD we employed an allogeneic minor antigen mismatched hematopoietic stem cell transplantation (AHSCT) model of GVHD involving an MHC (H2b) matched donor and recipient strain combination B6->C3H.SW [40, 41]. Groups of C3H.SW recipients exposed to 10.5 Gy TBI were injected with donor B6 T cell depleted bone marrow (TCD-BM) alone or together with 2.3×106 B6 T cells. Varying doses of PTC (data not shown) was examined in C3H.SW recipients over the course of several experiments (11– 66mg/kg per dose delivered at 1 or 2 doses which represents 0.275 to 3.3 mg total dose per 25g mice), and single dose 200mg/kg which equaled 5.0 mgs / 25g mice and was lethal in C3H.SW recipients, data not shown). In this AHSCT model, a dose of 33 but not 11mg/kg administered on Days 3 and 4 was found to significantly ameliorate GVHD associated weight loss induced by infusion of 2.3 × 106 sIg depleted B6 splenocytes when C3H.SW recipients were conditioned with lethal (10.5 Gy) TBI (Fig. A). Notably, improvement survival (Fig. S1) and GVHD clinical score (data not shown) was also observed when 33mg/kg × 2 (66mg total) was employed.
To more closely examine the effects of PTC on dividing donor cell populations, sIg depleted B6 splenocytes were labeled with a CFSE cell proliferation dye and transplanted into C3H.SW recipients. We then examined the effects of two doses of PTC, 2×11 and 2×33 mg/kg, on dividing donor CD8+ T-cells 5 days post-transplant. PTC administration caused depletion of peaks representing later generations of rapidly dividing cells in a dose dependent fashion. Notably, at a concentration of PTC that was unable to abrogate GVHD (2×11 mg/kg), a population of cells remained evident that divided greater than 5 times in the 5 day experimental period. In contrast, at the 2×33 mg/kg concentration which successfully diminished GVHD, the profile was distinctly different in that: 1) there was a more apparent diminution of cells in later (>2) generations and 2) only two to three generations of early dividing cells remained (Fig. 1B). In total, PTC diminished the percentage of proliferating donor T cells in a dose dependent fashion, however some slowly dividing (1–2 divisions) cells were not deleted even after the 2×33 mg/kg protocol. The loss of rapidly dividing cells after this PTC exposure was observed in many experiments using several distinct strain combinations (data not shown).
Fig. 1. PTC following MHC-matched allogeneic HSCT ameliorates GVHD and reduces the percentage of donor T cells in later divisions 5 days after transplant.
A) GVHD induced weight loss was reduced after 2 × 33mg/kg PTC (○, n=6) and not significantly different from recipients of TCD-BM only (●, (p>0.05; n=5). Recipients of 11mg/kg PTC (▲, n=5) exhibited slightly decreased weight loss vs. the non-PTC treated recipients (□, n=7) but did not differ statistically (p>0.5). A representative experiment of 3 is shown. B) To analyze how PTC impacts donor T cells early post-HSCT, B6 sIg depleted, CD4 and CD8 T cells were labeled with CFSE and transferred into lethal TBI (10.5Gy) conditioned C3H.SW recipients (n=5/group). Varying doses (11 mg/kg or 33 mg/kg) of cyclophosphamide (PTC) were administered i.p. at D.3 and 4 post-transplant. Data represents the percentage of donor T cells that had undergone at least 2 divisions at D4–5 post-HSCT. The CFSE dilution profiles indicated that there was greater reduction within the later generations of rapidly dividing donor CD8 T cells.
Donor cells dividing due to lymphopenia are essentially spared following exposure to PTC
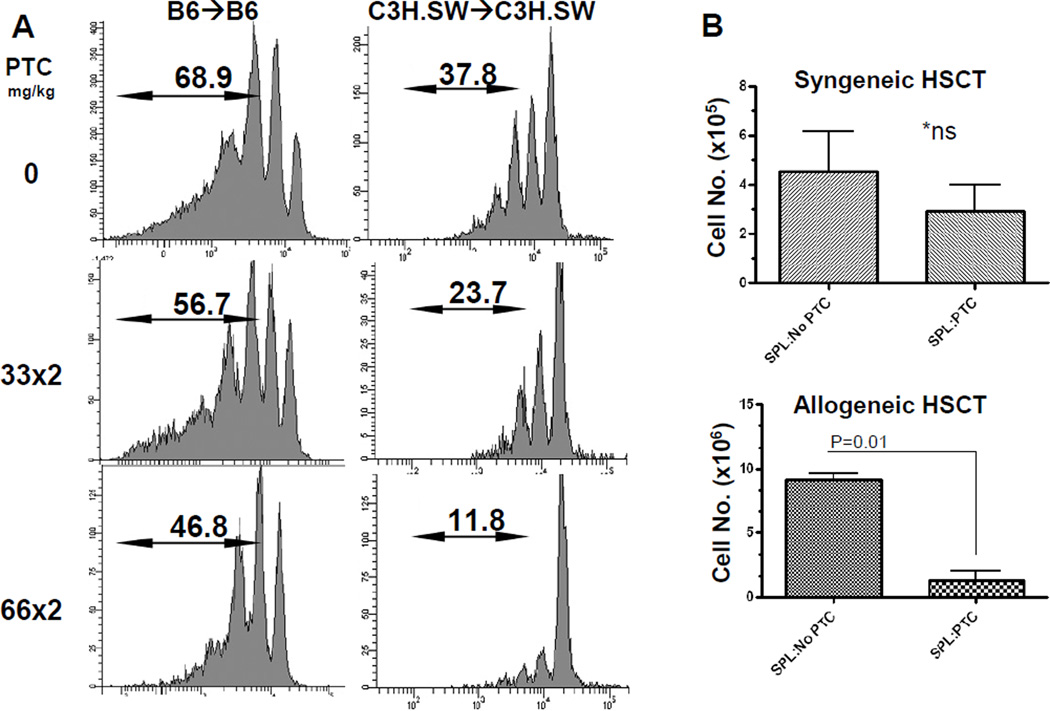
Previous experiments by ourselves (DR, RBL, unpublished observations) and others have shown that cells dividing due to lymphopenia divide approximately once per 24 hrs [42–44]. In order to elucidate whether 2× injection of 33 mg/kg PTC spared cells dividing solely due to lymphopenia, CFSE labeled T cells were infused into lethally conditioned syngeneic recipients. At a concentration of PTC that ameliorated GVHD following AHSCT in the allogeneic transplant (2×33 mg/kg), the effect of PTC in both B6 and C3H.SW recipients of syngeneic sIg depleted spleen cells was minimal to cells that had divided more than once, with more than 50% of cells remaining post-treatment. A more vigorous depletion of cells was observed at a higher concentration of PTC (i.e. 2×66mg/kg), however, a) T cells which had not undergone division were again present and b) some T cells which had undergone only 1–2 divisions were again observed (Fig. 2A).
Fig. 2. PTC significantly reduces transplanted donor T cell numbers following allogeneic but not syngeneic HSCT.
A) Cells responding to lymphopenia induced proliferation are susceptible to PTC in a dose dependent manner. Note that the loss is most apparent in the more rapidly dividing generations. B6-CD45.1 or C3H.SW sIg- CD4 and CD8 T cells were labeled with CFSE and injected into lethally conditioned (10.5Gy) syngeneic recipients. Results presented as representative histograms from individual mice (n=6, B6; n= 4, C3H.SW) B) Total cell numbers present in spleens of recipients 5–7 days post-syngeneic (upper panel) or allogeneic (lower panel) transplant. Three strains were utilized for the syngeneic transplants: B6, C3H.SW and BALB.B. Allogeneic transplants were performed using B6→BALB.B. Recipients received 2 doses of 33mg/kg PTC on days 3 and 4 post-transplant. Results represent the composite from 7 and 5 independent syngeneic and allogeneic experiments respectively. No significant decrease in cell numbers was observed in syngeneic recipients following administration of PTC. In contrast, cell numbers were significantly reduced in allogeneic recipients after treatment with the same PTC dose.
The numbers of donor cells present following PTC was then determined in syngeneic and allogeneic transplant recipients (Fig. 2B). No significant diminution in total cell numbers was observed in syngeneic recipients examined 5–7 days post-transplant after administration of 33 mg/kg on Days 3 and 4 (Fig. 2B, Top). In contrast, a significant loss of cell number was observed in the spleens of B6->BALB.B allogeneic transplant experiments after the identical Day 3 & 4 PTC regimen post-transplant (Fig. 2B, Bottom). These findings were consistent with the CFSE dilution patterns observed after syngeneic and allogeneic transplant.
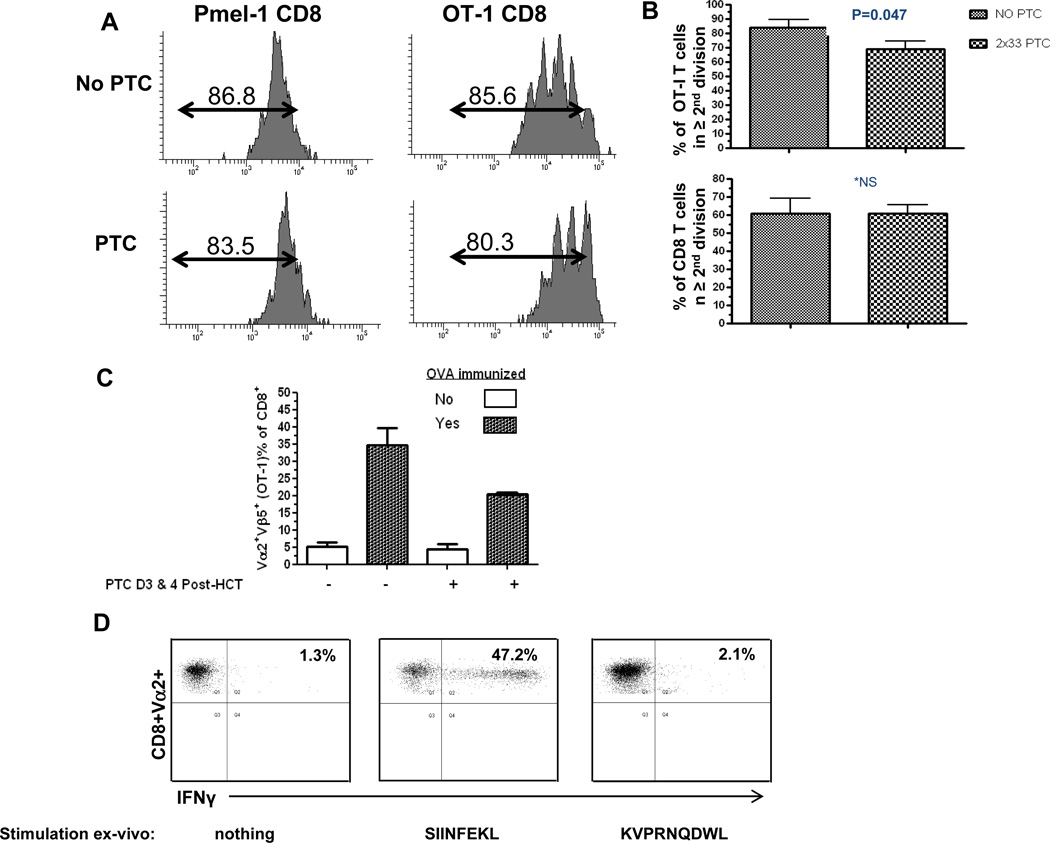
Based on the observations that donor CD8+ T cells dividing as a consequence of lymphopenia were diminished to a small degree, by PTC administered at a concentration (33mg/kg ×2) which diminished GVHD (Fig. 1), antigen specific T cells were subsequently included in the transplant inoculum to enable more precise monitoring of the impact of the PTC regimen on non-antigen driven donor T cell proliferation post-transplant. Purified CD8+ populations were obtained from B6-OT-I/Rag−/− and B6-Pmel-1/Rag−/− TcR transgenic mice, labeled with cell proliferation dye and co-administered with B6 T cells to lethally ablated syngeneic B6 recipients. We observed, as reported by others after sub-lethal irradiation [31], the rate of LIP by the CD8 OT-I population (~1/day) was significantly more rapid than that by the Pmel-1 (~1–2 divisions through 1 week post-transplant) CD8 T cells following transplant into syngeneic, lethally irradiated mice (Figure 3A). Following 2 × 33mg/kg PTC administration, minimal diminution of CD8+ Pmel-1 T cells was observed and a small, but consistent decrease was detected in the OT-I population (Fig. 3A). This effect on the OT-I population was further evidenced in several experiments in which examination of OT-I T cells identified a decrease - although just at the margin of statistical significance (p=0.047) - in the percentage of these CD8 T cells in the second or greater division after PTC was administered on Days 3 and 4 post-transplant in syngeneic recipients (85.6% → 77.9% with and without PTC, respectively, Fig. 3B, Upper). A similar pattern was also observed after co-infused polyclonal B6 CD8+ T cells were transplanted and analyzed in syngeneic recipients, i.e. no statistically significant decrease was observed whether or not 2×33mg/kg PTC was administered (Fig. 3B, Lower).
Fig. 3. Survival and function of donor T cells undergoing division in the lymphopenic environment following syngeneic HSCT and 33mg/kg ×2 PTC treatment.
A) 1×106 OT-I Rag−/− or Pmel-1 Rag−/− CD8 T cells were labeled with CFSE and co-injected with 2.3×106 sIg-polyclonal B6 T cells into lethally irradiated syngeneic recipients. PTC (33mg/kg) was administered on days 3 and 4 and mice were sacrificed at day 5. Representative flow analyses indicated there was virtually no and small reduction of CD8 Pmel-1 (n=2) and OT-I (n=6) cells respectively. B) Upper graph: Pooled results of three independent experiments (n=6 mice) illustrating a small decrease of OT-I T cells in the second or later division PTC (p=0.047). Lower graph: Results from two pooled independent experiments using CFSE labeled B6 polyclonal CD8 T cells. After transplant into B6 recipients, no significant differences were observed in animals who did not or did receive PTC (33mg/kg, Day 3,4) administration. C) OT-I donor T cells surviving PTC are functionally responsive to antigen 2 months post-HSCT. B6 recipients were transplanted with B6 T cells, OT-I and TCD-BM as in 3A and administered PTC (33mg/kg) on Days 3 and 4. After 60 days, mice were immunized with 20 ×106 splenocytes from B6-OVA tg mice. Three days later, the percentage of Vα2+ Vβ5+ OT-I cells in the blood had significantly increased in both non-PTC treated and PTC treated ova immunized recipients (P=0.02 & P=0.01, respectively). D) Donor OT-I T cells express effector cytokines when cultured in the presence of cognate antigen after PTC exposure. Animals were sacrificed and splenocytes obtained 4 weeks following ovalbumin immunization with B6-OVA tg cells. Unfractionated cells (1×106/well) were incubated for 6 hrs. in the presence of Brefeldin A and: no antigen, 1nM SIINFEKL (ova peptide: 257–264 chicken OVA, Kb binding) or 1nM KVPRNQDWL (human gp100 peptide: 25–33 of human melanin gp100, Db binding). Cells were then permeabilized and stained with anti-IFNγ mAb. Values represent % IFNγ positive staining cells gated on the Vα2+CD8+population.
We next asked whether cells that had been exposed to PTC and persisted in transplant recipients maintained functional responsiveness when stimulated by cognate antigen. Syngeneic transplants were therefore performed with co-administered CD8+ OT-I T cells, B6 T cells (sIg depleted splenocytes) and 5×106 B6 (CD45.1) TCD-BM. Six to eight weeks post-transplant, recipients that were untreated or treated with PTC were exposed to ovalbumin antigen following injection of splenocytes (2×107) from B6-OVA-transgenic. The presence and expansion (see below) of the transplanted donor OT-I (Vα2+Vβ5+CD8+ T cells) was evident in the blood of both untreated and PTC treated recipient groups (Fig. 3C). Additionally, splenocytes obtained after B6-ova (antigen) injection from these transplant groups of recipients were stimulated in-vitro with ova-peptide (SIINFEKL) for 6 hours and assessed for IFNγ production. Strong intracellular staining with anti-IFNγ mAb was observed in donor B6-CD8+Vα2+ T cells stimulated ex-vivo with SIINFEKL - but not an unrelated nonameric class I H-2Kb binding peptide (Fig. 3D).
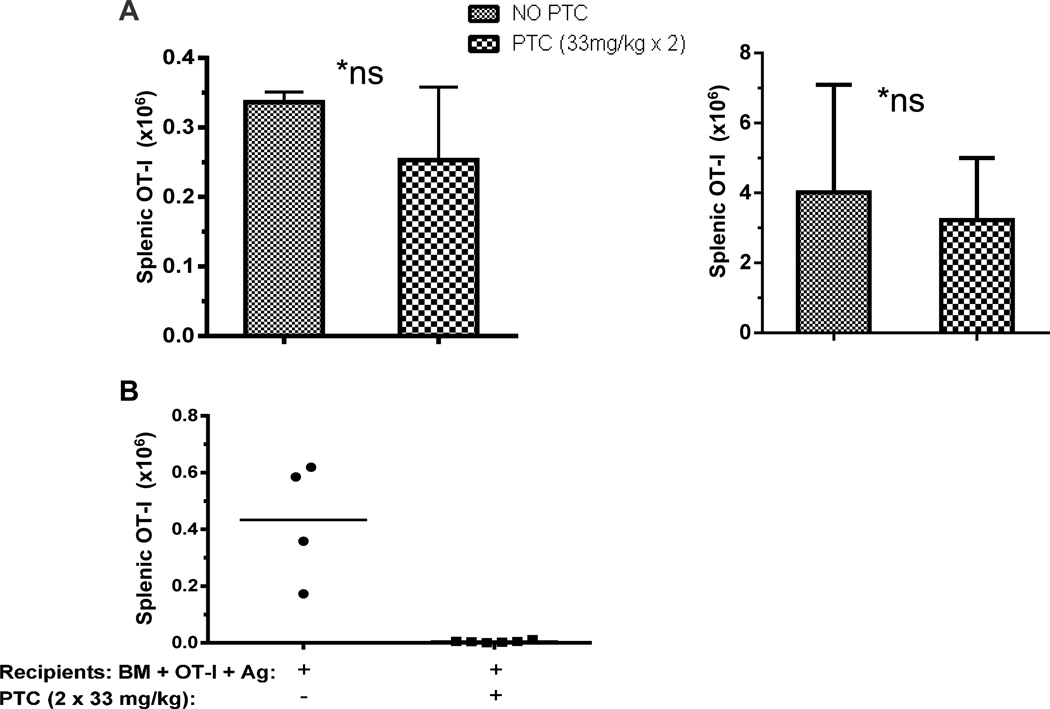
To more precisely quantify persistence and expansion of OT-I T cells after 2 × 33mg/kg PTC administration, syngeneic B6 mice transplanted with 2.3×106 polyclonal T cells and 1×106 CD8-OT-I cells were sacrificed and numbers of CD8+Vα2+Vβ5+ positive donor B6 T cells in the spleen were calculated. Two months post-transplant, the number of OT-I T cells present was not significantly different in groups that received D.3 and 4 PTC administration compared with the group that did not receive PTC (Fig. 4A). To determine the susceptibility of B6 CD8+OT-I T cells to this PTC regimen under antigen driven proliferation, these transgenic non-host reactive CD8 T cells together with B6 T cells were transplanted into syngeneic recipients and specific antigen introduced (Day 0) by injection of 2×107 splenocytes from B6-ova Tg mice (Fig. 4B). In contrast to findings obtained in the absence of ovalbumin antigen (Fig. 3), OT-I T cells were significantly depleted following Day 3 and 4 (2 × 33 mg/kg) PTC treatment (Fig. 4B). These findings demonstrated the effectiveness of the 2×33 mg/kg dose to deplete any donor T cell population following efficient antigen stimulation at the time of transplant prior to PTC exposure.
Figure 4. PTC reduces numbers of non-host reactive T cells in the presence but not absence of cognate antigen.
A) OT-I cell numbers recovered after recipient treatment with PTC are not significantly different in the absence of cognate antigen. B6 OT-I (1 or 5 ×106) T cells (Vα2+, CD90.2+ CD45.2+) were injected with 2.3×106 polyclonal B6 T cells (CD90.1+) and 6×106 TCD-BM (CD90.1+) into lethally irradiated (10.5Gy) syngeneic recipients (CD45.1+). PTC (2×33mg/kg) was administered at Day 3 & 4. OT-I T cells were identified in the spleens of PTC recipients 5 days (left panel) or 8 weeks (right panel) post-transplant. B) PTC administration depletes antigen specific CD8 OT-I T cells when cognate antigen is present. OT-I (1×106) cells were transferred into lethally irradiated syngeneic recipients (10.5 Gy). Antigen stimulation was initiated on Day 0 by infusion of 20 ×106 splenocytes from B6-OVA tg (1×107 sc and 1×107 i.p.). PTC (2× 33mg/kg) was administered on Days 3 & 4 post-transplant. Spleens were harvested three days later and Vα2+ Vβ5+ CD8+ OT-I T cells were stained and assessed by flow cytometric analysis. Data represents OT-I cell numbers per spleen determined from the percentage CD8 T cells expressing Vα2 and Vβ5 and multiplying this percentage by the calculated number of total CD8 T cells based on the numbers of viable (trypan blue negative) spleen cells (data not shown). Each data point represents an individual mouse (n=4, n=6/group).
In contrast to the two PTC dose injection regimen used in clinical transplants with a matched unrelated donor, haplo-identical HSCT is currently performed using a single injection of PTC [26]. To assess if a single, PTC dose containing the same total amount of cyclophosphamide resulted in similar persistence of non-host reactive donor CD8 T cells, B6-CD45.2+ OT-I cells were co-infused with B6 CD45.1+ T cells and bone marrow cells into lethally conditioned C3H.SW CD45.2+ recipients followed by injection of 66 mg/kg PTC at Day 3 post-transplant (Fig. 5). Recipients of donor T cells with or without co-infused B6 OT-I T cells that were treated with PTC did not exhibit loss of cell numbers in lymphoid tissue or weight loss reflective of GVHD in this strain combination (Fig. 5B,C). Notably, although few Vα2+ CD8+CD45.1− T cells were present in non-PTC treated recipients undergoing GVHD (graph per centages represent < 10 gated events / mouse), these T cells were readily detectable in lymph nodes in recipients treated on Day 3 with 66 mg/kg PTC ~ 1 month post-HSCT (Fig. 5A). The ability of PTC to facilitate persistence of non anti-host reactive OT-I T cells while concomitantly inhibiting GVHD was corroborated in an independent transplant in which recipients were examined >6 weeks post-HSCT (Fig. 6). PTC treatment again clearly prevented weight loss and clinical signs induced by GVHD (Fig. 6B, C). Importantly, only in PTC treated animals were transplanted Vα2+Vβ5+ CD8+ CD45.1− CD90.1− OT-I cells present, indicating that these cells survived the PTC treatment regimen which ameliorated GVHD (Fig. 6A).
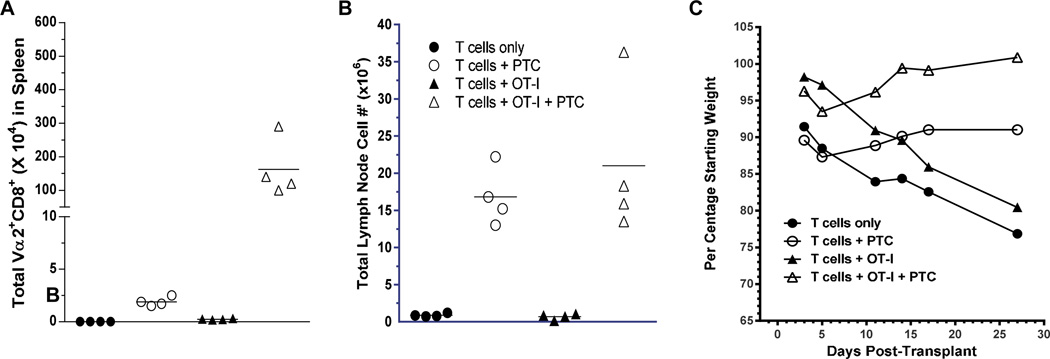
Figure 5. Persistence of non anti-host reactive donor T cells only in recipients treated with PTC and protected from GVHD.
Recipient C3H.SW were conditioned with a single dose of 10.5 Gy TBI on day 0 and 2.1×106 CD4+ and CD8+ B6-CD45.1+ T cells with or without 2.1×106 B6 CD45.2+ OT-I CD8 T cells were co-infused together with 6×106 B6-CD45.1+ TCD-BM (n=4 mice /group). Some groups received 66mg/kg ip injection of PTC on Day 3. Recipient tissues were harvested on Day 28 and assessed for total cell numbers and numbers of OT-I T cells. A) Total numbers of Vα2+CD8+ CD45.1− (OT-I) T cells in lymph nodes of mice at Day 29. p=0.003 and 0.03 for T cells only / T cells + PTC; and T cells + OT-I / T cells + OT-I + PTC, respectively; B) Total lymph node cell numbers at Day 29 post-transplant. p=0.003 and 0.03 for T cells only / T cells + PTC; and T cells + OT-I / T cells + OT-I + PTC respectively. One of 3 representative experiments.. C) Mean percentage weight loss vs. initial starting weight of each group of transplanted recipients. T cells only vs. T cells + PTC: p=0.09; T cells + OT-I vs. T cells + OT-I + PTC: p=0.11.
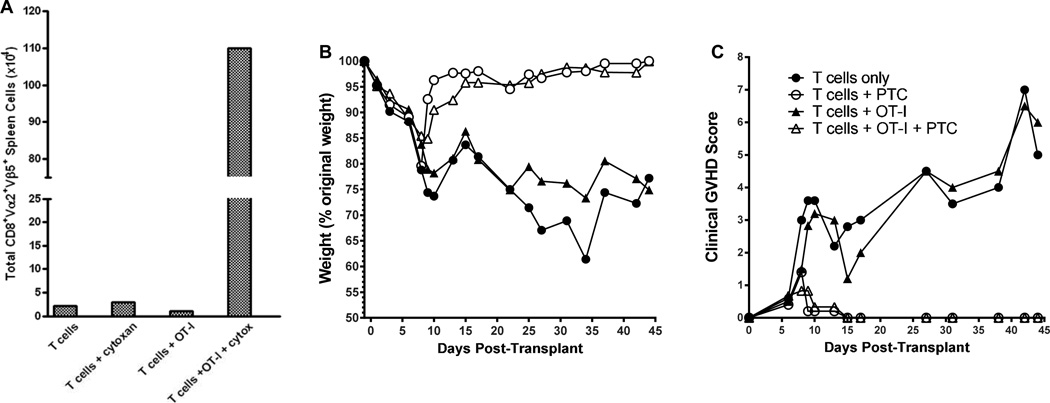
Figure 6. Non anti-host reactive donor T cells are present >6 weeks following PTC treatment in allogeneic HSCT recipients protected from GVHD.
Recipient C3H.SW were conditioned with a single dose of 10.5 Gy TBI on day 0 and 2.1×106 CD4+ and CD8+ B6-CD90.1+ T cells with or without 2.1×106 B6 CD45.2+CD90.2+ OT-I CD8 T cells were co-infused together with 6×6 B6-CD45.1 TCD-BM (n=4 mice /group). Some groups received 66mg/kg ip injection of PTC on Day 3. A) Total numbers of splenic Vα2+Vβ5+CD8+ CD45.1− CD90.1− (Ly9.1 which is expressed by C3H.SW T cells was not detected on these cells, data not shown) OT-I T cells in spleens of mice Day 44 post-transplant. B) Mean percentage weight loss vs. initial starting weight of each group of transplanted recipients. T cells only vs. T cells + PTC: p< 0.0001; T cells + OT-I vs. T cells + OT-I + PTC: p< 0.0001. C) Mean clinical score of each transplanted group based on monitoring changes including posture, fur texture and alopecia as described in Methods. One of 3 representative experiments. T cells only vs. T cells + PTC: p< 0.0001; T cells + OT-I vs. T cells + OT-I + PTC: p= 0.0005.
Discussion
Bacterial, viral and fungal infections are recurring complications associated with the diminished immune function following HSCT. Immune deficiency post-transplant can arise as a consequence of conditioning, GVHD prophylaxis and GVHD associated suppression. The use of methotrexate and cyclosporine to inhibit donor anti-host allo-reactive responses leading to GVHD following HSCT has been the standard of care for more than two decades [45, 46]. Strategies which could block GVHD inducing T cells while sparing other populations to provide immunity against pathogens and tumor antigens post-transplant would represent an advance in treatment. Recent clinical trials have provided evidence that administration of cyclophosphamide following T cell replete allogeneic HSCT may be a strategy to minimize long-term immunosuppression.[24] In the present study, we investigated the question of whether PTC results in a global and equivalent diminution of all dividing T cells, or whether anti-host alloantigen specific donor T cells may be more effectively depleted than other populations. The findings illustrate that although non-host alloantigen reactive donor T cells undergo some division early post-transplant in the lymphopenic setting, cyclophosphamide can be administered at Days 3–4 in a manner which predominantly spares such cells while concomitantly depleting a sufficient level of anti-host allo-reactive T cells to ameliorate GVHD.
In the 1970’s, PTC administration was reported to promote tolerance to allogeneic MHC-mismatched skin grafts in mice [18]. These results were followed by the observation that particular Vβ TCR families were deleted after MHC matched allogeneic skin grafting and cyclophosphamide infusion was performed, consistent with the hypothesis that the allo-reactive T cell response was inhibited by PTC [47]. Notably, these studies were not carried out under lymphopenic conditions. Subsequent work by Luznik and Fuchs demonstrated that PTC could promote engraftment following non-myeloablative HSCT supporting the notion that anti-host alloantigen reactive donor T cells elicited post-transplant could be eliminated [21]. Accordingly, the use of PTC in clinical allogeneic stem cell transplantation was performed based on the premise that this method of GVHD prophylaxis would specifically deplete activated donor anti-host specific T cells as initially proposed by Nomoto and colleagues after studies involving non-conditioned HSCT recipients [48]. However, whether or not PTC has a greater impact reducing host allo-antigen specific activity compared with non-host antigen reactive T cells undergoing LIP - signaled by excess cytokine (e.g. IL-7, IL-15) due to the marked loss of cytokine receptor binding T cells - has not been examined. While the abrogation of GVHD is a key goal following AHSCT, the utility of PTC would be diminished if a more global deletion of cells irrespective of their capacity to induce GVHD simultaneously took place.
When CFSE labeled B6 donor T cells were transplanted into lethally conditioned allogeneic minor histocompatibility mismatched C3H.SW recipients, a large percentage of rapidly dividing cells were identified. Following PTC (66 mg/kg total dose) administration, most of these rapidly dividing cells were eliminated and GVHD was significantly ameliorated. To assess how lymphopenic signals alone or together with antigen signals influenced susceptibility of transplanted T cells to PTC exposure, the addition of multiple TcR transgenic CD8+ T cell populations were included together with polyclonal donor CD8+ T cells. One population, OT-I, possesses a TcR specificity generated by engineering a RAG−/− B6 mouse to express a TcR Vα and Vβ segment that yields a specificity for a peptide of hen egg white lysoszyme (an antigen not normally found in the mouse) presented by the MHC class I H2Kb molecule. The second CD8 TcR transgenic population, i.e. PMEL-1 with specificity for the gp100 melanoma antigen is also presented by MHC Kb, and is an antigen that is expressed in the skin and ocular tissue at low levels. Notably, previous work by others reported more rapid expansion kinetics under lymphopenic conditions of OT-I CD8+ T cells (~1 division / 24 hrs.) vs. PMEL-1 CD8+ T cells (~1 division /34 hrs) [42, 44]. and those findings were corroborated in the present work. When co-transplanted into lethally conditioned syngeneic recipients, neither CD8+ T cell transgenic population was significantly diminished by the PTC dose employed as assessed by CFSE division and cell number (Figs. 3,4). However, the more rapid rate of LIP by OT-I than Pmel-1 T cells did appear to consistently, although modestly, increase their susceptibility to PTC. We interpret these findings to indicate that PTC susceptibility was markedly higher for T cells dividing more rapidly (multiple times per day), i.e. as a result of alloantigen stimulated activation post-transplant. Consistent with such a hypothesis was the finding that when OT-I CD8 T cells were subsequently co-infused with splenocytes from transgenic B6-OVA producing mice, the more rapid rate of OT-I proliferation demonstrated by dye dilution was accompanied by a large reduction in CD8 OT-I cell number following PTC administration (Fig. 4B). It is interesting that freshly obtained OT-I T cells expressing a predominantly naïve (i.e. CD44−CD62L+ Fig. S2) phenotype become highly susceptible to Day 3 and 4 PTC administration following antigen exposure because naïve donor T cells appear to mediate severe GVHD responses, and therefore deletion of responsive naïve T cells would presumably be important as well as effective for GVHD prevention [10, 49].
A key benefit of post-transplant usage of cyclophosphamide in hematopoietic cell transplants is that the pro-drug 4-hydroxycylcophosphamide is not converted to the alkylating phosphoromide mustard compound in cells expressing high levels of aldehyde dehydrogenase, which includes hematopoietic stem cells thus removing the necessity for additional stem cell infusion[50–54]. Although cyclophosphamide induced alkylation can occur throughout the cell cycle, it most effectively kills rapidly proliferating cells, especially those in the G1 and S phases. Resistance can result from several pathways including increased drug efflux, reaction of the drug with thiols (for example, glutathione) as well as increased DNA repair time. Successful DNA repair leading to survival requires the coordination of a number of protein factors to the damaged area, access to the strands, and sufficient time [55]. Since to some extent, both un-activated and activated T cells convert cyclophosphamide to the alkylating form, it is reasonable to propose that the more rapid the expansion kinetics by a T cell population - for example one driven by allogeneic antigen to divide multiple times per day - compared to division stimulated by cytokine under lymphopenic conditions i.e. ~ once / 18–36 hours - will result in less time for the former to repair damage caused in G1 / S phase resulting in death due to failure to replicate damaged DNA [56, 57]. Indeed, a consistent observation in our studies was the capacity of our PTC treatment regimens to prevent induction of GVHD mediated by donor T cells reactive with host alloantigen epitopes (Figs. 1, 5, 6, S1). The ability to enhance DNA repair pathways in selected cell populations could therefore provide approaches to minimize / abrogate the loss of slowly dividing cells with the objective to augment immediate immune responsiveness and protection in recipients following allogeneic hematopoietic stem cell transplantation.
The present study has important implications with regard to immune function / reconstitution following T cell replete allogeneic HSCT. Survival and responsiveness by those infused T cells which are not host allogeneic transplantation antigen reactive is crucial to provide patients with immediate immune function and protection against both pathogens and residual hematopoietic tumor cells. The findings here support the notion that the antigen and cytokine signals present which can rapidly induce T cell proliferation post-HSCT will have a major impact on determining the T cell populations which persist following administration of Day 3 / 4 post-transplant cyclophosphamide and accordingly, the cells which can be exploited via vaccination or other means to provide rapid adaptive immune function to protect individuals from opportunistic infection as well as relapse post-transplant. We posit that together with the drug concentration administered, development and application of strategies which diminish non-host alloantigen specific T cell proliferation and promote DNA repair can better protect ‘desired’ T cells and thereby successfully facilitate more rapid and effective immune function in such patients.
Supplementary Material
Groups of C3H.SW recipients (10.5 Gy TBI) were injected with donor B6 T cell depleted bone marrow (TCD-BM) alone or together with 2.3×106 B6 T cells (weight loss of these groups presented in Figure 1A). GVHD induced lethality was reduced after 2 × 33mg/kg PTC (○) (n=6) and not significantly different from recipients of TCD-BM only (p>0.05)(n=5). Recipients of 11mg/kg PTC (▲) exhibited slightly decreased lethality (n=5) vs. the non-PTC treated recipients (□) (n=7) but did not differ statistically (p>0.5).
OT-I T spleen cells were stained with anti-CD8, anti-CD44-(clone IM7) and anti-CD62L (Mel-14) mAbs. Values represent the positive cells in each quadrant based on analyses of CD8 stained cells. These cells are representative of those co-transplanted into recipients (Figures 3, 4 and 5).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED, Fefer A. Graft-versus-host disease. N Engl J Med. 1979;301:556. doi: 10.1056/NEJM197909063011017. [DOI] [PubMed] [Google Scholar]
- 2.Ueno NT, Rizzo JD, Demirer T, Cheng YC, Hegenbart U, Zhang M-J, Bregni M, Carella A, Blaise D, Bashey A, Bitran JD, Bolwell BJ, Elfenbein GJ, Fields KK, Freytes CO, Gale RP, Lazarus HM, Champlin RE, Stiff PJ, Niederwieser D. Allogeneic hematopoietic cell transplantation for metastatic breast cancer. Bone Marrow Transplant. 2008;41:537–545. doi: 10.1038/sj.bmt.1705940. [DOI] [PubMed] [Google Scholar]
- 3.Epstein RB, Graham TC, Buckner CD, Bryant J, Thomas ED. Allogeneic marrow engraftment by cross circulation in lethally irradiated dogs. Blood. 1966;28:692–707. [PubMed] [Google Scholar]
- 4.Buckner CD, Epstein RB, Rudolph RH, Clift RA, Storb R, Thomas ED. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. Blood. 1970;35:741–750. [PubMed] [Google Scholar]
- 5.Santos GW, Hess AD, Vogelsang GB. Graft-versus-host reactions and disease. Immunol Rev. 1985;88:169–192. doi: 10.1111/j.1600-065x.1985.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 6.Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 7.Mielke S, Solomon SR, Barrett AJ. Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy. 2005;7:109–115. doi: 10.1080/14653240510018172. [DOI] [PubMed] [Google Scholar]
- 8.Sprent J, Miller JFAP. Interaction of thymus lymphocytes with histoincompatible cells. I. Quantitation of the proliferative response of thymus cells. Cellular Immunology. 1972;3:361–384. doi: 10.1016/0008-8749(72)90244-4. [DOI] [PubMed] [Google Scholar]
- 9.Edinger M, Hoffmann P, Contag CH, Negrin RS. Evaluation of effector cell fate and function by in vivo bioluminescence imaging. Methods. 2003;31:172–179. doi: 10.1016/s1046-2023(03)00127-0. [DOI] [PubMed] [Google Scholar]
- 10.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao Y-A, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godfrey WR, Krampf MR, Taylor PA, Blazar BR. Ex vivo depletion of alloreactive cells based on CFSE dye dilution, activation antigen selection, and dendritic cell stimulation. Blood. 2004;103:1158–1165. doi: 10.1182/blood-2003-04-1098. [DOI] [PubMed] [Google Scholar]
- 12.Martins SLR, John LSS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ, Komanduri KV. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood. 2004;104:3429–3436. doi: 10.1182/blood-2004-05-1918. [DOI] [PubMed] [Google Scholar]
- 13.Spierings E, Hendriks M, Absi L, Canossi A, Chhaya S, Crowley J, Dolstra H, Eliaou J-F, Ellis T, Enczmann J, Fasano ME, Gervais T, Gorodezky C, Kircher B, Laurin D, Leffell MS, Loiseau P, Malkki M, Markiewicz M, Martinetti M, Maruya E, Mehra N, Oguz F, Oudshoorn M, Pereira N, Rani R, Sergeant R, Thomson J, Tran TH, Turpeinen H, et al. Phenotype Frequencies of Autosomal Minor Histocompatibility Antigens Display Significant Differences among Populations. PLoS Genet. 2007;3:e103. doi: 10.1371/journal.pgen.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bueger M, Bakker A, Van Rood JJ, Van der Woude F, Goulmy E. Tissue distribution of human minor histocompatibility antigens. Ubiquitous versus restricted tissue distribution indicates heterogeneity among human cytotoxic T lymphocyte-defined non-MHC antigens. J Immunol. 1992;149:1788–1794. [PubMed] [Google Scholar]
- 15.Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 16.Zong W-X, Ditsworth D, Bauer DE, Wang Z-Q, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owens AH, Jr, Santos GW. The effect of cytotoxic drugs on graft-versus-host disease in mice. Transplantation. 1971;11:378–382. doi: 10.1097/00007890-197104000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Glucksberg H, Fefer A. Chemotherapy of established graft-versus-host disease in mice. Transplantation. 1972;13:300–305. doi: 10.1097/00007890-197203000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Storb R, Buckner CD, Dillingham LA, Thomas ED. Cyclophosphamide regimens in rhesus monkey with and without marrow infusion. Cancer Res. 1970;30:2195–2203. [PubMed] [Google Scholar]
- 20.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nomoto K. Intrathymic clonal deletion of V beta 6+ T cells in cyclophosphamide-induced tolerance to H-2-compatible, Mls-disparate antigens. J Exp Med. 1990;171:97–113. doi: 10.1084/jem.171.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biology of Blood and Marrow Transplantation. 2002;8:131–138. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 22.Luznik L, Jones RJ, Fuchs EJ. High-dose cyclophosphamide for graft-versus-host disease prevention. Curr Opin Hematol. 2010;17:493–499. doi: 10.1097/MOH.0b013e32833eaf1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, Wang SA, Konopleva M, Fernandez-Vina M, Montes N, Bosque D, Chen J, Rondon G, Alatrash G, Alousi A, Bashir Q, Korbling M, Qazilbash M, Parmar S, Shpall E, Nieto Y, Hosing C, Kebriaei P, Khouri I, Popat U, de Lima M, Champlin RE. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luznik L, Bolaños-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, Huff CA, Borrello I, Matsui W, Powell JD, Kasamon Y, Goodman SN, Hess A, Levitsky HI, Ambinder RF, Jones RJ, Fuchs EJ. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayraktar UD, de Lima M, Ciurea SO. Advances in haploidentical stem cell transplantation. Rev Bras Hematol Hemoter. 2011;33:237–241. doi: 10.5581/1516-8484.20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolaños-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, Brodsky RA. HLA-haploidentical bone marrow transplantation with post-transplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012 doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai H-L, Bolanos-Meade J, Morris LE, Crilley PA, O’Donnell PV, Rossiter N, Huff CA, Brodsky RA, Matsui WH, Swinnen LJ, Borrello I, Powell JD, Ambinder RF, Jones RJ, Fuchs EJ. Nonmyeloablative HLA-Haploidentical BMT with High-Dose Posttransplantation Cyclophosphamide: Effect of HLA Disparity on Outcome. Biol Blood Marrow Transplant. 2010;16:482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosso D, Carabasi M, Filicko-O’Hara J, Kasner M, Wagner JL, Colombe B, Cornett Farley P, O’Hara W, Flomenberg P, Werner-Wasik M, Brunner J, Mookerjee B, Hyslop T, Weiss M, Flomenberg N. A 2-step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T-cell dosing. Blood. 2011;118:4732–4739. doi: 10.1182/blood-2011-07-365338. [DOI] [PubMed] [Google Scholar]
- 29.Bell EB, Sparshott SM, Drayson MT, Ford WL. The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. J Immunol. 1987;139:1379–1384. [PubMed] [Google Scholar]
- 30.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential Requirements for Survival and Proliferation of CD8 Naïve or Memory T Cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 31.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 32.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine Requirements for Acute and Basal Homeostatic Proliferation of Naive and Memory CD8+ T Cells. JExpMed. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkman R. Antigen-specific immunity following hematopoietic stem cell transplantation. Blood Cells Mol Dis. 2008;40:91–93. doi: 10.1016/j.bcmd.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23:783–788. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 35.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 36.Alpdogan O, Muriglan SJ, Eng JM, Willis LM, Greenberg AS, Kappel BJ, van den Brink MR. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J ClinInvest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012;19:324–335. doi: 10.1097/MOH.0b013e328353bc7d. [DOI] [PubMed] [Google Scholar]
- 38.Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again. Tissue Antigens. 2012;79:83–89. doi: 10.1111/j.1399-0039.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- 39.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte JJ, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 40.Baker MB, Riley RL, Podack ER, Levy RB. Graft-versus-host-disease-associated lymphoid hypoplasia and B cell dysfunction is dependent upon donor T cell-mediated Fas-ligand function, but not perforin function. Proc Natl Acad Sci USA. 1997;94:1366–1371. doi: 10.1073/pnas.94.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, Reddy P, Ferrara JLM. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naïve T cells. PNAS. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang LN, Yu Z, Palmer DC, Restifo NP. The In vivo Expansion Rate of Properly Stimulated Transferred CD8+ T Cells Exceeds That of an Aggressively Growing Mouse Tumor. Cancer Res. 2006;66:1132–1138. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson LDS, Jameson SC. Self-specific CD8+ T cells maintain a semi-naïve state following lymphopenia-induced proliferation. J Immunol. 2010;184:5604–5611. doi: 10.4049/jimmunol.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross M, Schmidt GM, Niland JC, Amylon MD, Dagis AC, Long GD, Nademanee AP, Negrin RS, O’Donnell MR, Parker PM, Smith EP, Snyder DS, Stein AS, Wong RM, Forman SJ, Blume KG, Chao NJ. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prevention of acute graft-vs.-host disease: effect on chronic graft-vs.-host disease and long-term survival. Biol Blood Marrow Transplant. 1999;5:285–291. doi: 10.1016/s1083-8791(99)70003-0. [DOI] [PubMed] [Google Scholar]
- 46.Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, Berenson R, Buckner CD, Clift R. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73:1729–1734. [PubMed] [Google Scholar]
- 47.Maeda T, Eto M, Nishimura Y, Nomoto K, Kong YY, Nomoto K. Direct evidence for clonal destruction of allo-reactive T cells in the mice treated with cyclophosphamide after allo-priming. Immunology. 1993;78:113–121. [PMC free article] [PubMed] [Google Scholar]
- 48.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Maeda T, Ando T, Nomoto K. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide-induced tolerant mice. J Immunol. 1991;146:1402–1409. [PubMed] [Google Scholar]
- 49.Juchem KW, Anderson BE, Zhang C, McNiff JM, Demetris AJ, Farber DL, Caton AJ, Shlomchik WD, Shlomchik MJ. A repertoire-independent and cell-intrinsic defect in murine GVHD induction by effector memory T cells. Blood. 2011;118:6209–6219. doi: 10.1182/blood-2011-01-330035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magni M, Shammah S, Schiró R, Mellado W, Dalla-Favera R, Gianni AM. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87:1097–1103. [PubMed] [Google Scholar]
- 51.Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, Nolta JA. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong L, Stojkovic M, Dimmick I, Ahmad S, Stojkovic P, Hole N, Lako M. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 53.Mirkes PE, Ellison A, Little SA. Role of aldehyde dehydrogenase (ALDH) in the detoxication of cyclophosphamide (CP) in rat embryos. Adv Exp Med Biol. 1991;284:85–95. doi: 10.1007/978-1-4684-5901-2_11. [DOI] [PubMed] [Google Scholar]
- 54.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 55.Kovarsky J. Clinical pharmacology and toxicology of cyclophosphamide: emphasis on use in rheumatic diseases. Semin Arthritis Rheum. 1983;12:359–372. doi: 10.1016/0049-0172(83)90016-1. [DOI] [PubMed] [Google Scholar]
- 56.Jones RJ. Haploidentical Transplantation - Repurposing Cyclophosphamide. Biol Blood Marrow Transplant. 2012;18:1771–1772. doi: 10.1016/j.bbmt.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Geva-Zatorsky N, Dekel E, Batchelor E, Lahav G, Alon U. Fourier analysis and systems identification of the p53 feedback loop. PNAS. 2010;107:13550–13555. doi: 10.1073/pnas.1001107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Groups of C3H.SW recipients (10.5 Gy TBI) were injected with donor B6 T cell depleted bone marrow (TCD-BM) alone or together with 2.3×106 B6 T cells (weight loss of these groups presented in Figure 1A). GVHD induced lethality was reduced after 2 × 33mg/kg PTC (○) (n=6) and not significantly different from recipients of TCD-BM only (p>0.05)(n=5). Recipients of 11mg/kg PTC (▲) exhibited slightly decreased lethality (n=5) vs. the non-PTC treated recipients (□) (n=7) but did not differ statistically (p>0.5).
OT-I T spleen cells were stained with anti-CD8, anti-CD44-(clone IM7) and anti-CD62L (Mel-14) mAbs. Values represent the positive cells in each quadrant based on analyses of CD8 stained cells. These cells are representative of those co-transplanted into recipients (Figures 3, 4 and 5).