Abstract
Objective:
The aim of this study is to detect breast cancer rate, nodal status, tumor size, and associated risk factors using clinical breast examination (CBE) and mammography as screening tools in women aged 40–49 years.
Materials and Methods:
A total of 500 women were screened in a time period of 2 years, between the ages of 40–49 years for breast cancer. Screening tools used were CBE and mammography. Clinical history and risk factors related to breast cancer were recorded. CBE was performed to detect any breast pathology followed by mammographic screening. Breast Imaging Reporting and Data System (BI-RADS) mammographic density categories were used for reporting breast imaging on mammography. For women with dense breasts or an inconclusive mammography report, ultrasonography was performed to assess the lesion/s. Suspicious lesion was subjected to fine-needle aspiration cytology or an open surgical biopsy for a confirmatory diagnosis. Women with history of breast cancer were excluded from the study.
Results:
CBE was normal in almost 90% of the women. Screening mammography revealed Breast Imaging Reporting and Data System (BI-RADS) I and BI-RADS II in 58.4% and 34.6% of women, respectively. Only 7% of women belonged to BI-RADS III and none in BI-RADS IV category.
Conclusion:
The study findings are in agreement with the recommendations of the World Health Organization, US preventive task force and UK guidelines that recommend screening mammography in women starting at 50 years.
Key Words: Clinical breast examination, mammography, screening
INTRODUCTION
Breast cancer is the most common cancer in females worldwide. It is the most frequent cancer in both developed and developing regions.[1,2] India is the largest developing country, has a steadily increasing incidence of breast cancer. At present in our country, cancer of the breast is the most common cancer among women in many regions and has overtaken cervical cancer.[3] The age-standardized incidence rate for breast cancer in India is 22.9/100,000, one-third that of Western countries, and the mortality rates are disproportionately higher.[4,5] The data from Atlas Project suggest that breast cancer in urban areas of India is three times higher than in rural parts of the country. In metropolitan city age adjusted incidence rates are, for example, Chandigarh 39.5, North Goa 36.8, New Delhi 28.9, Bengaluru 30.9, Chennai 33.0, Delhi 31.0, Mumbai 29.3, and Kolkata 20.6 per 100,000 whereas rates are much lower in rural areas such as the nonurban Ahmedabad district 9.2, Barshi 9.4 and Sikkim 6.8 per 100,000.[6,7] There is no organized, systematic, government funded screening program for breast cancer in India. The screening in developing countries can be considered as “Opportunistic Screening.” The World Health Organization (WHO) recommends mammography every 1–2 years for women aged 50–69 years.[8]
The breast cancer screening programs in the United Kingdom currently invites women aged 50–70 years for screening mammography every 3 years. Since the time the screening programs were established, there has been debate, at times sharply polarized, over the magnitude of their benefit and harm, and the balance between them. The expected major benefit of screening is reduction in mortality from breast cancer. The major harm is overdiagnosis and its consequences; overdiagnosis refers to the detection of cancers on screening, which would not have become clinically apparent in the woman's lifetime in the absence of screening.[9]
In India, breast cancer incidence peaks before the age of 50 years, and a recent review of the evidence in younger women (aged 39–49 years) based on eight trials conducted between 2001 and 2008, suggests that mammographic screening is also beneficial in this younger age group.[10] Hence, there is a need to screen women in the age group of 40–49 years to know the effectiveness of breast cancer screening in this age group.
Objectives
The aim of this study was to detect breast cancer rate, nodal status, tumor size, and associated risk factors using clinical breast examination (CBE) and mammography as screening tools in women between ages 40 and 49 years. Furthermore to know the false positive rate, false negative rate, sensitivity, and specificity of these screening methods.
MATERIALS AND METHODS
A cross-sectional study was conducted to screen 500 women for 2 years, between the ages of 40–49 years for breast cancer. Screening tools used were CBE and mammography. CBE was performed to detect any breast pathology followed by mammographic screening. BI-RADS mammographic density categories were used for reporting breast imaging on mammography.
Methodology
A cross-sectional study was conducted to screen 500 women in a time period of 2 years, between the ages of 40–49 years for breast cancer. Screening tools used were CBE and mammography. Women attending the gynecology outpatient department (OPD) clinic, ministerial staff, staff-nurses, and health-professionals of Government Medical College, Chandigarh between ages of 40 and 49 years and giving consent to participate in the study were chosen for breast cancer screening. Clinical history and risk factors related to breast cancer were recorded. CBE was performed to detect any breast pathology followed by mammographic screening. BI-RADS mammographic density categories were used for reporting breast imaging on mammography.[11] For women with dense breasts or an inconclusive mammography report, ultrasonography was performed to assess the lesion/s. Suspicious lesion was subjected to fine-needle aspiration cytology (FNAC) or an open surgical biopsy for a confirmatory diagnosis. Women with history of breast cancer were excluded from the study.
Statistical analysis
Categorical data were presented as number and percentage. Quantitative data were given as mean ± standard deviation and medians. As there was no positive case of breast cancer in the study, and hence sensitivity and specificity could not be assessed, hence no further statistical tools were applied.
Review of literature
Cancer screening is a preventive step towards a healthier life. Screening for cancer involves a process of assessing people for early signs of a certain type cancer even though they have no symptoms.
Recent recommendations of the United States Preventive Task Force (USPTF) have reignited the debate about mammographic screening in women below 50 years. The United States Preventive Services Task Force (USPSTF) recommends against routine screening mammography in women aged 40–49 years. The decision to start regular, biennial screening mammography before the age of 50 years should be an individual one and take into account patient context, including the patient's values regarding specific benefits and harms. The USPSTF recommends biennial screening mammography for women between the ages of 50 and 74 years.[12]
The American Cancer Society recommends yearly mammograms starting at age 40 and continuing for as long as a woman is in good health. Clinical breast exam (CBE) every year for women 40 and over. All major US medical organizations recommend screening mammography for women aged 40 years and older. Screening mammography reduces breast cancer mortality by about 20%–35% in women aged 50–69 years and slightly less in women aged 40–49 years at 14 years of follow-up.[13]
The WHO recommends mammography every 1–2 years for women aged 50–69 years.[8]
The effectiveness of mammographic screening in reducing mortality from breast cancer in women aged 50–69 is widely accepted.[1] However, debate continues regarding the risks and benefits of mammography, especially for women in their forties. Disadvantages of screening, which need to be weighed against any benefit include false-positive results; the recall of women for further investigations that do not result in a diagnosis of breast cancer. The UK age trial was the only trial designed specifically to investigate the effect of annual invitation to mammography starting at age 40. Results from the age trial do not suggest that the new national policy of inviting women for breast screening from age 47 by 2012 will result in a large increase in false-positive results. Whether screening should be implemented in this age group is a separate issue, but the question of greatly increased false-positive rates in this age group and of their compromising re-attendance is refuted by the findings of this study and should be taken into account when determining screening policy.[14]
The breast cancer screening programs in the United Kingdom currently invite women aged 50–70 years for screening mammography every 3 years. Breast screening extends lives. The panel's review of the evidence on benefit – the older randomized controlled trials (RCTs), and those more recent observational studies – points to a 20% reduction in mortality in women invited to screening. A great deal of uncertainty surrounds this estimate, but it represents the panel's overview of the evidence. This corresponds to one breast cancer death averted for every 235 women invited to screening for 20 years, and one death averted for every 180 women who attend screening. The panel's best estimate is that the breast screening program in the United Kingdom, inviting women aged 50–70 every 3 years, prevent about 1300 breast cancer deaths a year, a most welcome benefit to women and to the public health.[9]
The Korean National Cancer Screening Survey (KNCSS) is a nationwide survey conducted annually, since 2004. This study was conducted to report on trends in rates of cancer screening for five major cancers-stomach, liver, colorectal, breast, and cervix uteri in Korea. Data collected by the KNCSS between 2004 and 2011 were used in this study. Lifetime screening rates and screening rates with recommendation have increased since 2004. On average, screening rates with recommendation have shown an annual increase 4.0% (95% confidence interval [CI], 3.0%–4.9%) for breast cancer, and 0.2% (95% CI, −0.9%–1.3%) for cervical cancer. Screening rates for stomach and breast cancer in particular showed a marked increase.[15]
Knowledge and practices on screening methods of breast and cervical cancers among female health-care workers in Sri Lanka, in spite of having an organized screening program is land wide. A cross-sectional survey was conducted among 219 female health-care workers including public health midwives (68.9%) selected from six districts in Sri Lanka using convenient sampling methods. A self-administered questionnaire was used as a pretest in a capacity building training program to collect the data. Over 98% knew about self-breast examination. Even though 84.1% practiced it, only 47.9% practiced it on a monthly basis. CBE and mammography were known by 94.1% and 64.3%, respectively. Only 19.2% had undergone a CBE within 1 year and 3.6% had ever undergone a mammography.[16]
Known risk factors for breast cancer are grouped into modifiable and nonmodifiable factors given below:[17,18]
Modifiable risk factors
Age at first childbirth
Breastfeeding practices
Obesity
Physical activity
Menopausal hormone therapy
Alcohol intake.
Nonmodifiable risk factors
Age
Benign breast disease
BRCA 1 or 2 carrier
Family history
Early menarche/delayed menopause
Increased breast density
Chest irradiation.
A recent systematic review and meta-analysis on risk factors for breast cancer revealed that extremely dense breasts and first-degree relatives with breast cancer were each associated with at least a 2-fold increase in risk for breast cancer in women aged 40–49 years. The identification of these risk factors may be useful for personalized mammography screening.[19]
In India, breast cancer incidence peaks before the age of 50 years, and a recent review of the evidence (in younger women (aged 39–49 years) based on 8 trials conducted between 2001 and 2008, suggests that mammographic screening is also beneficial in this younger age group.[10] Hence, there is a need to screen women in the age group of 40–49 years to know the effectiveness of breast cancer screening in this age group.
RESULTS
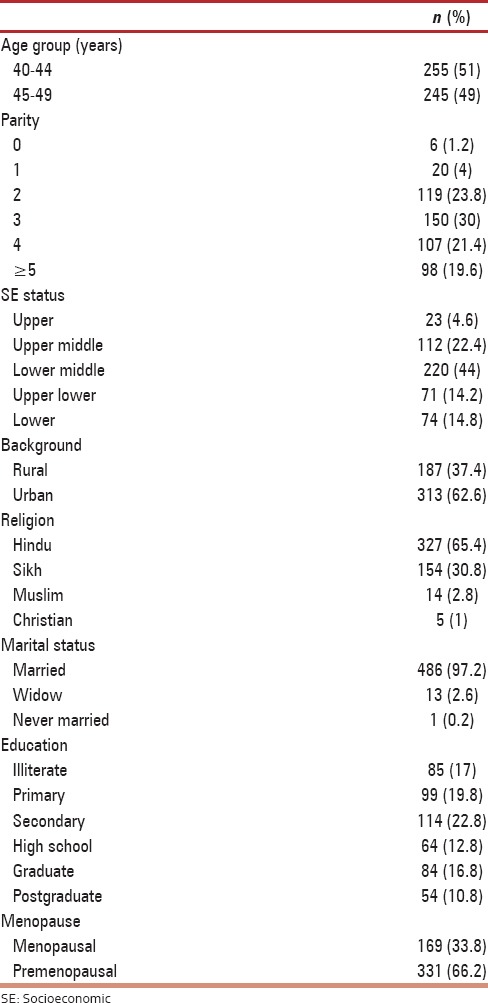
Five hundred women in the age group of 40–49 years underwent screening for breast cancer and the mean age of the study group was 44.1 ± 3.61 years. The demographic characteristics of the study group are shown in Table 1. Most of the women (44%) belonged to the lower middle socioeconomic status as per modified Kuppuswamy classification. About 62.6% of women were of urban background. All women except one were married and 98.8% of them were parous. Only one-third (33.8%) of the women in the study group had attained menopause. Among the menopausal women, 70% of them had natural menopause and the remaining 30% had surgical menopause.
Table 1.
Demographic characteristics (n=500)
The modifiable risk factors studied were age at first childbirth, breast feeding practices, obesity (in relation to body mass index [BMI]) use of menopausal hormone therapy and alcohol intake, depicted in Table 2. The mean age at first childbirth in the study group was 22 ± 3.1 years. Most of the women (97.6%) had their first childbirth before the age of 30 years and only 1.2% were nulliparous. As regards breast feeding practices, 36.6% of women breastfed their children up to 1 year, 50.3% of women breastfed their children between 1 and 2 years and 13.1% breastfed for more than 2 years. Using BMI as a marker for obesity, 40.8% of women were overweight (BMI 25–29.9), 13% had class I obesity (BMI 30–34.9), 3% had class II (BMI 35–40), and 0.6% had class III (BMI >40) obesity. None of the women in the study group used menopausal hormone therapy and neither of them had alcohol intake.
Table 2.
Risk factors (n=500)
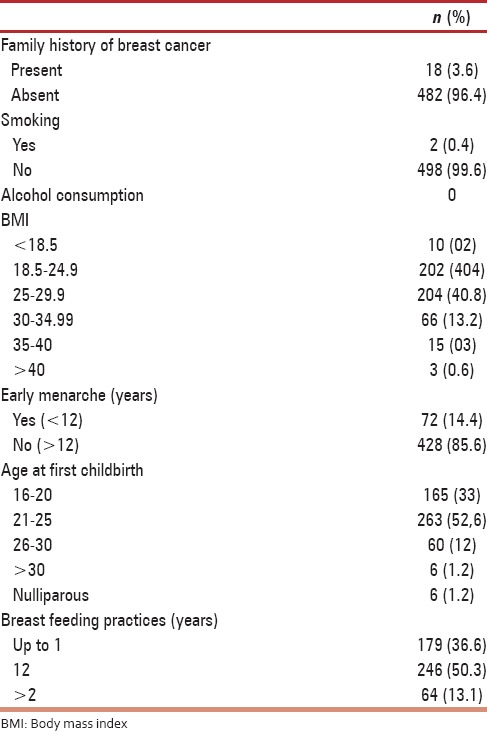
The nonmodifiable risk factors studied were benign breast disease, family history of breast cancer, early menarche, and age at menopause. Benign breast disease as detected on Mammography was present in 35.6% of women, but there was no prior history of breast disease in these patients. Family history of breast cancer was seen in 18 women (3.6%). In 14 of the 18 women the sister had history of breast cancer, in one case, mother had history of breast cancer whereas in the remaining three women, history of breast cancer was present in the daughter. None of the women with family history of breast cancer underwent BRCA testing. Early menarche, that is, below 12 years of age was observed in 72 (14.4%) women. As mentioned earlier 169 women, that is, 33% of women in the study group were menopausal. Among these women about 12.4% attained menopause below 40 years, 45.6% were menopausal between ages 40–44 years and 42% attained menopause between 45 and 49 years.
Screening for breast cancer was assessed by using CBE [Table 3] and mammography in 500 women, inclusive of women attending gynecology OPD clinic, ministerial staff and nursing staff who consented to be a part of this study. CBE was normal in 89.4% of women and in 7.2% of the women had breast nodularity suggestive of Fibroadenosis, Fibroadenoma was observed in 2.6% and palpable Axillary lymph node in 0.8% of the study population.
Table 3.
Clinical breast examination (n=500)

Using Breast Imaging Reporting and Data System (BI-RADS) classification [Table 4] for studying mammographic findings, 58.6% of the women belonged to BI-RADS I, 34.6% to BI-RADS II and 7% to BI-RADS III and none in BI-RADS IV. Benign lesions on mammography were seen in 35.6% of women [Table 5]. A total of four women were selected for interventions in the form of FNAC or smears from nipple discharge. In one patient with significantly enlarged axillary lymph node on mammography, underwent FNAC which was reported as granulomatous infection s/o tuberculosis. She underwent 6 months of directly observed treatment, short-course (DOTS) therapy and was disease free on follow up. Another patient with nipple discharge, Bilateral ductal ectasia and BI-RADS III on mammography underwent cytologic examination of nipple discharge which was negative for malignancy. The third patient had simple cyst with BI-RADS III, underwent FNAC which was reported as benign cyst. In the fourth patient, two fibroadenomas with BI-RADS II was the clinical diagnosis. The fibroadenomas measured (i) 3.2 cm × 1.3 cm × 1.4 cm and (ii) 2.0 cm × 0.8 cm × 1.6 cm, which did not increase in size on 6 months follow up and then she opted for Aryuvedic treatment elsewhere. Benign breast lesions detected on mammography are tabulated in Table 5, the common ones being axillary lymph nodes (33.3%), fibroadenoma (32.2%) and simple cyst (12%). Ultrasound of breast was used to complement mammography, was done in 322 women of the total 500 women screened. The findings of Ultrasound of the breast are charted in Table 6. The salient findings of Ultrasound breast examination are a normal examination in 152 women, fibroadenosis in 99 women, axillary lymph nodes in 23 women, and simple cyst in 17 women. All women (n = 35) who had BI-RADSIII on mammography underwent ultrasound examination as well. Ultrasound findings were normal in 13 women with BIRADS III on mammography, simple cyst was seen in 11 women, eight women had changes suggestive of fibroadenosis and axillary lymph node seen in three women [Table 7].
Table 4.
Mammography findings (n=500)

Table 5.
Benign breast lesions on mammography (n=183)

Table 6.
Findings of ultrasound breast (n=322)
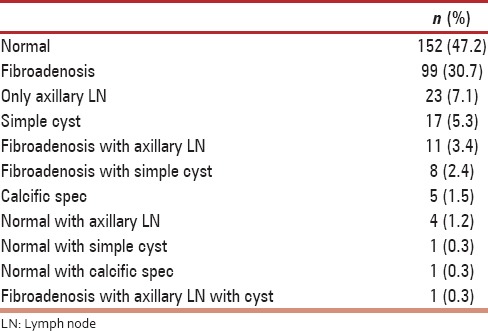
Table 7.
Breast Imaging Reporting and Data System III and ultrasound correlation (n=35)

DISCUSSION
The project included the study of risk factors for breast cancer, modifiable and nonmodifiable risk factors were taken into account.
The modifiable risk factors were age at first childbirth, breast feeding practices, obesity (in relation to BMI), use of alcohol, smoking, and hormone replacement therapy.
Age at first childbirth
Taking into consideration age at first childbirth, the Brinton studies demonstrated that the risk of breast cancer increased if a woman was nulliparous or experienced her first live birth at or after age of 30.[20] The minimum age at first childbirth in the study population was 16 years and maximum was 34 years. In 97.6% of the women studied, experienced their first childbirth before 30 years, only 1.2% of women had their first childbirth after 30 years of age and 1.2% were nulliparous. Due to early childbearing age in our country (mostly below 30 years), which is reflected in our study also it has a protective affect against development of breast cancer.
Breast feeding practices
Individual data from 47 epidemiological studies in thirty countries that included information on breastfeeding practices showed that relative risk of breast cancer decreased by 4.3% for every 12 months of breast feeding.[21] Breast feeding practices in our study showed that about half of the women (50.3%) breast fed their children for a period between 1 and 2 years and 13.1% of women breastfed their children for more than 2 years. As per the Lancet study, stated above, the longer the women breastfed, the more they are protected against breast cancer. It also states that the lack or short life time duration of breastfeeding, typical of women in developed countries makes a major contribution to high incidence of breast cancer in these countries.[21]
Obesity (in relation of body mass index)
In the study population, 40.4% of the women had a normal BMI, 40.8% of them were overweight and 16.8% were obese. A prospective study of 570,000 women aged 30–69 years in Norway studied the relationship between BMI and breast cancer.[22] In premenopausal women, BMI was not a risk factor for breast cancer, whereas in postmenopausal women, a high BMI was at least a minor risk factor for breast cancer.
The explanation for this higher risk with obesity in the postmenopausal women is that adipose tissue is an important extra gonadal source of bioavailable estrogens in postmenopausal women and exposure to these estrogens postmenopausally increases the time frame in which they may affect both initiation and promotion of breast cancer. In addition, a high BMI is associated with increased levels of insulin and insulin like growth factors, which have been associated with increased risk of breast cancer. Another feature of special importance is that accumulation of body fat in the peri and postmenopausal women is usually abdominal and abdominal obesity is strongly associated with hyperinsulinemia, a risk factors for breast cancer.
As the study group included women in the age group of 40–49 years, only 33.8% of the women were postmenopausal and 40.2% of these women were overweight 12% were obese.
Use of alcohol, smoking, and hormone replacement therapy
In the study population, use of alcohol and hormone replacement therapy was nil. Only two women (0.4%) in the study group were smokers, hence, these risk factors were of almost negligible significance.
The nonmodifiable risk factors were family history of breast cancer, early menarche, benign breast disease, and age at menopause.
Family history of breast cancer
A family history of breast cancer was present in 18 women, that is, 3.6% of the study population. Among the 18 women who had family history of breast cancer, 14 women had history of breast cancer in the sister, in three women history of breast cancer was in their daughters and in one case, the mother had history of breast cancer.
The first expert opinion on association of familial predisposition of breast cancer was published in 1866 by Broca et al.[23] Thereafter, 52 case control studies and 22 cohort studies quantified the risk associated with a family history of breast cancer.[24,25] These studies estimated that compared to individuals with no family history of breast cancer, a relative risk of 1.8 is associated with a first degree relative who developed breast cancer at 50 years or older as compared to a relative risk of 3.3 for the first degree relative who developed breast cancer at age <50 years. In this study, all women who had family history of breast cancer, the first degree relative affected were below the age of 50 years at the time of disease affection, hence, a relative risk of 3.3 will be awarded to these women with family history of breast cancer.
Early menarche
Early menarche, that is, menstruation below 12 years of age confers a relative risk for invasive breast cancer of 1.3 compared to those who began menstruation after age of 15. In our study, early menarche was observed in 72 women (14.4%) and hence, a relative risk of 1.3 as regards their risk quantification.[26] However, majority of the women (85.6%) had menarche after 12 years, therefore had a low relative risk and contributes toward a lower chance of malignancy in the study population.
Benign breast disease
As regards history of benign breast disease, 10.6% of the patients had a diagnosis of simple fibro adenoma and none of the patients had complex fibro adenoma or atypical hyperplasia or ductal carcinoma in situ.
Age at menopause
The age of at menopause was a study limitation since only 33% of women were menopausal and in 2/3rd of women the age of menopause was yet to be determined, hence, quantification of this risk factor was not possible.
CBE was one of the screening tools in the study and was performed after taking history and enumeration of the risk factors listed above.
A total of 500 women underwent CBE and in 89.4% of the women, no abnormalities were detected. In 36 women (7.2%), CBE revealed nodularity, in two women (0.4%) fibro adenomas were detected and no case of suspicious lesion was observed. Canadian National breast screening study evaluated the efficacy of combination of annual screening with mammography, CBE and teaching of breast self-examination in women aged 40–49 years and concluded that screening with yearly mammography and CBE detected considerably more node negative, small tumors than usual care, but had no impact on rate of death from breast cancer up to 7 years of follow up. The Canadian national breast screening study 1 used CBE and mammography in conjunction.[27] There are no randomized trials for CBE as per the Cochrane database systematic Reviews 2003.[28] The American Cancer Society guidelines recommend yearly CBE for women aged 40 years and over. Barton et al. in 1999 carried out a systematic review of CBE and from the studies found that CBE was found to detect between 3% and 4.5% of breast cancers missed by screening mammography.[29] The conclusion from this review was that, there is indirect evidence supporting the effectiveness of CBE as a screening tool. McDonald et al. in a more recent review in 2004, suggest that 4.6%–5.7% of breast cancers were solely identified by CBE and many women request CBE because of breast changes they had noted.[30] In Asian women, CBE is more sensitive, which may be related to their tendency to have smaller breasts than Caucasian women.[31] The sensitivity of CBE is low 54% whereas specificity is high 94%. In our study, the sensitivity and specificity of CBE could not be calculated, as we did not find any positive case for breast cancer in the 500 women screened over 2 years.
Screening mammography using quantitative criteria for the BI-RADS showed BI-RADS I (<25% dense for almost entirely fatty) in 58.4% of women and BI-RADS II (25%–50% dense for scattered fibrograndular densities) in 34.6% of women and BI-RADS III (51%–75% dense for heterogeneously dense category) in 7% of women and none in the BI-RADS IV (>75% dense for the extremely dense category).
Mammographic screening has been a subject of debate in the recent years as to when to start screening, the interval between two screening mammograms and endpoint of screening, that is, reduction in breast cancer detection mortality. As per the Canadian national breast screening study 1 breast cancer detection and death rates among women aged 40–49 years, the results confirm that there is no evidence that screening for breast cancer is effective in women aged 40–49 years, at least in the first 7 years after initiation of screening.[27] The Swedish two-county trial and the Stockholm trial initially showed no reduction in death rate among women aged 40–49 years on entry who were screened.[32,33] However, updated results published in 2011 with three decades of follow up, on the Swedish two county trial have suggested that mammographic screening results in a highly significant decrease in breast cancer-specific mortality and the number of breast cancer deaths prevented increased with the increasing time of follow-up. WHO position on mammographic screening in women aged 40–49 years, there is uncertainty as to balance the benefits and harms of mammographic screening in women aged 40–49 years. The reduction in breast cancer mortality is proven in RCT's, however, due to much lower incidence rate of breast cancer in this age group and somewhat lower sensitivity of mammography screening programs for women aged 50–69 years at a screening interval of 2 years.[34]
The USPTF recommends mammographic screening for women age 50–74 years every 2 years, requiring 13 mammograms during this time.[12] The breast cancer screening programs in the United Kingdom currently invite women aged 50–70 years every 3 years for screening mammography.
The UK age trial investigated the effect of annual mammography screening starting at age 40 up to the calendar year of their 40th birthday, were randomized into an intervention arm or a control arm, in a ratio of 1:2. There were 4.9% false positives at first screens and 3.2% false positives at subsequent screens. Results from the age trial do not suggest that a new policy for inviting breast screening from age 47 will result in large increase in false positives and will prevent women from re-attendance.[14]
The American Cancer Society guidelines for screening mammography and yearly mammograms starting at age 40 and continuing for as long as woman is in good health.[13] There is no organized breast cancer screening program in India, although in April 2013, the Indian Menopause Society has laid down guidelines, mammographic screening annually starting at age 40 years. For women between 50 and 70 years of age, selective use of mammography once in 3 years and decision to perform mammography should be determined with shared decision making about risks and benefits by individual patient values.[35] In our study of screening mammography of 500 women in age group of 40–49 years over a period of two years, no case of breast cancer was detected.
Ultrasound of the breast was performed in 322 women, revealed normal finding in 47.2%, fibroadenosis in 30.7% and in few women, fibroadenosis with axillary lymph nodes, fibroadenosis with simple cysts, calcific specs, and simple cysts. In all 35 women with BI-RADS III on Mammography, ultrasound correlation revealed, normal findings in 13, fibroadenosis in eight, axillary lymph nodes in three and simple cyst in 11 women. Ultrasound was thus used to complement screening mammography.
Additional benefit of screening mammography was diagnosis of benign lesions in 183 women (36.6%) as shown in Table 6. The common benign lesions were axillary lymph nodes seen in 61 (33.3%), fibro adenomas in 59 women (32.2%), and simple cyst in 22 women (12%).
FNAC were performed in four women, of which, three women underwent breast cytology and in one woman axillary lymph node cytology was performed. In the three women FNAC of the breast lesions, were negative for malignancy. In one patient with axillary lymph node biopsy revealed granulomatous inflammation suggestive tuberculosis, was given DOTS therapy and was cured.
CONCLUSION
Screening for breast cancer in women aged 40–49 years revealed few favorable factors like, most women experienced their first childbirth at or before 30 years, breastfed their children up to 2 years, did not have early menarche, no alcohol consumption, and minimal smoking. CBE was normal in almost 90% of the women. Screening mammography revealed BI-RADS I and BI-RADS II in 58.4% and 34.6% of women, respectively. Only 7% of women belonged to BI-RADS III and none in BI-RADS IV category. No case of breast cancer was detected in the women screened. The study findings are in agreement with the recommendations of WHO, US preventive task force and UK guidelines that recommend screening mammography in women starting at 50 years.
Study limitations
First, this is a cross-sectional study carried out over 2 years and long term follow up of women could not be assessed. Second, no patient of malignancy was detected in the 500 women screened, although the sample size was statistically sufficient. As there was no case of breast cancer detected in the women screened sensitivity, specificity, false positive, and false negative rates of CBE and mammography could not be calculated.
This study was funded by Department of Science and Technology, Chandigarh.
Financial support and sponsorship
This study was funded by Department of Science and Technology, Chandigarh.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferlay J, Bray F, Parkin DM, Pisani P. IARC Cancer Bases No. 5. Lyon: IARC Press; 2001. Globocan 2000: Cancer Incidence and Mortality Worldwide version 1.0. [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. 2010. [Last cited on 2011 Oct 25]. Available from: http://www.globocan.iarc.fr .
- 3.Murthy NS, Chaudhry K, Nadayil D, Agarwal UK, Saxena S. Changing trends in incidence of breast cancer: Indian scenario. Indian J Cancer. 2009;46:73–4. doi: 10.4103/0019-509x.48603. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal G, Ramakant P. Breast cancer care in India: The current scenario and the challenges for the future. Breast Care (Basel) 2008;3:21–27. doi: 10.1159/000115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal G, Ramakant P, Forgach ER, Rendón JC, Chaparro JM, Basurto CS, et al. Breast cancer care in developing countries. World J Surg. 2009;33:2069–76. doi: 10.1007/s00268-009-0150-z. [DOI] [PubMed] [Google Scholar]
- 6.Two-Year Report of the Population Based Cancer Registries 2004-2005, Incidence and Distribution of Cancer. 2008 [Google Scholar]
- 7.Yeole BB, Jayant K, Jussawalla DJ. Trends in breast cancer incidence in greater Bombay: An epidemiological assessment. Bull World Health Organ. 1990;68:245–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Screening for Breast Cancer. Geneva: World Health Organization; 2009. [Last accessed on 2015 Jun 28]. Available from: http://www.who.int/cancer/detection/breastcancer/en/index.html . [Google Scholar]
- 9.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: An independent review. Br J Cancer. 2013;108:2205–40. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. U.S. Preventive Services Task Force. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–37. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson BT, LoRusso AP, Smolkin M, Bovbjerg VE, Petroni GR, Harvey JA. Accuracy of assigned BI-RADS breast density category definitions. Acad Radiol. 2006;13:1143–9. doi: 10.1016/j.acra.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–26. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 13.Smith RA, Cokkinides V, Brooks D, Saslow D, Shah M, Brawley OW. Cancer Screening in the United States, 2011 A Review of Current American Cancer Society Guidelines and Issues in Cancer Screening. CA Cancer J Clin. 2011;61:8–30. doi: 10.3322/caac.20096. [DOI] [PubMed] [Google Scholar]
- 14.Johns LE, Moss SM. Age Trial Management Group. False-positive results in the randomized controlled trial of mammographic screening from age 40 (“Age” trial) Cancer Epidemiol Biomarkers Prev. 2010;19:2758–64. doi: 10.1158/1055-9965.EPI-10-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park B, Choi KS, Lee YY, Jun JK, Seo HG. Trends in cancer screening rates among Korean men and women: Results from the Korean National Cancer Screening Survey (KNCSS), 2004-2011. Cancer Res Treat. 2012;44:113–20. doi: 10.4143/crt.2012.44.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilaweera RI, Perera S, Paranagama N, Anushyanthan AS. Knowledge and practices on breast and cervical cancer screening methods among female health care workers: A Sri Lankan experience. Asian Pac J Cancer Prev. 2012;13:1193–6. doi: 10.7314/apjcp.2012.13.4.1193. [DOI] [PubMed] [Google Scholar]
- 17.Ewertz M, Duffy SW, Adami HO, Kvåle G, Lund E, Meirik O, et al. Age at first birth, parity and risk of breast cancer: A meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 1990;46:597–603. doi: 10.1002/ijc.2910460408. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Closas M, Brinton LA, Lissowska J, Chatterjee N, Peplonska B, Anderson WF, et al. Established breast cancer risk factors by clinically important tumour characteristics. Br J Cancer. 2006;95:123–9. doi: 10.1038/sj.bjc.6603207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson HD, Zakher B, Cantor A, Fu R, Griffin J, O'Meara ES, et al. Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis. Ann Intern Med. 2012;156:635–48. doi: 10.1059/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinton LA, Hoover R, Fraumeni JF., Jr Reproductive factors in the aetiology of breast cancer. Br J Cancer. 1983;47:757–62. doi: 10.1038/bjc.1983.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–95. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 22.Tretli S. Height and weight in relation to breast cancer morbidity and mortality. A prospective study of 570,000 women in Norway. Int J Cancer. 1989;44:23–30. doi: 10.1002/ijc.2910440105. [DOI] [PubMed] [Google Scholar]
- 23.Broca PP. Traité Des Tumeurs. Paris: Asselin; 1866. [Google Scholar]
- 24.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: A systematic review and meta-analysis. Int J Cancer. 1997;71:800–9. doi: 10.1002/(sici)1097-0215(19970529)71:5<800::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Goldgar DE, Stratton MR, Eeles RA. Familial breast cancer. In: Eeles RA, Ponder BA, Easton DF, Horwich A, editors. Genetic Predisposition to Cancer. London: Chapman and Hall; 1996. pp. 227–38. [Google Scholar]
- 26.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237:474–82. doi: 10.1097/01.SLA.0000059969.64262.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 1.Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ. 1992;147:1459–76. [PMC free article] [PubMed] [Google Scholar]
- 28.Kösters JP, Gøtzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003;2:CD003373. doi: 10.1002/14651858.CD003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton MB, Harris R, Fletcher SW. The rational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: Should it be done? How? JAMA. 1999;282:1270–80. doi: 10.1001/jama.282.13.1270. [DOI] [PubMed] [Google Scholar]
- 30.McDonald S, Saslow D, Alciati MH. Performance and reporting of clinical breast examination: A review of the literature. CA Cancer J Clin. 2004;54:345–61. doi: 10.3322/canjclin.54.6.345. [DOI] [PubMed] [Google Scholar]
- 31.Oestreicher N, White E, Lehman CD, Mandelson MT, Porter PL, Taplin SH. Predictors of sensitivity of clinical breast examination (CBE) Breast Cancer Res Treat. 2002;76:73–81. doi: 10.1023/a:1020280623807. [DOI] [PubMed] [Google Scholar]
- 32.Tabàr L, Fagerberg G, Duffy SW, Day NE, Gad A, Gröntoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am. 1992;30:187–210. [PubMed] [Google Scholar]
- 33.Frisell J, Eklund G, Hellström L, Lidbrink E, Rutqvist LE, Somell A. Randomized study of mammography screening – Preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat. 1991;18:49–56. doi: 10.1007/BF01975443. [DOI] [PubMed] [Google Scholar]
- 34.WHO Position Paper on Mammography Screening. 2014. Dec, [Last accessed on 2015 Jun 28]. Available from: http://www.who.int/cancer/publications/mammography_screening/en/
- 35.Meeta, Digumarti L, Agarwal N, Vaze N, Shah R, Malik S. Clinical practice guidelines on menopause: An executive summary and recommendations. J Midlife Health. 2013;4:77–106. doi: 10.4103/0976-7800.115290. [DOI] [PMC free article] [PubMed] [Google Scholar]


