Abstract
With the recent success of checkpoint inhibitors and other immune-modulating agents, there has been renewed interest in the combination of such agents with radiation. The biological premise behind such a strategy is that the tumor-antigen release achieved by localized radiation will promote specific tumor-targeting by the adaptive immune system, which can be augmented further by systemic immune-stimulating agents. In this manner, clinicians hope to induce a phenomenon known as the abscopal effect, whereby localized radiation results in immune-mediated tumor regression in disease sites well outside of the radiation field. In this Crossroads in Cancer Immunology article, we will present a comprehensive overview of the early clinical and pre-clinical evidence behind this approach.
Introduction
Immunomodulation as a means of cancer therapy has been studied in laboratory settings for many years. Cancer cells are known to have the ability to evade immunosurveillance through a variety of different mechanisms, including reduced expression of tumor antigens, downregulation of MHC class I and II molecules for reduced tumor antigen presentation, secretion of immunosuppressive cytokines such as tumor growth factor-beta (TGF-β), recruitment or induction of immunosuppressive cells such as regulatory T cells (Treg) or myeloid-derived suppressor cells (MDSC), and overexpression of certain ligands (e.g., programmed death ligand-1 [PD-L1]) that inhibit the host’s existing antitumor immunity. Recent advances in melanoma research have led to the development of immunotherapies that have substantial antitumor effects in other cancers including lymphoma, renal cell carcinoma, and non-small cell lung cancer (NSCLC) (1–5). These advances have been paradigm-shifting for several reasons. First, the observed immune response patterns have led to marked tumor regression that often outlasted the period of study (5,6). These responses are unprecedented for such a treatment-refractory patient population. Second, these new forms of immunotherapy have shown activity in tumors traditionally viewed as unresponsive to immune therapies, raising hope that any type of cancer might be “targetable” by immunotherapies if the right agent can be found. This antitumor activity has been most impressive in NSCLC (7), particularly among patients with unresectable disease treated with primary radiation therapy, a modality hypothesized to stimulate antigen production (8). It is conceivable that radiation therapy treatment acts as an “in situ vaccine” to prime the immune response. Nascent preclinical and early clinical findings have supported this possibility, suggesting that radiation, through its immune-stimulating properties, may be utilized as a systemic therapy in addition to a means of local tumor control (1,4,9,10).
Early in the history of immunomodulation and immunotherapy, irradiated tumor cells that had been engineered to secrete granulocyte-macrophage colony-stimulating factor were used broadly as anticancer vaccines for metastatic melanoma (11). The intent was to activate T cells that had been inactivated by the tumor’s immunosuppressive mechanisms (12). This nonspecific “shotgun” method occasionally caused tumor regression but did not provide clinical benefit consistently. Early vaccine trials focused on activating T cells without fully understanding the inhibitory pathways that control T-cell responses. The first molecule identified on T cells as an inhibitory checkpoint was cytotoxic T-lymphocyte antigen-4 (CTLA-4; ref.13). Allison and colleagues showed that CTLA-4 was a critical inhibitory molecule that controlled T-cell responses, thus preventing prolonged immune responses that could be detrimental to normal tissues. The Allison laboratory showed that an antibody blocking CTLA-4 elicited tumor regression in murine models (14). As a result, an antibody to human anti-CTLA-4 was developed, and its clinical success (15,16) opened a new field termed “immune checkpoint targeting”. Due to the groundwork laid by studies using anti-CTLA-4 mAb, and an improved understanding of how T-cell pathways could be targeted to promote antitumor T-cell responses, there was rapid development of additional antibodies to target T-cell pathways. Recently, antibodies targeting another inhibitory T-cell pathway known as PD-1/PD-L1 or -L2 have also shown clinical benefit (3,17). Furthermore, results from a combination therapy study showed that anti-CTLA-4 plus anti-PD-1 provided even greater clinical benefit than either alone as monotherapy (5). These so-called checkpoint inhibitors (anti-CTLA-4, anti-PD-1, and anti-PD-L1/L2) have led to renewed enthusiasm for immunotherapy as a treatment modality (12,18,19).
These antibodies were tested initially as monotherapy, but over time various combinations of these immunotherapeutic agents with current cytotoxic therapies are being investigated both in preclinical tumor models and in clinical trials. Recent studies of radiotherapy combined with immunotherapy have produced promising outcomes in animal models of various types of cancer. However, there is a considerable amount of work that remains to be done to create successful combinations of immunotherapeutics and radiation, which includes identifying the optimal radiation dose, fractionation, and sequence for use in combination with immune checkpoint inhibitors. This Crossroads article addresses the current understanding of these topics and highlights future directions for research.
Radiation in the Treatment of Solid Tumors
Historically, radiation was used as therapy for the majority of locally advanced solid tumors that are too extensive to be surgically resected. The noninvasive nature of radiation also quickly led to its becoming the de facto standard for patients whose age or general health status would make surgery risky. The technologies associated with tumor imaging and delineation have advanced greatly over the past 2 to 3 decades with the advent of new applications such as positron emission tomography and endobronchial ultrasonography. Such advances, in combination with similar advances in radiation planning and delivery, such as the use of 4-dimensional computed tomography (CT) to assess and account for tumor motion and intensity-modulated radiation therapy, have led to substantial improvements in the conformality of the radiation dose being delivered to the tumor. One example of this is in early-stage lung cancer with the advent of stereotactic ablative radiation therapy (20,21), which incorporates on-board imaging, management of tumor motion, and the highly precise delivery of relatively large radiation fractions. Findings from prospective trials have demonstrated that using this form of radiation therapy for tumors located in the periphery of the lung can lead to 3-year local control rates in excess of 98% (22). Successes such as these, in combination with the benefit of being noninvasive, have led to the emergence of radiation therapy as a key means of achieving local control of lung cancer. Radiation also has an integral role in the treatment of advanced cancers with involvement of mediastinal lymph nodes, often making complete surgical resection difficult. However, even though radiation offers clear benefits in terms of both local control and survival, disease recurrence outside the radiation field remains all too common, and most patients eventually die from progressive metastatic disease.
Moreover, despite the known benefit of ionizing radiation in local tumor control, it also enhances the release of cytokines such as tumor growth factor-β (TGF-β), a known inducer of tumor invasion and the epithelial-mesenchymal transition (23–25). On the other hand, radiation can also promote immune responses through the induction of neoantigens and the stimulation of factors such as interferon-γ (IFNγ) that can enhance T-cell infiltration (26). This dual ability to control local tumor progression and to influence metastatic spread and immune response constitute a compelling argument for including radiation in combination with the current arsenal of immune checkpoint inhibitors now entering the clinic.
The Abscopal Effect
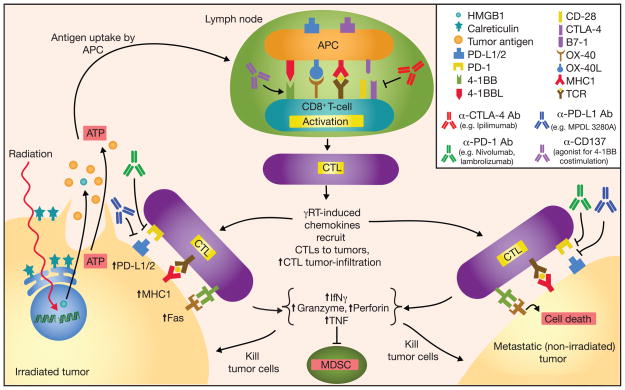
The abscopal effect refers to the ability of radiation delivered to a local site to minimize or eradicate metastases at distant sites. Although this phenomenon is not often described, it has led to complete regression of metastases at several anatomic sites in patients with cancer (10,27). Preclinical studies have provided insight on how localized radiation therapy can induce the abscopal effects and have implicated the immune system as a crucial mediator (reviewed by Frey and colleague in ref. 28). Figure 1 is a schematic diagram outlining the antitumor activity including the abscopal effect in combining radiotherapy with checkpoint immunotherapy. Local radiation therapy damages DNA within tumor cells leading to tumor-cell apoptosis/necrosis. Tumor antigens released from the dying tumor cells potentially can provide antigenic stimulation that induces antitumor-specific immune responses. This hypothesis is supported by the lack of the abscopal effect of radiotherapy in T cell-deficient (nude) mice or in mice with CD8+ T-cell depletion (29–31). Demaria and colleagues reported that the abscopal effect was tumor-specific in an elegant study. They administered the dendritic cell growth factor Flt3-L to mice that were implanted in one flank with mammary 67NR tumor cells alone and in the contralateral flank with both 67NR tumor cells and A20 lymphoma cells. They irradiated the flank with only the 67NR tumor cells, which led to significant regression in the contralateral flank of the non-irradiated, 67NR tumors but did not affect the growth of the antigenically unrelated A20 lymphoma (29). In contrast, Camphausen and colleagues found that irradiating Lewis lung carcinoma cells restricted the growth of non-irradiated T241 (fibrosarcoma) cells that had been inoculated in a second site, suggesting that abscopal effect of radiotherapy can also be mediated through other non-tumor-specific mechanisms, or that these two tumors are antigenically related (32). The systemic increase of many pro-inflammatory cytokines and chemokines after radiation, from both immune cells and tumor tissues, could account for the nonspecific eradication of distant tumors and metastases (33). Alternatively, the release of low-affinity tumor antigens prompted by local radiotherapy may also stimulate the release of cross-reactive tumor antigens.
Figure 1.
Schematic diagram outlining the antitumor activity and abscopal effect in combining checkpoint inhibitors with radiation-induced immune response. Radiation induces DNA damage and tumor cell death by promoting tumor cell expression of Fas and MHC class I; dying tumor cells release ATP, tumor antigens, and danger signals such as HMGB1 and calreticulin. Radiation also increases tumor cell expression of PD-L1 and secretion of TGF-β, and suppression of CD4+ Treg. Tumor antigens captured by antigen-presenting cells (APC) are processed and presented on MHC class I molecules in the draining lymph node to tumor antigen-specific T cells in conjunction with co-stimulation to promote activation and proliferation. CTLA-4 can bind B7-1 to downregulate T-cell activation. Activated CTLs leave the lymph node, follow inflammatory chemokines, and migrate to tumor sites. PD-L1 and PD-1 can interact to suppress CTL activation; various α–CTLA-4, α–PD-L1, and α-PD-1 mAbs have been developed and used successfully in cancer immunotherapies. CTL antitumor activity includes secretion of INFγ and TNF, suppression of myeloid derived suppressor cells (MDSC), expression of perforin and granzyme, and activation of Fas ligand-mediated tumor cell apoptosis.
Abscopal effects seem to depend on both the radiation dose and the delivery schedule for different types of tumors. In one mouse model involving the Lewis lung carcinoma and a murine fibrosarcoma, low-dose irradiation (2-Gy fractions given twice daily for 6 days) reduced the abscopal effect that had been evident after 5 daily fractions of 10 Gy each (32). Lee and colleagues reported that a single ablative dose of radiation (20 Gy) to murine B16 melanoma generated a strong antitumor T-cell response that was diminished by the use of fractionated radiotherapy or the addition of chemotherapy agents (34). In contrast, the combination of an antibody to CTLA-4 and fractionated radiation (but not single-dose radiation) resulted in abscopal effects in preclinical models of breast and colon cancer (35). Although radiation therapy causes tumor-cell apoptosis/necrosis, radiation alone is not sufficient to trigger antigenic signals and a second costimulatory signal is required to elicit systemic antitumor immune responses, especially in poorly immunogenic cancers (30). Moreover, radiotherapy alone can suppress the growth of primary breast, colon, and lung cancer tumors but not the appearance of lung metastasis in mouse models (30,35,36). Thus the combination of radiotherapy with immune modulators may be able to escalate antitumor responses to a level that could suppress or eliminate systemic metastasis.
Immune Checkpoint Inhibitors
During the past decade, the framework for treating systemic disease has gradually shifted from the broad approach of treating all dividing cells, especially tumor cells, to potentiating the immune system. Immunomodulators that target T-cell surface proteins have shown promise in mobilizing immune cells from a state of anergy to activation in response to the presence of cancer cells. Two T-cell surface proteins, CTLA-4 and PD-1, serve as “immune checkpoints” for T cells (37); when CTLA-4 or PD-1 interact with their cognate ligands, an inhibitory signal is conveyed to T cells, resulting in decreased cytokine production, inhibition of proliferation, and reduced cytotoxic function (38,39). CTLA-4 is activated by binding to B7-1 (CD80) or B7-2 (CD86), which are expressed predominantly by antigen-presenting cells (APC; ref. 39). PD-1 is activated through its interaction with PD-L1 and PD-L2, which are present on APCs, on non-neoplastic tumor stroma, and on tumor cells (38). Although the roles of these immune recognition molecules have been studied extensively in the context of host-pathogen defenses and autoimmune diseases, their roles in suppressing antitumor T-cell responses are now beginning to be understood. Evidence is mounting that during cancer development, ligands for both immune inhibitory molecules are upregulated in tumor-associated immune cells, in the surrounding tumor stromal environment, and in the tumor (37,40,41). Tumor cells capitalize on these immune tolerance mechanisms to facilitate their growth.
A humanized anti-CTLA-4 IgG1 antibody (ipilimumab; Bristol-Myers Squibb) has been tested in several multi-institutional, randomized control trials. Most of these studies have focused on patients with stage III or IV melanoma, for whom the prognosis is poor and few therapeutic options are available (42). Hodi and colleagues reported the results of a randomized, phase III clinical trial of 676 melanoma patients, who received ipilimumab with or without gp100, a well-studied cancer vaccine derived from the melanoma glycoprotein 100 (15). These investigators showed that the groups treated with ipilimumab alone or with ipilimumab plus gp100 had better overall survival (the primary study endpoint) than those treated with gp100 alone (15). This was the first phase III randomized clinical trial to report a survival benefit for melanoma patients. In a second phase III randomized clinical trial, Robert and colleagues (16) assigned 502 patients with previously untreated stage III or IV melanomas to receive dacarbazine, a chemotherapy agent approved by the FDA for advanced melanoma (43), or dacarbazine plus ipilimumab. The combination of dacarbazine plus ipilimumab led to improved overall survival relative to dacarbazine alone. In addition to melanoma, ipilimumab has been tested in phase I and II trials for other solid malignancies including small cell lung cancer (44), NSCLC (45), bladder cancer (46), pancreatic cancer (47), prostate cancer (48), and renal cell carcinoma (49). Lynch and colleagues reported results of a randomized phase II trial of 204 patients, who received paclitaxel and carboplatin either with or without ipilimumab, administered concurrently or in a phased manner, in which patients received alternating cycles of chemotherapy and ipilimumab. Patients treated with ipilimumab administered in a phased manner, not concurrently, with chemotherapy had significantly better progression free survival (PFS), providing evidence supporting sequenced therapy (44). A new phase III trial of 908 patients with squamous NSCLC and utilizing a similar design has begun recently (ClinicalTrials.gov number NCT01285609).
Based on the success of anti-CTLA-4 cancer immunotherapy, other antibodies have been developed to target additional T-cell molecules, including those involved in the PD-1 signaling pathway. Several large multi-institution trials have tested the use of these antibodies for various types of cancer. In two clinical studies for patients with advanced solid malignancies, Topalian and colleagues tested the anti-PD-1 antibody (nivolumab; Bristol-Myers Squibb) in 296 patients, and Brahmer and colleagues tested an anti-PD-L1 antibody (BMS-936559; Bristol-Myers Squibb) in 207 patients (3,17). Significant objective response rates were observed against melanomas, NSCLC, and renal cell carcinoma in both trials, and the anti-PD-1 antibody seemed to elicit higher response rates (17). Particularly notable was the response rate in NSCLC (5 out of 49), which unlike melanoma and renal cell carcinoma, was historically considered unresponsive to immunotherapy (7). This finding suggests that our previous thinking of defining cancers as “immunogenic” versus “non-immunogenic” was incorrect and in reality, many types of cancer can be treated with immunotherapy if we use the appropriate agents to overcome the inhibitory pathways that exist within the immune system. As a result, there are now multiple trials testing anti-PD-L1 or PD-1 antibodies in patients with solid tumors.
Since CTLA-4 provides inhibitory signals to T cells by binding to B7 molecules, which blocks appropriate co-stimulation of T cells by CD28 (13), and PD-1 provides inhibitory signals to T cells by interfering with T-cell receptor signaling, Allison and colleagues tested the combination of anti-CTLA-4 plus anti-PD-1 in tumor-bearing mouse models and showed improved antitumor responses in the combination therapy compared to monotherapy (37,50). As predicted by the murine studies, a phase I clinical trial combining anti-CTLA-4 plus anti-PD-1 was reported to provide dramatic antitumor responses in patients with metastatic melanoma. Wolchok and colleagues administered nivolumab and ipilumumab to patients with advanced melanoma and found that the 53 patients given the two-drug regimen had a higher objective response rate than did patients treated with either antibody alone (5). Moreover, many of these patients achieved “deep” responses, with greater than 80% tumor reduction that lasted for extended periods (5). In another study, Hamid and colleagues used a different humanized anti-PD-1 antibody, lambrolizumab (MK-3475; Merck), in 135 patients with melanoma and reported a high rate of sustained tumor regression, with no difference in response among patients who had or had not received prior ipilumumab therapy (6). Together, results from these two trials suggest that concurrent—but not sequential—use of anti-CTLA-4 and anti-PD-1 drugs can have clinically complementary effects.
Combining Immune Checkpoint Inhibitors with Radiation
Although anti-CTLA-4 and anti-PD-1 mAbs may overcome T-cell suppression, T-cell activation depends on the engagement of the antigen receptor and the activating costimulation molecule CD28 expressed by mature APCs (39). For this reason, ionizing radiation, which can increase the production and presentation of tumor antigens not only by immunogenic cancers like melanoma but also by poorly immunogenic tumors, could augment the antitumor immune responses elicited by checkpoint immunomodulators anti-CTLA-4 and anti-PD-L1 (30,51,52). Radiation may augment immunomodulation by increasing CTL activity and the antigenic peptide pool (4,51). New data from Deng and colleagues suggested that the combination of radiation and PD-L1 checkpoint blockade can synergistically reduce MDSCs (53). Preclinical studies of murine models have demonstrated that various immunomodulators benefit from combinations with radiation through antigen release (54). Postow and colleagues and Hiniker and colleagues each reported systemic responses in patients with melanoma treated with the combined regimen of anti-CTLA-4 mAb ipilimumab and radiation, suggesting that coupling radiation therapy with immunotherapy may hold promise for inducing powerful, long-term abscopal effects in human patients (10,55). Preclinical and clinical studies conducted to date on combinations of immune checkpoint modulators and radiation will be discussed below.
Anti-CTLA-4 and radiation: preclinical findings
In the first preclinical study of the CTLA-4 blocker ipilimumab with radiation, Demaria and colleagues tested the hypothesis that ipilimumab in combination with radiation therapy could elicit an abscopal antitumor response in a model of metastatic 4T1 breast cancer (30). In that study, tumors were injected in a primary and a distant site in mice and, as expected, ipilimumab alone did not stop the progression of this poorly immunogenic tumor. Similarly, although radiation alone delayed the growth of primary tumors, irradiated and control IgG-treated mice had similar OS rates owing to distant lung metastasis. However, mice that received the combined regimen of CTLA-4 blockade and local radiation therapy showed both tumor shrinkage and inhibition of lung metastasis, which was associated with a significant survival advantage (30). A similar study combining Flt3 therapy with radiation conducted with metastatic lung cancer cells in a tumor model produced the same results and suggested a long-term protective immune response (36). In another study of breast cancer cells, Mastsumura and colleagues provided the first evidence that T-cell recruitment by proinflammatory chemotactic factors overcame previous blocks at the effector phase by poorly immunogenic tumors (56). This study confirmed that the expansion of vaccine-specific T cells via the use of mAbs was not sufficient to elicit an antitumor immune response (57,58). Rather, the chemokines and their receptors induced by ionizing radiation were needed to recruit tumor-specific CTLs to the target (59) and, together with CTLA-4 blockers, break the pattern of immune escape and tumor tolerance by lymphocytes. Ruocco and colleagues reported that in mice anti-CTLA-4 mAbs used as monotherapy did not induce effective immune responses to poorly immunogenic tumors but it did when combined with local radiation, confirming the role of ipilimumab and radiation in overcoming the tumor-elicited MHC class I-dependent arrest (60). Although relatively immunogenic tumors such as melanoma have shown regression after antibody therapy (15), poorly immunogenic tumors may need the priming effects of radiotherapy to overcome blocks at the effector level (9,30). In other words, expansion of vaccine-specific T cells by CTLA-4 blockade alone may not be sufficient (57,58,61).
Anti-CTLA-4 and radiation: clinical findings
Postow and colleagues described a patient whose metastatic melanoma regressed upon treatment with ipilimumab and concurrent palliative radiation therapy (10). Specifically, a CT scan obtained several months after the patient had received a 28.5-Gy radiation dose (given in three 9.5-Gy fractions) to an area next to the spine revealed that masses elsewhere in the spleen and hilar lymph nodes had also regressed and eventually reached the point of stable minimal disease 10 months after the last dose of radiation. This case led to a pilot study at a different institution by Hiniker and colleagues to combine ipilimumab and concurrent radiotherapy for a patient with asymptomatic melanoma (55). That patient was given a higher radiation dose (54 Gy in three fractions) and showed a complete response in both the primary tumor and the metastatic lesions, which confirms the findings from preclinical studies indicating the importance of radiation dose (9,35). These results have led to interest in combining ipilimumab with radiation for other cancers, and recently there was a case report for a patient with NSCLC, who has also demonstrated an abscopal response (27).
In a phase I/II clinical study, Slovin and colleagues found that patients with metastatic castration-resistant prostate cancer responded to ipilimumab plus radiation (55). In that study of 50 men given ipilimumab (four 10-mg/kg doses) plus radiation (8-Gy fractions to each lesion for 3 weeks), one patient experienced a complete response, six had disease stabilization, and eight showed declines in prostate-specific antigen levels that mirrored findings from previous preclinical studies. This combination is now being tested in phase III trials, and interest has been spurred in its use to treat other types of tumors.
Anti-PD-1 and radiation: preclinical findings
With the anti-cancer efficacy of CTLA-4-blockers, anti-PD-1/-PD-L1 mAbs have drawn much interest for their potential use in lung or colon cancer (62) and in combination with CTLA-4 blockade for melanoma (5,6). The mechanism by which radiation augments the therapeutic effects of the anti-PD-1/PD-L1 mAbs was elucidated in a preclinical study of triple-negative breast cancer. In this study, neither anti-PD-1 mAb nor radiation when given alone was effective in a murine model of triple-negative breast cancer. However, the addition of anti-PD-1 mAb enhanced the curative capacity of radiotherapy and α-CD137 (an agonist antibody for costimulatory molecule 4-1BB) against both established tumors and secondary tumor challenge, indicating that the combined regimen conferred antitumor immune responses and memory (63). Moreover, a subset of tumor-specific CD8+ T cells expressing CD137, PD-1, or both was found to persist in the irradiated tumor tissues, suggesting that the synergistic effect of this triple combination was mediated by an activation or escalation of CD8+ T cell-mediated antitumor responses (63). Although the PD-1 axis did not appear to be the main contributor to the metastatic and neoplastic capability of AT-3 tumors, PD-1 signaling within those tumors was critical for limiting the effectiveness of α-CD137/α-CD40 immunotherapy, with or without adjuvant radiotherapy (63). Indeed, the combination of α-CD137, anti-PD-1, and radiation showed greater efficacy (40% rejection) than anti-PD-1 or radiation, given alone or in combination (63). Furthermore, in mice whose tumors regressed completely at the primary site when treated with this triple combination, growth of the AT-3 tumors at distant sites also was impaired, indicating an abscopal effect. This effect was dependent on the presence of CD8+ T cells, as mice lacking CD4+ T cells showed the abscopal effect but CD8+ T cell-depleted mice did not (63).
Results of a preclinical study of murine intracranial glioma treated with anti-PD-1 mAb plus radiation therapy showed not only long-term survival of the treated mice, but also robust systemic immunologic memory in the surviving mice, as they were able to reject a secondary challenge of glioma cells injected in the flank (18). Specifically, median survival periods were similar for control mice (25 days), and mice given only anti-PD-1 mAb (27 days) or radiation (28 days). However, the combination of radiation plus anti-PD-1 therapy extended the median survival to 53 days (P<0.05 by log-rank mantel-cox test), and 15% to 40% of mice survived more than 180 days after treatment (18). The combination therapy increased tumor infiltration by CD8+ CTLs and decreased the number of CD4+ Tregs. Finally, in a test of immunologic memory, naïve and long-term surviving mice were injected in the flanks with GL261-luc cells. All 8 naïve mice died from the growth of the challenged glioma cells, whereas mice that received prior treatment with the combined regimen rejected the glioma challenge (18).
Based on the results of these preclinical studies, several clinical trials have been initiated to assess the efficacy of combining anti-PD1 immunotherapy with radiation therapy. Reports of toxicity associated with immune checkpoint inhibitors in completed trials are summarized below.
Toxicity of Immune Checkpoint Inhibitors
Perhaps not surprisingly, rates of immune-related adverse events (AE) were high in the clinical trials completed to date. Although most of these AEs were mild (grade 1–2), some were severe, and a few were lethal. The most common toxic effects were considered of immunologic origin, and the sites most commonly affected were the skin, gastrointestinal system, liver, and lung (3,5,6,16,17).
Of particular importance for trials aiming to explore abscopal effects is the risk of radiation pneumonitis, especially for patients with thoracic disease. Rates of severe AEs (grade 3–4) including pneumonitis, dyspnea, or cough have ranged from 2% to 4% (5,6,15,17) among patients treated with agents targeting CTLA-4 and PD-1, and 3 deaths occurred in one study (17) and 1 in another study (6) were attributable to pulmonary toxicity. Radiation pneumonitis is thought to reflect an immune-mediated inflammatory reaction to radiation-induced lung damage (64,65). Severe forms can be lethal, and thus radiation pneumonitis is often considered as a radiation-dose-limiting toxicity (66). Results from retrospective analyses have indicated that the conformality of the radiation dose to be delivered is crucial to minimizing toxicity, and indeed significant reductions in toxicity have been achieved with the use of more sophisticated radiation planning and treatment modalities, such as protons (66–58). Similarly radiation treatment in the abdomen which could result in bowel radiation could similarly exacerbate colitis. Thus trials combining checkpoint immunotherapy with radiation for solid tumors must be conducted with great care, and with strict limits placed on the amounts of normal lung, bowel and other tissues that are exposed to radiation via the use of lung dose-volume histograms. Interestingly, immunotherapy with PD-L1 inhibitors was associated with much lower pneumonitis rates than that with PD-1 inhibitors (69). A final precaution is the need to account for both acute and late toxicity in designing trials. Most phase I trials involving radiation allow dose-escalation based on the appearance of acute toxicity; however, pneumonitis often does not appear until several months after radiation treatment is completed, and thus the follow-up period must be considerably longer before dose-escalation should be considered.
Conclusions
Cancer immunotherapy has come of age and is becoming one of the pillars, along with surgery, chemotherapy and radiation therapy, for the treatment of cancer patients. Combination therapies are also being investigated, including inhibition of both CTLA-4 and PD-1 or use of one immunotherapy agent with chemotherapy or with other molecular-targeted agents. Although such combinations may improve antitumor efficacy, their toxicity may prove to be a major obstacle. In contrast, combinations of radiation (preferably stereotactic ablative radiation therapy) with immune checkpoint inhibitors have the advantage that radiation can provide local control and may also prompt the release of tumor antigens that activate immune response and enhance immune recognition on a systemic level. Many questions remain including how to minimize overlapping toxic effects of radiation and immunotherapy, and how to optimize the sequencing of these two treatment modalities. Although there exists only a limited number of patients treated with this approach, these early results are promising, and warrants further investigation with a cautious eye on safety and toxicity.
Acknowledgments
This work was supported in part by Cancer Center Support (Core) Grant CA016772 and P30CA016672 to The University of Texas MD Anderson Cancer Center. We are grateful to Christine Wogan for her valuable input in developing this manuscript.
Footnotes
Conflicts of Interest: none
References
- 1.Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai Wz, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–63. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brody JD, Ai Wz, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Kluger H, Callahan MK, Postown MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt GE, Podack ER, Raez LE. Immunotherapy as a strategy for the treatment of non-small-cell lung cancer. Therapy. 2011;8:43–54. doi: 10.2217/thy.10.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y, Kepp O, Ghieinghelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 12.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 13.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice With Intracranial Gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 21.Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–52. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 23.Morrison CD, Parvani JG, Schiemann WP. The relevance of the TGF-beta Paradox to EMT-MET programs. Cancer Lett. 2013;341:30–40. doi: 10.1016/j.canlet.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andarawewa KL, Erickson AC, Chou WS, Costes SV, Gascard P, Mott JD, et al. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor beta induced epithelial to mesenchymal transition. Cancer Res. 2007;67:8662–70. doi: 10.1158/0008-5472.CAN-07-1294. [DOI] [PubMed] [Google Scholar]
- 25.Jung JW, Hwang SY, Hwang JS, Oh ES, Park S, Han IO. Ionising radiation induces changes associated with epithelial-mesenchymal transdifferentiation and increased cell motility of A549 lung epithelial cells. Eur J Cancer. 2007;43:1214–24. doi: 10.1016/j.ejca.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Baschnagel AM, Williams L, Hanna A, Chen PY, Krauss DJ, Pruetz BL, et al. c-Met Expression Is a Marker of Poor Prognosis in Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma Treated With Chemoradiation. Int J Radiat Oncol Biol Phys. 2014;88:701–7. doi: 10.1016/j.ijrobp.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non-Small Cell Lung Cancer. Cancer Immunol Res. 2013;1:365–72. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey B, Rubner Y, Wunderlich R, Weiss EM, Pockley AG, Fietkau R, Gaipl US. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Curr Med Chem. 2012;19:1751–64. doi: 10.2174/092986712800099811. [DOI] [PubMed] [Google Scholar]
- 29.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Lienes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 31.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190:5874–81. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camphausen K, Moses MA, Menard C, Sproull M, Beecken WD, Folkman J, O’Reilly MS. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–3. [PubMed] [Google Scholar]
- 33.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravarty PK, Guha C, Alfieri A, Beri V, Niazova Z, Deb NJ, et al. Flt3L therapy following localized tumor irradiation generates long-term protective immune response in metastatic lung cancer: its implication in designing a vaccination strategy. Oncology. 2006;70:245–54. doi: 10.1159/000096288. [DOI] [PubMed] [Google Scholar]
- 37.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 39.Lu P, Wang YL, Linsley PS. Regulation of self-tolerance by CD80/CD86 interactions. Curr Opin Immunol. 1997;9:858–62. doi: 10.1016/s0952-7915(97)80190-2. [DOI] [PubMed] [Google Scholar]
- 40.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84:57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 41.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 42.Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9:587–95. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- 43.Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19:21–34. [PubMed] [Google Scholar]
- 44.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 45.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 46.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 55.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5:404–7. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 58.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 59.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 60.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–7. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 63.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–74. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 64.Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995;31:361–9. doi: 10.1016/0360-3016(94)00477-3. [DOI] [PubMed] [Google Scholar]
- 65.Roberts CM, Foulcher E, Zaunders JJ, Bryant DH, Freund J, Caims D, et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med. 1993;118:696–700. doi: 10.7326/0003-4819-118-9-199305010-00006. [DOI] [PubMed] [Google Scholar]
- 66.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042–4. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 67.Yom SS, Liao Z, Liu HH, Tucker SL, Hu CS, Wei X, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 68.Tang C, Liao Zx, Gomez D, Levy LB, Zhuang Y, Gebremichael R, et al. Lymphopenia association with gross tumor volume and low lung dose and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014 doi: 10.1016/j.ijrobp.2014.04.025. In Press. [DOI] [PubMed] [Google Scholar]
- 69.Chow LQ. Exploring novel immune-related toxicities and endpoints with immune-checkpoint inhibitors in non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2013:280–285. doi: 10.14694/EdBook_AM.2013.33.e280. [DOI] [PubMed] [Google Scholar]