Abstract
Levosulpiride is the levorotatory enantiomer of sulpiride used in dyspeptic syndromes of various etiologies. The prokinetic effect of levosulpiride is mediated through the blockade of enteric inhibitory dopaminergic type 2 (D2) receptors. The antagonism of central D2 receptors leads to both therapeutic (e.g. antiemetic effect due to D2 receptor blockade in the chemoreceptor trigger zone) and adverse (including hyperprolactinemia) effects. Dopamine is the main endogenous inhibitor of prolactin synthesis and secretion in the anterior pituitary. Levosulpiride causes significant elevation of serum prolactin levels in significant number of patients. The resultant hyperprolactinemia often manifests as distressing menstrual abnormalities and galactorrhoea in females. A significant number of patients who use levosulpiride develop serum prolactin levels of > 200 ng/mL that goes against the classical textbook teaching where pituitary tumor is supposed to be the mostly likely cause. Careful drug history in patients presenting with high serum prolactin levels will be of great help in reaching the exact diagnosis and avoiding unnecessary brain imaging.
Keywords: Galactorrhoea, hyperprolactinemia, levosulpiride
INTRODUCTION
Gastroprokinetic agents are used frequently for dyspeptic symptoms of various etiologies. Initial prokinetic agents such as metoclopramide and domperidone caused hyperprolactinemia as an unavoidable side effect by blocking dopamine in the anterior pituitary. However, the effect of these drugs in raising serum prolactin levels was mild to moderate, usually <100 ng/mL. Based on this observation, the classical textbook teaching suggested pituitary tumor to be more likely cause if serum prolactin levels were >200 ng/mL. After the introduction of levosulpiride, a more potent gastroprokinetic, more and more patients are found to develop hyperprolactinemia of greater magnitude (>200 ng/mL). In this article, we are describing a typical case of hyperprolactinemia due to levosulpiride use. We are also discussing the unique features of lactotroph axis, its relation with dopamine and disruption of this axis by potent gastroprokinetic agents.
CLINICAL VIGNETTE
A 42-year-old female was referred to this hospital for a 2-month history of no menstrual cycles and 1-month history of galactorrhoea and breast tenderness. Initially, she had presented to a clinician with these symptoms and was advised to get her serum prolactin levels done apart from other routine investigations. She had no history of headaches or visual disturbances. Her serum prolactin levels were 273 ng/mL (reference range 3.7–17.9 ng/mL), done by immunometric assay. The clinician prescribed magnetic resonance imaging (MRI) of pituitary gland with gadolinium enhancement. The patient got her serum prolactin levels done again before going for MRI of the brain and that found to be 260 ng/dL. MRI of pituitary gland was unremarkable. She was referred to a higher center for evaluation. In this outpatient clinic, she was found to be on rabeprazole extended release (20 mg) plus levosulpiride sustained release (75 mg) combination pill (one tablet daily). This combination pill was started 2 months ago for dyspeptic symptoms by a gastroenterologist. We advised her stop the pill and get serum prolactin levels done after 1 week. Serum prolactin levels were 42 ng/mL. After further 2 weeks, she resumed her menstrual cycles and galactorrhoea/breast tenderness improved. Two months after stopping the drug, her serum prolactin levels were 23 ng/mL and her dyspeptic symptoms were under control on rabeprazole sustained release 20 mg twice daily.
CONNECTION BETWEEN DOPAMINE AND PROLACTIN
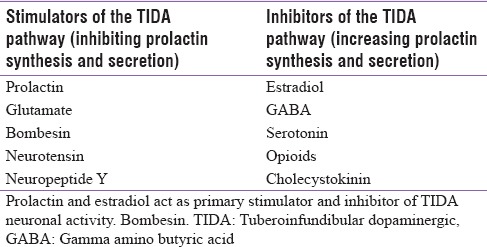
Dopamine is a small and simple molecule that accomplishes a number of functions in the brain. The extensively studied dopaminergic neuronal projections in the brain are nigrostriatal, mesolimbic, and mesocortical pathways that originate in the midbrain and project to the striatum, limbic system, and cortex, respectively. These neuronal pathways are involved in the control of motor, emotional, and cognitive functions. They have no direct role in the regulation of pituitary function. The dominant dopaminergic pathway that regulates prolactin secretion is the tuberoinfundibular dopaminergic (TIDA) pathway. Prolactin secretion is tonically inhibited by TIDA neuronal activity that provides dopamine to the anterior pituitary. The TIDA neurons have cell bodies in the arcuate nucleus of the hypothalamus. These neurons possess relatively short axons that terminate in the median eminence near the primary capillary loops of the hypophyseal portal vessels [Figure 1]. The TIDA neurons release dopamine into perivascular spaces surrounding the capillary loops and is carried by the portal blood to the anterior pituitary.[1] Dopamine acts on D2 dopaminergic receptors that are expressed on the cell membrane of the lactotrophs. There is a reduction of prolactin synthesis and secretion by the activation of these D2 receptors. The TIDA neurons are themselves regulated by feedback from prolactin through a “short-loop feedback” mechanism. A variety of other neurotransmitters and neuropeptides act at the hypothalamic level by either disinhibition of the TIDA neuronal activity (e.g., estradiol, serotonin, and opioids) or by reinforcing it (e.g., prolactin, neurotensin, and bombesin) as shown in Table 1.
Figure 1.
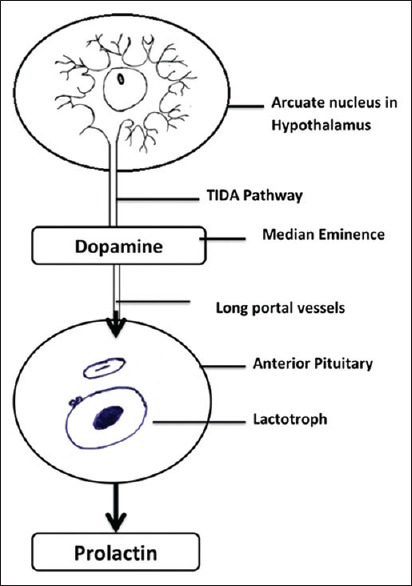
Prolactin axis
Table 1.
Selected compounds that stimulate or inhibit tuberoinfundibular dopaminergic neuronal activity
GENDER DIFFERENCES IN LACTOTROPH AXIS
There is a common observation that females are more prone to drug-induced hyperprolactinemia and its manifestations than males. This is because of the marked gender differences in the activity and responsiveness of the TIDA neurons to physiological and pharmacological stimuli.[2] The number of TIDA neurons in the hypothalamus does not differ between males and females. However, the basal TIDA neuronal activity is higher in females and lower in males. The lower basal activity of these neurons in males may be due to tonic inhibition by endogenous opioids.[3] The TIDA neurons in females are more sensitive to stress and to feedback stimulation by prolactin but less sensitive to bombesin and opioid antagonists.[4,5] Another reason could be that the manifestations of hyperprolactinemia such as menstrual abnormalities and galactorrhoea may be quite obvious in females and thereby diagnosed more frequently.
LACTOTROPH AS UNIQUE ENDOCRINE CELL
The lactotroph is a unique neuroendocrine cell in having a high basal secretory activity. Tonic inhibition by dopamine, which maintains low serum prolactin levels, needs a continuous high input of dopamine. This high output depends on a sustainable high rate of dopamine synthesis. To enable rapid prolactin surges, the dopaminergic input to the lactotrophs must be concomitantly decreased. This process is accomplished by a unique mechanism governing the regulation of hypothalamic tyrosine hydroxylase (TH) activity. TH in most tissues exists in an inactive, nonphosphorylated state. In response to stimuli, the enzyme is rapidly phosphorylated, resulting in increased hydroxylation of tyrosine to DOPA and its rapid conversion to dopamine that is immediately available for release.[6] Hypothalamic TH is an exception in that it is constitutively active and maintains the constant demand for high dopamine output.[7] In response to estrogen, hypothalamic TH is transiently and rapidly inactivated by dephosphorylation and lead to prolactin surge.[8,9] Furthermore, the absence of dopamine autoreceptors on the TIDA neurons assists in maintaining high dopamine output by eliminating the negative feedback by dopamine on TH activity.
PROKINETIC AGENTS AND SERUM PROLACTIN LEVELS
Currently, three antidopaminergic gastrointestinal prokinetic agents are in use. These are metoclopramide, domperidone, and levosulpiride. The prokinetic effect of these drugs is mediated through the blockade of enteric (neuronal and muscular) inhibitory D2 receptors. Justin-Besançon and Laville first described metoclopramide in 1964, as a first dopamine antagonist with antiemetic and gastroprokinetic properties.[10] It is a procainamide derivative that acts by both peripheral and central dopamine blockade. Antiemetic action of metoclopramide is thought to be on the chemoreceptor trigger zone (CTZ) as well as on the intracerebral vomiting center. Furthermore, an important component of its antiemetic action is related to its gastroprokinetic properties.[11] It increases basal lower esophageal sphincter pressure, inhibits relaxation of the gastric fundus, enhances antral contractility, and relaxes the pyloric sphincter. It also stimulates and coordinates gastroduodenal motility. It is indicated for many gastrointestinal motility disorders such as gastroparesis, dyspepsia, reflux esophagitis, nausea and vomiting.[12] Its use to some extent is limited by the side effect profile, which are mostly an unavoidable extension of its antidopaminergic properties. Among the side-effects, endocrinologic problems related to hyperprolactinemia are the most troublesome, especially in females. Mastalgia, galactorrhoea, and menstrual irregularities are due to the release of prolactin from the anterior pituitary by blocking D2 dopaminergic receptors.[13]
Domperidone was later introduced as a better gastroprokinetic agent, as unlike metoclopramide, it did not readily cross the blood–brain barrier (BBB). Therefore, it was claimed to have better side effect profile in comparison with metoclopramide.[13] Domperidone is a benzimidazole derivative that acts peripherally by dopamine blockade. The antiemetic action of domperidone is on the CTZ, which is on the blood side of the BBB. Like metoclopramide, its antiemetic action is also potentiated by its gastroprokinetic properties. Among the side effect profile, domperidone use is associated with an increased risk of sudden cardiac death most likely through prolongation of cardiac QT interval, thereby facilitating ventricular arrhythmias.[14,15] Domperidone also causes hyperprolactinemia by blocking D2 dopaminergic receptors on the cell membrane of lactotrophs. Prolactin response to both metoclopramide and domperidone is dose related and is more in women, especially in the hyperprolactinemic state after childbirth.[16]
Levosulpiride is the levo-enantiomer of sulpiride used for many conditions such as nausea and vomiting, dyspepsia, depression, and psychosis. The prokinetic effect of levosulpiride is mediated through the blockade of enteric (neuronal and muscular) inhibitory D2 receptors, and the ability to interact with Type 4 serotonergic (5-HT4) receptors. The serotonergic component of levosulpiride enhances its therapeutic efficacy in gastrointestinal disorders such as functional dyspepsia and diabetic gastroparesis.[17] The antagonism of central D2 receptors may lead to both therapeutic (e.g., antiemetic effect due to D2 receptor blockade in CTZ) and adverse (including hyperprolactinemia and extrapyramidal dystonic reactions) effects. Hyperprolactinemia is a side effect occurring with all antidopaminergic prokinetics. In a study by Lozano et al., galactorrhoea was reported in 26.7% of patients on levosulpiride.[18] There is no study that had looked at the effect of levosulpiride on lactotroph axis.
CONCLUSION
Levosulpiride is a potent inhibitor of D2 receptors in the anterior pituitary. Due to its increasing use as a prokinetic agent, more and more patients develop hyperprolactinemia as an unavoidable side effect. The magnitude of hyperprolactinemia is greater as compared to older antidopaminergic prokinetic agents. Awareness about levosulpiride and its effect on serum prolactin levels and careful drug history will help the clinician in reaching the exact diagnosis and avoiding unnecessary brain imaging.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: Structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 2.Pan JT. Neuroendocrine functions of dopamine. In: Stone TW, editor. CNS Neurotransmitters and Neuromodulators: Dopamine. Boca Raton, FL: CRC Press; 1996. pp. 213–31. [Google Scholar]
- 3.Manzanares J, Wagner EJ, LaVigne SD, Lookingland KJ, Moore KE. Sexual differences in kappa opioid receptor-mediated regulation of tuberoinfundibular dopaminergic neurons. Neuroendocrinology. 1992;55:301–7. doi: 10.1159/000126129. [DOI] [PubMed] [Google Scholar]
- 4.Toney TW, Manzanares J, Moore KE, Lookingland KJ. Sexual differences in the stimulatory effects of bombesin on tuberoinfundibular dopaminergic neurons. Brain Res. 1992;598:279–85. doi: 10.1016/0006-8993(92)90194-e. [DOI] [PubMed] [Google Scholar]
- 5.Moore KE, Demarest KT, Lookingland KJ. Stress, prolactin and hypothalamic dopaminergic neurons. Neuropharmacology. 1987;26:801–8. doi: 10.1016/0028-3908(87)90055-4. [DOI] [PubMed] [Google Scholar]
- 6.Haycock JW, Haycock DA. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multiple-site phosphorylation in vivo and in synaptosomes. J Biol Chem. 1991;266:5650–7. [PubMed] [Google Scholar]
- 7.Arbogast LA, Ben-Jonathan N. Tyrosine hydroxylase in the stalk-median eminence and posterior pituitary is inactivated only during the plateau phase of the preovulatory prolactin surge. Endocrinology. 1989;125:667–74. doi: 10.1210/endo-125-2-667. [DOI] [PubMed] [Google Scholar]
- 8.Arbogast LA, Voogt JL. Mechanisms of tyrosine hydroxylase regulation during pregnancy: Evidence for protein dephosphorylation during the prolactin surges. Endocrinology. 1991;129:2575–82. doi: 10.1210/endo-129-5-2575. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualini C, Guibert B, Leviel V. Short-term inhibitory effect of estradiol on tyrosine hydroxylase activity in tuberoinfundibular dopaminergic neurons in vitro. J Neurochem. 1993;60:1707–13. doi: 10.1111/j.1471-4159.1993.tb13394.x. [DOI] [PubMed] [Google Scholar]
- 10.Justin-Besancon L, Laville C. Antiemetic action of metoclopramide with respect to apomorphine and hydergine. C R Seances Soc Biol Fil. 1964;158:723–7. [PubMed] [Google Scholar]
- 11.Albibi R, McCallum RW. Metoclopramide: Pharmacology and clinical application. Ann Intern Med. 1983;98:86–95. doi: 10.7326/0003-4819-98-1-86. [DOI] [PubMed] [Google Scholar]
- 12.Reyntjens AJ. Clinical pharmacology and therapeutics of domperidone. Clin Res Rev. 1983;3:91–100. [Google Scholar]
- 13.Brogden RN, Carmine AA, Heel RC, Speight TM, Avery GS. Domperidone. A review of its pharmacological activity, pharmacokinetics and therapeutic efficacy in the symptomatic treatment of chronic dyspepsia and as an antiemetic. Drugs. 1982;24:360–400. doi: 10.2165/00003495-198224050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Leelakanok N, Holcombe A, Schweizer ML. Domperidone and risk of ventricular arrhythmia and cardiac death: A systematic review and meta-analysis. Clin Drug Investig. 2016;36:97–107. doi: 10.1007/s40261-015-0360-0. [DOI] [PubMed] [Google Scholar]
- 15.van Noord C, Dieleman JP, van Herpen G, Verhamme K, Sturkenboom MC. Domperidone and ventricular arrhythmia or sudden cardiac death: A population-based case-control study in the Netherlands. Drug Saf. 2010;33:1003–14. doi: 10.2165/11536840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.De Loose F. Domperidone in chronic dyspepsia: A pilot open study and a multicentre general practice crossover comparison with metoclopramide and placebo. Pharmacol Ther. 1979;2:140–6. [Google Scholar]
- 17.Tonini M, Cipollina L, Poluzzi E, Crema F, Corazza GR, De Ponti F. Review article: Clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004;19:379–90. doi: 10.1111/j.1365-2036.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 18.Lozano R, Concha MP, Montealegre A, de Leon L, Villalba JO, Esteban HL, et al. Effectiveness and safety of levosulpiride in the treatment of dysmotility-like functional dyspepsia. Ther Clin Risk Manag. 2007;3:149–55. doi: 10.2147/tcrm.2007.3.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]


