Abstract
Introduction:
Somatic B-type Raf kinase (BRAF) V600E mutation in exon 15 was frequently found in high frequencies associated with papillary thyroid cancer (PTC). The phenotype of these cancers expressed aggressive clinical and pathological features. The present study aimed to assess the prevalence of BRAF V600E mutation among conventional and follicular variants of PTC and its association with aggressive tumor factors and outcome.
Study Design:
Patients who were operated and received further treatment for PTC during 2012 were included in the study. BRAF V600E mutation analysis was done by extracting genomic DNA from tumor tissue.
Results:
Of the 59 patients included in the study, 51% harbored BRAF V600E mutation, but the mutation status was not associated with aggressive tumor factors and adverse outcome.
Conclusion:
BRAF V600E mutation was not significant predictor of aggressive tumor behavior in conventional and follicular variants of PTC.
Keywords: B-type Raf kinase V600E mutation, differentiated thyroid cancer, genetic features of thyroid cancer, papillary thyroid cancer
INTRODUCTION
Somatic activating mutation in the gene for the B-type Raf kinase (BRAF) was found in melanomas initially and later in many other human cancers.[1] Somatic BRAF V600E mutation in exon 15 was described in papillary thyroid cancers (PTCs) in 2002, and later, numerous studies had consistently shown a high prevalence of BRAF mutation in thyroid cancer, ranging from 29% to 83%.[2,3] Genetic evidence indicates that thyroid cell transformation to papillary cancers takes place through constitutive activation of effectors along the RET/PTC-RAS-BRAF signaling pathway and BRAF V600E mutations are the most common abnormality in adult population. RET/PTC, RAS, or BRAF mutations have independent effect with no overlap among them.[2]
BRAF V600E mutations are specific for PTC and so genetic study is useful as a diagnostic tool in suspicious aspiration cytology. The prognostic relevance of the mutation was tested in many observational studies. The recent systematic reviews observed that PTC-harboring BRAF V600E mutations were associated with increased prevalence of high-risk histological features and adverse outcome.[3,4,5] It was observed that PTC-harboring mutation showed decreased sodium/iodide symporter function and hence decreased I131 utility in treatment options.[6] However, few single institutional studies with large sample size did not find any significant difference in tumor characteristics or outcome.[7,8]
PTC is generally indolent with 93% cause-specific 10-year survival. PTC includes a spectrum histological subtypes differing markedly in biological behavior. The aggressive subtypes of PTC include tall cell variant, columnar cell variant, diffuse sclerosing variant, and hobnail variant.[9] Conventional and follicular variants account for 86%–90% of all PTC. Most common of aggressive subtype is tall cell variant accounting for 1.3%–12% of all PTCs and has poor 5-year disease-specific survival of 81.9%.[10] Recent data support that tall cell histology alone remains a significant prognostic factor independent of conventional high-risk clinicopathological features.[11] The high expression of Muc1 and type IV collagenase (matrix metalloproteinase-2) in tall cell subtype may allow for degradation of stroma and greater invasive properties.[12] The tall cell variant shows the highest prevalence of BRAF V600E mutation.
A SEER database analysis showed an appreciable increase in incidences of aggressive variants such as diffuse sclerosing and tall cell variants.[13] The prevalence of BRAF V600E mutations is less frequent with other aggressive variants as 33% in columnar cell variant, 50% in Hobnail variant, and absent in diffuse sclerosing variant.[14,15,16]
Conventional and follicular variants account for more than 80% of PTC and have favorable outcome. The present study is designed to assess the biological behavior of conventional and follicular variants of PTC with somatic BRAF V600E mutation. We hypothesized that patients with conventional and follicular variants of PTC with somatic BRAF V600E mutation show significantly high rate of factors predictive of adverse outcome and so have significantly low rate of disease-free survival (DFS) despite adequate treatment.
Study design
Case records of differentiated thyroid cancer patients who underwent treatment during January 2012 to December 2012 were reviewed. Patients with cytological diagnosis of Bethesda V and VI and a histological diagnosis of conventional and invasive follicular variant of PTC were included in the study. Patients with papillary microcarcinoma, incidental cancers, and collision tumors were excluded from the study.
Outline of treatment
Total thyroidectomy was the primary surgical treatment, but therapeutic central compartment dissection was carried out when suspicious lymph nodes (LMs) were found. Therapeutic lateral neck node dissection was done based on sonological and cytological evidence.
Patients were further categorized based on risk stratification criterion (American Thyroid Association [ATA] guidelines; 2009).[17] Patients in intermediate- and high-risk category received radioiodine remnant ablation.
All patients were followed at 6 monthly intervals with stimulated thyroglobulin and thyroglobulin antibody estimation. Neck ultrasonography was done routinely; I131 whole-body scan and fluorodeoxyglucose positron emission tomography were done selectively. Patients who were diagnosed to have locoregional or distant diseases were treated with metastatic doses of I131 or operative procedures as the case may be.
B-type Raf kinase V600E mutation analysis
Genomic DNA was extracted from the tumor tissues using the QIAamp DNA FFPE tissue kit according to the manufacturer's protocol (Qiagen, Germany). The DNA obtained was quantified using the Multiskan GO Microplate Spectrophotometer (Thermo Scientific, USA). For BRAF V600E mutation analysis, a 223 bp region flanking the T1799A mutation of the BRAF gene was amplified with the primers: B-Raf15F (5' CATAATGCTTGCTCTGATAGGA-3') and B-Raf15R (5'-GCCAAAAATTTAATCAGTGGA-3') in a 50 μl PCR reaction solution using ~50 ng of the genomic DNA isolated from the tumor tissues as template and EmeraldAmp GT PCR Master Mix (Takara Bio Inc., Japan). The thermocycling conditions for the amplification were 1 denaturing cycle at 94°C for 5 min followed by 40 cycles of denaturing at 94°C for 30 s, annealing at 58°C for 1 min, and extension at 72°C for 1 min. Final extension was at 72°C for 5 min. The PCR products obtained were electrophoresed in a 2% agarose gel to assess PCR efficacy and product size. Before sequencing, the PCR products were treated with ExoSAP-IT (Affymetrix, USA) to remove excess primers and dNTPs. For sequence analysis, cycle sequencing was performed with one of the PCR primers using BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, USA) where 5 μL of the purified PCR products served as template. The thermocycling conditions for the cycle sequencing consisted of 25 cycles of denaturing at 96°C for 10 s, annealing at 50°C for 5 s, and extension at 60°C for 4 min. The resulting cycle sequencing products were purified using the EDTA-ethanol method and were analyzed on the ABI 3130 XL Genetic Analyzer (Applied Biosystems, California, USA). The data obtained were analyzed with Sequencing Analysis Software, version 5.2 (Applied Biosystems, California, USA).
Outcome was measured based on ATA guidelines 2015.[18]
Excellent response: No clinical, biochemical, or structural evidence of disease
Biochemical incomplete response: Abnormal thyroglobulin or antithyroglobulin antibody levels in the absence of localizable disease
Structural incomplete response: Persistent or newly identified locoregional or distant metastases
Indeterminate response: Nonspecific biochemical or structural findings.
Statistics
The cohort was grouped based on BRAF V600E mutation status and group to group analysis was done to estimate association of aggressive features such as tumor size, multifocality (MF), extra-thyroid invasion, vascular invasion, and LM metastasis.
The variables used for outcome analysis included clinicopathological features, adequacy of primary surgery, and BRAF V600E mutation status. Inadequate primary surgery is defined as the presence of residual primary tumor (recorded by surgeon or detected by neck ultrasound), residual or incidental regional LM metastases, and residual or incidental distant metastases detected by I131 whole-body scan. For the comparison of categorical variables among the different groups, Chi-square test was used. Multivariable regression analysis was done if the univariate analysis showed significance (P< 0.05). SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.
RESULTS
We analyzed the data of 59 patients (females n: 33; 56% and males n: 26; 44%) with a mean age of 40.40 years (standard deviation 12.282). Surgical procedures included total thyroidectomy (n: 33, 56%), total thyroidectomy and Level VI dissection (n: 19, 32%), and total thyroidectomy Level VI and unilateral neck dissection (n: 7, 12%). One patient had clinically evident bone metastasis before thyroidectomy.
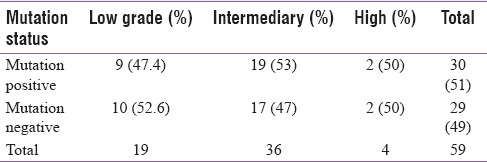
The group included conventional variant (n: 43; 73.8%) and infiltrating follicular variant (n: 16, 27.2%). When categorized based on risk stratifications, the group contained 19 (32%) in low grade, 36 (61%) in intermediary grade, and 4 (7%) in high grade.
We have analyzed samples that were pathologically characterized as PTC for the presence of BRAF T1799A (V600E) mutation by Sanger sequencing method. Of the 59 samples, 3 samples were homozygous positive for the BRAF T1799A (V600E) mutation, 27 samples were heterozygous positive, and 29 (49%) samples did not harbor the mutation [Figure 1].
Figure 1.
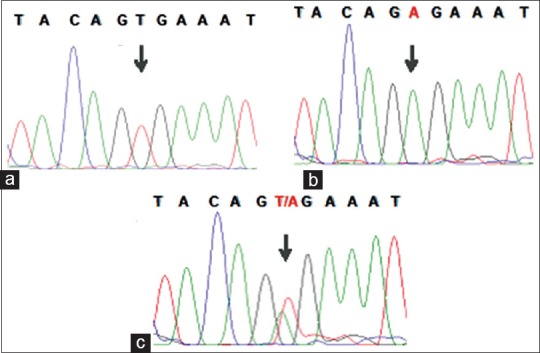
Sequence analysis of the exon 15 of the BRAF gene. Representative electropherograms for the wild type BRAF codon 600 (a), homozygous B-type Raf kinaseBRAF codon V600E mutation (b), and heterozygous B-type Raf kinaseBRAF codon V600E mutation (c)
In one patient, we have observed three mutations, a homozygous T1799A (V600E) missense mutation, a heterozygous nonsense mutation at the codon 603 (CGA→TGA) wherein the codon 603 coding for Arginine is mutated to TGA a stop codon (p.R603*/c.1807C>), and a third novel mutation A1757T (E586V) [Figure 2]. The novel mutation A1757T observed is a heterozygous A to T transversion at position 1757 affecting the codon 586 (GAA→GTA) resulting in the substitution of glutamic acid with valine (E586V).
Figure 2.
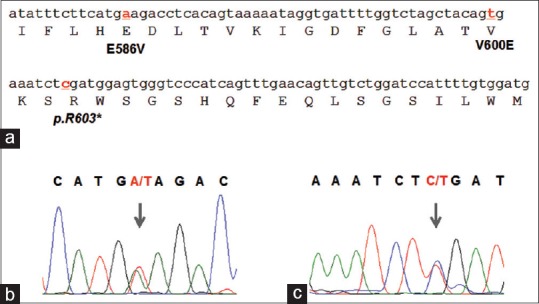
(a) Sequence of the exon 15 of the B-type Raf kinaseBRAF gene depicted with the amino acid translation. Nucleotides affected by mutations as observed in this study are depicted as bold and underlined. (b) Electropherogram of the novel mutation A1757T (E586V). (c) Electropherogram of the heterozygous nonsense mutation (p.R603* / c.1807C>) at the codon 603 (CGA®® TGA). * Indicates stop codon
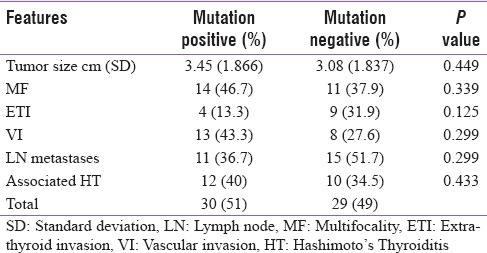
The cohort was grouped based on BRAF V600E mutation status and group to group analysis showed a trend toward higher prevalence among male patients to harbor the mutation (63.3% vs. 42%; P = 0.112), [Table 1].
Table 1.
Distribution of pathological features in the groups
Pretherapy 3 millicurie (mCi) I131 scan showed no significant residual thyroid tissue in any patient but showed unsuspected regional LM metastases in 15 (25.4%) and unsuspected distant metastases in two patients. Patients were grouped based on ATA guidelines (2009) [Table 2]. Forty-two patients received radioiodine ablation, but there was no difference in amount of I131 used in both groups (50.81 mCi vs. 51.31 mCi; P = 0.236).
Table 2.
Patients grouped based on risk stratification
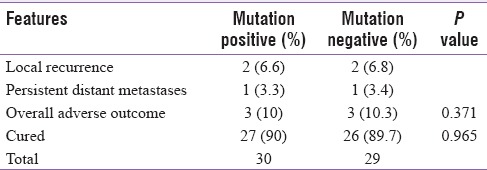
Mean duration of follow-up was 43.68 (36–48; median 48) months. Fifty-one patients (90%) showed excellent response and 6 (10%) had structural incomplete response [Table 3]. Untoward outcome was noted in 3 (10%) with mutation while 3 (10.3%) without mutation (P = 0.371). There was no difference in DFS between the groups.
Table 3.
Details of follow-up
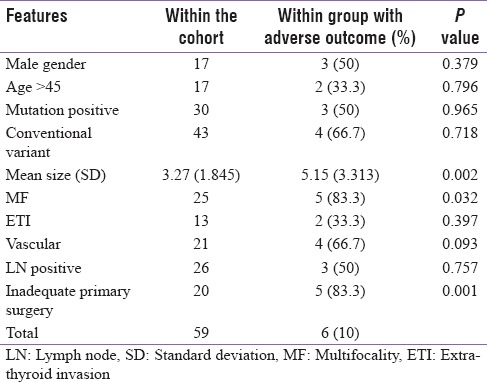
Tumor size, MF, and inadequate primary surgery showed association with adverse outcome [Table 4], but multiple regression model retained inadequate primary surgery as significant predictor adverse outcome (B = 0.502; standard error = 0.061; confidence interval 0.571–0.921; P = 0.000).
Table 4.
Association between clinicopathologic features and outcome
DISCUSSION
The age and gender distribution of the cohort are comparable to reported series.[19] We included patients with cytological reports of Bethesda V and VI categories to avoid possible primary surgery-related bias and to ensure that all patients received uniform pattern of treatment. The overall cure rate is lower than expected that could be explained by the fact that the cohort included 67.7% patients with intermediary and high-risk features.
The overall prevalence of BRAF V600E mutation in the present cohort is comparable to the previous reports.[3,4,5] The mean age of mutation positive patients was high but did not show statistical significance. The cohort did not have participants of pediatric age group. Follicular variant harbors BRAF V600E mutation infrequently,[20] but the high prevalence of the mutation in the present series may be due to infiltrating histology of all patients in this subgroup.
Many systematic reviews concluded that positive mutation status was a determinant factor of aggressive characteristics and adverse outcome. These studies analyzed pooled data of PTC encompassing all histological subtypes who underwent different treatment protocols.[4,5] Observations contradicting the negative influence of BRAF V600E mutation were blamed to have inadequate sample size.
Two single-center studies with high sample size from Japan and USA did not appreciate significant correlation between mutation status and high-risk features.[7,8] Gouveia et al. studied a cohort of 429 PTC patients among which 73.2% showed BRAF V600E positivity but was not significantly associated with aggressive clinicopathologic features.[7] Ito et al. concluded in their experience of 766 patients with 37% BRAF V600E positivity with average follow-up of 130 months that mutations' positivity was associated with poor cause-specific survival only with high- risk patients but not with low-risk category.[21] Niederer-Wüst et al. did not find correlation of BRAF V600E mutation status with clinical risk scores and survival.[22] Henke et al. analyzed 506 patients with 70% mutation positivity but was not correlated with recurrence-free survival or DFS.[23] However, recently concluded multicenter study to address the prognostic value of BRAF V600E mutation found strong association BRAF V600E mutation with PTC-associated patient mortality.[24]
There are no valid explanations to these conflicting observations other than possible heterogeneity of sample regarding tumor variants and treatment protocol. In the present study, we found completeness of thyroidectomy was the significant predictor of DFS. The tumor characteristics such as size and multifocal lesions were associated negatively with DFS but lost significance in multivariable regression model. We did not find association between positive mutation status and adverse tumor characteristics.
CONCLUSION
The prevalence of BRAF V600E mutation is comparable to reported series from other geographical region. The mutation status was not significantly associated with aggressive features of conventional and follicular variants of PTC. The mutation status was not a predictor of adverse outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 3.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 4.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: A systematic review and meta-analysis. Medicine (Baltimore) 2012;91:274–86. doi: 10.1097/MD.0b013e31826a9c71. [DOI] [PubMed] [Google Scholar]
- 6.Gao WL, Wie LL, Chao YG, Wie L, Song TL. Prognostic prediction of BRAF(V600E) and its relationship with sodium iodide symporter in classic variant of papillary thyroid carcinomas. Clin Lab. 2012;58:919–26. [PubMed] [Google Scholar]
- 7.Gouveia C, Can NT, Bostrom A, Grenert JP, van Zante A, Orloff LA. Lack of association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinoma: The University of California, San Francisco, experience. JAMA Otolaryngol Head Neck Surg. 2013;139:1164–70. doi: 10.1001/jamaoto.2013.4501. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: Its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009;56:89–97. doi: 10.1507/endocrj.k08e-208. [DOI] [PubMed] [Google Scholar]
- 9.DeLellis RA, Lloyd RD, Heitz PU, Eng C, editors. WHO: Pathology and Genetics. Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 10.LiVolsi VA. Papillary carcinoma tall cell variant (TCV): A review. Endocr Pathol. 2010;21:12–5. doi: 10.1007/s12022-010-9106-y. [DOI] [PubMed] [Google Scholar]
- 11.Ghossein RA, Leboeuf R, Patel KN, Rivera M, Katabi N, Carlson DL, et al. Tall cell variant of papillary thyroid carcinoma without extrathyroid extension: Biologic behavior and clinical implications. Thyroid. 2007;17:655–61. doi: 10.1089/thy.2007.0061. [DOI] [PubMed] [Google Scholar]
- 12.Wreesmann VB, Sieczka EM, Socci ND, Hezel M, Belbin TJ, Childs G, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res. 2004;64:3780–9. doi: 10.1158/0008-5472.CAN-03-1460. [DOI] [PubMed] [Google Scholar]
- 13.Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: Incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol. 2012;19:1874–80. doi: 10.1245/s10434-011-2129-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen JH, Faquin WC, Lloyd RV, Nosé V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod Pathol. 2011;24:739–49. doi: 10.1038/modpathol.2011.2. [DOI] [PubMed] [Google Scholar]
- 15.Sheu SY, Schwertheim S, Worm K, Grabellus F, Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: Lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol. 2007;20:779–87. doi: 10.1038/modpathol.3800797. [DOI] [PubMed] [Google Scholar]
- 16.Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, et al. Papillary thyroid carcinoma with prominent hobnail features: A new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol. 2010;34:44–52. doi: 10.1097/PAS.0b013e3181c46677. [DOI] [PubMed] [Google Scholar]
- 17.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 18.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. IARC Scientific Publications No 160. Vol. IX. Lyon, France: IARC Press; 2007. Cancer Incidence in Five Continents. [Google Scholar]
- 20.Ghossein R. Encapsulated malignant follicular cell-derived thyroid tumors. Endocr Pathol. 2010;21:212–8. doi: 10.1007/s12022-010-9141-8. [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Yoshida H, Kihara M, Kobayashi K, Miya A, Miyauchi A. BRAF(V600E) mutation analysis in papillary thyroid carcinoma: Is it useful for all patients? World J Surg. 2014;38:679–87. doi: 10.1007/s00268-013-2223-2. [DOI] [PubMed] [Google Scholar]
- 22.Niederer-Wüst SM, Jochum W, Förbs D, Brändle M, Bilz S, Clerici T, et al. Impact of clinical risk scores and BRAF V600E mutation status on outcome in papillary thyroid cancer. Surgery. 2015;157:119–25. doi: 10.1016/j.surg.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Henke LE, Pfeifer JD, Ma C, Perkins SM, DeWees T, El-Mofty S, et al. BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma. Cancer Med. 2015;4:791–9. doi: 10.1002/cam4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]


