Abstract
Aim:
To study the prevalence of cardiac autonomic neuropathy (CAN) in patients with Type 2 diabetes mellitus at high risk for foot ulcers.
Materials and Methods:
We screened patients attending diabetic clinic for identifying patients at high risk for foot ulcers. Those with foot risk category 1, 2 and 3 as per criteria of Foot Care Interest Group were subjected to battery of cardiovascular autonomic reflex tests. Those with one abnormal test were termed as probable CAN and those with two abnormal tests as definite CAN. Those with postural fall in blood pressure with one other abnormal test were termed to have advanced CAN.
Results:
A total of 74 patients were recruited in the study. The prevalence of abnormal cardiovascular autonomic reflex test was sustained hand grip 81%, E/I ratio 66.2%, 30:15 ratio 28.3% and orthostatic hypotension 13.5%. The prevalence of possible CAN was 31.0% (23/74) and definite CAN was 66.2% (49/74). Ten patients had advanced CAN. There was no observable difference in presence of probable or definite CAN in three risk category for foot ulcers.
Conclusion:
We found a high prevalence of CAN in subgroup of diabetic patients at increased risk for foot ulcer.
Keywords: Cardiac autonomic neuropathy, diabetes, foot ulcers
INTRODUCTION
Diabetic autonomic neuropathies are a heterogeneous group of complications occurring due to wide spread damage of autonomic nerves as a result of sustained hyperglycemia and resulting metabolic derangements. Cardiac autonomic neuropathy (CAN) is described as impairment of autonomic nerve fibers that innervate the heart and blood vessels resulting in abnormalities of heart rate control and vascular dynamics.[1] The presence of CAN increases the risk for severe hypoglycemia, silent myocardial ischemia, stroke, peroperative morbidity, and mortality even in minor surgical procedures'.[1,2,3] The CAN subcommittee of Toronto consensus panel on diabetic neuropathy suggests screening for CAN in asymptomatic Type 2 diabetic patients at diagnosis and Type 1 diabetic patients with disease duration ≥5 years who are at high risk. Patients at higher risk for CAN are those with poor glycemic control (HbA1c >7%), presence of one or more major cardiovascular risk factors (hypertension, dyslipidemia, smoking) or the presence of macrovascular and microvascular complications. The panel recommends diagnosis of CAN be based on the use of cardiovascular autonomic reflex tests such as heart response to breathing, standing, Valsalva maneuver, and blood pressure response to standing. Toronto consensus panel does not mention which patients are most likely to benefit from screening for due to the lack of sufficient data.[4]
The lifetime risk of a diabetic patient to develop foot ulcer is estimated to be 5–7%.[5] Patients with diabetes are ten times more likely to undergo lower extremity amputations as compared to those without and the risk increases further in those with foot ulcers.[6] According to a systematic review, the mortality after the onset of a diabetic foot ulcer is estimated to be 40% which is mainly attributed to heightened risk of cardiovascular disease.[7]
The prevalence of CAN in patients of diabetes with foot at risk for ulcer is not known despite the fact that this subgroup is more likely to undergo surgical intervention and have a heightened risk of cardiovascular disease.
MATERIALS AND METHODS
Study design
Patients of Type 2 diabetic mellitus undergo an annual foot examination at our clinic as outlined by the task force of the Foot Care Interest Group.[8] Key components of foot examination include history of foot ulceration and amputations, inspection of the foot for changes over skin and deformities of the foot. Neurological assessment is performed to look for the loss of protective sensation (LOPS) including 10 g monofilament test, vibration using 128 Hz tuning fork, pinprick sensation, and presence of ankle reflexes. LOPS is deemed to be present if one or more test is abnormal. Peripheral arterial disease (PAD) is deemed to be present if Ankle Brachial Pressure Index is < 0.8. After examination the patient are categorized as foot risk category 0 (no LOPS, PAD or deformity), 1 (LOPS ± deformity), 2 (PAD ± LOPS) and 3 (history of ulcer or amputation).[8] Higher the risk category higher is the risk for ulceration, hospitalization, and amputation.[9]
After an informed consent patients with foot risk category 1, 2, and 3 from March 2014 to February 2015 were recruited to undergo evaluation for the presence of CAN. The patients demographic details, medical, diabetic history, and results of laboratory investigations (HbA1c, low-density lipoprotein-cholesterol, serum creatinine) were noted. We estimated glomerular filtration rate (eGFR) by Modification of Diet in Renal Disease formula. Patients were excluded if they had the presence of cardiac arrhythmias, known coronary artery disease, thyroid disease (hyperthyroidism/hypothyroidism), poor glycemic control, severe systemic disease (cardiac, pulmonary, renal, malignancy), proliferative diabetic retinopathy, and other likely etiology for neuropathy (alcohol, neurotoxic medications). Those who had history of hypoglycemia in preceding 24 h before testing and those on medications likely to affect functioning of autonomic system (antiarrhythmics, antidepressants, antihistaminic, cough preparations) were also excluded from the study.
We assessed for CAN by performing a battery of four standard tests.[9,10]
E/I ratio: Each participant was asked to take deep breaths at a rate of six breaths per minute. During this deep breathing, the shortest R-R intervals during inspiration (I) and the longest R-R intervals during expiration (E) were measured. The mean values were calculated from the six inspirations and expirations, and the E/I ratio was calculated by dividing the mean value for the longest R-R intervals during expiration by the mean value for the shortest R-R intervals during inspiration. An E/I ratio below the age-related reference value age 20–24 years, 1.17; 25–29, 1.15; 30–34, 1.13; 35–39, 1.12; 40–44, 1.10; 45–49,1.08; 50–54, 1.07; 55–59, 1.06; 60–64, 1.04; 65–69, 1.03; and 70–75, 1.02) was scored as abnormal
30:15 ratio: Each participant was asked to rise from a supine to standing position. The ratio of the R-R interval measured at beat 30 to the R-R interval at beat 15 was measured. A 30/15 ratio below 1.03 was marked as abnormal
Systolic blood pressure (SBP) response to standing: Blood pressure was measured in the participant in supine position. The participant was asked to rise from a supine to standing position and the SBP was measured after 2 min. A fall of 30 mmHg was marked as abnormal
Diastolic blood pressure (DBP) response to sustained hand grip: The maximum voluntary contraction was first determined using a handgrip dynamometer. Handgrip was then maintained at 30% of that maximum for 5 min. Blood pressure was measured three times before and at 1-min intervals during handgrip. The result was expressed as the difference between the highest DBP during handgrip exercise and the DBP reading before handgrip began. A difference of ≤ 15 mmHg was marked as abnormal.
The presence of one abnormal cardiovascular autonomic reflex test result was termed as possible CAN, two abnormal tests was termed as definite CAN and presence of orthostatic hypotension in addition to one other abnormal test was termed as advanced CAN.[4]
Ethics committee
The study was approved by the institutional committee of the hospital and was conducted in accordance with Declaration of Helsinki. All patients signed written consent and approved the data collection for the study purposes.
Statistical analysis
The results are presented in mean ± standard deviation and percentages. The categorical variables were compared using Chi-square test. The continuous variables were compared by using unpaired t-test. The P < 0.05 was considered statistically significant. All the analysis was carried out using SPSS 16.0 version (SPSS Inc, Chicago, Illinois, USA).
RESULTS
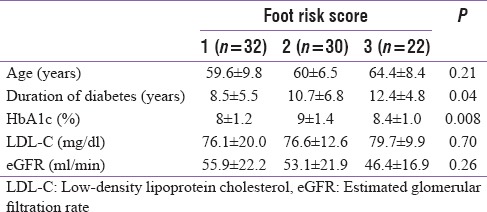
A total of 74 patients (male = 44, female = 30) participated in the study. Mean age of the patients was 61.1 ± 8.1 years (range 41–88) and duration of diabetes was 10.2 ± 5.8 years. The distribution of foot risk category 1, 2, and 3 in the participants was 32 (43.2%), 20 (27.0%) and 22 (29.7%), respectively. The mean duration of diabetes, HbA1c and eGFR values in each risk category is depicted in Table 1. Those with risk category 3 had longer duration of diabetes as compared with those with risk category 1.
Table 1.
Baseline data of patients according to foot risk category
The prevalence of abnormal cardiovascular autonomic reflex test was sustained hand grip 81%, E/I ratio 66.2%, 30:15 ratio 28.3% and orthostatic hypotension 13.5%. The prevalence of possible CAN was 31.0% (23/74) and definite CAN was 66.2% (49/74). Only two patients had no abnormality in cardiac autonomic function tests. The presence of probable and definite CAN in various foot risk category is depicted in Table 2.
Table 2.
Relationship between foot at risk and presence of cardiac autonomic neuropathy

The presence of advanced CAN (postural hypotension and one other abnormal test) was seen in 10 patients. The mean duration of diabetes in these patients was 14.7 ± 7.4 years. Number of patients with foot risk category 1, 2, and 3 in patients with advanced CAN was 3, 2, and 5 respectively.
DISCUSSION
We studied the prevalence of CAN in a subset of patients with diabetes at higher risk for developing foot ulcers. The prevalence of possible CAN and definite CAN in patients with newly diagnosed Type 2 diabetes has been reported to be 15.3% and 1.8%.[11] Low et al. studied the prevalence of autonomic impairment in a mixed population of patients with Type 1 and 2 diabetes. In patients with Type 2 diabetes mellitus the mean age and duration of disease was 64.1 ± 11.7 and 15.3 ± 8.6 years. Abnormal cardiovagal and sympathetic adrenergic tests (>2 tests abnormal) were present in 44% and 16% respectively.[12] Valensi et al. studied the association of CAN in a mixed population of Type 1 and 2 diabetes. They found the prevalence of moderate CAN (2 abnormal tests) and severe CAN (3 abnormal tests) to be 16.1% and 3.9% in the patients, respectively. They found the prevalence to be higher in those with longer duration of diabetes and those with retinopathy.[13] The studies indicate prevalence of CAN increases with age of patient and duration of diabetes indicating as expected a role for sustained hyperglycemia in development of CAN.
In the EURODIAB study, patients with preexisting Type 1 diabetes (age at baseline 31.3 ± 8.9 years, duration of diabetes 13.5 ± 8.3 years) with no CAN at baseline were followed up for 7.3 ± 0.6 years. During follow up, CAN developed in 17% of patients. The presence of cardiovascular disease and distal sensory polyneuropathy at baseline were strong predictors for development of CAN.[14] In our study, the mean age and duration of diabetes was 61.1 ± 8.1 years and 10.2 ± 5.8 years. Our patients were younger in age and with lesser duration of diabetes as compared to western patients. The demographic pattern of younger age of onset, lack of good glycemic control, and increased the occurrence of vascular complications in patients of diabetes in India has been described.[15] The prevalence of possible CAN and definite CAN (31% and 66.2%) in our subjects helps identify a subset of diabetic patients with the highest likelihood of presence of CAN. Diabetic foot ulcer result from a complex interplay of distal sensory polyneuropathy, peripheral autonomic neuropathy, and peripheral vascular disease.[16] EURODIAB study demonstrated distal sensory polyneuropathy as a strong predictor for development of CAN.[14] Hence it may not appear surprising that we had a large number of patients with CAN.
Toronto consensus panel has proposed screening of CAN for helping tailor therapeutic strategy, evaluation of perioperative anesthetic risk and for risk stratification before screening for CAD; most are level B or C recommendations. There are no studies which help identify patients who might benefit most with screening for CAN nor any assessing the cost effectiveness of testing for CAN.[4] Individuals with foot at risk for ulceration are at increased risk for cardiovascular events and mortality and also are more likely to undergo surgical procedures. A simple bedside examination helps stratify foot risk category and our study demonstrates they are likely to benefit most by screening for CAN.
The limitation of our study is the small sample size and lack of data to assess the strength of association of CAN with microvascular complications such as nephropathy and retinopathy.
CONCLUSION
Patients with Diabetes and Foot at risk for ulceration have high prevalence of CAN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4:4–18. doi: 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yun JS, Kim JH, Song KH, Ahn YB, Yoon KH, Yoo KD, et al. Cardiovascular autonomic dysfunction predicts severe hypoglycemia in patients with type 2 diabetes: A 10-year follow-up study. Diabetes Care. 2014;37:235–41. doi: 10.2337/dc13-1164. [DOI] [PubMed] [Google Scholar]
- 3.Kadoi Y. Anesthetic considerations in diabetic patients. Part I: Preoperative considerations of patients with diabetes mellitus. J Anesth. 2010;24:739–47. doi: 10.1007/s00540-010-0987-1. [DOI] [PubMed] [Google Scholar]
- 4.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–53. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–7. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 6.Siitonen OI, Niskanen LK, Laakso M, Siitonen JT, Pyorala K. Lower-extremity amputations in diabetic and nondiabetic patients: a population-based study in Eastern Finland. Diabetes Care. 1993;16:16–20. doi: 10.2337/diacare.16.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int Wound J. 2016;13:892–903. doi: 10.1111/iwj.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679–85. doi: 10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–8. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28:956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 11.Zoppini G, Cacciatori V, Raimondo D, Gemma M, Trombetta M, Dauriz M, et al. Prevalence of cardiovascular autonomic neuropathy in a cohort of patients with newly diagnosed type 2 diabetes: The Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) Diabetes Care. 2015;38:1487–93. doi: 10.2337/dc15-0081. [DOI] [PubMed] [Google Scholar]
- 12.Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, et al. Autonomic symptoms and diabetic neuropathy: A population-based study. Diabetes Care. 2004;27:2942–7. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 13.Valensi P, Pariès J, Attali JR. French Group for Research and Study of Diabetic Neuropathy. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications – The French multicenter study. Metabolism. 2003;52:815–20. doi: 10.1016/s0026-0495(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 14.Witte DR, Tesfaye S, Chaturvedi N, Eaton SE, Kempler P, Fuller JH. EURODIAB Prospective Complications Study Group. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48:164–71. doi: 10.1007/s00125-004-1617-y. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran A, Snehalatha C. Current scenario of diabetes in India. J Diabetes. 2009;1:18–28. doi: 10.1111/j.1753-0407.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 16.Boulton AJ. The pathway to foot ulceration in diabetes. Med Clin North Am. 2013;97:775–90. doi: 10.1016/j.mcna.2013.03.007. [DOI] [PubMed] [Google Scholar]


