Abstract
ATP-sensitive potassium channels (KATP channels) are critical nutrient sensors in many mammalian tissues. In the pancreas, KATP channels are essential for coupling glucose metabolism to insulin secretion. While orthologous genes for many components of metabolism–secretion coupling in mammals are present in lower vertebrates, their expression, functionality and ultimate impact on body glucose homeostasis are unclear. In this paper, we demonstrate that zebrafish islet β-cells express functional KATP channels of similar subunit composition, structure and metabolic sensitivity to their mammalian counterparts. We further show that pharmacological activation of native zebrafish KATP using diazoxide, a specific KATP channel opener, is sufficient to disturb glucose tolerance in adult zebrafish. That β-cell KATP channel expression and function are conserved between zebrafish and mammals illustrates the evolutionary conservation of islet metabolic sensing from fish to humans, and lends relevance to the use of zebrafish to model islet glucose sensing and diseases of membrane excitability such as neonatal diabetes.
Keywords: KATP, metabolism, pancreas, zebrafish, ion channels
1. Introduction
In the pancreatic β-cell, ATP-sensitive potassium (KATP) channels link glucose metabolism and insulin secretion and are essential to the normal regulation of plasma glucose and other nutrients [1,2]. At low glucose, intracellular [ATP]/[ADP] is low, and KATP channels are open, hyperpolarizing the cell membrane. As plasma glucose rises, it enters β-cells through glucose transporter 2 (GLUT2), increasing [ATP]/[ADP] which closes KATP channels, inducing plasma membrane depolarization and opening voltage-dependent Ca2+ channels (VDCCs). Calcium influx through VDCCs subsequently triggers insulin secretion (electronic supplementary material, figure S1a). The predominant role of KATP channels is illustrated by the striking disease consequences of KATP mutations. Loss-of-function mutations result in congenital hyperinsulinism [3], whereas gain-of-function (GOF) mutations cause neonatal diabetes mellitus (NDM) [4,5], and polymorphisms are associated with the development of type 2 diabetes [6].
KATP channels have been well characterized in multiple mammalian tissues, and mechanisms coupling metabolism to insulin secretion have been well established in humans and other mammals. However, whether KATP channel structure or function, as well as insulin secretion mechanisms, are conserved in and physiologically significant for lower vertebrates remains unclear. Studies in the zebrafish, Danio rerio, indicate that KATP channels may be physiologically significant in fishes: treatment of larvae with pharmacological activators of mammalian KATP channels or transgenic expression of mammalian KATP channels with GOF mutations is sufficient to raise larval whole-body glucose [7]. Conversely, treatment of larvae with compounds that can close mammalian KATP channels, or transgenic expression of mammalian KATP channels with dominant-negative mutations, is sufficient to lower whole-larval glucose [7,8].
However, there have been very few mechanistic studies of insulin secretion in fishes, only a handful of papers even mention KATP channels in zebrafish [7,9–11], and direct analysis of KATP expression and functional characterization is lacking. We have now developed approaches for efficiently identifying and isolating zebrafish islets, and for electrophysiological analysis of isolated β-cells. We show that zebrafish β-cells express functional KATP channels with similar regulation, subunit composition and pharmacology to their mammalian counterparts, and that pharmacologic KATP channel openers can disrupt glucose tolerance in adult fish. Our results indicate that KATP channels serve a highly conserved role in regulating metabolism in zebrafish, and that zebrafish may function as valuable models for metabolic studies.
2. Material and methods
2.1. Nucleotide and amino acid alignments and identity determination
Comparisons of nucleotide and amino acid sequences of the orthologues of mammalian KATP channel components were completed in DNASTAR Lasergene MegAlign using ClustalW alignment. Search query IDs giving the sequences analysed for the components studied are indicated in the electronic supplementary material, table S1 for the nucleotide alignments and electronic supplementary material, table S2 for amino acid alignments. Amino acid sequences were analysed using InterPro v. 5 [12] (https://www.ebi.ac.uk/interpro/) with Phobius [13] to determine predicted transmembrane, cytoplasmic and extracellular residues.
2.2. Animal lines and maintenance
Transparent Casper zebrafish [14] were used for injection experiments. Zebrafish expressing eGFP under the insulin promoter (Tg(−1.0ins:eGFP)sc1) were used for islet isolation studies [15]. These fish were crossed into the Casper background for three generations (until external pigmentation was lost) and were maintained in the Washington University zebrafish facility. Details of standard operating procedures for the facility can be found at http://zebrafishfacility.wustl.edu/documents.html. All procedures were approved by the Washington University in St Louis IACUC.
2.3. Islet and β-cell isolation
Zebrafish islets were isolated as described previously [15], with minor modifications. Briefly, fish were euthanized using cold-shock (8°C water immersion) followed by decapitation. Fish were rolled onto their right sides and the exterior skin, and scales were removed using surgical forceps to expose the abdomen. Visceral organs were removed by gently applying pressure using forceps until fully separated. The islets were identified at the intersection of hepatic and bile ducts with the intestine (located using the gall bladder and spleen as regional indicators) and confirmed by eGFP fluorescence. Islets were removed by gently pinching ducts with forceps and separating the islets from the surrounding tissues.
Exocrine tissues surrounding islets were digested with collagenase (Sigma C9263, 0.4 mg ml−1 in Hank's buffered salt solution, 0.5 ml/5–10 islets), during incubation at 29°C for 20 min, shaking gently every 5 min. Islets were then placed in RPMI (ThermoFisher 11875-093) supplemented with 1 mM HEPES, antibiotic solution (Sigma A5955, 10 ml l−1 solution), 10% fetal bovine serum and diluted with glucose-free RPMI to final glucose concentration of 6.67 mM.
For experiments involving individual β-cells, islets were dispersed with StemPro Accutase (ThermoFisher A11105) for 10 min at 37°C and clumps of cells were incubated a second time in the same conditions for 2 min. Dispersed cells were washed with media and re-suspended in less than or equal to 100 µl of media, then transferred to glass shards cut from coverslips. Cells were allowed to adhere for 30 min in incubator (28°C, 0% CO2) on shards before being completely covered with media and incubated overnight in the same conditions.
2.4. Chemicals
Salts and glucose were purchased from Sigma Aldrich. Diazoxide (D9035), pinacidil (P154), tolbutamide (T0891) and glibenclamide (G0639) were purchased from Sigma Aldrich.
2.5. Whole-cell voltage-clamp and excised inside-out patch-clamp experiments
Whole-cell and inside-out excised-patch voltage-clamp experiments were performed as described for mammalian β-cells [16], with minor modifications. Isolated β-cells adhering to glass shards were transferred to bath solutions. Bath solution for whole-cell experiments was Tyrode's solution containing 137 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.33 mM NaH2PO4, 5 mM HEPES and 1 mM glucose. Bath solution for inside-out excised patch experiments (K-INT) contained 140 mM KCl, 10 mM HEPES and 1 mM K-EGTA adjusted to pH 7.4 with KOH. In experiments testing, ADP or drug action on KATP channels, 0.5 mM free Mg2+ was added to K-INT except for high [ATP] lanes. The amount of MgCl2 used to reach this free Mg2+ concentration was calculated using the CaBuf program (no longer accessible at webpages previously cited in other articles (ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/cabuf.zip, as well as other sources). We are happy to provide this to any requestors. For drug studies, drugs were kept as 100 mM stock solutions in DMSO (except diazoxide, which was kept at 300 mM in DMSO). Drug stocks were diluted to 100 µM in K-INT, with DMSO added to K-INT and ATP solutions to match the drug solution (0.1% DMSO).
Glass electrodes were pulled from Kimble-Chase 2502 micro-haematocrit capillary tubes using a P-97 puller (Sutter instruments) to yield 2–4 MΩ tips, when filled with K-INT. Recordings of currents were made using an Axopatch1B or Axopatch 200B amplifier and Axon pCLAMP software from Molecular Devices. For excised patches (seal greater than 1 GΩ), membrane potential was kept constant at +50 mV. Once lifted, the pipet was moved through a mineral oil gate [17] to rip the cell free, leaving the patch in the micropipette. Patches were sequentially exposed to varying concentrations of ATP or ATP and activators, as noted. For whole-cell recordings, membrane potential was held at −70 mV and repeatedly ramped between −120 and +40 mV.
2.6. RNA isolation, cDNA preparation and channel subunit PCR
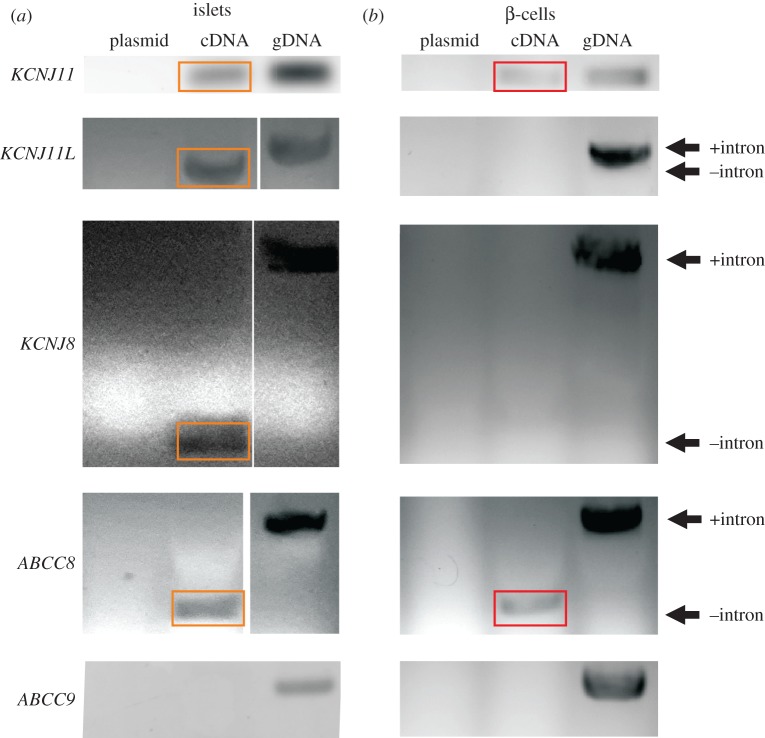
As young adult zebrafish pancreata typically contain only one to three large islets (approx. 10 000 cells), biological replicates were designated as pools of islets from eight to 15 fish each. RNA was isolated from pooled islets using the QIAGEN RNEasy mini-kit. cDNA was synthesized from isolated RNA with the ThermoFisher High-Capacity cDNA reverse transcription kit. Genomic DNA was isolated from zebrafish hearts using the gMax mini-kit (IBI scientific). Primers for ion channel PCRs are listed in the electronic supplementary material, table S5. Primers were designed using the Primer-BLAST NCBI online tool and checked for specificity for the selected genes (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) [18]. PCRs were run with Platinum Taq High-fidelity polymerase (ThermoFisher 11304–11) and products were separated on 1.5–2% agarose gels in TAE buffer and visualized with ChemiDoc. MP (Bio-Rad). PCRs on cDNAs from n > 5 separate pools of islets and n > 5 genomic DNAs were analysed, with figure 3a being a representative sample.
Figure 3.
Zebrafish β-cell KATP channels are similar in composition to mammalian β-cell KATP channels. (a) PCR of RNA-derived cDNA from different pools of islets shows bands for orthologues of KCNJ11 (Kir6.2), KCNJ8 (Kir6.1), KCNJ11 L (Kir6.3) and ABCC8 (SUR1), but no bands for ABCC9 (SUR2). Plasmid DNA is a negative control for non-specific replication by primer mix; genomic DNA (gDNA) is a positive control for presence of target genes. These reactions were repeated over n ≥ 5 separate pools of cDNA and gDNA for validation. Primers for KCNJ11 L, KCNJ8 and ABCC8 span exons to distinguish gDNA from cDNA. There are no introns in KCNJ11. (b) PCR of RNA-derived cDNA from eGFP-sorted β-cells shows bands for orthologues of KCNJ11 and ABCC8, but no bands for the other subunits. Images were cropped and resized, and in some cases contrast-enhanced to improve clarity. Original images are in the electronic supplementary material, figures S4 and S5. Orange and red boxes highlight transcript presence in islet and β-cell cDNAs, respectively.
For cell sorting, islets were dispersed as above and sorted using a BD FACSAria II (BD Biosciences) at the Washington University Flow Cytometry and Fluorescence Activated Cell Sorting Core (http://pathology.wustl.edu/Research/cores/facs/index.php). RNA was extracted from sorted cells as described [19] using TRIzol (ThermoFisher 15596026) and chloroform (Sigma C0549). DNA was removed from RNA samples using DNAseI (ThermoFisher 18068015) for islet samples and TURBO DNA-free kit (ThermoFisher AM1907) for sorted cell samples prior to reverse transcription. The FACSAria II data file is included as online material.
2.7. Adult zebrafish injection studies
Injections were performed as previously described [20], with modifications. Adult Casper zebrafish of both sexes, approximately six to eight months of age, were anaesthetized by cold water immersion. Animals were then transferred to pre-weighed cold water-soaked sponges in Petri dishes with indentations cut to maintain hydration while holding fish immobilized. Fish were injected (10 µl gBW−1) intraperitoneally (IP) using disposable 32 G needles (Acuderm) with Luer-Lock hubs on gas-tight 50 µl syringes (Hamilton 1705). For the IP glucose tolerance test, all solutions were prepared in 20% DMSO in 1 × PBS with 5 mg ml−1 phenol red. Following injection, animals were returned to warm water (28°C) for recovery. For plasma glucose measurements at indicated time points, individual fish were euthanized by immersion in cold water followed by decapitation across the gills. OneTouch Ultra glucometers were used to measure blood glucose by placing a glucometer strip at sectioned heart at time of decapitation.
2.8. Data analyses
Initial experiments with ATP inhibition on excised patches from zebrafish established a variability similar to that seen for mammalian channels under similar conditions [21]. [ATP]–response relationships were fitted with a modified Hill equation:
| 2.1 |
where Irel is the current relative to that in zero ATP; IC50 is the ATP concentration at which channels are half-maximally inhibited; [ATP] is the concentration of ligand; and nH is the Hill coefficient. Fitting was done with GraphPad Prism software, using least-squares variable-slope log([inhibitor]) versus normalized response function, with a resulting R-squared value of 0.97.
Statistical comparisons between the various datasets were performed in GraphPad Prism, and the specific tests are indicated in the relevant figure legends. Datasets were tested for normality (Shapiro–Wilk) and whether variances were statistically different between groups (Bartlett's test). Where normality and variance assumptions were met, ANOVA with Tukey's multiple comparisons tests (three or more groups) or Student's t-test with Welch's correction (two groups) was used. As some datasets showed non-normal data or significantly different variances within the groups, non-parametric tests were used (for tests of three or more groups, Kruskal–Wallis ranked test, with Dunn's multiple comparisons; Mann–Whitney for two-group analyses). To improve interpretability of glucose tolerance tests, the values were log-transformed before statistical analysis.
2.9. Online supplementary material
Included online are three electronic supplementary material tables and six electronic supplementary material figures. Electronic supplementary material, table S1: references for nucleotide sequence alignments and identity determination. Electronic supplementary material, table S2: references for amino acid sequence alignments and identity determination. Electronic supplementary material, table S3: sequences and relevant data for the primers used in PCR reactions for the KATP channel subunits in zebrafish. Electronic supplementary material, figure S1: amino acid and nucleotide identities for KATP channel subunits in humans and zebrafish. Electronic supplementary material, figure S2: the alignment of zebrafish Kir6.x subunits relative to their mammalian orthologues highlighting residues of interest and functional domains. Electronic supplementary material, figure S3: alignment of zebrafish SURx subunit sequences relative to mammalian sequences, highlighting residues of putative functional significance in mammalian subunits that are or are not conserved in zebrafish orthologues. Electronic supplementary material, figure S4: uncut images of gels for PCR of KATP channel subunit components in islets (cf. figure 3). Electronic supplementary material, figure S5: uncut images of gels for PCR of KATP channel subunits in sorted β-cells (cf. figure 3). Electronic supplementary material, figure S6: unmodified traces for tolbutamide and glibenclamide (cf. figure 4). Electronic supplementary material, figure S7: unmodified bright-field and fluorescence images used for the composite images in figure 1.
Figure 4.
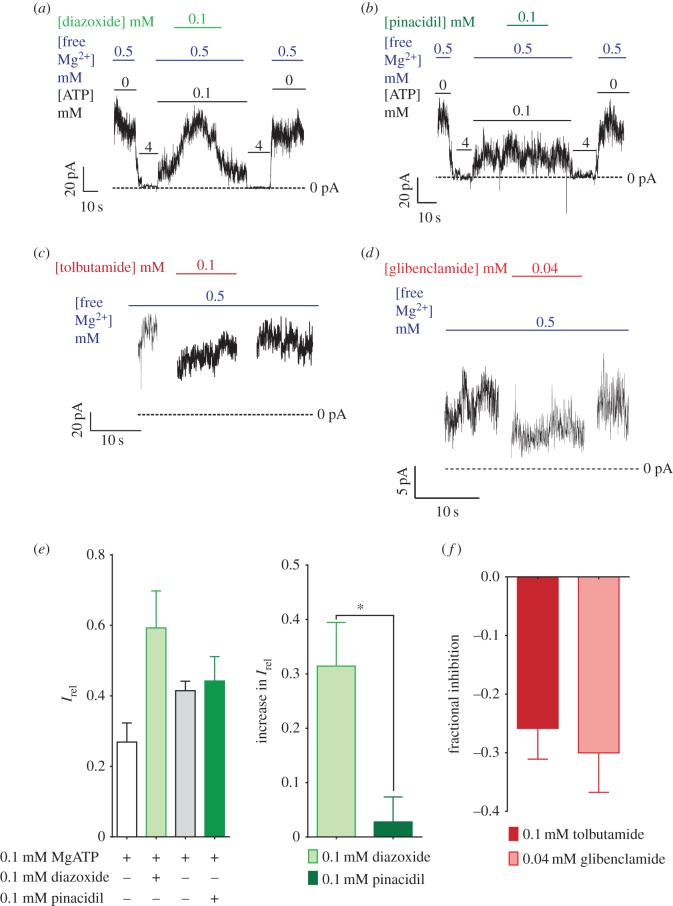
Zebrafish β-cell KATP channels exhibit similar in pharmacology to mammalian β-cell KATP channels. Zebrafish β-cell KATP channels show activation by diazoxide (a) but not by pinacidil (b) in excised patches. These channels also show inhibition by both tolbutamide (c) and glibenclamide (d). (e,f) Quantification of activation (e) and inhibition (f) of the KATP channels. Asterisk indicates p < 0.05 by the Mann–Whitney test. In (e), the right panel indicates the increase in Irel produced by each drug, whereas the left panel indicates the fraction of overall maximum current in each condition. These are quantified from recordings of six cells for diazoxide, four cells for pinacidil and glibenclamide, and five cells for tolbutamide.
Figure 1.
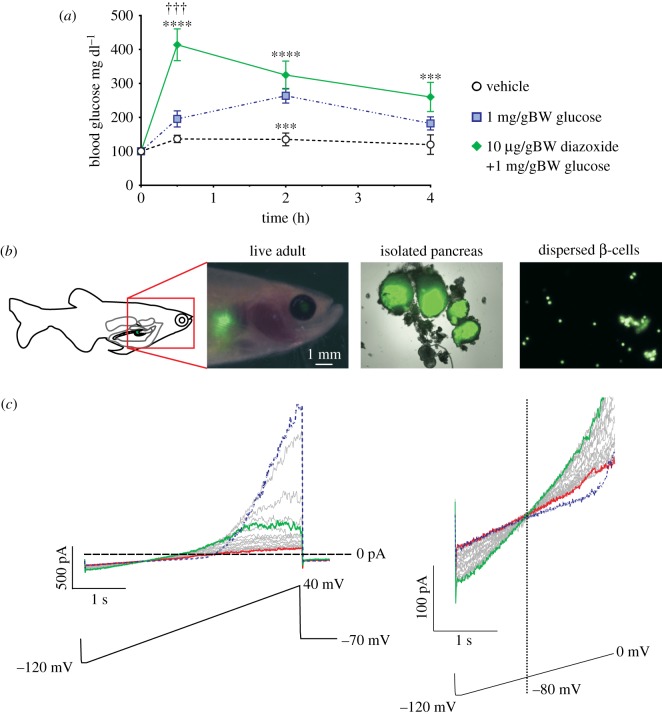
Whole-cell voltage-clamp of zebrafish β-cells reveals functional KATP channels. (a) Glucose tolerance in adult zebrafish. Blood glucose at each time point in (a) was compared between groups by ANOVA with Tukey's multiple comparisons on log-transformed datasets. n = 7–13 at each time point except for the baseline values which were 24. ***p < 0.001, group versus vehicle; ****p < 0.0001, group versus vehicle; p-values are from Tukey's multiple comparisons test following ANOVA of log-transformed values. †††p < 0.001, group versus glucose, also from Tukey's multiple comparisons test. Data in this panel are compiled from multiple injection experiments performed over several days. (b) Expression of eGFP in the fish pancreas allows visualization of β-cells in live adults (left image), isolated islets (middle image) and dispersed β-cells (right image). Scale is indicated for the live adult image. The middle and right images are at 20× and 40×, respectively. For image panels, bright-field and fluorescence images were superimposed for adult fish and whole islets. Adult fish bright-field image was contrast-enhanced prior to superimposing it with the fluorescence image to enhance visibility in the final combined image. (c) Whole-cell voltage-clamp detection of KATP in zebrafish β-cells. Voltage ramps (lower) were applied from −120 to +40 mV over 4 s. Following break-in, the initial ramp (blue) elicits large voltage-dependent K currents above −30 mV. These currents gradually run down in successive voltage ramps, and a weakly inwardly rectifying KATP conductance gradually increases to maximal (green) and then in turn runs down to baseline (red). Right panel shows currents between −120 and 0 mV for more clear visualization of KATP currents.
3. Results
3.1. Orthologues of major genes involved in mammalian insulin secretion exist in zebrafish
Key proteins involved in the electrical coupling of glucose metabolism to insulin secretion in mammals include glucose transporters (GLUT2), KATP channels and VDCCs (electronic supplementary material, figure S1a). Orthologues for each of these are present in the zebrafish genome, and predicted KATP channel subunit sequences are highly conserved (ClustalW alignments, electronic supplementary material, figure S1b,c). In mammals, KATP channels are generated as octameric complexes of four pore-forming Kir6.x subunits and four accessory sulfonylurea receptor (SURx) subunits [22]; Kir6.2 and SUR1 form KATP channels in pancreatic islets and in the central nervous system, whereas Kir6.1 and SUR2 form KATP channels in smooth and striated muscles [22]. The genes for Kir6.2 (KCNJ11) and SUR1 (ABCC8) are immediately adjacent to one another in both zebrafish and human chromosomes (chromosome 25 in zebrafish and 11 in humans). The genes for Kir6.1 (KCNJ8) and SUR2 (ABCC9) are also adjacent to one another in both species (located on chromosome 4 in zebrafish and 12 in humans). Kir6.1, Kir6.2, SUR1 and SUR2 all show more than 70% amino acid identity between humans and zebrafish [9] (electronic supplementary material, figure S1b), with functional domains in SUR1 being highly conserved (electronic supplementary material, figure S3). Kir6.3, a pore-forming subunit unique to zebrafish and likely to be derived from zebrafish Kir6.2 in a duplication event, is located on zebrafish chromosome 15 and has no SUR gene in its vicinity. Kir6.3 and SUR1 expression have previously been described in the zebrafish central nervous system [9], but expression of the different KATP channel subunits has not been examined in other tissues.
3.2. KATP channels regulate glucose homeostasis in adult zebrafish
We initially probed the glucose metabolism, and the role of KATP in glucose control, in adult zebrafish using a glucose tolerance test. IP injection of glucose elevates blood glucose in adult zebrafish beyond vehicle alone, and glucose gradually normalizes (figure 1a, blue dotted line). As shown in figure 1a, co-injection of diazoxide along with glucose significantly slows the return of glucose to baseline. These results are consistent with the effect of diazoxide on IP glucose tolerance in mammals, and with previous experiments on larval zebrafish [7].
3.3. Zebrafish β-cells express functional KATP channels
To examine KATP channel expression and function in zebrafish β-cells, we have developed approaches to isolate islets and individual β-cells for gene expression as well as whole-cell and excised-patch voltage-clamp techniques (figure 1b). Fish that express eGFP using the zebrafish insulin promoter allowed isolation and dispersion of pancreatic islets to yield individual β-cells as described in methods [15] (figure 1b). Whole-cell patch clamp of these isolated zebrafish β-cells (figure 1c), exhibited rapidly declining voltage-activated currents above ∼−40 mV, and activation of large, almost linear potassium conductances that are maximal within a few minutes after initial dialysis of the cell with zero ATP solution. The weak inward rectification (evident above approx. 0 mV, figure 1c, left), and reversal potential very close to EK (−80 mV, figure 1c, right), as well as the amplitude of this conductance, are indistinguishable from typical KATP currents activated in mammalian β-cells [23].
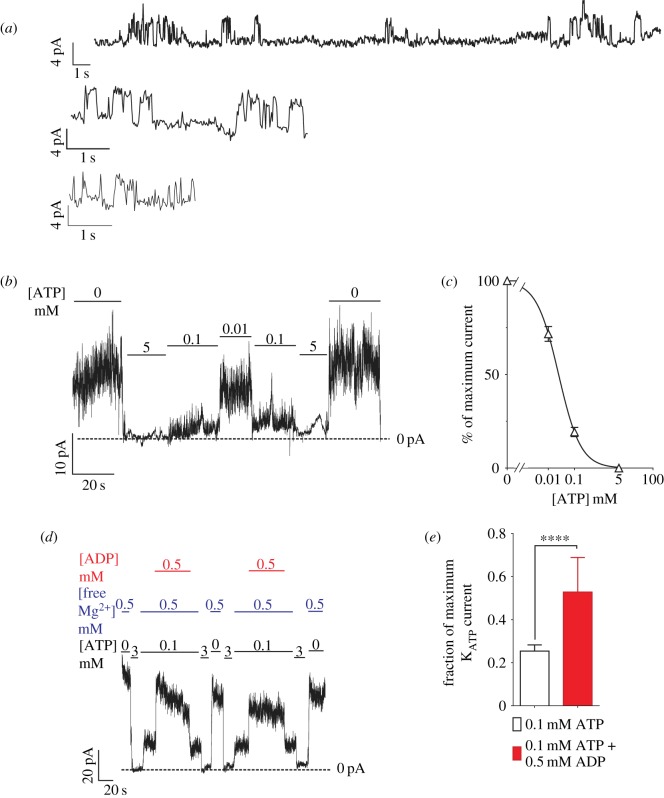
Inside-out excised-patch voltage-clamp experiments on zebrafish β-cells reveal potassium channels with single channel conductance of approximately 87 pS (4.35 pA at −50 mV driving force; figure 2a). Again, this property is indistinguishable that of from mammalian KATP channels formed form Kir6.2 + SUR1 subunits [24,25]. These channels are inhibited by increasing concentrations of ATP at the intracellular surface (figure 2b,c), with IC50 of 22.6 µM (nH = 1.01), very similar to reported values for mammalian β-cell KATP channels in the same conditions (IC50 ∼ 10–20 µM [21,24,26,27]). Furthermore, these channels are activated by addition of Mg-ADP to the cytoplasmic face (figure 2d,e), again similar to properties of mammalian KATP channels [28]. Taken together, these data show that zebrafish β-cells express functional KATP channels with activation and inhibition properties that are essentially the same as those expressed in mammalian β-cell KATP channels.
Figure 2.
Excised-patch clamp reveals functional properties of KATP channels in zebrafish β-cells. (a) Individual KATP channels (4.35 pA at −50 mV) are detected in three representative membrane patches excised from zebrafish β-cells. (b) These K+ currents are inhibited by ATP, with (c) IC50 = 22.6 mM, nH = 1.01. (d) These channels are also activated by increasing [Mg-ADP], quantified in (e). ****p < 0.0001 (Mann–Whitney test). The dose–response curve was generated from 16 cells derived from six pools of zebrafish islet (biological replicates). The ADP response graph comprises nine cells derived from four biological replicates. (a,b and d) Representative traces.
3.4. Zebrafish β-cell KATP channels show similar subunit composition and pharmacology to mammalian β-cell KATP channels
We performed PCR on genomic DNA (gDNA) and cDNA generated from RNA isolated from zebrafish islets, to characterize Kir6 and SUR subunit expression in zebrafish. Genes for Kir6.1, Kir6.2, Kir6.3, SUR1 and SUR2 were all detected in gDNA but only Kir6.2, Kir6.1, Kir6.3 and SUR1 were consistently detected in islet cDNA (figure 3a). The similarity of zebrafish KATP currents to those expressed in mammalian β-cells is consistent with both being formed of Kir6.2 and SUR1 subunits, raising the question of the relevance of Kir6.1 and Kir6.3 expression, Kir6.3 having been detected in fish neurons by RNA in situ hybridization studies [9]. cDNA derived from eGFP-sorted β-cells indicates transcription of only KCNJ11 (Kir6.2) and ABCC8 (SUR1) (figure 3). While β-cells form the majority of cells in the islet, islets are innervated and permeated by capillaries [29]; the presence of KCNJ8 (Kir6.1) and KCNJ11 L (Kir6.3) transcripts in whole islets may reflect the presence of these other cell types.
Mammalian SUR subunits respond differentially to activator and inhibitor compounds: the potassium channel opener (KCO) diazoxide is a more effective activator of SUR1-containing KATP channels and pinacidil is a more effective activator of SUR2-containing channels [30,31]. Sulfonylureas, furthermore, typically close SUR1-containing KATP channels approximately 100- to 1000-fold more effectively than SUR2-containing KATP channels in mammals [32]. Residues involved in drug sensitivity are conserved between zebrafish and mammalian SUR subunits (electronic supplementary material, figures S2 and S3). In excised zebrafish β-cell membranes, addition of Mg2+ and diazoxide is sufficient to activate KATP channels (figure 4a,e), whereas pinacidil is ineffective (figure 4b) at the same concentration. Two sulfonylurea drugs, tolbutamide (figure 4f) and glibenclamide (figure 4f), both inhibit the zebrafish β-cell KATP at relatively low concentrations in excised patches, similar to the level of inhibition seen for mammalian Kir6.2+SUR1 channels at the same drug concentrations in similar conditions [33]. Potent response to sulfonylureas and diazoxide, but not to pinacidil, is consistent with the expression data showing that SUR1 is the only SUR detected in islet cDNA. Taken together, these data indicate that the subunits comprising zebrafish β-cell KATP channels and the drug responsivity of these channels are essentially the same as their mammalian counterparts.
4. Discussion
4.1. Structure and functional properties of β-cell KATP channels are conserved between zebrafish and mammals
Mammalian KATP represents a family of potassium channels generated by various combinations of Kir6.1/2 and SUR1/2 subunits [34,35]. Expression patterns and functional properties have been extensively characterized, and shown to be generally well conserved between mammalian species [4,21,22,36–38]. KATP channel subunit orthologues are clearly present in all sequenced vertebrate genomes, but there have been surprisingly few studies of structural or functional properties of KATP channels in islets from non-mammalian vertebrate classes. Studies of KATP structure and function in fishes have been very limited, in part, owing to technical difficulties of identification and isolation of specific cell types. Zhang et al. identified a third, unique, pore-forming subunit, Kir6.3 [9], in zebrafish, and showed that this subunit is expressed in the central nervous system via RNA-in situ hybridization, but expression and potential roles in other tissues were not explored. Here, using fluorescently tagged β-cells in transparent Casper fish, we have succeeded in efficiently identifying, isolating and dissociating zebrafish islets. We show that zebrafish β-cells express functional KATP channels that exhibit very similar composition (Kir6.2 and SUR1) and pharmacology (activation by diazoxide, but not pinacidil) to those in mammalian β-cells, and that modulation of these channels affects adult fish glucose homeostasis similarly to the effects in mammals.
4.2. Conservation of KATP channel-dependent insulin secretion mechanisms between teleost fishes and mammals
Rapid responses to metabolic changes are challenges faced by all organisms, and the potential importance of insulin signalling in such responses is highlighted by the high conservation of insulin structure and insulin signalling pathways across vertebrates and invertebrates, with evolutionary lineages that diverged long ago [39,40]. However, the last common ancestor between teleost fishes and humans is estimated to have lived approximately 450 million years ago [41], and while insulin and other hormones are structurally conserved across the vertebrates, whether secretory regulation and functional consequences are as conserved is less clear.
The finely tuned properties and regulatory features of β-cell KATP channels are absolutely key to the regulation of mammalian insulin secretion [2,4]. A role for KATP channels in modulating glucose metabolism in zebrafish has been implied by the demonstration that treatment of fish larvae with the KATP opener diazoxide increases whole-larval glucose and that the inhibitor glibenclamide lowers whole-larval glucose [7]. Transgenic overexpression of mammalian KATP channels with gain-of-function mutations was also sufficient to increase larval glucose in these studies, whereas a dominant-negative mammalian KATP channel lowered larval glucose [7]. It has also been suggested that diazoxide interferes with regeneration of pancreatic islets in fishes after alloxan treatment, while glimepiride, a sulfonylurea, enhances recovery [10]. However, despite implicating KATP in metabolic control, none of these earlier studies characterized the properties of native zebrafish islet KATP channels or directly assessed their role in insulin secretion. We are unaware of ex vivo analyses of insulin secretion in zebrafish islets, but our findings suggest that KATP channels are conserved in both functional expression and properties between zebrafish and mammalian β-cells (figures 2, 3 and 4). Manipulation of KATP channels in vivo with the pharmacological activator diazoxide suggests that KATP channels are also key to normal glucose tolerance in adult fishes (figure 1a).
4.3. Zebrafish as model organisms for studying metabolic diseases
In addition to their use in tracking temporal expression patterns of transcription factors in endocrine development, zebrafish have been used to model atherosclerosis, the consequences of high fat diet feeding, hyperglycaemia and other metabolic interventions, as well as the regeneration of key endocrine organs, including the pancreas [42]. Our finding that zebrafish β-cells express functional KATP channels with very similar biophysical properties and pharmacology to mammalian channels, and that channel activation significantly impairs whole-body glucose clearance in adult fish, will lend further support to the use of these animals to model metabolic diseases.
Forward genetic screens can be powerful tools for unmasking subtle modifiers of disease phenotypes, but infrequent reproduction, low litter numbers, long maturation time and high cost limit the use of mammalian species for such studies. Zebrafish reproduce frequently and with large clutches (potentially hundreds of embryos per clutch), allowing analysis of thousands of individuals in short periods. The genome is fully sequenced, and techniques for introducing mutations in zebrafish have been well streamlined [43,44]. Larvae are transparent, allowing easy visualization of genetic markers or fluorescent dyes [14,45]. Zebrafish develop metabolic abnormalities when fed high-fat diets [46,47], show similar complications of persistently high glucose and have many of the same transcription factor pathways involved in development of endocrine, liver and other organs important in controlling metabolism [44,48]. While zebrafish may thus offer major advantages for screening diabetes modifiers, details of comparative organ biology must first be evaluated and further studies like those we describe here are required.
Supplementary Material
Supplementary Material
Acknowledgements
We acknowledge the excellent technical assistance of the Washington University in St Louis Zebrafish Facility (http://zebrafishfacility.wustl.edu/). Additionally, we thank the immunohistochemistry AMP core laboratory (https://pathology.wustl.edu/research/core-facilities/anatomic-molecular-pathology-core-lab) for their help in preparing zebrafish paraffin sections seen in the electronic supplementary material. We are also grateful for the two anonymous reviewers for RSOS whose comments substantially improved the manuscript.
Ethics
All zebrafish maintenance and experimental procedures were approved by the Washington University in St Louis Institutional Animal Care and Use Committee (IACUC; protocol number for approval: 20140193).
Data accessibility
Our data are deposited at Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1v074 [49]. Electronic supplementary material, including figures, legends and additional results, are also published on the Royal Society Open Science journal site.
Authors' contributions
C.H.E. carried out molecular laboratory work and electrophysiology studies, data analysis and sequence alignments, as well as design of the study and drafting of the manuscript; A.E., Z.Y., Y.W. and H.C. carried out molecular laboratory work; J.B.M. and L.G.M. generated molecular reagents for the study as well as participating in the design of the study and editing of the manuscript; M.S.R. participated in the molecular laboratory work, design of the study and drafting of the manuscript; C.G.N. participated in the electrophysiology studies, design of the study and drafting of the manuscript. All authors gave final approval for publication.
Competing interest
The authors declare no conflicts of interest.
Funding
These studies were supported by grants from the Washington University in St Louis Diabetes Research Center (Pilot feasibility grant ‘Zebrafish model of excitability driven Diabetes' 2014-present) and the National Institutes of Health (NIH) R01 DK069445 to C.G.N., NIH R01 DK 098584 to M.S.R., as well as NIH T32DK108742–01 (C.H.E.).
References
- 1.Keane K, Newsholme P. 2014. Metabolic regulation of insulin secretion. Vitamins Horm. 95, 1–33. (doi:10.1016/B978-0-12-800174-5.00001-6) [DOI] [PubMed] [Google Scholar]
- 2.Drews G, Krippeit-Drews P, Dufer M. 2010. Electrophysiology of islet cells. In The islets of langerhans (ed. Islam MS.), pp. 115–163. Dordrecht, The Netherlands: Springer. [DOI] [PubMed] [Google Scholar]
- 3.Remedi MS, Nichols CG. 2009. Hyperinsulinism and diabetes: genetic dissection of beta cell metabolism-excitation coupling in mice. Cell Metab. 10, 442–453. (doi:10.1016/j.cmet.2009.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koster JC, Permutt MA, Nichols CG. 2006. Diabetes and insulin secretion: the ATP-sensitive K+ channel (KATP) connection. Diabetes 54, 3065–3072. (doi:10.2337/diabetes.54.11.3065) [DOI] [PubMed] [Google Scholar]
- 5.Gloyn AL, et al. 2004. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 350, 1838–1849. (doi:10.1056/NEJMoa032922) [DOI] [PubMed] [Google Scholar]
- 6.Riedel M, Steckley D, Light P. 2005. Current status of the E23 K Kir6.2 polymorphism: implications for type-2 diabetes. Hum. Genet. 116, 133–145. (doi:10.1007/s00439-004-1216-5) [DOI] [PubMed] [Google Scholar]
- 7.Hodson DJ, et al. 2014. Incretin-modulated beta cell energetics in intact islets of langerhans. Mol. Endocrinol. 28, 860–871. (doi:10.1210/me.2014-1038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimmel RA, Dobler S, Schmitner N, Walsen T, Freudenblum J, Meyer D. 2015. Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment. Sci. Rep. 5, 14241 (doi:10.1038/srep14241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Miki T, Shibasaki T, Yokokura M, Saraya A, Seino S. 2006. Identification and characterization of a novel member of the ATP-sensitive K+ channel subunit family, Kir6.3, in zebrafish. Physiol. Genomics 24, 290–297. (doi:10.1152/physiolgenomics.00228.2005) [DOI] [PubMed] [Google Scholar]
- 10.Nam YH, Hong BN, Rodriguez I, Ji MG, Kim K, Kim U-J, Kang TH. 2015. Synergistic potentials of coffee on injured pancreatic islets and insulin action via KATP channel blocking in zebrafish. J. Agric. Food Chem. 63, 5612–5621. (doi:10.1021/acs.jafc.5b00027) [DOI] [PubMed] [Google Scholar]
- 11.Capiotti KM, Antonioli RJ, Kist LW, Bogo MR, Bonan CD, Da Silva RS. 2014. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 171, 58–65. (doi:10.1016/j.cbpb.2014.03.005) [DOI] [PubMed] [Google Scholar]
- 12.Mitchell A, et al. 2015. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res. 43, D213–D221. (doi:10.1093/nar/gku1243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kall L, Krogh A, Sonnhammer EL. 2004. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 338, 1027–1036. (doi:10.1016/j.jmb.2004.03.016) [DOI] [PubMed] [Google Scholar]
- 14.White RM, et al. 2008. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189. (doi:10.1016/j.stem.2007.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss JB, Koustubhan P, Greenman M, Parsons MJ, Walter I, Moss LG. 2009. Regeneration of the pancreas in adult zebrafish. Diabetes 58, 1844–1851. (doi:10.2337/db08-0628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koster JC, Remedi MS, Flagg TP, Johnson JD, Markova KP, Marshall BA, Nichols CG. 2002. Hyperinsulinism induced by targeted suppression of beta cell KATP channels. Proc. Natl Acad. Sci. USA 99, 16 992–16 997. (doi:10.1073/pnas.012479199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederer WJ, Nichols CG. 1989. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J. Physiol. 419, 193–211. (doi:10.1113/jphysiol.1989.sp017869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13, 134 (doi:10.1186/1471-2105-13-134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon KT, Li L, Janowski BA, Corey DR. 2014. Analysis of nuclear RNA interference in human cells by subcellular fractionation and argonaute loading. Nat. Protoc. 9, 2045–2060. (doi:10.1038/nprot.2014.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinkel MD, Eames SC, Philipson LH, Prince VE. 2010. Intraperitoneal injection into adult zebrafish. J. Vis. Exp. 42, e2126 (doi:10.3791/2126). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. 2000. Targeted overactivity of [Beta] cell KATP channels induces profound neonatal diabetes. Cell. 100, 645–654. (doi:10.1016/S0092-8674(00)80701-1) [DOI] [PubMed] [Google Scholar]
- 22.Nichols CG. 2006. KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476. (doi:10.1038/nature04711) [DOI] [PubMed] [Google Scholar]
- 23.Misler S, Gee WM, Gillis KD, Scharp DW, Falke LC. 1989. Metabolite-regulated ATP-sensitive K+ channel in human pancreatic islet cells. Diabetes 38, 422–427. (doi:10.2337/diab.38.4.422) [DOI] [PubMed] [Google Scholar]
- 24.Ashcroft FM. 1988. Adenosine 5'-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 11, 97–118. (doi:10.1146/annurev.ne.11.030188.000525) [DOI] [PubMed] [Google Scholar]
- 25.Tinker A, Aziz Q, Thomas A. 2014. The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Brit J. Pharmacol. 171, 12–23. (doi:10.1111/bph.12407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miki T, Nagashima K, Seino S. 1999. The structure and function of the ATP-sensitive K+ channel in insulin-secreting pancreatic beta-cells. J. Mol. Endocrinol. 22, 113–123. (doi:10.1677/jme.0.0220113) [DOI] [PubMed] [Google Scholar]
- 27.Mannikko R, Flanagan SE, Sim X, Segal D, Hussain K, Ellard S, Hattersley AT, Ashcroft FM. 2011. Mutations of the same conserved glutamate residue in NBD2 of the sulfonylurea receptor 1 subunit of the KATP channel can result in either hyperinsulinism or neonatal diabetes. Diabetes 60, 1813–1822. (doi:10.2337/db10-1583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunne MJ, Petersen OH. 1986. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 208, 59–62. (doi:10.1016/0014-5793(86)81532-0) [DOI] [PubMed] [Google Scholar]
- 29.Moss LG, Caplan TV, Moss JB. 2013. Imaging beta cell regeneration and interactions with islet vasculature in transparent adult zebrafish. Zebrafish 10, 249–257. (doi:10.1089/zeb.2012.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shyng S-L, Ferrigni T, Nichols CG. 1997. Regulation of KATP channel activity by diazoxide and MgADP: distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 110, 643–654. (doi:10.1085/jgp.110.6.643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inagaki N, Gonoi T, Iv JPC, Wang C-Z, Aguilar-Bryan L, Bryan J, Seino S. 1996. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron 16, 1011–1017. (doi:10.1016/S0896-6273(00)80124-5) [DOI] [PubMed] [Google Scholar]
- 32.Vila-Carriles WH, Zhao G, Bryan J. 2007. Defining a binding pocket for sulfonylureas in ATP-sensitive potassium channels. FASEB J. 21, 18–25. (doi:10.1096/fj.06-6730hyp) [DOI] [PubMed] [Google Scholar]
- 33.Koster JC, Sha Q, Nichols CG. 1999. Sulfonylurea and K+-channel opener sensitivity of K(ATP) channels. Functional coupling of Kir6.2 and SUR1 subunits. J. Gen. Physiol. 114, 203–213. (doi:10.1085/jgp.114.2.203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flagg TP, Nichols CG. 2011. ‘Cardiac KATP’: a family of ion channels. Circulation: Arrhyth. Electrophysiol. 4, 796–798. (doi:10.1161/CIRCEP.111.968081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler A, et al. 2008. Coassembly of different sulfonylurea receptor subtypes extends the phenotypic diversity of ATP-sensitive potassium (KATP) channels. Mol. Pharmacol. 74, 1333–1344. (doi:10.1124/mol.108.048355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dean M, Annilo T. 2005. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum.Genet. 6, 123–142. (doi:10.1146/annurev.genom.6.080604.162122) [DOI] [PubMed] [Google Scholar]
- 37.Brereton MF, Ashcroft FM. 2013. Mouse models of β-cell KATP channel dysfunction. Drug Discov. Today: Disease Mod. 10, e101–e109. (doi:10.1016/j.ddmod.2013.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seino S, Iwanaga T, Nagashima K, Miki T. 2000. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes 49, 311–318. (doi:10.2337/diabetes.49.3.311) [DOI] [PubMed] [Google Scholar]
- 39.Das R, Dobens LL. 2015. Conservation of gene and tissue networks regulating insulin signalling in flies and vertebrates. Biochem. Soc. Transact. 43, 1057–1062. (doi:10.1042/BST20150078) [DOI] [PubMed] [Google Scholar]
- 40.Ellsworth DL, Hewett-Emmett D, Li WH. 1994. Evolution of base composition in the insulin and insulin-like growth factor genes. Mol. Biol. Evol. 11, 875–885. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JS, Van de Peer Y, Braasch I, Meyer A. 2001. Comparative genomics provides evidence for an ancient genome duplication event in fish. Phil. Trans. R. Soc. Lond. B 356, 1661–1679. (doi:10.1098/rstb.2001.0975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seth A, Stemple DL, Barroso I. 2013. The emerging use of zebrafish to model metabolic disease. Dis. Models Mech. 6, 1080–1088. (doi:10.1242/dmm.011346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maddison LA, Li M, Chen W. 2014. Conditional gene-trap mutagenesis in zebrafish. In Gene function analysis (ed. Ochs MF.), pp. 393–411. New York, NY: Humana Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lieschke GJ, Currie PD. 2007. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 8, 353–367. (doi:10.1038/nrg2091) [DOI] [PubMed] [Google Scholar]
- 45.Patton EE, Zon LI. 2001. The art and design of genetic screens: zebrafish. Nat. Rev. Genet. 2, 956–966. (doi:10.1038/35103567) [DOI] [PubMed] [Google Scholar]
- 46.Fang L, Liu C, Miller YI. 2014. Zebrafish models of dyslipidemia: relevance to atherosclerosis and angiogenesis. Transl. Res. 163, 99–108. (doi:10.1016/j.trsl.2013.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oka T, et al. 2010. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 10, 1–13. (doi:10.1186/1472-6793-10-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tehrani Z, Lin S. 2011. Endocrine pancreas development in zebrafish. Cell Cycle 10, 3466–3472. (doi:10.4161/cc.10.20.17764) [DOI] [PubMed] [Google Scholar]
- 49.Emfinger CH, Welscher A, Yan Z, Wang Y, Conway H, Moss JB, Moss LG, Remedi MS, Nichols CG. 2017. Data from: Expression and function of ATP-dependent potassium channels in zebrafish islet β-cells. Dryad Digital Repository. http://dx.doi.org/10.5061/dryad.1v074 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Emfinger CH, Welscher A, Yan Z, Wang Y, Conway H, Moss JB, Moss LG, Remedi MS, Nichols CG. 2017. Data from: Expression and function of ATP-dependent potassium channels in zebrafish islet β-cells. Dryad Digital Repository. http://dx.doi.org/10.5061/dryad.1v074 [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Our data are deposited at Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1v074 [49]. Electronic supplementary material, including figures, legends and additional results, are also published on the Royal Society Open Science journal site.