Abstract
Background and Objectives:
Insulin resistance (IR) is frequent in human immunodeficiency virus (HIV) infection and may be related to antiretroviral therapy (ART). Increased oxidative stress parameters and carbonyl protein are linked to insulin sensitivity. The present study is aimed to determine IR, its association with oxidative deoxy nucleic acid (DNA) damage in HIV-1-infected patients with different ART status.
Materials and Methods:
In this case–control study, a total 600 subjects were included. We used plasma levels of the oxidized base, 8-hydroxy-2-deoxyguanosine (8-OHdG), as our biomarker of oxidative DNA damage. 8-OHdG was measured with the highly sensitive 8-OHdG check enzyme-linked immunosorbent assay kit. IR was determined using homeostasis model assessment.
Results:
All subjects were randomly selected and grouped as HIV-negative (control group) (n = 300), HIV-positive without ART (n = 100), HIV-positive with ART first line (n = 100), and HIV-positive with ART second line (n = 100). IR and oxidative DNA damage were significantly higher in HIV-positive patients with second-line ART and HIV-positive patients with first-line ART than ART-naive patients. In a linear regression analysis, increased IR was positively associated with the increased DNA damage (odds ratio: 3.052, 95% confidence interval: 2.595–3.509) P < 0.001.
Interpretation and Conclusions:
In this study, we observed that ART plays a significant role in the development of IR and oxidative DNA damage in HIV-positive patients taking ART. Awareness and knowledge of these biomarkers may prove helpful to clinicians while prescribing ART to HIV/AIDS patients. Larger studies are warranted to determine the exact role of ART in the induction of IR and DNA damage.
Keywords: 8-hydroxy-2-deoxyguanosine, deoxy nucleic acid damage, human immunodeficiency virus infection, insulin resistance
INTRODUCTION
The AIDS epidemic continues to spread in the SouthEast Asia (SEA) Region. The SEA is the second most affected WHO region in the world, after Sub-Saharan Africa. To date, close to 40 million people throughout the world have been infected with human immunodeficiency virus (HIV). Of these, almost 6.4 million are in the SEA Region. Over 99% of cases have been reported from four countries – Thailand, India, Indonesia, and Myanmar.[1] Combination antiretroviral therapy (ART) has positively modified the natural history of HIV infection, leading to a significant reduction in morbidity and mortality. However, long-term toxicity is becoming recognized, treated patients are at a significant risk for a number of diseases typically associated with older age, including insulin resistance (IR), cardiovascular disease, osteoporosis, cancer, neurocognitive impairment, and frailty, particularly when therapy contains protease inhibitors (PIs).[1,2,3,4,5]
However, a growing body of evidence suggests that up to 60% of treated patients develop a complex of metabolic alterations, to varying degrees, which is emerging as a significant medical concern. These alterations include fat tissue redistribution (peripheral lipodystrophy and increased central adiposity), dyslipidemia, and systemic IR. The last is characterized clinically by elevated fasting insulin and/or C-peptide levels and was confirmed using euglycemic–hyperinsulinemic clamp studies. The clinical significance of these side effects is the exposure of patients to an apparent increased risk for developing premature cardiovascular morbidity, as well as to new-onset or aggravated type 2 diabetes.[6,7,8] Although metabolic alterations have also been described in (HIV PI[HPI]) HPI sparing highly active antiretroviral therapy (HAART) regimens, an increasing amount of data attributes a role for this class of drugs in the induction of IR.[9,10,11,12] HIV-positive subjects receiving HPI became insulin-resistant even in the absence of changes in body composition.[1,13] PIs acutely and reversibly inhibit the insulin-responsive glucose transporter Glut 4, leading to peripheral IR and impaired glucose tolerance.[3,6,14]
The drugs implicated include nucleoside reverse transcriptase inhibitors (major components of first-line therapy in India) and also PIs (components of current second-line therapy in India). In India, where there is a genetic predisposition to IR and cardiovascular risk, this impact may be significant.[5,15,16,17,18,19,20,21] IR plays a key role in the pathogenesis of the metabolic syndrome and is frequently detected among HIV patients on ART.[22,23,24] Biochemistry of IR: pancreatic b-cells release insulin in response to hyperglycemia, stimulating muscle and adipose tissue to store sugar, and blocking the liver from releasing glucose into circulation. IR occurs when glucose uptake is insufficiently stimulated and hepatic glucose release is permitted. Persistent hyperglycemia results with the eventual development of Type 2 diabetes mellitus. The estimated incidence of glucose metabolism disorders in the HIV-infected population is between 2% and 25% with a 2.2-fold increase in the relative risk of Type 2 diabetes mellitus.[6,25,26]
Oxidative damage results from biochemical interactions between reactive oxygen species (ROS) and target biomolecules. ROS can damage nucleic acids, lipids, and proteins; this damage figures prominently in the etiology and progression of numerous cancers as well as coronary and carotid atherosclerosis. Although many damaged deoxy nucleic acid (DNA) lesions have been identified, we have chosen 8-hydroxy-2-deoxyguanosine (8-OHdG) as our biomarker of oxidative damage.[27] The importance of this lesion stems from the fact that it is both abundant in DNA and it is mutagenic. The current evidence suggests that 8-OHdG lesions present in DNA during cellular replication result in somatic mutation, the driving force behind carcinogenesis.[28,29]
MATERIALS AND METHODS
Subject selection
A case–control study was carried out on HIV-1-infected patients at the Outpatient Infectious Disease Unit (OPD) and ART center of the Sir J J Hospital and Grant Government Medical College, Mumbai, over a period of 1 year, from February 2014 to March 2015. We have selected 300 subjects from OPD after evaluation of their medical records (negative serial enzyme-linked immunosorbent assay [ELISA]/Western blot for HIV before 3 months of sample collection) as HIV-negative controls and 300 HIV-positive subjects detected by serial ELISA/Western blot method from ART center. We used power analysis method for sample selection at 5% significance level for 95% confidence.
Ethical approval
The protocol study was approved by the Institutional Ethics Committee (No. IEC/Pharm/902/2013) and the National AIDS Control Organisation Delhi, India (T-11020/67/2011-NACO).
Inclusion criteria
All participants were 20 years of age or older HIV-positive patients detected by serial ELISA/Western blot method and were included in this study after getting their informed consent. The family history of all subjects was recorded, and the subjects without any diabetes history were chosen. Normal control HIV-negative subjects (n = 300), who are negative to ELISA/Western blot test for HIV before 3 months of sample collection, were selected from the outpatient department of Sir J J Group of Hospitals, Mumbai, Maharashtra, India.
Exclusion criteria
Exclusion criteria included pregnant women, patients with chronic diseases such as hepatitis, diabetes or family history of diabetes, renal impairment, cardiovascular comorbidities, neurological psychiatric disorders, various malignancies, as well as heavy smokers, alcoholics, and tobacco-chewers, and HIV patients with the withdrawal of combination ART. The demographic details were collected from each patient and entered into the pro forma. Subsequent to this, a detailed history was taken.
Sample collection
Venous blood samples were collected in plain and lithium heparin vacutainers as an anticoagulant. Blood was centrifuged (4000 g, 10 min, 4°C) to separate the plasma. The collected plasma was stored at −70°C with aseptic precautions. Plain blood samples 2 h after collection were centrifuged at 3000 rpm for 5 min; serum was separated and collected in sterile tubes.
Different treatment regimens as per the National AIDS Control Organisation guidelines
The list of ART administered to Indian HIV-1 patients is As shown in Table 1.
Table 1.
List of Antiretroviral Therapy used in HIV/AIDS patients

Biochemical methods
Determination of blood glucose
Fasting blood glucose levels were estimated using the hexokinase (enzymatic) method spectrophotometrically in R × L dimension automated equipment.
Determination of fasting insulin
Fasting insulin was determined using Immulite 1000 Insulin kit from Siemens using chemiluminescence. We used the homeostasis model assessment-IR (HOMA-IR) index,[16,30] first described by Matthews et al. in 1985 to determine IR. The formula used to calculate HOMA-IR was:
HOMA-IR = Fasting insulin (mU/L) × fasting plasma glucose (mmol/L)/22.5
We assumed an HOMA-IR value of >2.8 to define IR, from previous studies on IR.[26]
Determination of deoxy nucleic acid damage marker 8-hydroxy-2-deoxyguanosine
We used plasma levels of the oxidized base, 8-OHdG, as our biomarker of oxidative damage.[27] 8-OHdG was measured with the highly sensitive 8-OHdG check ELISA kit (StressXpress ELA Kit). StressMarq's 8-OHdG ELA is a competitive assay that can be used for the quantification of 8-OHdG in urine, cell culture, plasma, and saliva. The ELA utilizes an anti-mouse IgG-coated plate and tracer consisting of an 8-OHdG enzyme conjugate. It is important to note that the OHdG antibody used in this assay recognizes both free 8-OHdG and DNA-incorporated 8-OHdG. Since samples such as plasma, cell lysates and tissues contains mixtures of DNA incorporated 8 OHdG fragments and free 8 OHdG. The assay is based on the competition between 8-OHdG and an 8-OHdG-acetylcholinesterase (AChE) conjugate (8-OHdG tracer) for a limited amount of 8-OHdG monoclonal antibody. Because the concentration of 8-OHdG tracer is held constant while the concentration of 8-OHdG varies, the amount of 8-OHdG tracer that can bind to the 8-OHdG monoclonal antibody will be inversely proportional to the concentration of 8-OHdG in the well. This antibody-8-OHdG complex binds to goat polyclonal anti-mouse IgG that is previously attached to the well. The plate is washed to remove any unbound reagent and then Ellman's Reagent (which contains the substrate to AChE) is added to the well. The product of this enzymatic reaction has a distinct yellow color and absorbs strongly at 412 nm. The intensity of this color, determined spectrophotometrically, is proportional to the amount of 8-OHdG tracer bound to the well, which is inversely proportional to the amount of free 8-OHdG present in the well during the incubation. Procedures were followed as manufacturer's instructions.
Preparation of data
Average absorbance reading of the Non-Specific Binding (NSB) well and average absorbance reading of B0 wells were determined. Then, we subtracted the average NSB readings from average B0 readings and calculated %B/B0 (% of Sample or Standard Bound/Maximum Bound). Then, we obtained a standard curve plot %B/B0 for standards using 4-parameter logistic equations. The sample concentration was determined using above equation.
Statistical methods
Student's t-test was performed to assess differences between two means. Statistical analysis was performed using Epi-Info 7 statistical software for medical research studies (Atlanta, Georgia, USA). Differences in means between groups were tested using independent-sample t-tests. We calculated the 95% confidence interval (CI) for the difference between the population means. Mann–Whitney test for nonparametric continuous variables, Chi-square or Fisher's exact test for categorical variables were used to test for statistical significance. We used linear regression method for correlation of IR and DNA damage marker 8-OHdG.
RESULTS
A total of 600 (300 HIV-1 Positive and 300 HIV Negative) subjects were included in this study. All the subjects were divided into two age groups of 20–40 years and 40–60 years.
Insulin resistance
IR was significantly higher in HIV-1-positive patients with ART than HIV-negative subjects. In this study, IR was measured by HOMA-IR that is homeostatic model assessment IR index. The mean HOMA for HIV-positive patients was higher than that for HIV-negative subjects. The mean HOMA for HIV-positive and ART not started in the age group of 20–40 years was 2.33 and in the age group of 40–60 years was 2.90 (P < 0.01). The mean HOMA for HIV-positive in both age groups of 20–40 years and 40–60 years with ART (first line) were 5.79 and 8.78 and (second line) 7.00 and 8.10 (P < 0.01).
The prevalence of IR in ART-naive HIV-positive patients with the age group of 20–40 years was 11 (22%) and in the age group of 40–60 years was 19 (38%). The IR was significant in HIV-positive patients in both age groups of 20–40 years and 40–60 years with ART (first line) were 27 (54%) and 34 (68%) and (second line) 31 (62%) and 35 (70%) [Figure 1]. These results show there was an increase in IR in HIV-positive patients with ART than HIV-negative subjects.
Figure 1.
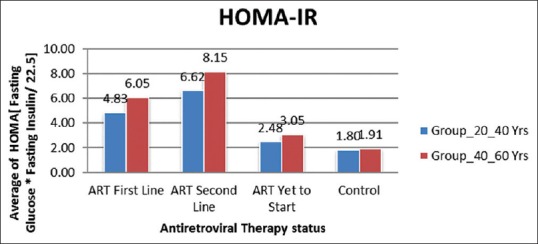
Average HOMA-IR in Group 20–40 years and Group 40–60 years
Deoxy nucleic acid damage marker: 8-hydroxy-2- deoxyguanosine
DNA damage was significantly higher in HIV-1-positive patients with ART than HIV-negative subjects. In this study, DNA damage marker 8-OHdG was measured by ELISA. The mean 8-OHdG for HIV-positive patients was higher than that for HIV-negative subjects. The mean 8-OHdG for HIV-positive and ART not started in the age group of 20–40 years was 1.81 ng/ml and in the age group of 40–60 years was 1.95 ng/ml (P < 0.01). The mean 8-OHdG for HIV-positive in both age groups of 20–40 years and 40–60 years with ART (first line) were 2.50 ng/ml and 3.13 ng/ml and (second line) 2.79 ng/ml and 3.27 ng/ml (P < 0.01). ART accelerates DNA damage in HIV-positive patients [Figure 2]. These results show there was an increase in DNA damage in HIV-positive patients with ART.
Figure 2.
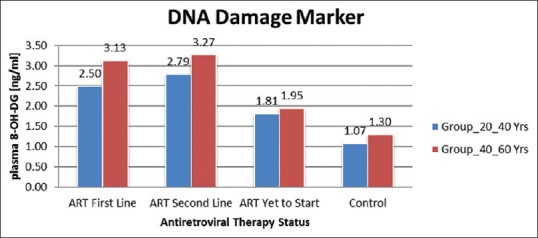
Average DNA damage marker 8 OHdG Group 20–40 years and Group 40–60 years
Correlation of homeostasis model assessment-insulin resistance and deoxy nucleic acid damage marker
In this study, we used linear regression method for the correlation of IR with DNA damage. We observed that increased IR was positively associated with the increased DNA damage (odds ratio: 3.052, 95% CI: 2.595–3.509) P < 0.001. Increased mean IR values were observed in HIV-positive patients with ART first line and second line in both age groups (20–40 years and 40–60 years) than ART-naive, and controls similar pattern was observed with DNA damage Marker 8-OHdG in HIV-positive patients with ART first line and second line in both age groups (20–40 and 40–60 years).
DISCUSSION
As HIV-positive people survive longer, they are at increased risk for a variety of non-AIDS conditions even when their CD4 cell counts are high. IR has been increasingly recognized in HIV-infected individuals since the introduction of effective ART.[13,26,31] IR (impaired insulin action) contributes many disease processes. These include type 2 diabetes and cluster of cardiovascular risk factors known as “metabolic syndrome,” as well as polycystic ovarian syndrome, nonalcoholic fatty liver disease.[26,31] Adipose tissue is a key target for ART in inducing IR, as the metabolic syndrome described in patients taking HAART consists of gross alterations in fat tissue distribution and dyslipidemia, including elevated circulating free fatty acid and glycerol levels. Adipose tissue is now believed to play a major role in the pathogenesis of systemic IR. In particular, free fatty acid and various adipose-derived peptides (adipokines) have been identified as modulators of whole-body insulin sensitivity. The systemic IR seen in patients taking HAART also involves impaired insulin responsiveness in skeletal muscle and liver.[32] In addition, PI use among individuals from developed countries, usually older males, has been associated with the development of diabetes and hypertriglyceridemia.
In the pre cART era, several cross sectional studies reported slightly increased or normal insulin sensitivity when compared to uninfected controls.[33] ART introduction led to increases in fasting insulin and decreases in insulin sensitivity, effects dependent both on the class of the antiretroviral used and the different antiretrovirals within each class.[16,34,35,36,37,38]
As IR presence is associated with an increased risk of diabetes, cardiovascular events, stroke, and death, the reduction of its prevalence must be considered as an essential therapeutic target in HIV patients undergoing ART.
Under normal physiological conditions in all aerobic organisms, there is a balance maintained between endogenous oxidants and numerous enzymatic and nonenzymatic antioxidant defenses. When an imbalance occurs, oxidants produce extensive oxidative damage to DNA, which, in turn, contributes to aging, malignant tumors, and other degenerative diseases. In all living cells, damaged DNA is repaired enzymatically so that they regain their normal function, whereas misrepaired DNA can result in mutations (base substitution, deletions, and strand fragmentation) leading to carcinogenesis. Although a broad range of DNA products are produced during oxidative damage to DNA (bases and sugar modifications, covalent cross-links, single and double-stranded breaks), most interest focused on nucleobase modifications and especially on the abundant lesion of 8-oxo-2-deoxyguanosine because it is formed in vivo and can be measured quantitatively in cells following hydrolysis of the DNA to component bases.
That oxidative stress-induced DNA lesions may contribute to carcinogenesis is suggested by the increased cancer susceptibility of persons with a variety of chronic inflammatory diseases, such as ulcerative colitis, viral hepatitis, prostatitis, Helicobacter pylori infection, parasitic diseases, and others. In these diseases, cancer induction may be a pathological consequence of elevated ROS levels which lead to increased steady-state levels of oxidative DNA damage which in turn leads to a higher risk of mutations that may activate oncogenes or inactivate tumor suppressor genes.[23]
The presence of persistent DNA lesions in tumor cells has led many to propose that DNA damage markers are potential biomarkers for screening ongoing oxidative stress and for cancer diagnostics and prediction.[29]
In our study, we assessed IR and oxidative DNA damage marker 8-OHdG in the HIV-infected population treated with ART and compared them with those of untreated HIV patients and HIV-negative subjects. The important observation of our study was significantly increased IR and DNA damage in HIV-positive patients taking first line ART and second line ART than ART-naive and controls. [Figure 3]. Thus, there was a positive correlation between these two biomarkers in HIV-positive patients with ART. ART-induced increased oxidative stress may be the cause of the IR and DNA damage in HIV-positive patients. A better understanding of the mechanism of ROS-induced IR and DNA damage will help develop effective strategies to prevent and/or treat HIV infection.
Figure 3.
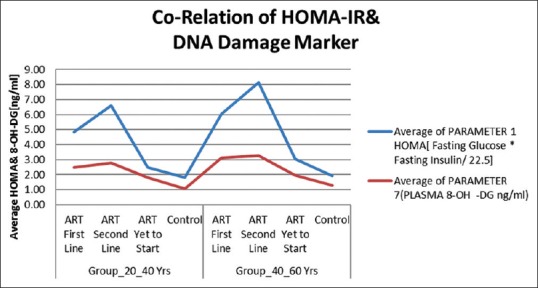
Correlation of HOMA-IR with DNA damage marker
Our study has some limitations as we could not do viral load and follow-up study because of limited time and funding source.
CONCLUSIONS
Although the substantial benefits of combination ART clearly outweigh the increase in cardiovascular risk associated with this therapy, it must be borne in mind that with progressive aging of the HIV-infected population and the expected long-term use of combination ART, the need will arise to prevent an increased incidence of IR in this population. Increased IR increases risk of type 2 diabetes and cardiovascular risk factors similarly increase in DNA damage increases the opportunity of various types of cancers. Hence, management of IR and DNA damage should be a prime concern for health-care physicians.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was supported by the National AIDS Control Organisation (NACO), Mumbai District AIDS Control Society (MDACS) and Department of Biochemistry, Department of Medicine and Antiretroviral Therapy Centre of Grant Government Medical College and Sir JJ Group of Hospitals, Mumbai.
REFERENCES
- 1.Vigouroux C, Gharakhanian S, Salhi Y, Nguyen TH, Chevenne D, Capeau J, et al. Diabetes, insulin resistance and dyslipidaemia in lipodystrophic HIV-infected patients on highly active antiretroviral therapy (HAART) Diabetes Metab. 1999;25:225–32. [PubMed] [Google Scholar]
- 2.van der Valk M, Bisschop PH, Romijn JA, Ackermans MT, Lange JM, Endert E, et al. Lipodystrophy in HIV-1-positive patients is associated with insulin resistance in multiple metabolic pathways. AIDS. 2001;15:2093–100. doi: 10.1097/00002030-200111090-00004. [DOI] [PubMed] [Google Scholar]
- 3.Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes. 2003;52:1695–700. doi: 10.2337/diabetes.52.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florescu D, Kotler DP. Insulin resistance, glucose intolerance and diabetes mellitus in HIV-infected patients. Antivir Ther. 2007;12:149–62. doi: 10.1177/135965350701200214. [DOI] [PubMed] [Google Scholar]
- 5.Mbunkah HA, Meriki HD, Kukwah AT, Nfor O, Nkuo-Akenji T. Prevalence of metabolic syndrome in human immunodeficiency virus – Infected patients from the South West region of Cameroon, using the adult treatment panel III criteria. Diabetol Metab Syndr. 2014;6:92. doi: 10.1186/1758-5996-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Li X, Cole SR, Kingsley LA, Palella FJ, Riddler SA, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–83. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 7.Ledergerber B, Furrer H, Rickenbach M, Lehmann R, Elzi L, Hirschel B, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45:111–9. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- 8.Rotger M, Gsponer T, Martinez R, Taffé P, Elzi L, Vernazza P, et al. Impact of single nucleotide polymorphisms and of clinical risk factors on new-onset diabetes mellitus in HIV-infected individuals. Clin Infect Dis. 2010;51:1090–8. doi: 10.1086/656630. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Romano R, Rudich A, Török D, Vanounou S, Riesenberg K, Schlaeffer F, et al. Agent and cell-type specificity in the induction of insulin resistance by HIV protease inhibitors. AIDS. 2003;17:23–32. doi: 10.1097/00002030-200301030-00005. [DOI] [PubMed] [Google Scholar]
- 10.Mondy KE, de las Fuentes L, Waggoner A, Onen NF, Bopp CS, Lassa-Claxton S, et al. Insulin resistance predicts endothelial dysfunction and cardiovascular risk in HIV-infected persons on long-term highly active antiretroviral therapy. AIDS. 2008;22:849–56. doi: 10.1097/QAD.0b013e3282f70694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV-infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serraino D, Bruzzone S, Zucchetto A, Suligoi B, De Paoli A, Pennazza S, et al. Elevated risks of death for diabetes mellitus and cardiovascular diseases in Italian AIDS cases. AIDS Res Ther. 2010;7:11. doi: 10.1186/1742-6405-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tien PC, Schneider MF, Cole SR, Levine AM, Cohen M, DeHovitz J, et al. Antiretroviral therapy exposure and insulin resistance in the Women's Interagency HIV study. J Acquir Immune Defic Syndr. 2008;49:369–76. doi: 10.1097/qai.0b013e318189a780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aukrust P, Luna L, Ueland T, Johansen RF, Müller F, Frøland SS, et al. Impaired base excision repair and accumulation of oxidative base lesions in CD4+ T cells of HIV-infected patients. Blood. 2005;105:4730–5. doi: 10.1182/blood-2004-11-4272. [DOI] [PubMed] [Google Scholar]
- 15.Vajpayee M, Mohan T. Current practices in laboratory monitoring of HIV infection. Indian J Med Res. 2011;134:801–22. doi: 10.4103/0971-5916.92627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheney L, Hou JC, Morrison S, Pessin J, Steigbigel RT. Nef inhibits glucose uptake in adipocytes and contributes to insulin resistance in human immunodeficiency virus type I infection. J Infect Dis. 2011;203:1824–31. doi: 10.1093/infdis/jir170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valcour V, Maki P, Bacchetti P, Anastos K, Crystal H, Young M, et al. Insulin resistance and cognition among HIV-infected and HIV-uninfected adult women: The Women's Interagency HIV Study. AIDS Res Hum Retroviruses. 2012;28:447–53. doi: 10.1089/aid.2011.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandina Ndona M, Longo-Mbenza B, Wumba R, Tandu Umba B, Buassa-Bu-Tsumbu B, Mbula Mambimbi M, et al. Nadir CD4+, religion, antiretroviral therapy, incidence of type 2 diabetes mellitus, and increasing rates of obesity among black Africans with HIV disease. Int J Gen Med. 2012;5:983–90. doi: 10.2147/IJGM.S32167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dusingize JC, Hoover DR, Shi Q, Mutimura E, Kiefer E, Anastos K. Associations of HIV infection with insulin and glucose levels in antiretroviral-naive Rwandan women: A cross-sectional analysis. BMJ Open. 2013;3:e003879. doi: 10.1136/bmjopen-2013-003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesfaye DY, Kinde S, Medhin G, Megerssa YC, Tadewos A, Tadesse E, et al. Burden of metabolic syndrome among HIV-infected patients in Southern Ethiopia. Diabetes Metab Syndr. 2014;8:102–7. doi: 10.1016/j.dsx.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Kagaruki GB, Mayige MT, Ngadaya ES, Kimaro GD, Kalinga AK, Kilale AM, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: A cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health. 2014;14:904. doi: 10.1186/1471-2458-14-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan S, Schouten JT, Atkinson B, Brown T, Wohl D, McComsey GA, et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J Acquir Immune Defic Syndr. 2012;61:381–9. doi: 10.1097/QAI.0b013e3182690e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gundurao Sreekantamurthy G, Singh NB, Singh TB, Singh TS, Singh KR. Study of body composition and metabolic parameters in HIV-1 male patients. J Nutr Metab. 2014;2014:498497. doi: 10.1155/2014/498497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jantarapakde J, Phanuphak N, Chaturawit C, Pengnonyang S, Mathajittiphan P, Takamtha P, et al. Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDS. 2014;28:331–40. doi: 10.1089/apc.2013.0294. [DOI] [PubMed] [Google Scholar]
- 25.Idiculla J, Ravindra'n GD, D'Souza J, Singh G, Furruqh S. Diabetes mellitus, insulin resistance, and metabolic syndrome in HIV-positive patients in South India. Int J Gen Med. 2011;4:73–8. doi: 10.2147/IJGM.S15818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arama V, Tiliscan C, Streinu-Cercel A, Ion D, Mihailescu R, Munteanu D, et al. Insulin resistance and adipokines serum levels in a Caucasian cohort of HIV-positive patients undergoing antiretroviral therapy: A cross sectional study. BMC Endocr Disord. 2013;13:4. doi: 10.1186/1472-6823-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 28.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2' deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 29.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki Sasaki R, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–5. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 31.Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Salmon-Ceron D, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26:303–14. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 32.Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158. doi: 10.1186/1471-2288-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adeyemi OM, Livak B, Orsi J, Glesby MJ, Villacres MC, Weber KM, et al. Vitamin D and insulin resistance in non-diabetic women's interagency HIV study participants. AIDS Patient Care STDS. 2013;27:320–5. doi: 10.1089/apc.2012.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitazawa T, Yoshino Y, Suzuki S, Koga I, Ota Y. Lopinavir inhibits insulin signaling by promoting protein tyrosine phosphatase 1B expression. Exp Ther Med. 2014;8:851–5. doi: 10.3892/etm.2014.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathi A, Liese AD, Jerrell JM, Zhang J, Rizvi AA, Albrecht H, et al. Incidence of diabetes mellitus in a population-based cohort of HIV-infected and non-HIV-infected persons: The impact of clinical and therapeutic factors over time. Diabet Med. 2014;31:1185–93. doi: 10.1111/dme.12455. [DOI] [PubMed] [Google Scholar]
- 36.Shikuma CM, Chow DC, Gangcuangco LM, Zhang G, Keating SM, Norris PJ, et al. Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS One. 2014;9:e90330. doi: 10.1371/journal.pone.0090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiozzo E, Konefal J, Adwan S, Martinez LA, Villabona J, Lopez J, et al. A cross-sectional assessment of metabolic syndrome in HIV-infected people of low socio economic status receiving antiretroviral therapy. Diabetol Metab Syndr. 2015;7:15. doi: 10.1186/s13098-015-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–80. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]


