Abstract
The targeting of 5-aminosalicylic acid (5-ASA), a first-line therapeutic agent for mild to moderate active ulcerative colitis (UC), to the site of inflammation has remained a challenge and an unmet requirement in the treatment of UC. However, nanoscale carriers for targeted drug delivery are promising for pharmacotherapy, and nanoparticles improve the pharmacokinetics of the loaded therapeutics based on their physical properties. To design and prepare 5-ASA-loaded silicon dioxide nanoparticles (5-ASA-SiO2 NPs), a micro-emulsion method was conducted, and their respective therapeutic effects were validated in a mouse model of UC. Cytotoxicity of 5-ASA-SiO2 NPs was detected in vitro using the Cell Counting Kit-8 method. The therapeutic effect of 5-ASA-SiO2 NPs was assessed based on their disease activity index (DAI), colon histopathology, myeloperoxidase (MPO) and levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). SiO2 NPs were successfully prepared, and cytotoxicity of 5-ASA-SiO2 NPs was identified as being similar to 5-ASA and SiO2 NPs. DAI and colonic histopathology scores in the normal dosage, high dosage and the 5-ASA-SiO2 NP groups demonstrated a significant improvement when compared with the model group. DAI in the high dosage and 5-ASA-SiO2 NP groups also demonstrated a significant improvement when compared with the normal dosage group. However, MPO, serum IL-6 and TNF-α levels in normal dosage, high dosage and 5-ASA-SiO2 NPs groups were significantly lower than in the model group, and these indexes in the high dosage group and 5-ASA-SiO2 NP group were significantly lower than that in the normal dosage group. Expression of IL-6 and TNF-α mRNA in colonic mucosa in the normal dosage, high dosage and 5-ASA-SiO2 NP group was significantly lower than that in the model group. Colonic mucosal IL-6 and TNF-α mRNA expression in the high dosage and 5-ASA-SiO2 NP groups was significantly lower than that in the normal dosage group (P<0.05). In conclusion, 5-ASA-SiO2 NPs are a selective drug release system that target the inflamed colon, characteristics of UC, and can greatly increase therapeutic efficacy in UC.
Keywords: 5-aminosalicylic acid, silicon dioxide, nanoparticles, ulcerative colitis
Introduction
Ulcerative colitis (UC) is progressively emerging as a global threat (1), and an intractable disease by the World Health Organization (2). Colonic mucosal healing is currently considered the gold standard for treatment (3). 5-aminosalicylic acid (5-ASA), a conventional anti-inflammatory drug, has been a first-line therapeutic agent in the treatment of mild to moderate active UC for several decades, which allows for remission to be maintained (4). However, when 5-ASA is orally administered, a large quantity of the drug is absorbed by the upper gastrointestinal (GI) tract and enters into the systemic circulation, with only a fraction of the active compound reaching the inflamed target colon areas. As a result, targeting 5-ASA to the site of inflammation has remained a challenge and an unmet requirement in the treatment of UC. In the current study, 5-ASA-loaded silicon dioxide nanoparticles (5-ASA-SiO2 NPs) were designed and prepared, and their therapeutic efficacy in a dextran sodium sulfate (DSS)-induced mice model of UC was investigated.
Materials and methods
Preparation and characterization of SiO2 NPs and 5-ASA-SiO2 NPs
SiO2 NPs and 5-ASA-SiO2 NPs were prepared and synthesized by the Dalian Institute of Chemical Physics, Chinese Academy of Sciences (Dalian, China). The brief procedures were illustrated in Fig. 1. The micro-emulsion method was used to prepare the SiO2 NPs according to the literature (5). This method involved use of an appropriate amount of cyclohexane (31 ml), butanol (8 ml), polyoxyethylene nonlyphenol ether (15 ml), ammonia water (0.5 ml), and distilled H2O (6 ml) were mixed thoroughly for 5 min. Then, 20 mmol tetraethyl orthosilicate was added to the mixture and reacted for 12 h. A total of 20 ml alcohol was then added into the mixture to stop emulsification. The NPs were collected by centrifuging the mixture at 8,000 × g for 5 min at room temperature, prior to being re-suspended in ethanol. The NPs suspension was added into a pressure bottle and mixed for 5 min in an 80°C oil bath; and this step was repeated four times. The SiO2 NPs were obtained by drying the suspension. After reaction with APTES and dehydrated toluene, the amination of SiO2 NPs were obtained; then the amination of SiO2 NPs (0.2 g) were anhydride modified by reacting with succinic anhydride (1 g), acetonitrile (10 ml) in a pressure bottle for oil bath at 80°C with magnetic stirring. After reaction for 24 h, the anhydride modified SiO2 NPs were collected with centrifuging (8,000 × g, 5 min), washing with acetonitrile and drying. SiO2 NPs were dispersed in deionized water to prepare 1 mg/ml suspension. Following ultrasonic dispersion for 30 min, the suspension was taken out, and a small amount of dispersion suspension was drained by copper mesh. The morphology of the nanoparticles was observed by transmission electron microscopy (TEM). The anhydride-SiO2 NPs (0.2 g), 5-ASA (0.2 g; TCI, Tokyo, Japan) and acetonitrile (20 ml) were mixed in a pressure bottle for another 24 h oil bath at 90°C; and the drying precipitate was washed with dimethyl formamide (30 ml); then the target agent of 5-ASA-SiO2 NPs were obtained after drying.
Figure 1.
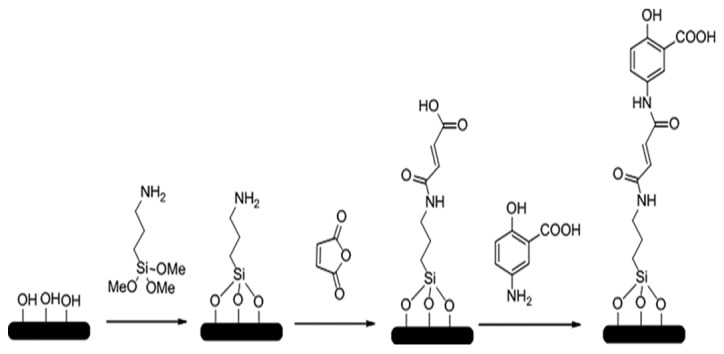
Typical reactions illustrating the preparation of 5-ASA-SiNPs. The brief procedures of 5-ASA-SiO2 NPs, which were prepared and synthesized by Dalian Institute of Chemical Physics China Academy of Sciences (Dalian, China). 5-ASA-SiO2 NPs, 5-aminosalicylic acid-loaded silicon dioxide nanoparticles.
Assessment of drug loading efficiency and 5-ASA-SiO2 NPs toxicity in Caco-2 cells
5-ASA-SiO2 NPs were dissolved in 1 M NaOH solution (pH=9.0) and mixed at 15 × g in a 50°C oil bath for 12 h. The suspension was filtered with a 0.22 µm filter membrane (Thomas Scientific, Swedesboro, NJ, USA) and detected using high performance liquid chromatography (HPLC; Agilent Technologies, Inc., Santa Clara, USA). The loading efficiency of 5-ASA-SiO2 NPs was calculated using the following equation: (Total 5-ASA volume/total 5-ASA-SiO2 NPs volume) × 100%. The detection was performed in triplicate.
Concentration of the logarithmic growth of Caco-2 cells (Cell Resource Center/PUMC, Beijing, China). in Minimum Essential Medium (MEM) was adjusted to 2×106/ml. Cell suspension (100 µl) was added to one well of the 96-well plate and cultured in CO2 incubator for 48 h. The medium in the plate was discarded and the cells washed with PBS twice. Then, 100 µl 5-ASA, 100 µl SiO2 NPs and 100 µl 5-ASA-SiO2 NPs solution were added into different wells and incubated for 12 h. For different treatments, the working concentrations of the solutions were adjusted by MEM to 15.6 µg/ml, 31.2 µg/ml, 62.5 µg/ml, 125 µg/ml, 250 µg/ml, 500 µg/ml and 1 mg/ml. Following the incubation, the medium was replaced by 90 µl new medium with 10 µl Cell Counting Kit-8 (CCK-8) solution (Beijing Zoman Biotehnlogy Co., Ltd., Beijing, China) and incubated for another 2 h before recording the optical density (OD). The negative control group (wells with Caco-2 cells and CCK-8 solution) and the blank control group (wells with CCK-8 solution only) were also set up as described above. Each treatment was represented by three replicates.
The OD values in different wells were recorded using a microplate reader at 450 nm. The survival rates (%) of different treatments were calculated as (OD value in treatment group-OD value in blank control group)/(OD value in negative control group-OD value in blank control group) × 100%.
Colitis model
A total of 60 male healthy BALB/c mice (8–9 weeks of age, body weight 20–25 g) were provided by the Animal Center, Dalian Medical University (Dalian, China). Colitis in mice was induced by 5% (w/v) DSS supplemented in the drinking water for seven days (6,7). Mice were maintained in a room with constant temperature (22±1°C) and a dark-light cycle (12/12 h), and housed in cages (<5 mice per cage). They were fed with standard laboratory food and water for one week before the experiments. All animal experiments were conducted in the accordance with the Institutional Animal Ethics Committee and Animal Care Guidelines of Dalian Medical University (Dalian, China) governing the use of experimental animals.
Mice were randomly divided into six groups (10 per group): Group A, control group; group B, model group (UC model mice); group C, normal dose 5-ASA group (200 mg/kg.day−1 for 7 days); group D, high dose 5-ASA group (400 mg/kg.day−1 for 7 days); group E, SiO2 NPs group (100 mg/kg.day−1 for 7 days); group F, 5-ASA-SiO2 NPs group (100 mg/kg day−1 for 7 days).
Evaluation of colitis severity by disease activity index (DAI), colon mucus histological assessment and measurements of myeloperoxidase activity and cytokines
The DAI scores of different treatments were recorded every day, according to previous studies (Table I) (8,9). On the eighth day of the treatment, animals were anesthetized using 1% pentobarbital sodium (40 mg/kg; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) through intraperitoneal injection and sacrificed by cervical dislocation. Eyeball blood samples were then collected and colon samples were obtained. Hematoxylin and eosin staining was conducted on colon samples. Histology of the colon was evaluated using a microscope. Levels of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) in blood samples were detected utilizing IL-6 (cat. no. EK0411) and TNF-α (cat. no. EK0527) ELISA kits (Wuhan Boster Biological Technology., Ltd, Wuhan, China); and myeloperoxidase (MPO) activity in colon mucosa samples were measured with an MPO ELISA kit (cat. no. DRE 30329; Xinfan Biomart Co., Ltd., Shanghai, China) following the manufacturer's instructions. Total RNA in colon tissue samples was extracted by miRCURY™ RNA Isolation kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's protocol, and reverse transcribed into cDNA using a PrimeScript™ RT reagent kit (Takara Bio, Inc.) according to the manufacturer's protocol. The expression of IL-6 and TNF-α was determined using GAPDH as a loading control. The reaction mixture totaled 25 µl and included, 12.5 µl of 2X SYBR® Premix Ex Taq (Takara Bio, Inc.), 0.5 µl of each primer (Table II), 0.5 µl of 50X ROX Reference Dye II (Takara Bio, Inc.), 2 µl DNA template and 9 µl distilled H2O. One quantitative reverse transcriptase PCR (RT-qPCR) cycle consisted of 95°C for 30 sec, 95°C 5 sec, 55°C for 30 sec and 72°C for 30 sec. This was repeated for 40 cycles. The program was conducted in Agilent 3500-qPCR System (Agilent Technologies, Inc.).
Table I.
Disease activity index scoring criteria.
| Score | Weight loss (%) | Shape of feces | Hematochezia |
|---|---|---|---|
| 0 | 0 | Normal | Negative |
| 1 | 1–5 | ||
| 2 | 6–10 | Slight loose | Occult blood |
| 3 | 11–15 | ||
| 4 | >15 | Loose | Hematochezia |
Table II.
Primer information.
| Gene | Sequence (5′-3′) | Tm (°C) | Production length (bp) |
|---|---|---|---|
| IL-6 | Forward: CCACTTCACAAGTCGGAGGCTTA | 78 | 169 |
| Reverse: CCAGTTTGGTAGCATCCATCATTTC | |||
| TNF-α | Forward: TATGGCCCAGACCCTCACA | 80.6 | 199 |
| Reverse: GGAGTAGACAAGGTACAACCCATC | |||
| GAPDH | Forward: AAATGGTGAAGGTCGGTGTGAAC | 75.6 | 90 |
| Reverse: CAACAATCTCCACTTTGCCACTG |
IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Statistical analysis
All the data are expressed as mean ± standard deviation. Analysis of variance and Fisher's least significant difference tests were performed and P<0.05 was considered to indicate a statistically significant difference. All the statistical analysis was conducted using SPSS, Inc. (version, 16.0; Chicago, IL, USA).
Results
Morphology and drug loading efficiency of the SiO2 NPs
Morphological characteristics of SiO2 NPs were presented in Fig. 2. SiO2 NPs were uniformly distributed and the surface of the NPs was smooth with an average diameter of 90 nm. Based on the detection of HPLC, the average loading efficiency of 5-ASA-SiO2 NPs was 13.79±2.50 (wt%).
Figure 2.
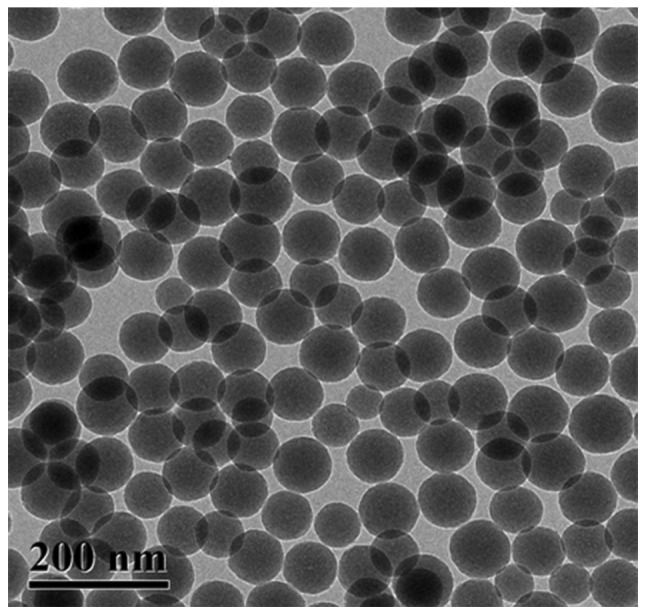
Transmission electron microscopy image of 5-ASA-SiO2 NPs. Surface of the NPs was smooth and the average diameter of the NPs was 90 nm. Based on the detection of HPLC, the average loading efficiency of 5-ASA-SiO2 NPs was 13.79±2.50 (wt%). 5-ASA-SiO2 NPs, 5-aminosalicylic acid-loaded silicon dioxide nanoparticles; HPLC, high performance liquid chromatography.
Cytotoxicity of the SiO2 NPs in Caco-2 cells
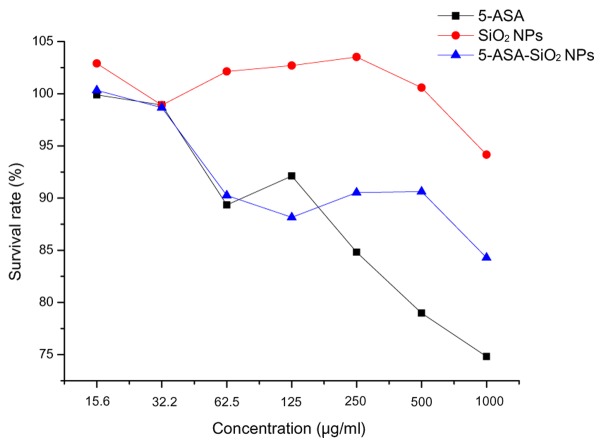
Survival rates of the Caco-2 cells decreased with the increase in the concentration of the treatment agents (Fig. 3). There had no significant difference in survival rate following treatment with SiO2, 5-ASA and 5-ASA-SiO2 NPs in the 15.6, 31.25 and 62.5 µg/ml groups, respectively. But when the concentration was above 250 µg/ml, a significant difference could be observed between the three treatments, SiO2 NPs had the strongest cytotoxicity and 5-ASA had the weakest cytotoxicity. However, even for SiO2 NPs treatment of 1,000 µg/ml, the survival rate was still more than 70%.
Figure 3.
Effect of different agents on Caco-2 cell survival rate. Effect of different agents on Caco-2 cell survival rate upon 12 h of exposure to a range from 15.6 to 1,000 µg/ml. (■), SiO2 NPs; (●), 5-ASA; (▲), 5-ASA-SiO2 NPs. The survival rate was assessed by Cell Counting Kit-8 assay. Results are expressed as percentage (mean ± standard deviation).
Evaluation of 5-ASA-SiO2 NPs in DDS-induced UC in mice
The result of DAI was showed in Fig. 4. There was no significant difference in DAI scores between groups B and E throughout the entire treatment period (Fig. 4). On the 7th day, all 5-ASA treatment groups (groups C, D and F) demonstrated reduced clinical activity compared with group B; and all differences were statistically significant when compared with the control group (group A) on the 7th day (Fig. 4).
Figure 4.
DAI score in the DSS mice model in 6 different groups. DAI scores in groups B and D were similar during the entire treatment period. All 5-ASA treatment groups (group C, D and F) reduced clinical activity as compared to the groups B and C. Similar results were noted in groups D and F. All differences were statistically significant in comparison with the untreated control group (group A). DAI, disease activity index.
H&E staining demonstrated that the colon mucosa was injured as congestion, edema and a number of shallow ulcers; and the structure of the glands were destroyed and disordered in the model group (Fig. 5). Similar pathological changes were observed in the colons of animals treated with SiO2 NPs. The degree of leukocyte infiltration and the mucosal edema as well as destroyed glands were reduced in the case of the normal dose 5-ASA group (group C). Conversely, the colon samples of animals treated with high dose 5-ASA group (group D) and 5-ASA-SiO2 NPs group (group E) exhibited less severe mucosal injury compared with the model and SiO2 NPs groups.
Figure 5.
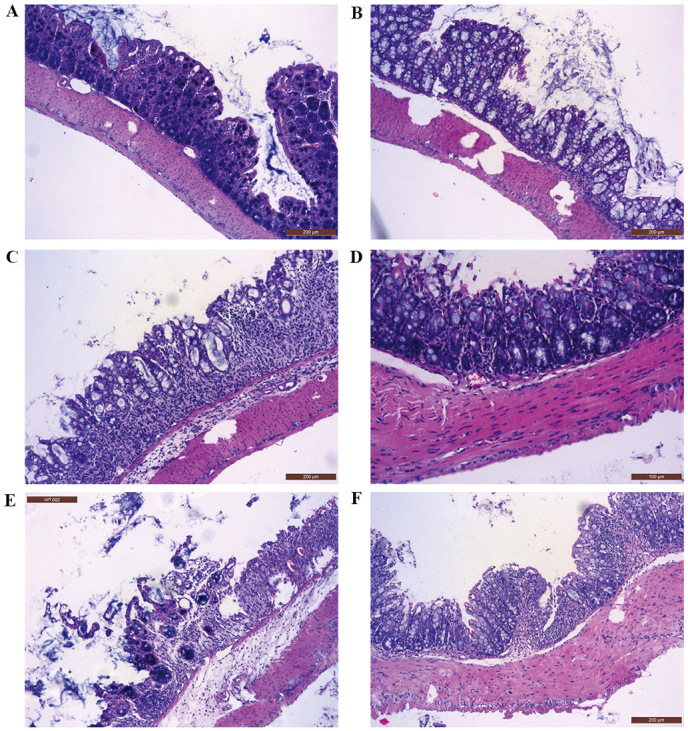
Histology image of colon issues obtained from different groups. The colons of animals in group E presented a similar degree of mucosal injury as group B. The degree of colon injury was reduced in group C. Conversely, the injured colon mucosa of animals in groups D and F was relieved significantly compared with the model group, with fewer edema and shallow ulcers. A, control group; B, model group (UC model mice); C, normal dose 5-ASA group (200 mg/kg.day−1 for 7 days); D, high dose 5-ASA group (400 mg/kg.day−1 for 7 days); E, SiO2 NPs group (100 mg/kg.day−1 for 7 days); F, 5-ASA-SiO2 NPs group (100 mg/kg day−1 for 7 days).
ELISA results of IL-6 and TNF-α measurements taken from eyeball blood samples and MPO from colon samples were presented in Table III. The IL-6, TNF-α and MPO levels in group B significantly increased compared with group A. There was no significant difference between groups B and E. The IL-6, TNF-α and MPO levels were significantly reduced by administration of normal dose 5-ASA (group C) compared with the control group; and the levels were lower in groups D and F when compared with group C.
Table III.
Protein levels of IL-6, TNF-α and MPO after treatment of 5-ASA-SiO2 NPs.
| Group | n | IL-6 (pg/ml) | TNF-α (pg/ml) | MPO (U/mg) |
|---|---|---|---|---|
| Group A | 10 | 53.2±9.0 | 42.2±7.0 | 2.0±0.8 |
| Group B | 9 | 315.8±31.1a | 284.5±27.1a | 34.5±2.2a |
| Group C | 10 | 226.2±17.5a,b | 153.3±17.9a,b | 25.7±2.0a,b |
| Group D | 10 | 126.5±8.9a–c | 84.0±9.3a–c | 10.3±1.3a–c |
| Group E | 9 | 317.8±18.9a | 282.0±22.7a | 34.1±1.7a |
| Group F | 10 | 135.1±10.9a,d,b,c | 87.5±7.0a,d,b,c | 10.9±1.4a,d,b,c |
P<0.01 vs. group A
P<0.01 vs group E
P<0.05 vs. group B
P<0.05 vs. group C. IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; MPO, myeloperoxidase; 5-ASA-SiO2 NPs, 5-aminosalicylic acid-loaded silicon dioxide nanoparticles.
Results of IL-6 and TNF-α mRNA expressions in colonic samples of different groups have been presented in Table IV. IL-6 and TNF-α mRNA expressions in group B were significantly higher than that of group A. In group E that received SiO2 NPs, IL-6 and TNF-α mRNA expressions were not significant to that of group B. IL-6 and TNF-α in groups C-E were significantly reduced when compared with group B. However, IL-6 and TNF-α were lower in groups D and F when compared with group C.
Table IV.
mRNA expression of IL-6, TNF-α in colonic mucosa.
| Group | n | IL-6 | TNF-α |
|---|---|---|---|
| Group A | 10 | 1.1±0.3 | 1.2±0.3 |
| Group B | 9 | 26.1±1.6a | 15.0±1.6a |
| Group C | 10 | 10.3±1.0a,b | 4.7±0.9a,b |
| Group D | 10 | 4.6±0.7a–c | 2.0±0.5a–c |
| Group E | 9 | 25.5±1.7a | 14.7±1.3a |
| Group F | 10 | 4.7±0.5a,d,b,c | 1.8±0.4a,d,b,c |
P<0.01 vs. group A
P<0.01 vs group E
P<0.05 vs. group B
P<0.05 vs. group C. IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Discussion
The colon is the terminal section of the digestive tract, and thus orally administered drugs targeting the colon must pass through a detrimental environment including digestive enzymes, pH and bacteria. Use of a novel oral colon-specific drug delivery system has confirmed that it is difficult to release a drug in the upper GI tract, and thus, the drug is released rapidly into the colon following oral administration, allowing for the accumulation of the drug in the inflamed target regions (10). Investigating this system would hopefully result in a higher local drug concentration at the target site and, consequently, reduced required doses, toxicity and systemic side effects. Nanopharmacology has gained interest in the clinic, and nanomedicines are promising therapeutics employed in the treatment of diseases at the molecular level (11). NPs have received considerable attention as a novel medical technology due to their non-toxic, biocompatible, biodegradable and mucoadhesive properties. Among the various pharmaceutical excipients, the SiO2 NPs are the most common. Their particle size is adjustable, they can be easily prepared and they are toxicologically safe, thus are favored by numerous researchers (12). NPs are an encouraging strategy to enhance the therapeutic benefit of UC (13–16), however, NPs in UC therapy remain in the initial stages.
The silica NPs demonstrated a mean size distribution and NP diameter of 90 nm in the present study, which is appropriate for uptake of NP in inflamed colonic mucosa (17). The particle size of the targeted delivery system is a major parameter to control and affect the particle transition into the mucosal tissue. As recorded in a recent study, small sized NPs may be able to more rapidly transit from the surface to deeper layer of intestinal wall, which reflects a size-dependency (18). The NPs in the current study had good spherical geometry, with transmission electron microscopy images of SiO2 NPs demonstrating that they have a solid and near consistent structure. The data obtained suggested that mixing 5-ASA with SiO2 NPs had no effect on the size and yield of 5-ASA-SiO2 NPs. 5-ASA-SiO2 NPs presented good loading efficiency through detection using HPLC, with findings similar to those of Pertuit et al (13), and with a loading efficiency much higher than poly (ε-caprolactone) NPs (15).
Survival rates were investigated in order to choose a safe range of concentrations to use for the transport experiments. For all exposures, >70% survival percentage proved to be safe. In vitro cytotoxicity investigations demonstrated that there was only marginal difference between the three agents when the concentration was lower than 62.5 µg/ml. A significant difference could be detected when the concentration was higher than 250 µg/ml, with the SiO2 NPs having the strongest cytotoxicity. The lower toxicity of 5-ASA SiO2 NPs compared to SiO2 NPs may be explained by the toxicity limiting the availability of bound compared to free drug. The general toxicity of the new system could be considered as minimal. However, we did not come to the conclusion that the toxicity of 5-ASA SiO2 NPs or SiO2 NPs was lower compared with 5-ASA, as previously indicated in the study of Pertuit et al (13). Together, the results of the present study demonstrate that 5-ASA-SiO2 NPs were successfully prepared, which may be considered for use in animal experimentation.
The severity of disease can be assessed using different parameters. The therapeutic effects of IBD can be assessed with IBD disease activity, including clinical, endoscopic, histological and radiological assessment tools (19). The clinical activity score allows the assessment of the severity of the diseases in the living animal. The treatment efficacy of 5-ASA-SiO2 NPs was assessed in UC model mice. The DAI scores was significantly decreased in the 5-ASA-SiO2 NPs group; the efficacy was even comparable to 5-ASA dose of 400 mg/kg day−1. Although the conditions of model mice did not return to normal levels during the experiment period, the high efficacy of 5-ASA-SiO2 NPs on UC was still evident. Furthermore, in histopathological examination, the injury to the colonic mucosa, including edema, shallow ulcers and destroyed glands, relieved in groups D and F. In the current study, 5-ASA SiO2 NPs with a 5-ASA dose of 100 mg/kg indicated a comparable efficacy to a 5-ASA solution at a dose of 400 mg/kg. The 5-ASA-SiO2 NPs with its low drug dosage can achieve similar effect of high dosage of 5-ASA, which is 32 times as much as that of 5-ASA-SiO2 NPs.
After the resection of the colon, different inflammatory markers can be determined to evaluate the therapeutic efficacy. A frequently used parameter is the activity of MPO. MPO is an enzyme that is observed in mammalian granulocytes and the MPO activity is used to determine the infiltration of tissue sections with these cells. Inflammation can be assessed by measuring cytokine levels, for example, TNF-α and IL-6 are suitable, as their expression levels (as well as MPO expression) are closely associated with the occurrence and development of UC. These mediators have been demonstrated as being highly expressed in UC samples (20) with a several-fold increase of MPO levels also reported throughout the colonic mucosa in patients with UC (21). Thus, these three factors are excellent indicators for the severity of inflammation. In the current study, the significant decrease of IL-6, TNF-α and MPO in UC model mice after exposure to 5-ASA SiO2 NPs was observed. The therapeutic potential of this delivery system appears similar to the application of high dose 5-ASA. It was concluded that a much lower dose of 5-ASA SiO2 NPs achieved similar results of high dose of 5-ASA in treating UC. NPs delivered through oral administration were determined to accumulate in the inflamed tissues and therefore developed a more local effect through their accumulation at the site of action (14,17,22). Due to the tighter bond between the drug and carrier system, 5-ASA SiO2 NPs present the advantage of targeting the drug to the inflamed area. The efficient binding of the 5-ASA to the SiO2 NPs is the key issue in the process. Additional associated studies confirmed the therapeutic benefits of targeting drug-loaded nanoparticles to the site of the disease and to mitigate the clinical symptoms compared with conventional dosage forms (23,24). In recent years, the incidence rate of UC in China has increased markedly (25), and therefore, the application 5-ASA-SiO2 NPs would decrease the financial cost and increase the safety of the treatment.
In conclusion, 5-ASA-SiO2 NPs is a selective drug release strategy that targets the inflamed tissue and highly increases therapeutic efficacy. The 5-ASA-SiO2 NPs at low dosage can achieve similar effects to high dosages of 5-ASA. As this drug delivery system is transposable to the majority of drugs in this therapeutic context, it represents a promising alternative for future innovative treatments of colon diseases.
Acknowledgements
The current study was supported by Science and Technology Agency of Liaoning Province (grant no. 2012225018) and the Science and Technology Bureau of Dalian (grant no. 2013E15SF154).
References
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimiol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematice review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Lakatos L, Lakatos LP. Is the incidence and prevalence of inflammatory bowel diseases increasing in Eastern Europe? Postgrad Med J. 2006;82:332–337. doi: 10.1136/pgmj.2005.043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Pineton Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the mangement of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 4.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 5.Min Wang, Chen Chen, Jiping Ma, Jie Xu. Preparation of superhydrophobic cauliflower-like silica nanosheres with tunable water adhesion. J Mater Chem. 2011;21:6962–6967. doi: 10.1039/c1jm10283d. [DOI] [Google Scholar]
- 6.Hoffmann JC, Pawlowski NN, Kühl AA, Höhne W, Zeitz M. Animal models of inflammatory bowel disease: An overview. Pathobiology. 2002;70:121–130. doi: 10.1159/000068143. -2003. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann G, Bidlingmaier C, Siegmund B, Albrich S, Schulze J, Tschoep K, Eigler A, Lehr HA, Endres S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J Pharmacol Exp Ther. 2000;292:22–30. [PubMed] [Google Scholar]
- 8.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 9.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–58. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamprecht A. IBD: Selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol and Hepatolo. 2010;7:311–312. doi: 10.1038/nrgastro.2010.66. [DOI] [PubMed] [Google Scholar]
- 11.Hock SC, Ying YM, Wah CL. A review of the current scientific and regulatory status of nanomedicines and the challenges ahead. PDA J Pharm Sci Technol. 2011;65:177–195. [PubMed] [Google Scholar]
- 12.Borbat PP, Costa-Filho AJ, Earle KA, Moscicki JK, Freed JH. Electron spin resonance in studies of membranes and proteins. Science. 2001;291:266–269. doi: 10.1126/science.291.5502.266. [DOI] [PubMed] [Google Scholar]
- 13.Pertuit D, Moulari B, Betz T, Nadaradjane A, Neumann D, Ismaïli L, Refouvelet B, Pellequer Y, Lamprecht A. 5-amio salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J Control Release. 2007;123:211–218. doi: 10.1016/j.jconrel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J Pharmacol Exp Ther. 2005;315:196–202. doi: 10.1124/jpet.105.088146. [DOI] [PubMed] [Google Scholar]
- 15.Moulari B, Pertuit D, Pellequer Y, Lamprecht A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials. 2008;29:4554–4560. doi: 10.1016/j.biomaterials.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Kshirsagar SJ, Bhalekar MR, Patel JN, Mohapatra SK, Shewale NS. Preparation and characterization of nanocapsules for colon-targeted drug delivery system. Pharm Dev Technol. 2012;17:607–613. doi: 10.3109/10837450.2011.557732. [DOI] [PubMed] [Google Scholar]
- 17.Lamprecht A, Schäfer U, Lehr CM. Size-dependent bioadhesion of micro-and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18:788–793. doi: 10.1023/A:1011032328064. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt C, Lautenschlaeger C, Collnot EM, Schumann M, Bojarski C, Schulzke JD, Lehr CM, Stallmach A. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa: A first in vivo study in human patients. J Control Release. 2013;165:139–145. doi: 10.1016/j.jconrel.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13:567–579. doi: 10.1038/nrgastro.2016.128. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, An P, Liu SQ, Yu HG. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. 2006;2006:92642. doi: 10.1155/MI/2006/92642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izzo RS, Witkon K, Chen AI, Hadjiyane C, Weinstein MI, Pellecchia C. Interleukin-8 and neutrophil markers in colonic mucosa from patients with ulcerative colitis. Am J Gastroenterol. 1992;87:1447–1452. [PubMed] [Google Scholar]
- 22.Lamprecht A, Ubrich N, Yamamoto H, Schäfer U, Takeuchi H, Maincent P, Kawashima Y, Lehr CM. Biodegradable nanoparticles for the targeted drug delivery in treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2001;299:775–781. [PubMed] [Google Scholar]
- 23.Niebel W, Walkenbach K, Béduneau A, Pellequer Y, Lamprecht A. Nanoparticle-based clodronate delivery mitigates murine experimental colitis. J Control Release. 2012;160:659–665. doi: 10.1016/j.jconrel.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Meissner Y, Pellequer Y, Lamprecht A. Nanoparticles in inflammatory bowel disease: Particle targeting versus pH-sensitive delivery. Int J Pharm. 2006;316:138–143. doi: 10.1016/j.ijpharm.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Ng SC. Epidemiology of inflammatory bowel disease: Focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363–372. doi: 10.1016/j.bpg.2014.04.003. [DOI] [PubMed] [Google Scholar]