Abstract
Liver disease is an important cause of mortality in type 2 diabetes mellitus (T2DM). It is estimated that diabetes is the most common cause of liver disease in the United States. Virtually, entire spectrum of liver disease is seen in T2DM including abnormal liver enzymes, nonalcoholic fatty liver disease, cirrhosis, hepatocellular carcinoma, and acute liver failure. The treatment of diabetes mellitus (DM) in cirrhotic patients has particular challenges as follows: (1) about half the patients have malnutrition; (2) patients already have advanced liver disease when clinical DM is diagnosed; (3) most of the oral antidiabetic agents (ADAs) are metabolized in the liver; (4) patients often have episodes of hypoglycemia. The aim of this consensus group convened during the National Insulin Summit 2015, Puducherry, was to focus on the challenges with glycemic management, with particular emphasis to safety of ADAs across stages of liver dysfunction. Published literature, product labels, and major clinical guidelines were reviewed and summarized. The drug classes included are biguanides (metformin), the second- or third-generation sulfonylureas, alpha-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, sodium-glucose co-transporter 2 inhibitors, glucagon-like peptide-1 receptor agonists, and currently available insulins. Consensus recommendations have been drafted for glycemic targets and dose modifications of all ADAs. These can aid clinicians in managing patients with diabetes and liver disease.
Keywords: Antidiabetic agents, hepatic impairment, type 2 diabetes mellitus
INTRODUCTION
The liver has an important role in carbohydrate metabolism. It is responsible for the balance of blood glucose levels by means of neoglucogenesis and glycogenolysis.[1] The metabolic homeostasis of glucose is impaired in the presence of chronic liver disease (CLD) resulting in insulin resistance (IR), glucose intolerance, and diabetes.[1,2,3] According to a report, the prevalence of diabetes mellitus (DM) in patients with CLD is reportedly 18%–71%.[4] In another report, glucose intolerance is seen in up to 80% of patients with CLD and diabetes in 30%–60%.[5] Moreover, in case of liver cirrhosis, glucose intolerance and diabetes is present in approximately 96% of the patients.[6] Hence, diabetes and CLD often coexist and existing evidence suggests that CLD increases complications and premature mortality in patients with diabetes.[7] Association between diabetes and CLD is given in Figure 1.
Figure 1.
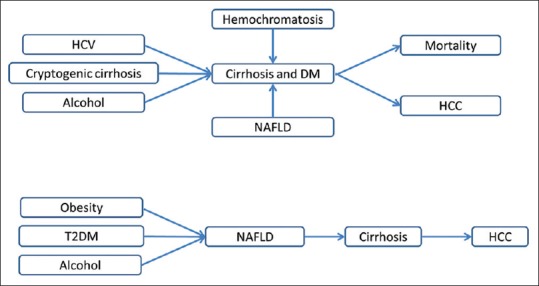
Relationship between diabetes and chronic liver disease. HCV: Hepatitis C Virus; HCC: Hepatocellular Carcinoma; NAFLD: Nonalcoholic Fatty Liver Disease; DM: Diabetes Mellitus; T2DM: Type 2 Diabetes Mellitus
In contrast to the involvement of liver disease in causing diabetes, diabetes has also been proposed as a risk factor for both CLD and hepatocellular carcinoma (HCC). In fact, diabetes, by most estimates, is now the most common cause of liver disease cryptogenic cirrhosis and has become the third leading indication for liver transplantation in the United States. DM has been commonly associated with nonalcoholic fatty liver disease (NAFLD), including its most severe form, nonalcoholic steatohepatitis (NASH). NASH is a chronic necroinflammatory condition that can lead to liver fibrosis, cirrhosis, and subsequently to HCC.[8] In addition, there is an unexplained association of diabetes with hepatitis C. Recent studies suggest that the core protein of hepatitis C virus (HCV) impairs insulin receptor substrate 1 (IRS-1) signaling, which plays an important role in the metabolic effects of insulin.[7]
NAFLD, a common cause of CLD in diabetic patients, is characterized by excess fat in liver. NAFLD increases the risk of mortality, estimated to be prevalent in 30%–74% of patients with diabetes.[7,9] Despite unclear pathogenesis, IR in diabetes is considered to be a critical contributing factor of NAFLD. IR causes downregulation of IRS-1 signaling, thereby leading to excess free fatty acids accumulation in the liver.[10] Another factor which contributes to NAFLD in patients with type 2 diabetes mellitus (T2DM) is dyslipidemia. The risk of all-cause mortality is found to be 2.2-fold more in T2DM patients with NAFLD.[11] In India, various cohort studies had shown that NAFLD is highly prevalent, i.e., 30.5%–64.2% in patients with T2DM.[12,13,14] Etiology of liver disease associated with DM can be classified into three groups as shown in Figure 2.
Figure 2.
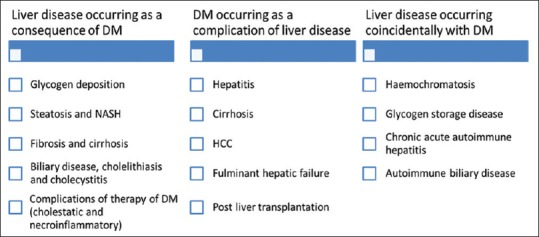
Etiology of liver disease associated with diabetes mellitus. DM: Diabetes Mellitus; HCC: Hepatocellular Carcinoma; NASH: Nonalcoholic Steatohepatitis
Management of diabetes in patients with CLD is challenging because the liver is the major site of metabolism for most of the antidiabetic agents (ADAs). Moreover, CLD was associated with complications such as impaired renal function, hypoalbuminemia, lactic acidosis, and hypoglycemia. In addition, more than 50% of patients with liver disease were malnourished and were at higher risk of developing hypoglycemia in the presence of predisposing factors.[7,15,16] Data on efficacy and safety of commonly prescribed glucose-lowering agents such as metformin, sulfonylureas (SUs), alpha-glucosidase inhibitors (AGIs), and thiazolidinedione's (TZDs) in patients with DM and CLD are very limited. To derive a consensus towards optimal dose modifications with ADAs in patients with T2DM with coexisting CLD, a group of experts from India held a consensus meeting at the National Insulin Summit in Puducherry, India, on November 8, 2015. The objectives of the meeting were to:
Examine the published evidence and product prescribing information (PI) of each antidiabetic therapy on dose modification and their pharmacokinetic/pharmacodynamics (PK/PD) behavior in hepatic impairment (HI) patients
Examine algorithms published as part of the established treatment guidelines from globally recognized professional bodies such as the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), the American Association of Clinical Endocrinologists, the American College of Endocrinology and International Diabetes Federation as well as other reports and guidelines published within India and other countries related to the management of glycemic control in this patient population
Propose consensus recommendations for the dose modifications of different ADAs based on published guidelines, evidence, and clinical experience.
METHODS
The expert group identified the following classes of drugs for proposed recommendations upon consensus: biguanides (metformin), SUs (glipizide, glimepiride, glyburide, and gliclazide), TZDs (pioglitazone), AGIs (acarbose and miglitol), dipeptidyl peptidase 4 (DPP-4) inhibitors (sitagliptin, saxagliptin, vildagliptin, and linagliptin), sodium-glucose co-transporter 2 (SGLT2) inhibitors (dapagliflozin, canagliflozin, and empagliflozin), glucagon-like peptide-1 receptor agonists (GLP1-RAs) (liraglutide, exenatide, and dulaglutide), and various types of insulin products.
Each class of drugs was subsequently evaluated for relevant and published clinical and epidemiological evidence as well as defined place in guidelines/algorithms from the national and global professional associations. These evaluations were then factored into the national context based on inputs from the consensus committee members. The final proposed consensus recommendation captured the collective outcome of the above process in easily implementable steps under each therapeutic drug class.
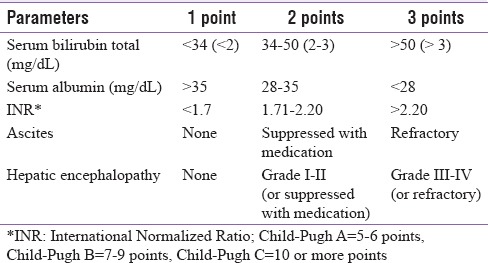
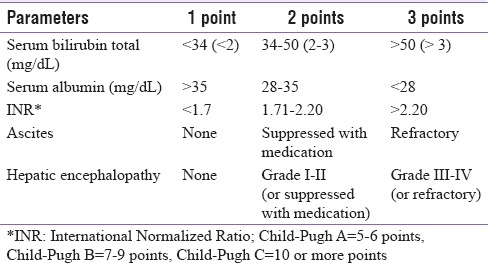
The Child-Pugh (also called as Child-Turcotte-Pugh score) classification [Table 1], commonly used to represent severity of CLD, is the basis for the proposed recommendations.[17] Patients are classified into three classes (A–C) with different expected survival: Child-Pugh A = 5–6 points, Child-Pugh B = 7–9 points, and Child-Pugh C = 10 or more points.[18,19]
Table 1.
Child-Pugh score parameters
Challenges in the management of diabetes in patients with chronic liver disease
Renal impairment
Patients with CLD are more prone to acute kidney injury.[20] Liver disease can sometimes alter kidney function, leading to accumulation of drugs/metabolites even if they are not eliminated by the liver.[20]
Altered metabolism
The liver is a primary site of drug metabolism, and the impairment of drug metabolism is proportional to the liver dysfunction. CLD is characterized by numerous metabolic alterations, predominantly catabolic.[21] Hepatic blood flow, liver enzyme activity, and plasma protein concentration can be impacted, and this in turn affects hepatic metabolism of drugs.[22] Bioavailability of drugs, metabolized by cytochromes P450 (CYP 450) enzyme system, may also be altered.[23]
Insulin resistance and hypoglycemia
Increased IR is frequently associated with CLD.[4] It significantly affects endogenous glucose production, oxidative and nonoxidative glucose disposal, lipolysis, and lipid oxidation.[24] The rate at which insulin is degraded in the liver is reduced resulting in peripheral hyperinsulinemia and hypoglycemia.[25,26,27]
Lactic acidosis
Patients with decompensated liver disease, in the setting of sepsis or hemorrhage, may have increased serum lactate levels[28] due to poor utilization and metabolism.[29] Lactic acidosis may be precipitated by biguanides as they inhibit mitochondrial respiration predominantly in the liver.[30]
Malnutrition
Patients with CLD develop malnutrition as the liver plays a key role in carbohydrate, protein, lipid, vitamin, and mineral metabolism and energy balance.[31,32] Hypoalbuminemia results from protein deficiency in malnutrition.[33] Highly protein bound drugs may cause toxicity in the presence of hypoalbuminemia as their free plasma concentrations increase.
Tools to measure long-term glycemic control in patients with chronic liver disease
Glycosylated hemoglobin (HbA1c), serum fructosamine, self-monitoring of blood glucose (SMBG), and continuous glucose monitoring (CGM) are some of the available tools to measure long-term glycemic control in patients with CLD.
Of all the tools, serum fructosamine seems to be more suitable test for monitoring blood glucose levels as HbA1c levels are frequently falsely low in patients with diabetes and liver cirrhosis.[34,35] Repeated SMBG shows short-term glycemic control with limited clinical effectiveness and is less cost-effective.[36] On the other hand, though CGM is recommended for those on insulin treatment or those with recurrent hypoglycemic attacks, it is not specified for Patients with diabetes and liver disease.[37] It is suggested to use indicators of glycemic control compositely to reduce the discrepancy between different parameters of glycemic control.
Dose modification of oral antidiabetic drugs in chronic liver disease
Biguanides (metformin)
Published scientific evidence
Metformin is first-line therapy for T2DM patients.[16] It does not undergo hepatic metabolism and is excreted unchanged by tubular secretion and glomerular filtration in the urine.[38]
Metformin may cause lactic acidosis in predisposed patients.[38] Lactic acidosis can occur through two mechanisms. Metformin shifts intracellular oxidation-reduction potential toward anaerobic metabolism augmenting lactate production. It also suppresses conversion of glucose from lactate in liver and hence decreases lactate clearance.[39] However, there are only a few reported cases of hepatotoxic side effects for metformin. Nevertheless, there may be an enhanced risk of developing lactic acidosis in the clinical setting of impaired liver function.[38] It is estimated that incidence of lactic acidosis is 0.03–0.5 cases/1000 patient-years in metformin-treated population. However, the incidence of lactic acidosis among patients with T2DM who consume metformin does not differ from the incidence in T2DM patients not receiving metformin.[39] Systematic review and meta-analysis of 194 comparative trials revealed no cases of fatal or nonfatal lactic acidosis in metformin group compared to nonmetformin group (36,893 vs. 30,109 patient-years). Moreover, there was no difference in lactate levels for metformin compared to placebo or other nonbiguanide therapies.[40] Hence, there is no evidence to date that metformin therapy is associated with an increased risk of lactic acidosis compared to other antihyperglycemic treatments.
Metformin is not expected to cause or exacerbate liver injury. It is probably beneficial in patients with NAFLD.[15] However, metformin has not been studied in patients with liver disease to date. All available information about liver dysfunction predisposing to metformin-associated lactic acidosis is drawn from case reports and postulated mechanisms.[39] Most of those patients had cirrhosis, with some degree of renal impairment. For this reason, it seems reasonable to use metformin with caution in patients with moderate CLD and to avoid its use in patients with severe CLD. Furthermore, identifying patients with cirrhosis and controlling renal function before initiating metformin seem prudent. Any circumstance favoring dehydration should promote the interruption of metformin, especially in such fragile patients.[15] Patients with multiple comorbidities, such as renal, liver, and cardiac diseases, particularly in acute deterioration, when treated with metformin, appear to be at higher risk of lactic acidosis.[16]
Tissue hypoxia is supposed to be a risk factor for lactic acidosis. In practice, not all cases of CLD are associated with hypoxia. Instances where concurrent pulmonary or heart diseases are present may be considered as hypoxic states.[39]
Current place in guidelines/recommendations
The Canadian Diabetes Association (CDA) recently recommended the avoidance of metformin in hepatic failure patients.[41] The Australian Diabetes Society also restricts the use of metformin in patients with severe hepatic failure.[42] In addition, ADA also recommends avoidance of metformin in patients with severe liver disease or in binge drinkers due to incidence of lactic acidosis.[43] The Indian Council of Medical Research (ICMR) proposes to avoid metformin usage in patients with hepatic insufficiency.[44] The British National Formulary (BNF) recommends to withdraw metformin if tissue hypoxia is likely in HI patients to reduce the risk of lactic acidosis.[45]
Consensus guidelines by the Egyptian Association for the Study of Liver and Gastrointestinal Disease (EASLGD) elaborates that metformin is an appropriate first-line therapy for most patients, except those with advanced liver disease who have an increased risk of lactic acidosis.[38] A review by Khan et al. suggested that if a patient has stable CLD and few other comorbidities, metformin is likely to be reasonably safe, but the dose should be decreased to a maximum of 1500 mg daily, and the drug should be withdrawn if liver or renal function is deteriorating, or in the setting of acute illness or decompensation.[16]
Prescribing information
Warning section of manufacturer's PI states, “Since impaired hepatic function has been associated with some cases of lactic acidosis, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease.” In addition, it also quotes that metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis.[46]
Based on the available evidence, we recommend that metformin should be used with caution up to a maximum dose of 1500 mg/day. It may be advisable to either reduce the dose or to avoid metformin in patients with moderate liver disease. The agent is better avoided in patients with severe hepatic dysfunction [Table 2].
Table 2.
Expert group recommendation 2: Dose modification of metformin in chronic liver disease

Sulfonylureas (second or third generation)
Published scientific evidence
The first-generation SUs are rarely used and are not discussed. The second- and third-generation SUs are currently positioned as the second-line treatment options after failure of metformin therapy. Glyburide/glibenclamide, glipizide, gliclazide, and glimepiride belong to second- and third-generation SUs, respectively.[47] The liver is the major site of biotransformation for all SUs. They are metabolized into active and inactive metabolites through hepatic oxidative enzymes (CYP P450s). SUs are extensively bound to serum proteins and excreted mainly through renal pathway.[48,49,50,51] The major risk associated with SUs is hypoglycemia.[15]
PK studies regarding the use of SUs in patients with hepatic insufficiency are lacking. However, the use of SUs in hepatic failure may be challenging as most of the SUs are metabolized in liver.[52] Furthermore, SUs exert their hypoglycemic effects by stimulating insulin secretion from the pancreatic beta-cell.[53] Hence, there may be chances of drug-induced hypoglycemia due to reduced inactivation of SUs in liver. In addition, protein binding of SUs may also be reduced due to hypoalbuminemia. This in turn enhances free drug plasma concentrations resulting into frequent hypoglycemia.[52] In severe HI, there may be diminished gluconeogenic capacity.[49] Moreover, SUs may also exhibit additive hypoglycemia in case of decompensated liver cirrhosis which is characterized by peripheral hyperinsulinism, resulting from both reduced insulin hepatic clearance and higher insulin secretion rate.[54] NASH can also be associated with impaired defense in countering hypoglycemia.[38,52] CLD patients who are malnourished are at higher risk of hypoglycemia.[15] Alcohol-induced enzyme degradation of SUs in patients with alcoholic liver disease may manifest decreased clinical effectiveness of SUs.[52] Therefore, SUs may also not be suitable where both IR and defects in insulin secretion are coexistent. It has been suggested that SUs with a short half-life such as glipizide or glyburide are preferred in CLD patients.[7]
Current place in guidelines/recommendations
Position statement of the ADA and EASD states that in severe hepatic disease, insulin secretagogues should be avoided due to the risk of hypoglycemia.[55] CDA suggests the use of alternate agents in hepatic failure patients.[41] BNF also mentions, “Insulin secretagogues should be avoided or used with caution at low doses in patients with T2DM and CLD.” However, glimepiride must be avoided in severe hepatic failure patients.[45] Guidelines from ICMR also recommend avoidance of SUs in hepatic insufficiency and acute hepatitis.[44] Consensus guidelines by EASLGD restricted the usage of SUs in severe hepatic disease due to high risk of hypoglycemia.[38]
Khan et al. recommended that dosage of SUs should be halved, especially if patients do not abstain from alcohol.[16] Expert opinion by Scheen suggests the use of SUs with caution in patients with advanced CLD as hypoglycemia may be a concern.[15]
Prescribing information
Manufacturer's PI of all SUs suggests cautious use in patients at higher risk of hypoglycemia such as hepatic and renal impairment, the elderly, malnourished, and patients on other antidiabetic medications. Initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemia. However, gliclazide is contraindicated in severe hepatic insufficiency.[48,49,50,51]
Based on the available evidence, we recommend that SUs should be avoided in patients with CLD due to greater risk of hypoglycemia and paucity of evidence. If used, lower doses should be preferred in Child-Pugh Class A and B. SUs should be avoided in patients with Child-Pugh Class C [Table 3].
Table 3.
Expert group recommendation 3: Dose modification of SU in chronic liver disease
Meglitinides
Published scientific evidence
Meglitinides have the potential to produce a rapid, short-lived insulin output. Two analogs are currently available for clinical use: repaglinide and nateglinide. Meglitinides are extensively (>98%) bound to serum albumin protein. They are completely metabolized by oxidative biotransformation (CYP 450) and conjugation with glucuronic acid in liver.[56,57] Meglitinides are characterized by shorter half-lives and absence of significant renal excretion.[15]
PK characteristics of glinides have been evaluated in patients with HI. Drug exposure and elimination of repaglinide are significantly affected by HI, whereas in the case of nateglinide, HI has only minimal impact upon these parameters. The reasons of this discrepancy are not clear. However, the two meglitinides are metabolized by different CYP isoforms, 2C8 for repaglinide and 2C9 for nateglinide. Besides, solute carrier organic anion transporter family member 1B1 (SLCO1B1) polymorphism exerts different effects on the PK/PD of repaglinide (significant PK changes) and nateglinide (PK unaffected).[15]
PKs and tolerability of a single 4 mg dose of repaglinide were compared in an open-label, parallel-group study in healthy controls and patients with CLD. Values for area under the concentration-time curve (AUC) and maximum plasma concentration (Cmax) were significantly higher in CLD patients compared to healthy controls. Values for time to reach Cmax (Tmax) did not differ between the groups, but terminal elimination half-life (t1/2) was significantly prolonged in CLD patients compared with previously determined values in healthy controls. Since AUC was inversely correlated with caffeine clearance in CLD patients but not in healthy control, repaglinide clearance is significantly reduced in HI patients. Therefore, it should be used with caution in patients with CLD and is contraindicated in patients with diabetes and severe HI.[58] As with SUs, rare case reports of either acute hepatotoxicity or cholestatic hepatitis have also been reported with repaglinide.[59,60]
On the contrary, no significant PK alteration of nateglinide occurs in patients with mild HI. PKs of nateglinide was compared in individuals with cirrhosis and matched healthy controls in a single-dose, open-label, and parallel-group study. In groups, Tmax (0.5 h) and mean t1/2 values were comparable. However, exposure was slightly increased (+30% for AUC and + 37% for Cmax). Mean apparent total clearance and mean renal clearance of nateglinide were comparable in both groups. Fractions bound to protein were also equivalent. Hence, no statistically significant or clinically relevant alterations in PK parameters of nateglinide were observed in hepatic dysfunction; therefore, adjustment of nateglinide dosage is not required in individuals with mild to moderate cirrhosis.[61] No data are available in patients with severe HI.
Furthermore, nateglinide was tested in a pilot 20-week study in diabetic patients with NASH who were randomly divided into two groups, with and without nateglinide treatment. Postprandial blood glucose, oral glucose tolerance test, HbA1c, abdominal ultrasound and computerized tomography imaging tests, liver function, and liver histological findings were improved after treatment with nateglinide. Hence, nateglinide was considered as useful and safe in the treatment of NASH in patients with T2DM.[62]
Current place in guidelines/recommendations
No specific guidelines are available pertaining to meglitinides. Expert opinion by Scheen suggests that meglitinides may represent an alternative to SUs, with a preference for nateglinide compared to repaglinide.[15] Moreover, Khan et al. recommended that dosage of repaglinide should be halved, especially if the patient is not abstinent from alcohol.[16]
Prescribing information
Repaglinide PI suggests caution due to elevation in plasma levels, and therefore, risk of added hypoglycemia may be there while prescribing repaglinide to HI patients. It emphasizes that proper patient selection, dosage, and instructions to the patients are important to avoid hypoglycemic episodes. In addition, rare adverse events such as severe hepatic dysfunction including jaundice and hepatitis have also been observed in patients taking repaglinide. Nateglinide PI advises cautious use in patients with moderate to severe liver disease.[56,57]
On the basis of available evidence, we recommend that repaglinide should be cautiously used in CLD patients. Although nateglinide may be used in Child-Pugh Class A patients, it is not preferred in Child-Pugh Class B and C patients [Table 4].
Table 4.
Expert group recommendation 4: Dose modification of meglitinides in chronic liver disease

Thiazolidinediones
Published scientific evidence
Pioglitazone is the only drug of this class available for clinical use in India.[63] It is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to glucuronide or sulfate conjugates. It is excreted primarily as metabolites and their conjugates in bile and feces.[64] Edema is one of the most frequent side effects of TZDs.[65] TZDs are also thought to increase vascular permeability in several tissues.[66] On the other hand, TZDs improve insulin sensitivity.[16] They exert improved insulin sensitivity through diverse mechanisms, both direct and indirect, and both at the level of the liver and extrahepatic tissues.[38]
Hepatic safety of pioglitazone in more than 20,000 patients with T2DM in Japan was evaluated in a prospective observational study. No case of hepatic failure was reported, and neither temporal nor dose relations were found between pioglitazone and alanine aminotransferase (ALT) abnormalities.[67]
Pioglitazone may also have a specific role in patients with NAFLD and NASH. There is preliminary evidence that patients with fatty liver may get benefitted from pioglitazone. A randomized study, on patients with NASH treated with pioglitazone, showed improvement in histological indices. Serum ALT and aspartate aminotransferase (AST) levels were reduced with pioglitazone as compared with placebo (P < 0.001). Moreover, hepatic steatosis and lobular inflammation were also reduced after pioglitazone treatment.[68] In addition, it is evident from several clinical trials that TZD treatment can prevent subsequent events, such as increase in oxidative stress, lipid peroxidation, and pro-inflammatory cytokines that contribute to the development of NAFLD to NASH.[65]
Current place in guidelines/recommendations
The guidelines from ADA and the Association of Physicians of India-Indian College of Physicians suggest that in case of active liver disease or clinical evidence of serum ALT level exceeding 2.5 times of upper normal limit, glitazones should be avoided.[43,69] However, ADA highlighted importance of TZDs in the treatment of NASH.[43] Consensus guideline by EASLGD recommends undergoing periodic monitoring of liver enzymes and glitazones not be initiated in patients exhibiting clinical evidence of active liver disease or increased serum transaminase levels (ALT >2.5 times the upper limit of normal [ULN]). It also suggests using glitazones with caution in HI patients. Also states that pioglitazone may be beneficial for fatty liver disease patients.[38] However, according to BNF, liver function test should be performed before the treatment with pioglitazone and periodically thereafter. It advises to discontinue treatment if jaundice occurs. Pioglitazone is also contraindicated in case of HI.[45] Khan et al. recommended maximum, 30 mg daily dose of pioglitazone with careful monitoring of liver function in CLD patients.[16]
Prescribing information
Pioglitazone PI recommends patient assessment and a group of liver tests such as serum ALT, AST, alkaline phosphatase, and total bilirubin before initiating therapy. Caution is advised in patients with abnormal liver tests. Patients who have serum ALT >3 times the reference range and serum total bilirubin >2 times the reference range without alternative etiologies are at risk for severe drug-induced liver injury and should not be restarted on pioglitazone.[64]
On the basis of available evidence, we recommend that pioglitazone should be used with caution in CLD patients. It should be avoided in patients whose liver enzymes are >3 times ULN range. Pioglitazone may be used in Child-Pugh Class A patients. However, it should be avoided in Class B and C patients. It is better to avoid pioglitazone in case of edema [Table 5].
Table 5.
Expert group recommendation 5: Dose modification of pioglitazone in chronic liver disease

Alpha-glucosidase inhibitors
Published scientific evidence
Acarbose and miglitol are available in India. Acarbose exerts its action within the gastrointestinal tract and is characterized by a low systemic bioavailability.[15] It is exclusively metabolized within the gastrointestinal tract.[38] However, miglitol is not considered to be metabolized in humans and it is eliminated by renal excretion as unchanged drug.[70] Therefore, AGIs may be particularly useful in patients with liver disease.
Due to lack of intestinal absorption and hepatic metabolism, several documents clearly report good tolerability and the absence of toxic effects of acarbose on liver. Because of these characteristics, no PK studies are available with this compound in patients with CLD. However, clinical studies demonstrated that acarbose can be safely and effectively used in diabetic patients with CLD, alcoholic cirrhosis, well-compensated nonalcoholic cirrhosis, and low-grade hepatic encephalopathy.[71,72,73,74] Acarbose is considered a promising therapeutic strategy for the treatment of patients with NASH.[75] However, there may be a possibility of hyperammonemia when acarbose is prescribed to the patients with DM and advanced liver cirrhosis.[71] Moreover, acarbose frequently causes mild transient elevations of ALT and, on rare occasions, severe liver disease.[7]
Current place in guidelines/recommendations
BNF states that liver function should be monitored while using acarbose and restricts its use in patients with liver disease. It warns about risk of hypoglycemia in case of liver dysfunction.[45] However, consensus guidelines by EASLGD and ADA recommend that acarbose is safe, useful, and well tolerated in CLD patients.[38,43]
Prescribing information
PI of acarbose reveals that a treatment emergent increase in serum transaminase levels was observed in postmarketing study. However, these elevations were symptomatic, reversible upon discontinuation, more common in females and in general were not associated with other evidence of liver dysfunction. In addition, these serum transaminase elevations appeared to be dose related. However, acarbose is contraindicated in diabetic cirrhosis.[76] Miglitol PI states that PK of miglitol is not altered in cirrhotic patients as compared to control. Since it is not metabolized by liver, therefore, no influence of hepatic function on the kinetics of miglitol is expected.[70]
On the basis of available evidence, we recommend that AGIs are relatively safe and could be used without dose modification in CLD patients. Hence, AGIs are safe in Child-Pugh Class A and B. However, they are not preferred in Class C patients [Table 6].
Table 6.
Expert group recommendation 6: Dose modification of alpha-glucosidase inhibitors in chronic liver disease

Dipeptidyl-peptidase-4 inhibitors
Published scientific evidence
Hepatic metabolism is a minor pathway for sitagliptin and vildagliptin, and major part of the administered drug is either excreted unchanged by renal pathway or through hydrolysis by multiple tissues/organs, respectively.[77,78] Similarly, metabolism also represents minor pathway for linagliptin and ~80% of administered dose is eliminated through enterohepatic recycling. On the other hand, saxagliptin is primarily metabolized by hepatic CYP3A4/5 and eliminated through renal and hepatic routes.[77]
Higher serum DPP-4 activity has been reported in few liver disorders such as chronic hepatitis-C and NAFLD patients, known to be associated with T2DM and IR. Therefore, DPP-4 inhibitors might offer the prevention of further metabolic deterioration, especially in NAFLD.[38] It has also been reported that NAFLD may worsen glycemic control afforded by sitagliptin.[15] All four DPP-4 inhibitors are particularly well studied in patients with various degrees of HI as far as PK characteristics are concerned. However, no clinical study with a chronic administration of a DPP-4 inhibitor in patients with CLD is yet available.[15]
In an open-label, single-dose study in patients with moderate HI (Child-Pugh's scores ranged from 7 to 9) and matched healthy controls, the mean AUC∞ and Cmax for sitagliptin were numerically, but not significantly, higher in patients with moderate HI compared with healthy matched controls. These small differences were not considered to be clinically significant. Furthermore, Tmax, apparent terminal t1/2, and renal clearance were not statistically significant.[79] In an another reported case–control study, efficacy and safety of sitagliptin was assessed for patients with diabetes complicated by CLD caused by HCV. There were no significant changes of average AST and ALT level reported during follow-up of 48 weeks in both sitagliptin group and control group. The study concluded that sitagliptin is effective and safe for the treatment of T2DM complicated with HCV positive CLD.[80] In an observational pilot study, paired liver biopsies from 15 patients with diabetes and NASH before and after 1 year of therapy with sitagliptin 100 mg once daily were studied. Sitagliptin resulted in a significant decrease in ballooning and NASH scores, while the reduction in the steatosis score was of borderline statistical significance. These effects were accompanied by a significant reduction in body mass index, AST, and ALT levels.[81]
PKs of vildagliptin (100 mg) was assessed in an open-label, parallel-group study in patients with mild, moderate, or severe HI and healthy controls. There was no significant difference in exposure to vildagliptin in patients with mild, moderate, or severe HI compared to healthy controls; therefore, it was concluded that no dose adjustment of vildagliptin is necessary in patients with HI.[82]
Linagliptin undergoes enterohepatic cycling; therefore, a potential effect of HI on the PKs of linagliptin may have important implications for dosing recommendations. An open-label, parallel-group, study enrolled patients with mild, moderate, or severe CLD and healthy controls to investigate whether HI affects linagliptin PKs, PDs, and tolerability. Healthy controls and patients with mild and moderate HI received 5 mg linagliptin for 7 days, whereas patients with severe HI received linagliptin 5 mg single dose. Compared to healthy controls, steady state exposure (AUCss) of linagliptin was slightly lower in mild and moderate HI patients. However, in severe HI patients, single-dose AUC(0-24) was similar to that in healthy controls whereas Cmax was lower. Accumulation of linagliptin (≤7%) based on AUC or Cmax and renal excretion of unchanged linagliptin were comparable across the groups. Median plasma DPP-4 inhibition at steady state trough concentration for healthy controls and mild and moderate HI patients was also similar, i.e., 91%, 90%, and 89%, respectively. However, it was 84% in patients with severe HI after 24 h of a single-dose administration. Thus, mild, moderate, or severe HI did not result in any increase in linagliptin exposure after single and multiple dosing compared with normal hepatic function. The authors suggested that dose adjustment with linagliptin is not required in patients with HI.[83]
The PK of saxagliptin (10 mg) and its pharmacologically active metabolite, 5-hydroxy saxagliptin, were compared in individuals with mild, moderate, or severe CLD without DM and in healthy adults in an open-label, parallel-group, and single-dose study. Compared to healthy controls, the AUC∞ values for saxagliptin were 10%, 38%, and 77% higher in patients with mild, moderate, or severe HI, respectively. The corresponding values were 22%, 7%, and 33% lower, respectively, for 5-hydroxy saxagliptin, compared with matched healthy controls. Saxagliptin Cmax values were 8% higher, 16% higher, and 6% lower in patients with mild, moderate, and severe HI, respectively, compared to controls (corresponding values for 5-hydroxy saxagliptin: −17%, −16%, and − 59%, respectively). Hence, increase of saxagliptin exposure was compensated by a corresponding decrease of the exposure to its active metabolite. Therefore, dose adjustment is recommended for patients with any degree of HI.[84]
There is little evidence that DPP-4 inhibitors such as sitagliptin and vildagliptin may be associated with hepatic risk. Pooled analysis of 12 large, double-blind, Phase IIb and III studies shows that sitagliptin treatment is associated with ALT elevations with concomitant increase in bilirubin. However, there was no meaningful difference between sitagliptin exposed and nonexposed patients. These cases resolved on treatment and overall no increased risk of hepatic events was reported.[85,86]
Further, in a meta-analysis of safety data, pooled from 38 Phase II and III clinical trials, greater proportion of vildagliptin recipients were found to have mild elevations in liver enzymes compared to comparators. Vildagliptin was also not associated with an increased risk of hepatic adverse events. Only two patients experienced severe elevations in liver enzymes (AST/ALT ≥10x ULN or AST/ALT ≥3x ULN and bilirubin ≥2x ULN) attributable to vildagliptin treatment. Both cases were asymptomatic and resolved upon discontinuation of treatment.[87,88]
Current place in guidelines/recommendations
A consensus guideline by EASLGD mentions that DPP-4 inhibitors, especially sitagliptin, demonstrated effectiveness and safety in T2DM patients with HCV-positive CLD.[38] However, vildagliptin should be avoided in liver disease patients.[45]
Prescribing information
The United States PI of sitagliptin, saxagliptins, and linagliptin recommends no dosage adjustments in patients with HI.[89,90,91] However, summary of product characteristic of vildagliptin suggests that it should not be used in patients with HI, including patients with pretreatment ALT or AST >3x the ULN.[92]
On the basis of available evidence, we recommend that except for vildagliptin, DPP-4 inhibitors can be used with caution without dose modification. More specifically, DPP-4 inhibitors may be used in Child-Pugh Class A patients while their use requires caution in Class B patients. Administration of DPP-4 inhibitors is not preferred in Class C patients [Table 7].
Table 7.
Expert group recommendation 7: Dose modification of dipeptidyl peptidase 4 inhibitors in chronic liver disease

Sodium-glucose co-transporter 2 inhibitors
Published scientific evidence
Canagliflozin, dapagliflozin, and empagliflozin are agents currently available in India. SGLT2 inhibitors share similar PK characteristics. They possess long elimination half-life allowing once-daily administration and undergo extensive hepatic metabolism mainly through glucuronidation, and small proportions of the parent drug are eliminated through renal route.[93]
Single-dose PKs of canagliflozin (300 mg) was studied in mild and moderate HI patients and compared with healthy volunteers. Mean Cmax and AUC0-∞ values differed by <11% between the group with normal hepatic function and those with mild and moderate HI. It was concluded that PKs of canagliflozin was not affected by mild or moderate HI and that single dose was well tolerated.[94] There is no clinical experience in patients with severe HI.
Plasma concentrations of dapagliflozin could be affected by HI. A study with 10 mg single dose of dapaglifozin showed that mean Cmax was 12% lower in patients with mild HI compared to healthy controls. However, in moderate and severe HI patients, Cmax was 12% and 40% higher, respectively. Mean exposure AUC(0-∞) of dapagliflozin was 3%, 36%, and 67% higher in mild, moderate, and severe HI patients, respectively, compared to healthy controls. As long-term safety and efficacy of dapagliflozin have not been studied, benefit: risk ratio should be individually assessed due to its greater exposure in severe HI patients.[95] Caution has been advised when HI is combined with some degree of renal impairment.[96]
The PK profile of empagliflozin in HI patients was investigated in an open-label and parallel-group study. A single dose of 50 mg empagliflozin was administered to patients with mild, moderate, and severe HI and to the matched controls with normal hepatic function. Exposure to empagliflozin (both Cmax and AUC∞) progressively increased with the severity of HI compared with individuals with normal hepatic function. However, as the increase in empagliflozin exposure was less than 2-folds in patients with impaired liver function, it was concluded that no dose adjustment of empagliflozin is required in these patients.[97]
Meta-analysis and review reports from large Phase II–III trials showed that dapagliflozin, canagliflozin, and empagliflozin do not cause hepatotoxicity.[98,99,100] No case reports describing alterations of liver tests with SGLT-2 inhibitors have been reported so far.
Current place in guidelines/recommendations
Guidelines are inconclusive regarding the use of SGLT2 inhibitors in patients with liver dysfunction.
Prescribing information
PI available with dapagliflozin and canagliflozin recommends no dosage adjustment for patients with mild, moderate, or severe HI.[101,102] However, it is suggested that benefit-risk for the use in patients with severe HI should be individually assessed since the safety and efficacy had not been specifically studied in this population. Empagliflozin may be used in patients with HI.[103]
On the basis of available evidence, we recommend that SGLT-2 inhibitors can be used with caution and lower doses should be considered during initiation of therapy in CLD patients. These agents are contraindicated in severe liver dysfunction. The risk of dehydration and hypotension is associated with the use SGLT-2 inhibitors; hence, caution is required. Precisely, SGLT-2 inhibitors are safe in Child-Pugh Class A patients; however, they should be used with caution in Class B patients. Agents of this class should better be avoided in Class C patients [Table 8].
Table 8.
Expert group recommendation 8: Dose modification of sodium-glucose co-transporter 2 inhibitors in chronic liver disease

Dose modification of injectable drugs in chronic liver disease
Glucagon-like peptide-1 receptor agonists
Published scientific evidence
Hepatic metabolism is not the main pathway for the elimination of GLP-1RAs. Exenatide is primarily eliminated by kidney. Liraglutide and dulaglutide are endogenously metabolized into their component amino acids by general protein catabolism pathways. No specific organ is presumed to be major route of elimination for GLP-1RAs.[104,105,106]
No PK study has been performed in patients with a diagnosis of acute or chronic HI for exenatide.[15,104] An interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials examined the metabolic effects of 2 years of exenatide treatment in patients with T2DM. Patients with normal baseline ALT had no significant ALT change. However, patients with elevated ALT at baseline had a slight but significant reduction of ALT from baseline and 39% achieved normal ALT by week 104. Hence, adjunctive exenatide treatment was well tolerated and caused improvements in hepatic biomarkers, ALT, and AST.[107]
PKs of liraglutide was studied in an open-label trial in which a single-dose (0.75 mg injected subcutaneously) of liraglutide was compared in four groups of six patients each with healthy, mild, moderate, and severe HI.[108] In this study, mean AUC∞ was highest for healthy controls and lowest for patients with severe HI (severe/healthy: 0.56, with 90% confidence interval [CI] 0.39, 0.81). Cmax also tended to decrease with HI (severe/healthy: 0.71, with 90% CI 0.52, 0.97), but Tmax was similar across groups (11.3–13.2 h). However, half-life was not affected by HI. It was concluded that differences in the overall exposure (AUC∞) of liraglutide might result primarily from differences in absorption of the drug from the subcutaneous depot rather than differences in its subsequent metabolism. Nevertheless, a decrease in albumin concentration in CLD may also result in an increased rate of metabolism of liraglutide by various enzymes as it is majorly bound to plasma albumin. However, this PK effect, resulting in lower plasma levels, might be compensated for by a possible enhanced PD effect in the setting of reduced circulation. Thus, the results indicate that patients with T2DM and CLD can use standard treatment regimens of liraglutide. There is, however, currently limited clinical experience with liraglutide in patients with HI.[108]
The effectiveness of liraglutide was studied in Japanese patients with NAFLD. Liraglutide not only improved T2DM but also resulted in improvement of liver inflammation, alteration of liver fibrosis, and reduction of body weight.[109] A meta-analysis of the “Liraglutide Effect and Action in Diabetes” program concluded that a 26-week therapy with liraglutide 1.8 mg is safe, well tolerated and improves liver enzymes in patients with T2DM.[110]
Dulaglutide is now available for use in India. Not enough information is available about its PK behavior in HI patients. PI states that systemic exposure of dulaglutide decreased by 23%, 33%, and 21% for mild, moderate, and severe HI groups, respectively, compared to individuals with normal hepatic function, and Cmax was decreased by a similar magnitude.[106]
Current place in guidelines/recommendations
A review article by Khan et al. recommends no dose adjustment of GLP-1RAs as there is limited experience of use in HI patients. However, it should be used with caution.[16]
Prescribing information
There is limited information available about the safety and efficacy of GLP-1RAs in HI patients; hence, PI of respective products advises cautious use in this patient population. However, there is no dosage adjustment recommended.[104,105,106]
On the basis of available evidence, we recommend that GLP-1RAs should be used with caution without dose modification in CLD patients. Drugs of this class can be administered to Child-Pugh Class A patients. However, due to scarcity of data in HI patients, GLP-1RAs should be avoided in Class B and C patients [Table 9].
Table 9.
Expert group recommendation 9: Dose modification of glucagon-like peptide-1 receptor agonists in chronic liver disease

Insulin and insulin analogs
Published scientific evidence
The major site of the metabolism for circulating insulin is liver. Approximately 40%–50% of the endogenous insulin produced by the pancreas is metabolized by the liver.[111] Insulin therapy is considered as the safest and most effective therapy in patients with liver dysfunction, with the limitation of increased risk of hypoglycemia.
Hyperinsulinemia and peripheral IR are frequent and well-documented complications of advanced liver fibrosis and cirrhosis. In cirrhosis, higher insulin secretion rate and markedly reduced hepatic clearance may be responsible for hyperinsulinemia. However, insulin requirement may vary in decompensated liver disease patients. It may be decreased due to reduced capacity for gluconeogenesis and reduced hepatic breakdown of insulin; however, it can be increased to compensate for IR. Due to these opposing factors, daily dose required to control blood glucose is difficult to predict in case when exogenous insulin is necessary.[15,38]
Insulin is the first-line agent to treat diabetes in CLD such as cirrhosis or chronic hepatitis. Short-acting insulins are preferred because the duration of action may vary in such situations.[112] Without increasing costs, insulin analogs may offer equivalent or improved glycemic control compared to standard insulin while being associated with a lower risk for hypoglycemia, particularly nocturnal and severe hypoglycemia.
Insulin therapy can be used at any stage of HI although clinical studies are scarce in insulin-treated diabetic patients with CLD. There is no single study reporting extensive experience with insulin analogs in patients with CLD.[15] In a study with insulin degludec, 24 individuals (normal hepatic function, Child-Pugh Grade A, B, or C) were administered a single subcutaneous dose of 0.4 U/kg insulin degludec. No difference was observed in area under the 120-h serum insulin degludec concentration–time curve (AUC0-120), Cmax, and apparent clearance (CL/F) for individuals with impaired versus normal hepatic function. It was concluded that ultra-long PK properties of insulin degludec were preserved in patients with HI and there were no statistically significant differences in absorption or clearance compared to normal controls.[113]
PKs of insulin aspart was studied in patients with varying degrees of HI without diabetes. There was no clinically significant impact of HI on PKs of insulin aspart.[114] In a case report, efficacy of insulin (detemir) was studied in two patients with significant NAFLD and hypertriglyceridemia. Insulin (detemir) appeared less efficacious in achieving glycemic control in such patients. Very high insulin doses were required, and weight gain was problematic.[115]
The PKs and PDs of rapid-acting insulin analogs suggest that they can be given just after meals. This is of benefit to many patients with advanced CLD as they may have nausea and reduced appetite and hence have the option of using rapid-acting insulin analogs just after their meals depending on their intake.
Current place in guidelines/recommendations
Guidelines from the ADA highlight the importance of insulin and suggest frequent dose adjustment and careful glucose monitoring for T2DM and CLD patients.[43] Similarly, consensus guideline by EASLGD recommends use of insulin with caution and suggests that dose should be adjusted frequently in CLD patients.[38] Indian guidelines also supported the use of insulin in hepatic decompensation patients.[44,69] Expert opinion by Scheen et al. mentions that insulin can be used in patients with all stages of CLD and does not exert hepatotoxic effects. However, the dose of insulin required in cirrhotic patients for optimal glucose control without hypoglycemia should be carefully adjusted upon individual basis and blood glucose monitoring. Insulins may be the safest agents and dose adjustment should be individualized.
Prescribing information
PI of insulin glargine, insulin glulisine, insulin aspart 30/70, insulin degludec, and insulin degludec/insulin aspart was reviewed for dose modification or special recommendations in HI patients. It is suggested that frequent glucose monitoring and dose adjustments are required to minimize the risk of hypoglycemia or hyperglycemia in this patient population.[116,117,118,119]
Based on the available evidence, we recommend that dose of insulin should be titrated to requirements to reduce risk of hypoglycemia. Moreover, newer insulin analogs are preferred in CLD patients as their PK is unaltered and possesses low risk of hypoglycemia [Table 10].
Table 10.
Expert group recommendation 10: Dose modification of insulin in chronic liver disease

CONCLUSION
T2DM and CLD may need to be managed together. The major challenge is the risk of altered metabolism of ADAs in case of HI; hence, there are chances of exaggerated response to a standard dose of medication and a higher risk of side effects. Therefore, careful and judicial selection of an antidiabetic agent is important in patients with associated CLD.
Older antidiabetic drugs such as metformin and SUs have been least investigated in HI patients; hence, their use is contraindicated in patients with moderate to severe HI. Such patients may be at higher risk of lactic acidosis (with metformin) and hypoglycemia (with SUs). Next generation SUs, i.e. glinides, have been well studied in patients with CLD; however, they are not preferred choice in Child-Pugh Class B and C due to risk of hypoglycemia. Similarly, safety data of pioglitazone indicate that it can be used in Child Pugh class A and should be avoided in Class B and C patients. Fluid retention is one of the frequently occurring side effects with pioglitazone. Therefore, its use should be avoided in edema. Nateglinide and pioglitazone have favorable effects on NAFLD (steatosis and NASH) due to possible hepatoprotective effects. Alpha-glucosidase inhibitors are considered entirely safe in HI patients due to the absence of hepatic metabolism. They can be used safely in Child-Pugh Class A and B and are considered as a promising therapeutic strategy for the treatment of patients with NASH. However, AGIs are not preferred choice in Class C patients due to risk of hyperammonemia. PKs of DPP-4 and SGLT-2 inhibitors has been very well reported in patients with various degrees of HI, and largely, the results were almost supportive, with limited PK changes probably without any clinical relevance in most of the cases. Drugs of both these classes can be used in Child-Pugh Class A whereas they should be used with caution in Class B patients. These agents are contraindicated in Class C or patients with severe liver dysfunction due to limited information in such patients. Vildagliptin should not be used in HI patients due to mild elevations in liver enzymes.
Among injectable preparations, no signs of hepatotoxicity have been reported so far with incretin-based therapies, contrasting with some concern and controversy regarding exocrine pancreas. Hence, use of GLP-1RAs should be avoided in Class B and C patients due to limited data in this class of patients. Insulin is the first-line agent to treat diabetes in severe CLD. It can be used in all classes of HI patients. However, dose of insulin should be titrated as per individual requirements to reduce risk of hypoglycemia. Moreover, newly discovered insulin analogs such as insulin degludec may be preferred, as it is observed that its PK properties are preserved in HI patients with reduced risk of hypoglycemia.
Finally, the management of patients with diabetes and CLD represents a challenge for the clinical practitioner. Clinical success may be achieved using a multidisciplinary approach if necessary and selecting the most appropriate glucose-lowering medications. This consensus guideline will facilitate clinicians an easy guide to check appropriate treatment option for diabetes in T2DM patients suffering from variety of liver disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Picardi A, D'Avola D, Gentilucci UV, Galati G, Fiori E, Spataro S, et al. Diabetes in chronic liver disease: From old concepts to new evidence. Diabetes Metab Res Rev. 2006;22:274–83. doi: 10.1002/dmrr.636. [DOI] [PubMed] [Google Scholar]
- 2.Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30:398–408. doi: 10.1016/s1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- 3.Tappy L, Minehira K. New data and new concepts on the role of the liver in glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2001;4:273–7. doi: 10.1097/00075197-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi T, Taniguchi E, Itou M, Sakata M, Sumie S, Sata M. Insulin resistance and chronic liver disease. World J Hepatol. 2011;3:99–107. doi: 10.4254/wjh.v3.i5.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blendea MC, Thompson MJ, Malkani S. Diabetes and chronic liver disease: Etiology and pitfalls in monitoring. Clin Diabetes. 2010;28:139. [Google Scholar]
- 6.Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120:829–34. doi: 10.1016/j.amjmed.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–43. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 9.Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol. 2014;109:1020–5. doi: 10.1038/ajg.2014.132. [DOI] [PubMed] [Google Scholar]
- 10.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: A community-based cohort study. Am J Gastroenterol. 2010;105:1567–73. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SV, Sikariya KK. Prevalence and risk factors of nonalcoholic fatty liver disease in type 2 diabetes mellitus in a tertiary care centre in Western India. IOSR J Dent Med Sci. 2006;15:1–7. [Google Scholar]
- 13.Sharavanan TK, Premalatha E. Prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus patients in a rural health care hospital. Sch J Appl Med Sci. 2015;3:1834–7. [Google Scholar]
- 14.Kalra S, Vithalani M, Gulati G, Kulkarni CM, Kadam Y, Pallivathukkal J, et al. Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes patients in India (SPRINT) J Assoc Physicians India. 2013;61:448–53. [PubMed] [Google Scholar]
- 15.Scheen AJ. Pharmacokinetic and toxicological considerations for the treatment of diabetes in patients with liver disease. Expert Opin Drug Metab Toxicol. 2014;10:839–57. doi: 10.1517/17425255.2014.902444. [DOI] [PubMed] [Google Scholar]
- 16.Khan R, Foster GR, Chowdhury TA. Managing diabetes in patients with chronic liver disease. Postgrad Med. 2012;124:130–7. doi: 10.3810/pgm.2012.07.2574. [DOI] [PubMed] [Google Scholar]
- 17.Papatheodoridis GV, Cholongitas E, Dimitriadou E, Touloumi G, Sevastianos V, Archimandritis AJ. MELD vs. Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol. 2005;11:3099–104. doi: 10.3748/wjg.v11.i20.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedeto-Stojanov D, Nagorni A, Bjelakovic G, Stojanov D, Mladenovic B, Djenic N. The model for the end-stage liver disease and Child-Pugh score in predicting prognosis in patients with liver cirrhosis and esophageal variceal bleeding. Vojnosanit Pregl. 2009;66:724–8. doi: 10.2298/vsp0909724b. [DOI] [PubMed] [Google Scholar]
- 19.Starr SP, Raines D. Cirrhosis: Diagnosis, management, and prevention. Am Fam Physician. 2011;84:1353–9. [PubMed] [Google Scholar]
- 20.Slack A, Yeoman A, Wendon J. Renal dysfunction in chronic liver disease. Crit Care. 2010;14:214. doi: 10.1186/cc8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolte W, Hartmann H, Ramadori G. Glucose metabolism and liver cirrhosis. Exp Clin Endocrinol Diabetes. 1995;103:63–74. doi: 10.1055/s-0029-1211331. [DOI] [PubMed] [Google Scholar]
- 22.Rodighiero V. Effects of liver disease on pharmacokinetics. An update. Clin Pharmacokinet. 1999;37:399–431. doi: 10.2165/00003088-199937050-00004. [DOI] [PubMed] [Google Scholar]
- 23.DeLeve LD. Alterations in Hepatic Metabolism of Drugs. 6th ed. Hamilton, ON: BC Decker; 2003. [Last accessed on 2016 Jun 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK12508/ [Google Scholar]
- 24.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 25.Imazeki F, Yokosuka O, Fukai K, Kanda T, Kojima H, Saisho H. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: Comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008;28:355–62. doi: 10.1111/j.1478-3231.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki Y, Ohkubo A, Kajinuma H, Akanuma Y, Kosaka K. Degradation and secretion of insulin in hepatic cirrhosis. J Clin Endocrinol Metab. 1978;47:774–9. doi: 10.1210/jcem-47-4-774. [DOI] [PubMed] [Google Scholar]
- 28.Ahya SN, José Soler M, Levitsky J, Batlle D. Acid-base and potassium disorders in liver disease. Semin Nephrol. 2006;26:466–70. doi: 10.1016/j.semnephrol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Nandwani S, Saluja M, Vats M, Mehta Y. Lactic acidosis in critically ill patients. Peoples J Sci Res. 2010;3:43–7. [Google Scholar]
- 30.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016;65:20–9. doi: 10.1016/j.metabol.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Butt S, Ahmed P, Liaqat P, Ahmad H. A study of malnutrition among chronic liver disease patients. Pak J Nutr. 2009;8:1465–71. [Google Scholar]
- 32.Purnak T, Yilmaz Y. Liver disease and malnutrition. Best Pract Res Clin Gastroenterol. 2013;27:619–29. doi: 10.1016/j.bpg.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Mobarhan S. The role of albumin in nutritional support. J Am Coll Nutr. 1988;7:445–52. doi: 10.1080/07315724.1988.10720260. [DOI] [PubMed] [Google Scholar]
- 34.Trenti T, Cristani A, Cioni G, Pentore R, Mussini C, Ventura E. Fructosamine and glycated hemoglobin as indices of glycemic control in patients with liver cirrhosis. Ric Clin Lab. 1990;20:261–7. doi: 10.1007/BF02900711. [DOI] [PubMed] [Google Scholar]
- 35.Youssef D, El Abbassi A, Jordan RM, Peiris AN. Fructosamine – An underutilized tool in diabetes management: Case report and literature review. Tenn Med. 2008;101:31–3. [PubMed] [Google Scholar]
- 36.Clar C, Barnard K, Cummins E, Royle P, Waugh N. Aberdeen Health Technology Assessment Group. Self-monitoring of blood glucose in type 2 diabetes: Systematic review. Health Technol Assess. 2010;14:1–140. doi: 10.3310/hta14120. [DOI] [PubMed] [Google Scholar]
- 37.Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5:39. doi: 10.1186/1758-5996-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamed AE, Abas B, Shaltout I, Esmt G, Gomez R. Managing diabetes and liver disease association, guidelines (consensus) development. J Endocrinol Diabetes Obes. 2015;3:1–19. [Google Scholar]
- 39.Brackett CC. Clarifying metformin's role and risks in liver dysfunction. J Am Pharm Assoc. 2010;50:407–10. doi: 10.1331/JAPhA.2010.08090. [DOI] [PubMed] [Google Scholar]
- 40.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: Systematic review and meta-analysis. Arch Intern Med. 2003;163:2594–602. doi: 10.1001/archinte.163.21.2594. [DOI] [PubMed] [Google Scholar]
- 41.Canadian Diabetes Association. Antihyperglycemic Agents for Use in Type 2 Diabetes. 2016. [Last accessed on 2016 Jun 15]. Available from: http://www.guidelines.diabetes.ca/cdacpg_resources/Ch13_Table1_Antihyperglycemic_agents_type_2_2016.pdf .
- 42.Gunton JE, Cheung NW, Davis TM, Zoungas S, Colagiuri S. Australian Diabetes Society. A new blood glucose management algorithm for type 2 diabetes: A position statement of the Australian Diabetes Society. Med J Aust. 2014;201:650–3. doi: 10.5694/mja14.01187. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. Standards of medical care in diabetes-2007. Diabetes Care. 2007;30(Suppl 1):S4–41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 44.Indian Council of Medical Research (ICMR) Pharmacological Treatment for Diabetes: Section 7. 2005. [Last accessed on 2016 Jun 15]. Available from: http://www.icmr.nic.in/guidelines_diabetes/section7.pdf .
- 45.British National Formulary 2009. [Last accessed on 2016 Jun 15]. Available from: http://www.bnf.com .
- 46.Glucophage (Metformin Hydrochloride) Tablet, Prescribing Information. Princeton, NJ: Bristol-Myers Squibb; 2008. Aug, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020357s031,021202s016lbl.pdf . [Google Scholar]
- 47.American Diabetes Association (ADA). Standards of medical care in diabetes-2015. Diabetes Care. 2015;38(Suppl 1):S41–8. [Google Scholar]
- 48.Gliclazide Tablets, Prescribing Information. Whiddon Valley, Barnstaple: Actavis UK Ltd.; [Last accessed on 2016 Jun 15]. Available from: http://www.medicines.org.uk/emc/medicine/24126/SPC/Gliclazide + Tablets + BP + 80mg . [Google Scholar]
- 49.Diaßeta (Glyburide) Tablet, Prescribing Information. Bridgewater, NJ: Sanofi; 2013. Oct, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017532Orig1s034lbl.pdf . [Google Scholar]
- 50.Amaryl (Glimepiride) Tablet, Prescribing Information. Bridgewater, NJ: Sanofi; 2013. Oct, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020496s027lbl.pdf . [Google Scholar]
- 51.Glucotrol (Glipizide) Tablet, Prescribing Information. New York: Pfizer Inc.; 2013. Oct, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017783s025lbl.pdf . [Google Scholar]
- 52.Kalra S, Aamir AH, Raza A, Das AK, Azad Khan AK, Shrestha D, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: A consensus statement. Indian J Endocrinol Metab. 2015;19:577–96. doi: 10.4103/2230-8210.163171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashcroft FM. Mechanisms of the glycaemic effects of sulfonylureas. Horm Metab Res. 1996;28:456–63. doi: 10.1055/s-2007-979837. [DOI] [PubMed] [Google Scholar]
- 54.Letiexhe MR, Scheen AJ, Gérard PL, Bastens BH, Pirotte J, Belaiche J, et al. Insulin secretion, clearance, and action on glucose metabolism in cirrhotic patients. J Clin Endocrinol Metab. 1993;77:1263–8. doi: 10.1210/jcem.77.5.8077319. [DOI] [PubMed] [Google Scholar]
- 55.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 56.Prandin (Repaglinide) Tablet, Prescribing Information. Princeton, NJ: Novo Nordisk Inc.; 2012. Mar, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020741s040lbl.pdf . [Google Scholar]
- 57.Nateglinide Tablet, Pescribing Information. Spring Valley, NY: Par Pharmaceutical Companies, Inc.; 2009. Jun, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/077463s000lbl.pdf . [Google Scholar]
- 58.Hatorp V, Walther KH, Christensen MS, Haug-Pihale G. Single-dose pharmacokinetics of repaglinide in subjects with chronic liver disease. J Clin Pharmacol. 2000;40:142–52. doi: 10.1177/00912700022008793. [DOI] [PubMed] [Google Scholar]
- 59.Nan DN, Hernández JL, Fernández-Ayala M, Carrascosa M. Acute hepatotoxicity caused by repaglinide. Ann Intern Med. 2004;141:823. doi: 10.7326/0003-4819-141-10-200411160-00024. [DOI] [PubMed] [Google Scholar]
- 60.López-García F, Borrás J, Verdú C, Salazar VR, Ruiz JA, Sales J, et al. Cholestatic hepatitis associated with repaglinide. Diabetes Care. 2005;28:752–3. doi: 10.2337/diacare.28.3.752-a. [DOI] [PubMed] [Google Scholar]
- 61.Choudhury S, Hirschberg Y, Filipek R, Lasseter K, McLeod JF. Single-dose pharmacokinetics of nateglinide in subjects with hepatic cirrhosis. J Clin Pharmacol. 2000;40:634–40. [PubMed] [Google Scholar]
- 62.Morita Y, Ueno T, Sasaki N, Tateishi Y, Nagata E, Kage M, et al. Nateglinide is useful for nonalcoholic steatohepatitis (NASH) patients with type 2 diabetes. Hepatogastroenterology. 2005;52:1338–43. [PubMed] [Google Scholar]
- 63.Jadhav SS, Shivane VK, Lila AR, Bandgar TR, Shah NS. Pioglitazone: Hype and hope. J Postgrad Med. 2014;60:293–6. doi: 10.4103/0022-3859.138765. [DOI] [PubMed] [Google Scholar]
- 64.Actos (Pioglitazone Hydrochloride) Tablet, Prescribing Information. Deerfield, IL: Takeda Pharmaceuticals; 2011. Jul, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021073s043s044lbl.pdf . [Google Scholar]
- 65.Chang E, Park CY, Park SW. Role of thiazolidinediones, insulin sensitizers, in non-alcoholic fatty liver disease. J Diabetes Investig. 2013;4:517–24. doi: 10.1111/jdi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horita S, Nakamura M, Satoh N, Suzuki M, Seki G. Thiazolidinediones and edema: Recent advances in the pathogenesis of thiazolidinediones-induced renal sodium retention. PPAR Res. 2015;2015:646423. doi: 10.1155/2015/646423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawamori R, Kadowaki T, Onji M, Seino Y, Akanuma Y. PRACTICAL Study Group. Hepatic safety profile and glycemic control of pioglitazone in more than 20,000 patients with type 2 diabetes mellitus: Postmarketing surveillance study in Japan. Diabetes Res Clin Pract. 2007;76:229–35. doi: 10.1016/j.diabres.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 68.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, Vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahay BK. API-ICP guidelines on diabetes 2007. J Assoc Physicians India. 2007;55:1–50. [Google Scholar]
- 70.Glyset (Miglitol) Tablet, Prescribing Information. Bayer HealthCare Pharmaceuticals Inc.; 2012. Aug, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020682s010lbl.pdf . [Google Scholar]
- 71.Kihara Y, Ogami Y, Tabaru A, Unoki H, Otsuki M. Safe and effective treatment of diabetes mellitus associated with chronic liver diseases with an alpha-glucosidase inhibitor, acarbose. J Gastroenterol. 1997;32:777–82. doi: 10.1007/BF02936954. [DOI] [PubMed] [Google Scholar]
- 72.Zillikens MC, Swart GR, van den Berg JW, Wilson JH. Effects of the glucosidase inhibitor acarbose in patients with liver cirrhosis. Aliment Pharmacol Ther. 1989;3:453–9. doi: 10.1111/j.1365-2036.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 73.Gentile S, Turco S, Guarino G, Oliviero B, Annunziata S, Cozzolino D, et al. Effect of treatment with acarbose and insulin in patients with non-insulin-dependent diabetes mellitus associated with non-alcoholic liver cirrhosis. Diabetes Obes Metab. 2001;3:33–40. doi: 10.1046/j.1463-1326.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- 74.Gentile S, Guarino G, Romano M, Alagia IA, Fierro M, Annunziata S, et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3:184–91. doi: 10.1016/s1542-3565(04)00667-6. [DOI] [PubMed] [Google Scholar]
- 75.Yamagishi S, Nakamura K, Inoue H. Acarbose is a promising therapeutic strategy for the treatment of patients with nonalcoholic steatohepatitis (NASH) Med Hypotheses. 2005;65:377–9. doi: 10.1016/j.mehy.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 76.Precose (Acarbose) Tablets, Prescribing Information. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; 2015. Mar, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020482s027lbl.pdf . [Google Scholar]
- 77.Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. 2012;51:501–14. doi: 10.1007/BF03261927. [DOI] [PubMed] [Google Scholar]
- 78.He YL. Clinical pharmacokinetics and pharmacodynamics of vildagliptin. Clin Pharmacokinet. 2012;51:147–62. doi: 10.2165/11598080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 79.Migoya EM, Stevens CH, Bergman AJ, Luo WL, Lasseter KC, Dilzer SC, et al. Effect of moderate hepatic insufficiency on the pharmacokinetics of sitagliptin. Can J Clin Pharmacol. 2009;16:e165–70. [PubMed] [Google Scholar]
- 80.Arase Y, Suzuki F, Kobayashi M, Suzuki Y, Kawamura Y, Matsumoto N, et al. Efficacy and safety in sitagliptin therapy for diabetes complicated by chronic liver disease caused by hepatitis C virus. Hepatol Res. 2011;41:524–9. doi: 10.1111/j.1872-034X.2011.00798.x. [DOI] [PubMed] [Google Scholar]
- 81.Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012;75:240–4. [PubMed] [Google Scholar]
- 82.He YL, Sabo R, Campestrini J, Wang Y, Ligueros-Saylan M, Lasseter KC, et al. The influence of hepatic impairment on the pharmacokinetics of the dipeptidyl peptidase IV (DPP-4) inhibitor vildagliptin. Eur J Clin Pharmacol. 2007;63:677–86. doi: 10.1007/s00228-007-0312-6. [DOI] [PubMed] [Google Scholar]
- 83.Graefe-Mody U, Rose P, Retlich S, Ring A, Waldhauser L, Cinca R, et al. Pharmacokinetics of linagliptin in subjects with hepatic impairment. Br J Clin Pharmacol. 2012;74:75–85. doi: 10.1111/j.1365-2125.2012.04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boulton DW, Li L, Frevert EU, Tang A, Castaneda L, Vachharajani NN, et al. Influence of renal or hepatic impairment on the pharmacokinetics of saxagliptin. Clin Pharmacokinet. 2011;50:253–65. doi: 10.2165/11584350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 85.Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, et al. Safety and tolerability of sitagliptin in clinical studies: A pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7. doi: 10.1186/1472-6823-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams-Herman D, Round E, Swern AS, Musser B, Davies MJ, Stein PP, et al. Safety and tolerability of sitagliptin in patients with type 2 diabetes: A pooled analysis. BMC Endocr Disord. 2008;8:14. doi: 10.1186/1472-6823-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ligueros-Saylan M, Foley JE, Schweizer A, Couturier A, Kothny W. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials. Diabetes Obes Metab. 2010;12:495–509. doi: 10.1111/j.1463-1326.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- 88.Kalra S. Emerging role of dipeptidyl peptidase-IV (DPP-4) inhibitor vildagliptin in the management of type 2 diabetes. J Assoc Physicians India. 2011;59:237–45. [PubMed] [Google Scholar]
- 89.JANUVIA® (Sitagliptin) Tablets, Prescribing Information. Whitehouse Station, NJ: Merc and Co., Inc.; 2015. Aug, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021995s034lbl.pdf . [Google Scholar]
- 90.Onglyza (Saxagliptin) Tablets, Prescribing Information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016. Apr, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022350s014lbl.pdf . [Google Scholar]
- 91.Tradjenta® (Linagliptin) Tablets, Prescribing Information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015. Aug, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/201280s012lbl.pdf . [Google Scholar]
- 92.Galvus (Vildagliptin) Tablets, Prescribing Information. Novartis Pharmaceuticals UK Ltd.; 2015. Dec, [Last accessed on 2016 Jun 15]. Available from: http://www.medicines.org.uk/emc/medicine/20734 . [Google Scholar]
- 93.Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015;54:691–708. doi: 10.1007/s40262-015-0264-4. [DOI] [PubMed] [Google Scholar]
- 94.Devineni D, Curtin CR, Marbury TC, Smith W, Vaccaro N, Wexler D, et al. Effect of hepatic or renal impairment on the pharmacokinetics of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Ther. 2015;37:610–8.e4. doi: 10.1016/j.clinthera.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 95.Kasichayanula S, Liu X, Zhang W, Pfister M, LaCreta FP, Boulton DW. Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: An open-label, parallel-group, single-dose study. Clin Ther. 2011;33:1798–808. doi: 10.1016/j.clinthera.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 96.Kasichayanula S, Liu X, Lacreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014;53:17–27. doi: 10.1007/s40262-013-0104-3. [DOI] [PubMed] [Google Scholar]
- 97.Macha S, Rose P, Mattheus M, Cinca R, Pinnetti S, Broedl UC, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16:118–23. doi: 10.1111/dom.12183. [DOI] [PubMed] [Google Scholar]
- 98.Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2014;30:204–21. doi: 10.1002/dmrr.2479. [DOI] [PubMed] [Google Scholar]
- 99.Nigro SC, Riche DM, Pheng M, Baker WL. Canagliflozin, a novel SGLT2 inhibitor for treatment of type 2 diabetes. Ann Pharmacother. 2013;47:1301–11. doi: 10.1177/1060028013503626. [DOI] [PubMed] [Google Scholar]
- 100.Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53:213–25. doi: 10.1007/s40262-013-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Farxiga (Dapagliflozin) Tablets, Prescribing Information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Dec, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/202293s008lbl.pdf . [Google Scholar]
- 102.Invokana (Canagliflozin) Tablets, Prescribing Information. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016. May, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204042s015s019lbl.pdf . [Google Scholar]
- 103.Jardiance (Empagliflozin) Tablets, Prescribing Information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2016. Mar, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204629s005lbl.pdf . [Google Scholar]
- 104.Bydureon (Exenatide Extended-release) Injectable Suspension, Prescribing Information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Sep, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022200s015s016s017s018lbl.pdf . [Google Scholar]
- 105.Victoza (Liraglutide) Injection, Prescribing Information. Bagsvaerd, Denmark: Novo Nordisk A/S; 2016. Apr, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022341s025lbl.pdf . [Google Scholar]
- 106.Trulicity (Dulaglutide) Injection, Prescribing Information. Indianapolis, IN: Eli Lilly and Company; 2014. Sep, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125469Orig1s000Lbl.pdf . [Google Scholar]
- 107.Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: An interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29:139–53. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 108.Flint A, Nazzal K, Jagielski P, Hindsberger C, Zdravkovic M. Influence of hepatic impairment on pharmacokinetics of the human GLP-1 analogue, liraglutide. Br J Clin Pharmacol. 2010;70:807–14. doi: 10.1111/j.1365-2125.2010.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ohki T, Isogawa A, Iwamoto M, Ohsugi M, Yoshida H, Toda N, et al. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. ScientificWorldJournal. 2012;2012:496453. doi: 10.1100/2012/496453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234–42. doi: 10.1111/apt.12149. [DOI] [PubMed] [Google Scholar]
- 111.Iglesias P, Díez JJ. Insulin therapy in renal disease. Diabetes Obes Metab. 2008;10:811–23. doi: 10.1111/j.1463-1326.2007.00802.x. [DOI] [PubMed] [Google Scholar]
- 112.Mukhopadhyay J. Use of Insulin in Chronic Liver Disorders. Medicine Update. 2005. [Last accessed on 2016 Jun 15]. Available from: http://www.apiindia.org/pdf/medicine_update_2005/chapter_43.pdf .
- 113.Kupcová V, Arold G, Roepstorff C, Højbjerre M, Klim S, Haahr H. Insulin degludec: Pharmacokinetic properties in subjects with hepatic impairment. Clin Drug Investig. 2014;34:127–33. doi: 10.1007/s40261-013-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holmes G, Galitz L, Hu P, Lyness W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol. 2005;60:469–76. doi: 10.1111/j.1365-2125.2005.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whyte M, Quaglia A, Hopkins D. Insulin detemir may be less efficacious in patients with nonalcoholic fatty liver disease and hypertriglyceridemia. Clin Case Rep. 2015;4:83–6. doi: 10.1002/ccr3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lantus (Insulin Glargine [rDNA origin]) Injection, Prescribing Information. Bridgewater, NJ: Sanofi; 2015. Jul, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021081s063lbl.pdf . [Google Scholar]
- 117.Apidra (Insulin Glulisine [rDNA origin]) Injection, Prescribing Information. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2015. Jul, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021629s030lbl.pdf . [Google Scholar]
- 118.NovoLog (Insulin Aspart [rDNA origin]) Injection, Prescribing Information. Princeton, NJ: Novo Nordisk; 2015. Feb, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021172s064lbl.pdf . [Google Scholar]
- 119.Ryzodeg 70/30 (Insulin Degludec and Insulin Aspart) Injection, Prescribing Information. Bagsvaerd, Denmark: Novo Nordisk; 2015. Sep, [Last accessed on 2016 Jun 15]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203313lbl.pdf . [Google Scholar]


