Abstract
This study examined the influence of the elongation of attached crossbridges and residual force enhancement on joint torque enhancement by the stretch-shortening cycle (SSC). Electrically evoked submaximal tetanic plantar flexions were adopted. Concentric contractions were evoked in the following three conditions: after 2 s isometric preactivation (ISO condition), after 1 s isometric then 1 s eccentric preactivation (ECC condition), and after 1 s eccentric then 1 s isometric preactivation (TRAN condition). Joint torque and fascicle length were measured during the concentric contraction phase. While no differences in fascicle length were observed among conditions at any time points, joint torque was significantly higher in the ECC than TRAN condition at the onset of concentric contraction. This difference would be caused by the dissipation of the elastic energy stored in the attached crossbridges induced by eccentric preactivation in TRAN condition due to 1 s transition phase. Furthermore, joint torques observed 0.3 and 0.6 s after concentric contraction were significantly larger in the ECC and TRAN conditions than in the ISO condition while no difference was observed between the ECC and TRAN conditions. Since the elastic energy stored in the attached crossbridges would have dissipated over this time frame, this result suggests that residual force enhancement induced by eccentric preactivation also contributes to joint torque enhancement by the SSC.
Keywords: muscle, crossbridge, titin, elastic energy, eccentric contraction
1. Introduction
In almost all human movements, each joint moves dynamically through the actions of concentric and eccentric contractions. Thus, to understand the mechanism of human movements, the force-generating capability of these contractions during complicated tasks should be examined. One of the most interesting themes related to these processes is the stretch-shortening cycle (SSC) [1–3]. Generally, the SSC is comprised of a concentric contraction phase (main contraction) and a preceding eccentric contraction phase (preliminary contraction). In addition, there is an isometric contraction phase (transition period) between the eccentric and concentric contraction phases, which is sometimes called the amortization phase and/or coupling time [4–6]. Because of the preceding eccentric contraction, which is called countermovement [7–9], muscle force/joint torque attained during the concentric contraction phase is increased [10,11].
To understand the mechanism of muscle force/joint torque enhancement by the SSC (i.e. SSC effect), many studies have been conducted in vivo [12,13], in situ [1,14,15] and in silico [16,17]. Taken together, these studies suggest that the SSC effect may be caused by the following factors: the stretch reflex [18,19], tendon elongation [11,13], preactivation [16,20] and residual force enhancement [21,22]. However, there are other studies that have reported that some of these factors may not contribute to the SSC effect [16,23,24]. Further investigation is therefore needed for a clearer understanding of the factors contributing to the SSC effect.
Recently, Fukutani et al. [25] suggested that preactivation and residual force enhancement contribute to the SSC effect in plantar flexors in humans. In particular, the authors found that joint torque observed during the concentric contraction phase was larger when isometric preactivation was conducted than when it was not. In addition, a larger SSC effect was observed when preactivation was conducted by eccentric rather than isometric contraction. Since residual force enhancement is evoked by eccentric contraction [21,26], the authors concluded that this factor may also contribute to the SSC effect.
However, these results should be interpreted with caution. In addition to between-group differences in whether or not eccentric contraction was included as preactivation, joint torque at the onset of concentric contraction was also different, i.e. larger in the condition where eccentric rather than isometric preactivation was conducted. This difference in joint torque, which has also been confirmed in the previous study [1], may therefore contribute to a larger SSC effect following eccentric preactivation rather than residual force enhancement.
Regarding this point, the following concept should be useful to solve the above problem. Ruiter et al. [27] separated the components that contributed to the joint torque enhancement by active lengthening into a crossbridge-related component and a non-crossbridge-related component. The first component is related to the elongation of attached crossbridges and the second component is related to residual force enhancement [28–30]. Because the former is caused by elongation and rotation of attached crossbridges by active lengthening, that is, eccentric contraction [29,31], this component is dissipated quickly due to the short time frame over which the crossbridge cycle occurs. Specifically, an attached crossbridge is detached from an actin filament, resulting in the dissipation of elastic energy stored in the attached crossbridge. On the other hand, the latter component would be caused by titin–actin interaction and/or increased titin stiffness in the presence of Ca2+ [32,33], and consequently, would last several seconds [34,35]. Thus, these components can be distinguished from one other by the time after active lengthening in which they occur, that is, whether they are a short (elongation of attached crossbridges-related) or long-lasting (residual force enhancement-related) component.
Therefore, the purpose of this study was to distinguish the influences of the elongation of attached crossbridges and residual force enhancement on the SSC effect. To this end, the following three conditions were set: (1) concentric contraction just after isometric preactivation, (2) concentric contraction just after eccentric preactivation, (3) concentric contraction after eccentric preactivation then 1 s isometric preactivation, i.e. where a substantial transition period was included between eccentric preactivation and concentric contraction. In condition 3, joint torque enhancement obtained by an elastic energy stored in the attached crossbridges at the onset of concentric contraction should disappear during the transition period. Thus, if joint torque enhancement is caused by the elongation of attached crossbridges only, joint torque during concentric contraction should be similar between conditions 1 and 3. On the other hand, if the influence of residual force enhancement is substantial, joint torque during concentric contraction would be larger in condition 3 than in condition 1.
2. Material and methods
2.1. Subjects
Twelve healthy young men (age, 24.9 ± 3.8 years; height, 1.73 ± 0.04 m; body mass, 67.5 ± 7.7 kg; all values presented as mean ± s.d.) voluntarily participated in this study.
2.2. Experimental set-ups
Similar to a previous study [25], plantar flexion (PF) with a dynamometer (Biodex; SAKAImed, Tokyo, Japan) was adopted as the tested motion. Subjects lay on their back on the dynamometer, and the knee and hip joints were fixed at 0 degrees (i.e. anatomical position). The ankle joint was fixed by the attachment of the dynamometer, with the centres of rotation of the attachment and ankle joint aligned as precisely as possible.
All muscle contractions were evoked by electrical stimulation (SEN-3401; Nihon Kohden, Tokyo, Japan) to rule out the influence of the stretch reflex, which is one of the mechanisms proposed to contribute to the SSC effect [18,19]. Electrical stimulation electrodes were placed on the belly of the triceps surae. Specifically, an anode (4 × 5 cm) was placed on the proximal side of the triceps surae, while a cathode (4 × 5 cm) was placed on the distal side of the soleus. The parameters of electrical stimulation were as follows: pulse frequency, 50–100 Hz which was sufficient to evoke a fully fused contraction; pulse duration, 0.5 ms; and train duration, 4.0 s. To determine the intensity of electrical stimulation to be used, maximal voluntary isometric contraction in PF was performed with the ankle joint angle at PF0° (anatomical position). The peak joint torque recorded in this contraction was set as 100% intensity. The intensity of electrical stimulation was adjusted to evoke 25% intensity at the identical ankle joint angle. This electrical stimulation intensity was applied to all contractions. Joint torque and joint angle were recorded with a sampling frequency of 4000 Hz (Power lab 16/30; ADInstruments, Bella Vista, Australia).
2.3. Motion control of the dynamometer and settings of electrical stimulation
Three conditions were tested in which the following was carried out: (1) a 1 s concentric contraction was conducted immediately after 2 s isometric preactivation (ISO condition hereafter), (2) a 1 s concentric contraction was conducted after 1 s isometric then 1 s eccentric preactivation (ECC condition hereafter) and (3) a 1 s concentric contraction was conducted after 1 s eccentric then 1 s isometric preactivation so that there was a transition period (i.e. an isometric contraction phase) between eccentric and concentric contractions (TRAN condition hereafter; figure 1). The range of motion of the ankle joint was from PF15° to dorsiflexion 15°. Joint angular velocities of the eccentric and concentric contractions were set at 45° s−1. Owing to the acceleration and deceleration phase of the attachment movements, the duration of the eccentric and concentric contractions was 1 s.
Figure 1.
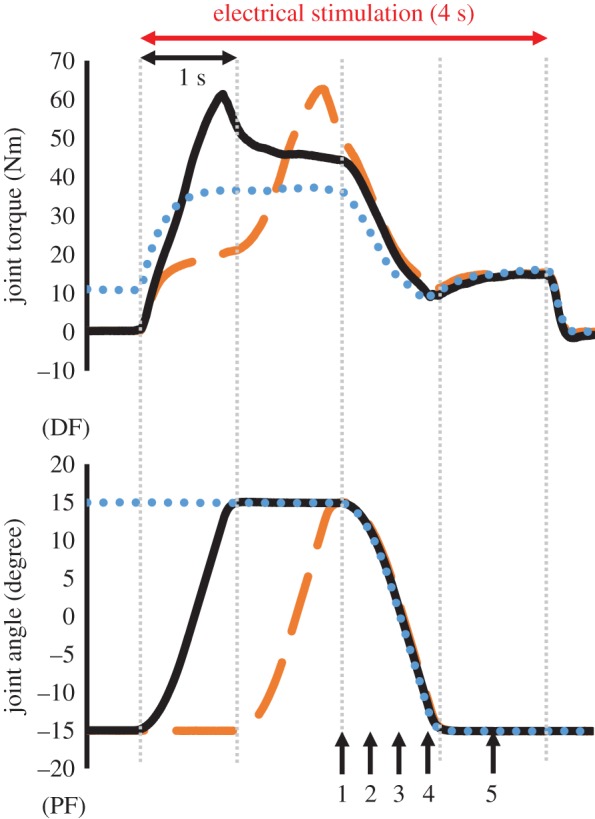
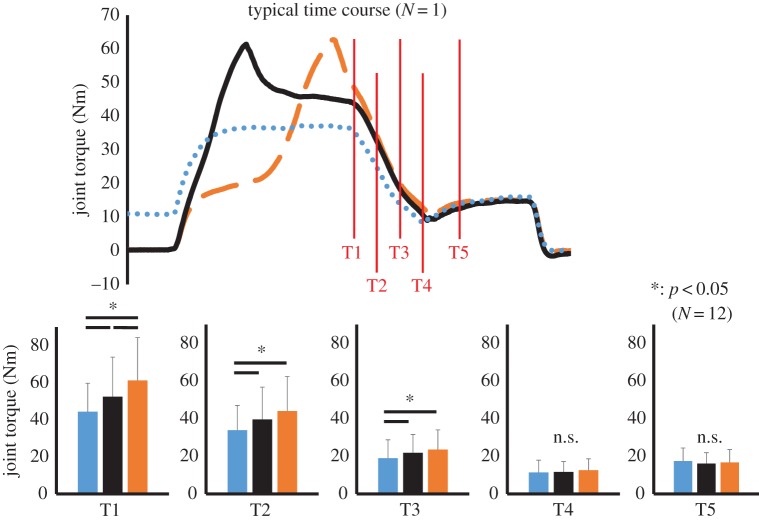
Typical time courses of joint torque and joint angle changes in the three conditions tested (blue dotted line, ISO condition; orange dashed line, ECC condition; black solid line, TRAN condition). The time points at which joint torque, fascicle length and pennation angle were measured (T1–T5) are shown by black arrows.
In the ISO condition, muscle contraction was started at DF15° in an isometric contraction state. After 2 s passed, the ankle joint was moved to PF15° with a shortening duration of 1 s. Then, the ankle joint angle was held at PF15° for 1 s before stimulation was stopped. In the ECC condition, muscle contraction was started at the PF15° in an isometric contraction state. After 1 s passed, the ankle joint was moved to DF15° with a 1 s lengthening duration. Immediately after reaching DF15°, the ankle joint was moved to PF15° with a shortening duration of 1 s. The ankle joint angle was held at PF15° for 1 s, and then the stimulation was stopped. Finally, in the TRAN condition, muscle contraction was started at PF15°. Immediately after the onset of stimulation, the ankle joint was moved to DF15° with a lengthening duration of 1 s. Then, the joint angle was held at DF15° for 1 s (i.e. the transition period). After 1 s passed, the joint was moved to PF15° with a 1 s shortening duration. The ankle joint angle was then held at PF15° for 1 s before stimulation was stopped.
2.4. Ultrasonographic measurement
Ultrasonographic measurement (SSD-3500; Aloka, Tokyo, Japan) was performed simultaneously during the above three trials to examine whether changes in architectural characteristics (i.e. fascicle length and pennation angle) were related to the SSC effect. A linear array probe (7.5 MHz, field of view: 6 × 6 cm, UST-5710; Aloka, Tokyo, Japan) was used to obtain images of the belly of the medial gastrocnemius muscle. The ultrasonographic probe was fixed onto the skin by using underwrap and surgical tape. Fascicle length was defined as the distance between the intersection of the superficial aponeurosis and fascicle and the intersection of the deep aponeurosis and fascicle, while pennation angle was defined as the acute angle formed by the fascicle and deep aponeurosis. Sampling frequency of ultrasonography was 30 Hz. Synchronization of joint torque and joint angle was done by inserting a pulse into the ultrasonographic recording machine. Acquired images were analysed by ImageJ 1.47v software (National Institute of Health, Bethesda, MD, USA).
2.5. Analyses and measurements
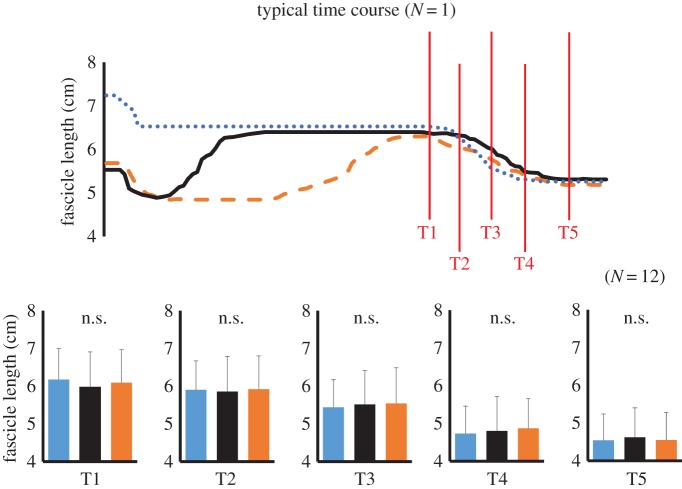
Because we focused on the joint torque attained during the concentric contraction phase, we compared the joint torques recorded at the following five points: at the onset of concentric contraction (T1), 300 ms after the onset of concentric contraction (T2), 600 ms after the onset of concentric contraction (T3), 900 ms after the onset of concentric contraction (T4), and 1400 ms after the onset of concentric contraction (T5) (see figure 1, black arrows). Fascicle length and pennation angle were also measured at these time points. In addition, to confirm whether elongation of the fascicle occurred in TRAN and ECC conditions, the magnitude of elongation of the fascicle was examined by subtracting the fascicle length at the onset of stimulation from that at the end of eccentric contraction. Furthermore, because the fascicle shortened after inserting the stimulation in TRAN and ECC conditions (figure 2), the shortest fascicle length observed after inserting stimulation was subtracted from the fascicle length at the end of eccentric contraction to calculate the actual elongation of the fascicle.
Figure 2.
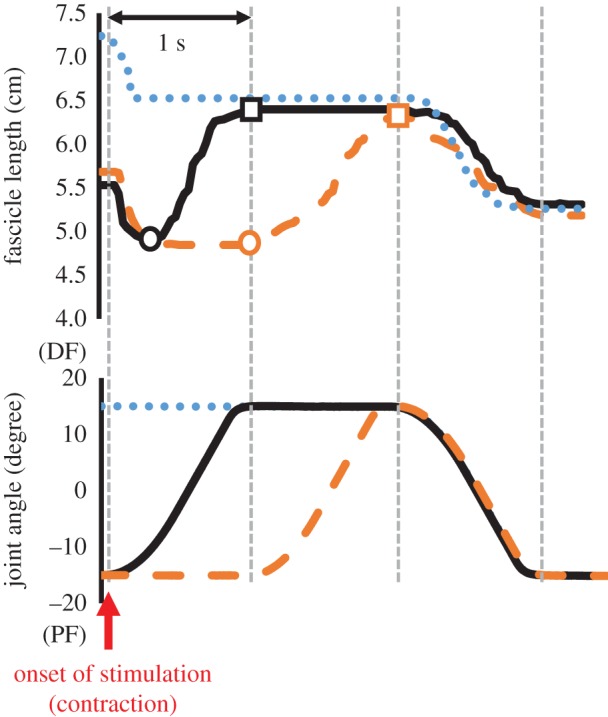
Typical time courses of fascicle length and joint angle changes in the three conditions tested (blue dotted line, ISO condition; orange dashed line, ECC condition; black solid line, TRAN condition). Circles indicate the timing at which fascicle length was the shortest during the eccentric contraction, and squares indicate the timing at which fascicle length was the longest (i.e. end of the eccentric contraction). Note that fascicle length shortened in the initial phase of contractions in all conditions, and fascicle was elongated during eccentric contraction phase in ECC and TRAN conditions.
In our previous study [25], which adopted similar experimental settings, coefficients of variation for joint torque, fascicle length and pennation angle were evaluated. Coefficients of variation for these were 2.3%, 1.1% and 1.6%, respectively, and their intraclass correlations were 0.995, 0.993 and 0.989, respectively.
2.6. Statistics
A two-way analysis of variance (ANOVA) with repeated measures was adopted to examine the interaction and main effect of condition and time on joint torque, fascicle length and pennation angle. If the interaction was significant, a one-way ANOVA with repeated measures followed by a post hoc test (Bonferroni's correction) was conducted. Effect size for ANOVA was calculated as the partial η2. To confirm whether the fascicle was elongated or not during the eccentric contraction phase, and to compare the magnitude of elongation of the fascicle between TRAN and ECC conditions, paired t-test was conducted. Statistical analyses were performed using SPSS version 20 software (IBM, Tokyo, Japan), with the level of statistical significance set at p < 0.05.
3. Results
For joint torque, a two-way ANOVA with repeated measures revealed a significant interaction between condition and joint angle (F = 16.505, partial η2 = 0.600, p < 0.001). Subsequent analyses showed that force was significantly larger in the order of ECC, TRAN and ISO conditions at T1 (p = 0.001–0.028). At T2 and T3, joint torque was larger in the ECC and TRAN conditions than in the ISO condition (p = 0.001–0.042), but was not different between ECC and TRAN (p = 0.131 for T2 and p = 0.378 for T3). No differences were observed among conditions at T4 and T5 (p = 0.169–0.999; figure 3).
Figure 3.
Joint torque observed at the following five points: the onset of concentric contraction (T1), and 300, 600, 900 and 1400 ms after the onset of concentric contraction (T2, T3, T4 and T5, respectively). The blue dotted line and bar show joint torque in the ISO condition, the orange dashed line and bar show joint torque in the ECC condition, and the black solid line and bar show joint torque in the TRAN condition. Asterisk indicates a significant difference between conditions (p < 0.05).
Although a significant interaction was found for fascicle length and pennation angle (fascicle length: F = 2.656, partial η2 = 0.195, p = 0.012; pennation angle: F = 2.160, partial η2 = 0.164, p = 0.038), subsequent analyses showed that no significant difference was observed among conditions for these variables (figure 4 and table 1).
Figure 4.
Fascicle length observed at the following five points: the onset of concentric contraction (T1), and 300, 600, 900 and 1400 ms after the onset of concentric contraction (T2, T3, T4 and T5, respectively). The blue dotted line and bar show joint torque in the ISO condition, the orange dashed line and bar show joint torque in the ECC condition and the black solid line and bar show joint torque in the TRAN condition.
Table 1.
Pennation angle measured at the onset of concentric contraction (T1), and 300, 600, 900 and 1400 ms after the onset of concentric contraction (T2, T3, T4 and T5, respectively). No significant differences (p < 0.05) among conditions.
| pennation angle (degree) | |||||
|---|---|---|---|---|---|
| Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | |
| ISO | 21.7 ± 3.1 | 22.8 ± 3.7 | 24.5 ± 3.8 | 28.3 ± 4.6 | 29.5 ± 4.2 |
| TRAN | 22.6 ± 2.5 | 23.1 ± 2.8 | 24.3 ± 3.1 | 27.7 ± 3.7 | 28.9 ± 3.1 |
| ECC | 22.1 ± 2.4 | 22.8 ± 2.4 | 24.0 ± 2.6 | 27.0 ± 3.0 | 29.1 ± 2.6 |
The magnitude of elongation of the fascicle calculated by subtracting the fascicle length at the onset of stimulation from that at the end of eccentric contraction was not different between TRAN (1.0 ± 0.5 cm) and ECC (1.0 ± 0.6 cm) conditions (p = 0.901). Furthermore, the magnitude of elongation of the fascicle calculated by subtracting the shortest fascicle length observed after inserting stimulation from the fascicle length at the end of eccentric contraction was not different between TRAN (1.6 ± 0.5 cm) and ECC (1.7 ± 0.4 cm) conditions (p = 0.564; figure 5).
Figure 5.
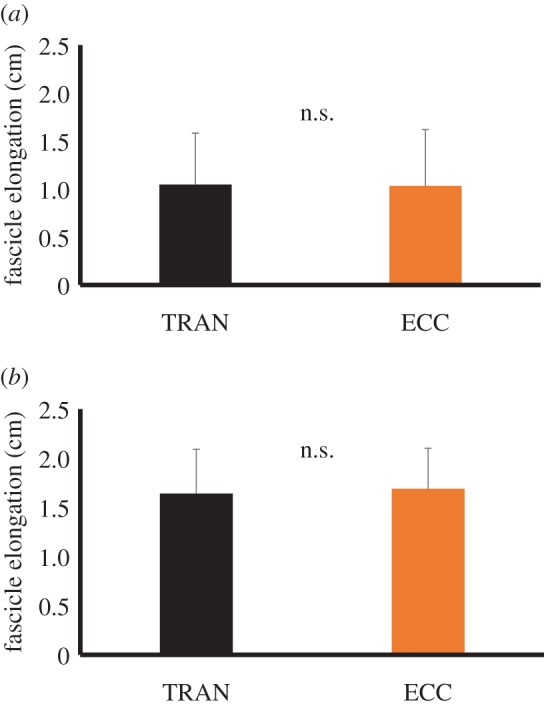
Magnitude of the fascicle elongation in TRAN and ECC conditions. (a) The magnitude of fascicle elongation calculated by subtracting the fascicle length before stretch from that at the end of eccentric contraction. (b) The magnitude of fascicle elongation calculated by subtracting the shortest fascicle length observed during the eccentric contraction phase from the fascicle length at the end of eccentric contraction (figure 2).
4. Discussion
The primary purpose of this study was to distinguish between the influence of the elongation of attached crossbridges and residual force enhancement on joint torque enhancement by the SSC. A significant difference in joint torque was found between the ECC and TRAN conditions at the onset of concentric contraction even though a similar magnitude of active lengthening, which determines the magnitude of residual force enhancement [21,36], was included in preactivation. This difference should be caused by an elongation of attached crossbridges induced by the eccentric contraction conducted just before the concentric contraction. Although the extent of elongation of attached crossbridges (i.e. elastic energy stored in the attached crossbridges) at the end of eccentric contraction should be the same between ECC and TRAN conditions, elastic energy stored in the attached crossbridges induced by eccentric contraction in TRAN condition disappeared due to the transition period between eccentric and concentric contraction. On the other hand, it was observed in this study that joint torque attained during the latter phase of concentric contraction (300 and 600 ms after the onset of concentric contraction) was still significantly larger in the ECC and TRAN conditions than in the ISO condition, despite the elastic energy stored in the attached crossbridges already having dissipated. Thus, a mechanism other than elongation of attached crossbridges should contribute to the joint torque enhancement by SSC. This mechanism would be residual force enhancement.
Joint torque at the onset of concentric contraction was larger in the ECC than in the TRAN conditions. This would be caused by elastic energy stored in the attached crossbridges [29,31]. In the ECC condition, concentric contraction was commenced ‘immediately after’ the eccentric contraction. During eccentric contraction, attached crossbridges are elongated so that the magnitude of force per attached crossbridge is increased due to an increase in X-distance (i.e. distance from the equilibrium position) [37,38]. This causes larger joint torque at the onset of concentric contraction. However, this effect should be quickly dissipated because elastic energy stored in the attached crossbridges is released due to the detachment of crossbridges from actin filaments. The duration of the attached state would be at most 200–300 ms [39–41]. Indeed, the difference in joint torque between the ECC and TRAN conditions, which appears to be attributable to the elastic energy stored in the attached crossbridges, disappeared within the early part of concentric contraction (within 300 ms) in this study. This result is in line with previous studies demonstrating that joint torque/force/power enhancement by active lengthening was dissipated within 300 ms [42,43]. Thus, it is reasonable to consider that the elastic energy stored in the attached crossbridges contributes to force enhancement by the SSC, especially during the early phase of concentric contraction.
On the other hand, joint torque enhancement observed in the latter phase (300 ms (T2) and 600 ms (T3) after the onset of concentric contraction) in the ECC and TRAN conditions than in ISO condition cannot be explained by the elastic energy stored in the attached crossbridges, because the influence of the elastic energy stored in the attached crossbridges should have disappeared in the early phase of concentric contraction as described above. In addition, Bosco et al. [2] discussed in their manuscript that it was somewhat surprising that large mechanical work by active lengthening was observed despite a long transition period (coupling time) of about 150 ms between eccentric and concentric contractions. This unexplained joint torque enhancement is likely to have instead been caused by residual force enhancement. Residual force enhancement is defined as the increase in muscle force (joint torque) induced by active lengthening [21,22]. Although the definition of residual force enhancement is that the force attained during ‘isometric contraction’ after active lengthening is higher than the force attained during isometric contraction without preliminary active lengthening at the same muscle length [21,26,44], this phenomenon should be applicable even for force attained during ‘concentric contraction’ after active lengthening. This is because residual force enhancement occurs through titin–actin interactions and/or increased titin stiffness [32,33], and increased force through this mechanism should affect not only isometric contractions but also concentric contractions. Therefore, it is reasonable to consider that residual force enhancement would be involved in joint torque enhancement by the SSC [1,25,45].
In the current experiment, the isometric joint torque after eccentric contraction (i.e. transition period) in the TRAN condition was larger than the isometric joint torque at the same joint angle in the ISO condition (figure 1). Therefore, it is reasonable to consider that substantial residual force enhancement occurred in the TRAN condition, and that same amount of residual force enhancement occurred in the ECC condition because the same magnitude of active lengthening was included in the TRAN and ECC conditions. Because residual force enhancement lasts for several seconds [34,35], this effect can explain the joint torque enhancement observed in the latter phase of the concentric contraction in the ECC and TRAN compared to the ISO condition. On the other hand, joint torque enhancement disappeared by 900 ms (T4) and 1400 ms (T5) after the onset of concentric contraction. Because residual force enhancement should be caused by enhanced titin stiffness and should continue several seconds [32–35], it may be reasonable to expect that joint torque enhancement (SSC effect) would continue even in the T4 and T5. Possible explanations about this unexpected result are interaction with residual force depression [28,46] and short muscle length (plantar flexed position) at T4 and T5. First, residual force depression induced by active shortening (negative effect) can mask the effect of residual force enhancement (positive effect). For example, previous studies conducted active shortening after active lengthening (i.e. SSC), and reported similar results; residual force enhancement disappeared after active shortening [47,48]. Second, short muscle fibre length can also explain the observed result. Because muscle fibre length should be short at T4 and T5, in other words, muscle should operate in the ascending limb in this phase, the influence of residual force enhancement should become small due to small or negligible elongation of titin. This can explain the no-SSC effect at T4 and T5. The latter point can be clarified by conducting a similar experiment adopting much longer muscle length even after the end of active shortening, because this experiment can emphasize the influence of residual force enhancement (i.e. titin stiffness).
Although the same amount of joint angle changes (i.e. muscle–tendon complex elongation) was imposed during the eccentric contraction phase in TRAN and ECC conditions, there is a possibility that magnitude of fascicle elongation was different due to tendon elongation. Regarding this point, the magnitude of fascicle elongation was confirmed by ultrasonographic data. As a result, fascicle elongation did occur during the eccentric contraction phase in both conditions, and the magnitude of fascicle elongation was similar between TRAN and ECC conditions. Thus, it is reasonable to assume that elongation of attached crossbridges and residual force enhancement induced by fascicle elongation occurred in our experimental settings.
It is widely considered that tendon elongation is the primary mechanism of the SSC effect [12,13,49]. According to the widely accepted method of evaluating tendon length changes, tendon length change can be estimated by fascicle length changes corrected by pennation angle and joint angle changes [13,50,51]. Considering the fact that joint angle change during the concentric contraction phase was identical among conditions and that no significant differences were observed in fascicle length and pennation angle among conditions (table 1) in our study, tendon length change was also considered to be the same among conditions. Therefore, the difference in joint torque observed among conditions in this study cannot be explained by elastic energy stored in the tendon [52,53] and/or muscle–tendon interaction [54,55]. The negligible contribution of tendon elongation may have been due to weak contraction intensity, which corresponded to 25% of the intensity of maximal voluntary isometric contraction at PF0°. This study adopted submaximal electrically evoked contractions instead of maximal electrically evoked contractions due to intolerable pain caused by the latter, and instead of maximal and/or submaximal voluntary contractions to avoid complicated neural modulations that affect joint torque [56–58]. As a result of adopting relatively low intensity, the magnitude of joint torque difference was not so large among condition (e.g. the largest difference observed at the T1 was about 10 Nm). This joint torque difference should not be enough to induce substantial tendon elongation. Although further studies are needed to clarify the influence of tendon elongation on the SSC effect in higher intensity contraction conditions, we can say based on the current data that differences in joint torque among current experimental conditions were not caused by tendon elongation and/or muscle architecture.
A limitation of this study is that we measured fascicle length from the medial gastrocnemius only. Previous studies reported that medial gastrocnemius and soleus behaved differentially [59]. Thus, there is a possibility that behaviour of soleus and lateral gastrocnemius which contribute to the PF torque made the interpretation more complicated. However, although the magnitude of length changes of these three muscles at a given joint angle changes may be different, the behaviours of these three muscles should be the same between TRAN and ECC conditions. Therefore, this factor would not affect our main result.
When considering the practical application of the current results, the influence of fibre-type dependence on the relative contribution of the elongation of attached crossbridges and residual force enhancement to the SSC effect should be mentioned. Regarding the elongation of attached crossbridges, the duration of the attached state (ATPase rate) is shorter in fast-twitch than slow-twitch fibres [60,61]. Thus, elastic energy stored in the attached crossbridge by active lengthening is released more quickly in fast-twitch fibres. Consequently, the duration over which the elastic energy stored in the attached crossbridges contributes to joint torque enhancement should be shorter in fast-twitch than in slow-twitch fibres. In addition, residual force enhancement, which is caused by titin elasticity [32,33], should also be affected by fibre type because the titin isoform found in slow-twitch fibres is longer than the isoform found in fast-twitch fibres [62,63]. Specifically, the shorter titin isoform produces a larger force at a shorter sarcomere length (muscle length) compared with the longer titin isoform. Thus, even in the same absolute muscle length (joint angle), the influence of residual force enhancement should vary among humans depending on which fibre type is more abundant [64]. Taking these into account, the influence of fibre type on the relative contribution of the elongation of attached crossbridges and residual force enhancement on the SSC effect should be examined in the future.
5. Conclusion
In conclusion, it was found that joint torque was larger in the ECC than in the TRAN condition despite both conditions involving the same magnitude of active lengthening. Thus, the joint torque enhancement observed in these conditions can be attributable to the elongation of attached crossbridges. In addition, the joint torque enhancement seen in these conditions during the latter phase of concentric contraction, after the influence of the elongation of attached crossbridges had dissipated, points to the involvement of residual force enhancement. Therefore, both the elongation of attached crossbridges and residual force enhancement contribute to joint torque enhancement by the SSC.
Supplementary Material
Acknowledgements
We thank all the subjects who participated in this study.
Ethics
Ethical approval was granted by the Ethics Committee on Human Research of Ritsumeikan University (IRB-2014-026). The purpose and risks of this study were explained to each volunteer, and written informed consent was obtained from all participants. This study conformed to the Declaration of Helsinki.
Data accessibility
From the view of the protection of personal information, data accessibility is partly restricted by the ethical committee. Individual values of the joint torque, fascicle length and pennation angle were provided in the electronic supplementary material while individual values of year, height and body mass were not provided.
Authors' contributions
All authors contributed to the design of this experiment. A.F. and J.M. performed the experiments. A.F. analysed the data. All authors were involved in the interpretation of the data and the writing and editing of the manuscript. All authors approved the final version of the manuscript. This work was done at the Ritsumeikan University, Kusatsu, Shiga, Japan.
Competing interests
The authors report no competing interests for this work.
Funding
This study was partly supported by a Grant-in-Aid for Japan Society for the Promotion of Science Fellows (13J03159), a Grant-in-Aid for Young Scientists B (26750313).
References
- 1.Cavagna GA, Dusman B, Margaria R. 1968. Positive work done by a previously stretched muscle. J. Appl. Physiol. 24, 21–32. [DOI] [PubMed] [Google Scholar]
- 2.Bosco C, Ito A, Komi PV, Luhtanen P, Rahkila P, Rusko H, Viitasalo JT. 1982. Neuromuscular function and mechanical efficiency of human leg extensor muscles during jumping exercises. Acta. Physiol. Scand. 114, 543–550. (doi:10.1111/j.1748-1716.1982.tb07022.x) [DOI] [PubMed] [Google Scholar]
- 3.Komi PV. 2000. Stretch-shortening cycle: a powerful model to study normal and fatigued muscle. J. Biomech. 33, 1197–1206. (doi:10.1016/S0021-9290(00)00064-6) [DOI] [PubMed] [Google Scholar]
- 4.Bosco C, Rusko H. 1983. The effect of prolonged skeletal muscle stretch-shortening cycle on recoil of elastic energy and on energy expenditure. Acta Physiol. Scand. 119, 219–224. (doi:10.1111/j.1748-1716.1983.tb07331.x) [DOI] [PubMed] [Google Scholar]
- 5.Aura O, Komi PV. 1987. Coupling time in stretch shortening cycle: influence on mechanical efficiency and elastic characteristics of leg extensor muscles. Biomechanics XA, 507–512. [Google Scholar]
- 6.Toumi H, Thiery C, Maitre S, Martin A, Vanneuville G, Poumarat G. 2001. Training effects of amortization phase with eccentric/concentric variations—the vertical jump. Int. J. Sports Med. 22, 605–610. (doi:10.1055/s-2001-18525) [DOI] [PubMed] [Google Scholar]
- 7.Komi PV. 1984. Physiological and biomechanical correlates of muscle function: effects of muscle structure and stretch-shortening cycle on force and speed. Exerc. Sport Sci. Rev. 12, 81–121. (doi:10.1249/00003677-198401000-00006) [PubMed] [Google Scholar]
- 8.Svantesson U, Ernstoff B, Bergh P, Grimby G. 1991. Use of a Kin-Com dynamometer to study the stretch-shortening cycle during plantar flexion. Eur. J. Appl. Physiol. Occup. Physiol. 62, 415–419. (doi:10.1007/BF00626613) [DOI] [PubMed] [Google Scholar]
- 9.Kubo K, Kawakami Y, Fukunaga T. 1999. Influence of elastic properties of tendon structures on jump performance in humans. J. Appl. Physiol. 87, 2090–2096. [DOI] [PubMed] [Google Scholar]
- 10.Ettema GJ, Huijing PA, de Haan A. 1992. The potentiating effect of prestretch on the contractile performance of rat gastrocnemius medialis muscle during subsequent shortening and isometric contractions. J. Exp. Biol. 165, 121–136. [DOI] [PubMed] [Google Scholar]
- 11.Finni T, Ikegawa S, Komi PV. 2001. Concentric force enhancement during human movement. Acta Physiol. Scand. 173, 369–377. (doi:10.1046/j.1365-201X.2001.00915.x) [DOI] [PubMed] [Google Scholar]
- 12.Kubo K, Kanehisa H, Takeshita D, Kawakami Y, Fukashiro S, Fukunaga T. 2000. In vivo dynamics of human medial gastrocnemius muscle-tendon complex during stretch-shortening cycle exercise. Acta Physiol. Scand. 170, 127–135. (doi:10.1046/j.1365-201x.2000.00768.x) [DOI] [PubMed] [Google Scholar]
- 13.Kawakami Y, Muraoka T, Ito S, Kanehisa H, Fukunaga T. 2002. In vivo muscle fibre behaviour during counter-movement exercise in humans reveals a significant role for tendon elasticity. J. Physiol. 540, 635–646. (doi:10.1113/jphysiol.2001.013459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ettema GJ, Huijing PA, van Ingen Schenau GJ, de Haan A. 1990. Effects of prestretch at the onset of stimulation on mechanical work output of rat medial gastrocnemius muscle-tendon complex. J. Exp. Biol. 152, 333–351. [DOI] [PubMed] [Google Scholar]
- 15.Ettema GJ, van Soest AJ, Huijing PA. 1990. The role of series elastic structures in prestretch-induced work enhancement during isotonic and isokinetic contractions. J. Exp. Biol. 154, 121–136. [DOI] [PubMed] [Google Scholar]
- 16.Bobbert MF, Gerritsen KG, Litjens MC, Van Soest AJ. 1996. Why is countermovement jump height greater than squat jump height? Med. Sci. Sports Exerc. 28, 1402–1412. (doi:10.1097/00005768-199611000-00009) [DOI] [PubMed] [Google Scholar]
- 17.Nagano A, Komura T, Fukashiro S. 2004. Effects of the length ratio between the contractile element and the series elastic element on an explosive muscular performance. J. Electromyogr. Kinesiol. 14, 197–203. (doi:10.1016/S1050-6411(03)00085-3) [DOI] [PubMed] [Google Scholar]
- 18.Nichols TR, Houk JC. 1973. Reflex compensation for variations in the mechanical properties of a muscle. Science 181, 182–184. (doi:10.1126/science.181.4095.182) [DOI] [PubMed] [Google Scholar]
- 19.Dietz V, Schmidtbleicher D, Noth J. 1979. Neuronal mechanisms of human locomotion. J. Neurophysiol. 42, 1212–1222. [DOI] [PubMed] [Google Scholar]
- 20.Bobbert MF, Casius LJ. 2005. Is the effect of a countermovement on jump height due to active state development? Med. Sci. Sports Exerc. 37, 440–446. (doi:10.1249/01.MSS.0000155389.34538.97) [DOI] [PubMed] [Google Scholar]
- 21.Edman KA, Elzinga G, Noble MI. 1982. Residual force enhancement after stretch of contracting frog single muscle fibers. J. Gen. Physiol. 80, 769–784. (doi:10.1085/jgp.80.5.769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joumaa V, Leonard TR, Herzog W. 2008. Residual force enhancement in myofibrils and sarcomeres. Proc. R. Soc. B 275, 1411–1419. (doi:10.1098/rspb.2008.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Ingen Schenau GJ, Bobbert MF, de Haan A. 1997. Does elastic energy enhance work and efficiency in the stretch-shortening cycle? J. Appl. Biomech. 13, 389–415. (doi:10.1123/jab.13.4.389) [Google Scholar]
- 24.Walshe AD, Wilson GJ, Ettema GJ. 1998. Stretch-shorten cycle compared with isometric preload: contributions to enhanced muscular performance. J. Appl. Physiol. 84, 97–106. [DOI] [PubMed] [Google Scholar]
- 25.Fukutani A, Kurihara T, Isaka T. 2015. Factors of force potentiation induced by stretch-shortening cycle in plantarflexors. PLoS ONE 10, e0120579 (doi:10.1371/journal.pone.0120579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edman KA, Tsuchiya T. 1996. Strain of passive elements during force enhancement by stretch in frog muscle fibres. J. Physiol. 490, 191–205. (doi:10.1113/jphysiol.1996.sp021135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiter CJ, Didden WJ, Jones DA, de Haan A. 2000. The force-velocity relationship of human adductor pollicis muscle during stretch and the effects of fatigue. J. Physiol. 526, 671–681. (doi:10.1111/j.1469-7793.2000.00671.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott BC, Aubert XM. 1952. The force exerted by active striated muscle during and after change of length. J. Physiol. 117, 77–86. (doi:10.1113/jphysiol.1952.sp004755) [PMC free article] [PubMed] [Google Scholar]
- 29.Edman KA, Elzinga G, Noble MI. 1978. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J. Physiol. 281, 139–155. (doi:10.1113/jphysiol.1978.sp012413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nocella M, Cecchi G, Bagni MA, Colombini B. 2014. Force enhancement after stretch in mammalian muscle fiber: no evidence of cross-bridge involvement. Am. J. Physiol. Cell. Physiol. 307, C1123–C1129. (doi:10.1152/ajpcell.00290.2014) [DOI] [PubMed] [Google Scholar]
- 31.Lombardi V, Piazzesi G. 1990. The contractile response during steady lengthening of stimulated frog muscle fibres. J. Physiol. 431, 141–171. (doi:10.1113/jphysiol.1990.sp018324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers K, Schappacher-Tilp G, Jinha A, Leonard T, Nishikawa K, Herzog W. 2014. Titin force is enhanced in actively stretched skeletal muscle. J. Exp. Biol. 217, 3629–3636. (doi:10.1242/jeb.105361) [DOI] [PubMed] [Google Scholar]
- 33.Cornachione AS, Leite F, Bagni MA, Rassier DE. 2016. The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms. Am. J. Physiol. Cell. Physiol. 310, C19–C26. (doi:10.1152/ajpcell.00156.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HD, Herzog W. 2002. Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J. Physiol. 545, 321–330. (doi:10.1113/jphysiol.2002.018010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rassier DE, Herzog W. 2004. Effects of shortening on stretch-induced force enhancement in single skeletal muscle fibers. J. Biomech. 37, 1305– 1312. (doi:10.1016/j.jbiomech.2003.12.033) [DOI] [PubMed] [Google Scholar]
- 36.Bullimore SR, MacIntosh BR, Herzog W. 2008. Is a parallel elastic element responsible for the enhancement of steady-state muscle force following active stretch? J. Exp. Biol. 211, 3001–3008. (doi:10.1242/jeb.021204) [DOI] [PubMed] [Google Scholar]
- 37.Huxley AF. 1957. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255–318. [PubMed] [Google Scholar]
- 38.Huxley AF, Simmons RM. 1971. Proposed mechanism of force generation in striated muscle. Nature 233, 533–538. (doi:10.1038/233533a0) [DOI] [PubMed] [Google Scholar]
- 39.Stienen GJ, Blangé T, Schnerr MC. 1978. Tension responses of frog sartorius muscle to quick ramp-shaped shortenings and some effects of metabolic inhibition. Pflugers. Arch. 376, 97–104. (doi:10.1007/BF00581573) [DOI] [PubMed] [Google Scholar]
- 40.Curtin NA, Gilbert C, Kretzschmar KM, Wilkie DR. 1974. The effect of the performance of work on total energy output and metabolism during muscular contraction. J. Physiol. 238, 455–472. (doi:10.1113/jphysiol.1974.sp010537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finer JT, Simmons RM, Spudich JA. 1994. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119. (doi:10.1038/368113a0) [DOI] [PubMed] [Google Scholar]
- 42.Wilson GJ, Wood GA, Elliott BC. 1991. Optimal stiffness of series elastic component in a stretch-shorten cycle activity. J. Appl. Physiol. 70, 825–833. [DOI] [PubMed] [Google Scholar]
- 43.Cronin JB, McNair PJ, Marshall RN. 2001. Magnitude and decay of stretch-induced enhancement of power output. Eur. J. Appl. Physiol. 84, 575–581. (doi:10.1007/s004210100433) [DOI] [PubMed] [Google Scholar]
- 44.Sugi H, Tsuchiya T. 1988. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J. Physiol. 407, 215–229. (doi:10.1113/jphysiol.1988.sp017411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seiberl W, Power GA, Herzog W, Hahn D. 2015. The stretch-shortening cycle (SSC) revisited: residual force enhancement contributes to increased performance during fast SSCs of human m. adductor pollicis. Physiol. Rep. 3, e12401 (doi:10.14814/phy2.12401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maréchal G, Plaghki L. 1979. The deficit of the isometric tetanic tension redeveloped after a release of frog muscle at a constant velocity. J. Gen. Physiol. 73, 453–467. (doi:10.1085/jgp.73.4.453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HD, Herzog W, Leonard T. 2001. Effects of cyclic changes in muscle length on force production in in-situ cat soleus. J. Biomech. 34, 979–987. (doi:10.1016/S0021-9290(01)00077-X) [DOI] [PubMed] [Google Scholar]
- 48.Herzog W, Leonard TR. 2000. The history dependence of force production in mammalian skeletal muscle following stretch-shortening and shortening-stretch cycles. J. Biomech. 33, 531–542. (doi:10.1016/S0021-9290(99)00221-3) [DOI] [PubMed] [Google Scholar]
- 49.Kubo K, Kanehisa H, Fukunaga T. 2005. Effects of viscoelastic properties of tendon structures on stretch-shortening cycle exercise in vivo. J. Sports Sci. 23, 851–860. (doi:10.1080/02640410400022029) [DOI] [PubMed] [Google Scholar]
- 50.Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN. 2001. In vivo behaviour of human muscle tendon during walking. Proc. R. Soc. Lond. B 268, 229–233. (doi:10.1098/rspb.2000.1361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichtwark GA, Bougoulias K, Wilson AM. 2007. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J. Biomech. 40, 157–164. (doi:10.1016/j.jbiomech.2005.10.035) [DOI] [PubMed] [Google Scholar]
- 52.Alexander RM, Bennet-Clark HC. 1977. Storage of elastic strain energy in muscle and other tissues. Nature 265, 114–117. (doi:10.1038/265114a0) [DOI] [PubMed] [Google Scholar]
- 53.Morgan DL, Proske U, Warren D. 1978. Measurements of muscle stiffness and the mechanism of elastic storage of energy in hopping kangaroos. J. Physiol. 282, 253–261. (doi:10.1113/jphysiol.1978.sp012461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffiths RI. 1991. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J. Physiol. 436, 219–236. (doi:10.1113/jphysiol.1991.sp018547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts TJ, Marsh RL, Weyand PG, Taylor CR. 1997. Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113–1115. (doi:10.1126/science.275.5303.1113) [DOI] [PubMed] [Google Scholar]
- 56.Westing SH, Seger JY, Karlson E, Ekblom B. 1988. Eccentric and concentric torque-velocity characteristics of the quadriceps femoris in man. Eur. J. Appl. Physiol. Occup. Physiol. 58, 100–104. (doi:10.1007/BF00636611) [DOI] [PubMed] [Google Scholar]
- 57.Westing SH, Cresswell AG, Thorstensson A. 1991. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur. J. Appl. Physiol. Occup. Physiol. 62, 104–108. (doi:10.1007/BF00626764) [DOI] [PubMed] [Google Scholar]
- 58.Webber S, Kriellaars D. 1997. Neuromuscular factors contributing to in vivo eccentric moment generation. J. Appl. Physiol. 83, 40–45. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann GP. 1985. Muscle-tendon interaction and elastic energy usage in human walking. J. Appl. Physiol. 99, 603–608. (doi:10.1152/japplphysiol.00189.2005) [Google Scholar]
- 60.Goldspink G, Larson RE, Davies RE. 1970. The immediate energy supply and the cost of maintenance of isometric tension for different muscles in the hamster. Zeitschrift. Für. Vergleichende. Physiologie. 66, 389–397. (doi:10.1007/BF00299938) [Google Scholar]
- 61.Millar NC, Homsher E. 1992. Kinetics of force generation and phosphate release in skinned rabbit soleus muscle fibers. Am. J. Physiol. 262, C1239–C1245. [DOI] [PubMed] [Google Scholar]
- 62.Neagoe C, Opitz CA, Makarenko I, Linke WA. 2003. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J. Muscle. Res. Cell. Motil. 24, 175–189. (doi:10.1023/A:1026053530766) [DOI] [PubMed] [Google Scholar]
- 63.Prado LG, Makarenko I, Andresen C, Krüger M, Opitz CA, Linke WA. 2005. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol. 126, 461–480. (doi:10.1085/jgp.200509364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramsey KA, Bakker AJ, Pinniger GJ. 2010. Fiber-type dependence of stretch-induced force enhancement in rat skeletal muscle. Muscle Nerve 42, 769–777. (doi:10.1002/mus.21744) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
From the view of the protection of personal information, data accessibility is partly restricted by the ethical committee. Individual values of the joint torque, fascicle length and pennation angle were provided in the electronic supplementary material while individual values of year, height and body mass were not provided.