Abstract
Moscatilin is a bibenzyl derivative extracted from the Dendrobium aurantiacum var. denneanum, which has traditionally been used as an immunomodulatory treatment in China. The present study was designed to determine whether moscatilin is a pro-apoptotic agent in pancreatic cancer, and to elucidate the underlying mechanisms. The apoptotic and anti-proliferative effects of moscatilin on pancreatic cancer cells were determined in vitro using biochemical assays, such as the MTT assay, colony formation assay, Hoechst staining and DNA fragmentation assay, and in vivo using Panc-1 pancreatic cancer xenografts. Western blotting was also conducted to evaluate the expression levels of B-cell lymphoma 2 (Bcl2), Bcl2-associated X protein (Bax), Bcl2 homologous antagonist killer (Bak), caspase 3, cleaved-caspase 3, poly (ADP-ribose) polymerase, p-c-Jun N-terminal kinase (JNK)/stress-activated protein kinases (SAPK) and JNK/SAPK in response to moscatilin. We used DCFH-DA to detect the production of reactive oxygen species (ROS) induced by moscatilin. The present study demonstrated that moscatilin markedly inhibited pancreatic cancer cell viability and induced cell apoptosis in a concentration-dependent manner. Conversely, moscatilin did not affect the cell viability of human umbilical vein endothelial cells at the comparable dosage. Treatment with moscatilin suppressed clonogenicity of Panc-1 cells in a concentration-dependent manner. Furthermore, a decrease in Bcl2 expression, and an increase in the expression levels of Bak and Bax, was detected following treatment with moscatilin, resulting in an increase in the proapoptotic/anti-apoptotic expression ratio (Bax/Bcl2) in Panc-1 cells. Moscatilin also induced activation of the caspase-dependent mitochondrial apoptotic pathway. In addition, moscatilin enhanced cellular ROS production and induced activation of JNKSAPK signaling pathway. Conversely, pretreatment with the ROS scavenger N-acetylcysteine or the JNK/SAPK-specific inhibitor SP600125 prevented moscatilin-mediated reductions in cell viability. Furthermore, moscatilin inhibited tumor growth in nude mice bearing Panc-1 cells, without apparent toxicity. In conclusion, these results demonstrated that moscatilin may induce pancreatic cell apoptosis, and therefore may be considered a potential therapeutic agent for the treatment of pancreatic cancer.
Keywords: moscatilin, pancreatic cancer, reactive oxygen species, JNK/SAPK, mitochondrial apoptotic pathway
Introduction
Pancreatic cancer is a fatal malignancy, which has been predicted to become the second most common cause of cancer-associated mortality within the next two decades (1). The incidence of pancreatic cancer has gradually increased during recent years, predominantly due to the increased rates of obesity, smoking, alcohol abuse, pre-existing chronic pancreatitis and prior abdominal radiotherapy (2). At present, surgery and chemotherapy during the early stages of the disease provide the best prognosis for long-term survival; however, the 5-year survival rate is still <5% and the median survival rate is rarely >20 months (3,4). Due to the persistent poor prognosis associated with pancreatic cancer, it is clear that more effective agents are required, and novel therapeutic strategies should be developed for the prevention and treatment of pancreatic cancer.
Oxidative stress occurs as a result of an imbalance between excessive reactive oxygen species (ROS) production and antioxidant depletion. ROS exert a critical role in several cellular processes associated with cancer development, metastasis, progression and survival (5). The majority of chemotherapeutic and radiotherapeutic agents may be selectively toxic to tumor cells by increasing oxidative stress beyond their limit, which is thought to be contribute to sustained activation of cell-cycle inhibitors, cell death induction, and macromolecular damage-induced senescence. At present, augmentation of oxidative stress represents the best opportunity for the development of novel therapeutic strategies for cancer treatment (6–8).
Natural products from traditional Chinese medicine (TCM) exhibit marked potential as anticancer drugs, including paclitaxel (6), camptothecin (7) and vincristine (8). The Dendrobium species (Orchidaceae) has been widely used in TCM, since members of this species exert a broad spectrum of beneficial health effects, including antipyretic, eye health-promoting, immunomodulatory and anti-aging activities. This species has been used in China, India, and other countries in subtropical and Southeast Asia, for >2,000 years (9–11). For decades, bibenzyls, which are the main bioactive components derived from Dendrobium species, have been subjected to extensive investigation as likely candidates for cancer treatment (12–19). For example, erianin exhibits anti-angiogenic activity via inducing endothelial cytoskeletal disorganization and activating the c-Jun N-terminal kinase (JNK)/stress-associated protein kinases (SAPK) signaling pathway (12,13). Dendrofalconerol A exerts antimetastatic effects via the suppression of epithelial-to-mesenchymal transition and integrin proteins in lung cancer (14). In addition, gigantol inhibits migration of non-small cell lung cancer cells via a decrease in caveolin-1 protein expression, and the activation of Akt and cell division cycle 42 (15). In our preliminary study, moscatilin, which was isolated from the Dendrobium aurantiacum var. denneanum, was revealed to exert proapoptotic effects in pancreatic cancer cells. Moscatilin has been reported to exert proapoptotic effects in esophageal cancer, antimetastatic effects in lung and breast cancer, and anti-angiogenic activities against malignant tumors (16–19). The present study aimed to investigate whether the cytotoxicity of moscatilin on Panc-1 cells was associated with intracellular ROS production, and to determine the underlying mechanisms.
Materials and methods
Chemicals and reagents
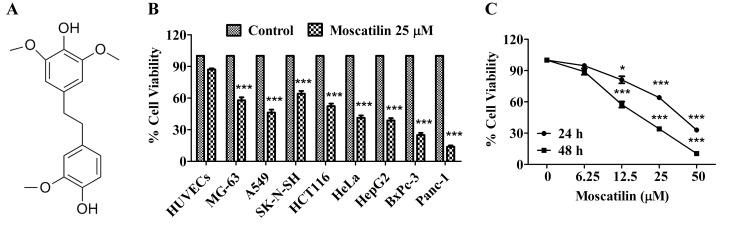
Moscatilin (Fig. 1A) was isolated from the Dendrobium aurantiacum var. denneanum. Its structure was determined using 1H-nuclear magnetic resonance (NMR) and 13C-NMR spectral analyses, and its purity was >98%, as determined by high-performance liquid chromatography analysis as described (20). Moscatilin was dissolved in absolute dimethyl sulfoxide (DMSO), and was further diluted with culture medium on the experimental day.
Figure 1.
Moscatilin inhibited cell viability in vitro. (A) Chemical structure of moscatilin. (B) Cell viability was measured following treatment with moscatilin (25 µM) for 48 h in various cell lines, including HUVECs, MG-63, A549, SK-N-SH, HCT116, HeLa, HepG2, BxPc-3 and Panc-1. Cell viability was determined using MTT assay. (C) Cell viability was measured following treatment with moscatilin (0–50 µM) for 24 and 48 h. Cell viability was determined using MTT assay. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05, ***P<0.001, compared with the control group. HUVECs, human umbilical vein endothelial cells.
Antibodies against phosphorylated (p)-JNK/SAPK (cat. no. 9251), JNK/SAPK (cat. no. 9252), B-cell lymphoma 2 (Bcl2) (cat. no. 2872), Bcl2-associated X protein (Bax) (cat. no. 2772), Bcl2 homologous antagonist killer (Bak; cat. no. 12105), caspase 3 (cat. no. 9662), cleaved-caspase 3 (cat. no. 9661), poly (ADP-ribose) polymerase (PARP) (cat. no. 9542) and GAPDH (cat. no. 2118) were all purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Peroxidase-conjugated goat anti-rabbit immunoglobulin G (H+L; cat. no. 111-065-003) was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Cell culture reagents were obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) and other reagents, unless otherwise indicated, were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Cell lines and cell culture conditions
The MG-63 human osteosarcoma cancer cell line, the A549 human lung cancer cell line, the SK-N-SH human neuroblastoma cancer cell line, the HCT116 human colon cancer cell line, the HeLa human cervical cancer cell line, the HepG2 human hepatic cancer cell line, and the Panc-1 and BxPc-3 human pancreatic cancer cell lines were purchased from the Chinese Academy of Science Committee Type Culture Collection Cell Bank (Shanghai, China). Panc-1 cells were cultured in Dulbecco's modified Eagle' medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. MG-63, SK-N-SH, HCT116, HepG2 and HeLa cells were cultured in minimum essential medium (MEM) supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. BxPc-3 cells were cultured in RPMI-1640 containing 10% (v/v) FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. A549 cells were cultured in F12K medium supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Human umbilical vein endothelial cell (HUVEC) was supplied by Dr. Wentao Zhou, from Institute of Nutrition Science, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China) in 2014. HUVECs were cultured as previously described (21). Cell viability assay. Cells were seeded at a density of 5×103 cells/well into 96-well plates in DMEM supplemented with 10% FBS. Following attachment, cells were treated with moscatilin (0–50 µM) for 24 and 48 h. Following treatment, MTT solution was added and incubated for 4 h at 37°C. The medium was then removed, 100 µl DMSO was added and absorbance was quantified at 570 nm. Cell viability was normalized as a percentage of control. For the blocking study, cells were pretreated with 5 mM N-acetylcysteine (NAC; Sigma-Aldrich; Merck Millipore), 50 µM Z-VAD-FMK (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or 20 µM SP600125 (Cell Signaling Technology, Inc.) for 30 min, and then treated with moscatilin (25 µM) for 24 h at 37°C.
Colony formation assay
Cells were resuspended in 1.5 ml growth media containing 0.3% low-melting temperature agarose, and were plated in 6-well plates over a base layer of 1.5 ml growth media containing 0.6% low-melting temperature agarose. The cells were allowed to form colonies for 15 days at 37°C in a humidified atmosphere containing 5% CO2. The colonies were fixed with 4% paraformaldehyde for 30 min at room temperature and were then stained with 0.04% crystal violet for 30 min at room temperature. Colonies with >50 cells were counted as one positive colony under a microscope (IX81; Olympus Corporation, Tokyo, Japan). The inhibition of colony formation was exhibited as a percentage of vehicle control.
Hoechst 33342 staining assay
Cells (1×105 cells/well) were seeded into 6-well plates in DMEM supplemented with 10% FBS. After attachment, cells were treated with various concentrations of moscatilin (0–25 µM) for 24 h. After treatment, the cells were incubated with Hoechst 33342 (10 µg/ml) for 5 min at room temperature in the dark. After incubation, stained cells were observed under a fluorescent microscope.
DNA fragmentation assay
Cells (3×105 cells/well) were seeded into 60 mm plates in DMEM supplemented with 10% FBS. After attachment, cells were treated with moscatilin (0–25 µM) for 24 h. After treatment, cells were lysed and the fragmented DNA in the lysate was extracted using a DNA extraction kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer's protocols. Briefly, the fragmented DNA in the lysate was extracted with phenol/chloroform/isopropyl alcohol (25:24:1, v/v), and then precipitated for 10 min in liquid nitrogen with chilled 100% ethanol and 3 M sodium acetate. The DNA pellet produced by centrifuging at 12,000 × g for 15 min at 4°C, the pellet was then washed with 70% ethanol and resuspended in Tris-HCl (pH 8.0) with 100 µg/ml RNase A for 1 h at 37°C. The DNA fragments were separated by 1.5% agarose gel electrophoresis, stained with ethidium bromide and images were captured under ultraviolet light.
Measurement of ROS formation
After treatment with moscatilin (0–25 µM), the cells were stained with DCFH-DA for 30 min at 37°C in the dark. Subsequently, stained cells were observed under a fluorescent microscope and absorbance was measured at 488 nm (excitation wavelength) and 525 nm (emission wavelength).
Western blotting
After treatment with moscatilin (0–25 µM), the cells were lysed in lysis buffer containing 50 mM Tris (pH 7.5), 1 mM EDTA, 150 mM NaCl, 20 mM NaF, 0.5% NP-40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 10 µg/ml leupeptin and 10 µg/ml pepstatin A. Protein concentration was determined using Bradford protein assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. Protein samples (30 µg) were separated using 10 or 12% premade SDS-PAGE gels, and were then transferred to a nitrocellulose filter membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was blocked with 10% bovine serum albumin (Santa Cruz Biotechnology, Inc.) in 1X Tris-buffered saline-0.05% Tween-20 (TBST) for 2 h at room temperature. Following blocking, the membrane was incubated with primary antibody (1:1,000) at 4°C overnight. The membrane was washed three times with TBST and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5,000) for 1 h at room temperature. The protein bands were visualized by RapidStep™ ECL reagent (Merck Millipore) and semi-quantified with ImageJ version 1.6.0 (imagej.nih.gov/ij/).
Xenograft mouse model
Specific pathogen-free nude male mice (age, 8 weeks; weight, 18–20 g) were provided by Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China). Mice were housed under specific pathogen-free conditions according to the guidelines of the association for Assessment and Accreditation of Laboratory Animal Care (Shanghai, China). They were housed under standard conditions (25°C, 12-h light/dark cycle) with access to food and water ad libitum. All studies were conducted in a manner aiming to minimize animal suffering and to reduce the number of animals used. Nude mice (n=6/group) were subcutaneously injected with Panc-1 cells (1×106 cells/mouse) into the left front leg. After tumors had been established (~30 mm3), the mice were administered intraperitoneal injections of vehicle control (0.5% DMSO and 0.5% Tween-80 in normal saline) or moscatilin (25 mg/kg in vehicle control) every day. The body weight and tumor sizes of all mice were recorded every 4 days. Tumor sizes were determined by Vernier caliper measurements and were calculated as follows: [(length × width2)/2]. After 21 days of treatment, mice were sacrificed with pentobarbital sodium (150 mg/kg; delivered by intraperitoneal injection), and their tumors were removed, weighed and photographed.
Statistical analysis
Data are presented as the mean ± standard error of the mean. Normal distributed data with equal variance were analyzed using one- or two-way analysis of variance followed by Fisher's LSD multiple comparisons test or Student's unpaired t-test for single comparisons using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Moscatilin inhibits viability of pancreatic cancer cells
It is well known that cancer cell viability is an important feature of cancer growth and development. The present study initially investigated the cytotoxicity of moscatilin in a panel of cell lines using the MTT assay. Following treatment with moscatilin (25 µM) for 48 h, increased cytotoxicity was detected in all tested cancer cell lines. These results indicate that cytotoxicity was observed in all of the following tested cancer cell lines: MG-63 (osteosarcoma), A549 (lung), SK-N-SH (neuroblastoma), HCT116 (colon), HeLa (cervical), HepG2 (hepatic), BxPc-3 and PanC-1 (pancreatic), following treatment with moscatilin (25 µM) for 48 h (Fig. 1B). Among the cell lines, the pancreatic cell lines were most sensitive to moscatilin (Fig. 1B). Conversely, moscatilin had little effect on the cell viability of HUVECs (Fig. 1B). Subsequently, the inhibitory effects of various concentrations of moscatilin were detected on Panc-1 cell viability. As shown in Fig. 1C, 24 or 48 h treatment with moscatilin (0–50 µM) markedly inhibited the viability of Panc-1 cells in a concentration-dependent manner.
Moscatilin suppresses clonogenicity of pancreatic cancer cells
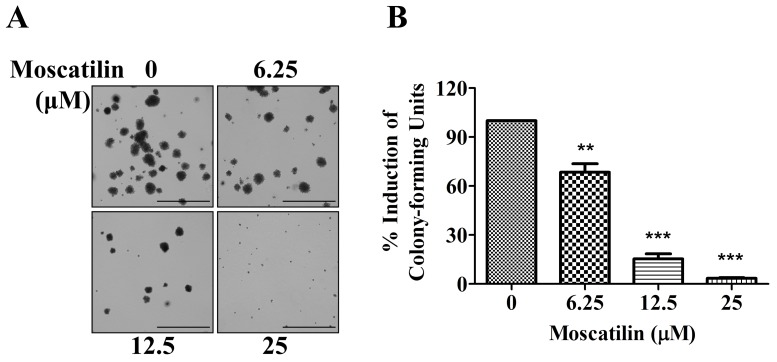
To determine the potential effects of moscatilin on long-term proliferation, a colony formation assay was conducted. Panc-1 cells were exposed to moscatilin (0–25 µM) for 15 days. In Panc-1 cells, colony formation was significantly decreased following treatment with moscatilin. An initial decrease in clonogenicity was observed following treatment with 6.25 µM moscatilin, and a maximal response was detected following treatment with 25 µM moscatilin (Fig. 2A and B). These results suggest that treatment with moscatilin suppressed clonogenicity in a dose-dependent manner compared with the control group.
Figure 2.
Moscatilin suppressed clonogenicity of Panc-1 cells. Colony formation was detected in Panc-1 cells following treatment with moscatilin (0–25 µM) for 15 days using the colony formation assay. (A) Representative micrographs of colony formation following treatment with 0, 6.25, 12.5 and 25 µM. Scale bar, 1 mm. (B) Data are expressed as the percentage induction compared to the control group. Data are presented as the mean ± standard error of the mean (n=3). **P<0.01, ***P<0.001, compared with the control group.
Moscatilin induces apoptosis of pancreatic cancer cells
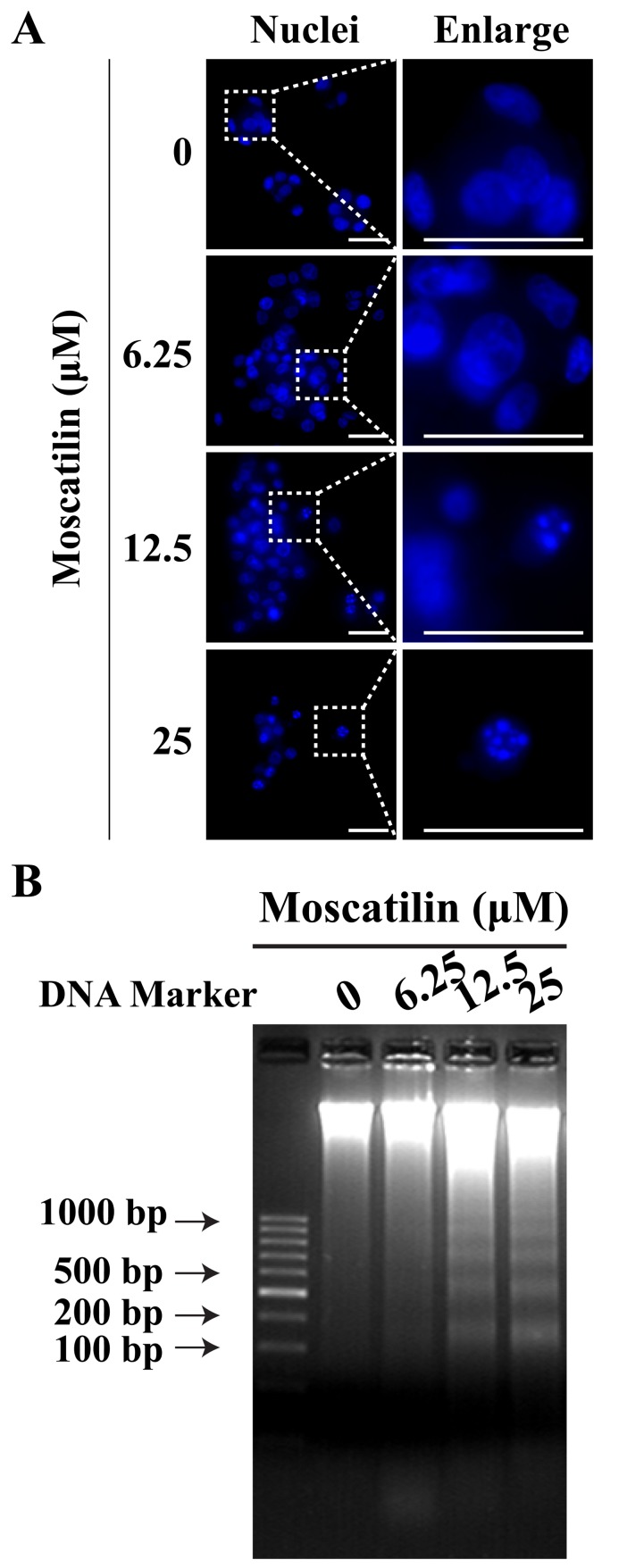
To determine whether the reduction in cell viability by moscatilin was due to the induction of apoptosis, Hoechst staining and a DNA fragmentation assay were conducted. In Panc-1 cells, treatment with moscatilin for 24 h led to nuclear fragmentation and chromatin condensation in a concentration-dependent manner, as determined by Hoechst staining (Fig. 3A). Similarly, the present study observed an induction in apoptotic DNA fragmentation following treatment with moscatilin (0–25 µM) for 24 h, as evidenced by the DNA fragmentation assay (Fig. 3B).
Figure 3.
Moscatilin induced cell apoptosis of Panc-1 cells. (A) Morphological alterations were observed in the nucleolus of Panc-1 cells treated with moscatilin (0–25 µM) for 24 h using Hoechst staining. Scale bar, 20 µm. (B) Apoptotic DNA fragmentation was observed following treatment with moscatilin (0–25 µM) for 24 h. n=3.
Moscatilin induces activation of the mitochondrial apoptotic pathway
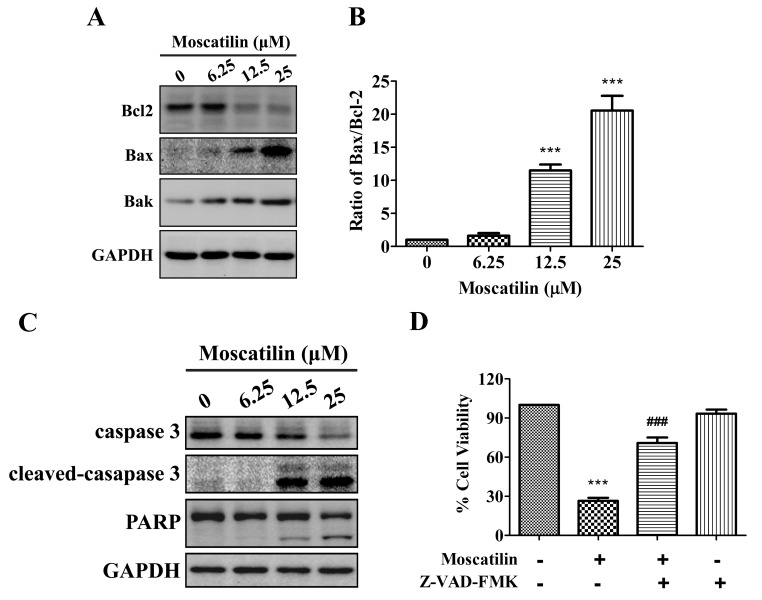
To investigate whether moscatilin induced apoptosis via triggering the mitochondrial apoptotic pathway, the expression levels of Bcl2 family proteins, including Bcl2, Bax and Bcl2 homologous antagonist killer (Bak), were detected in the presence of moscatilin. The results indicated that treatment with moscatilin induced a decrease in Bcl2 expression, and an increase in the expression levels of Bak and Bax, which led to an increase in the Bax/Bcl2 ratio (Fig. 4A and B). In addition, treatment with moscatilin led to the cleavage of caspase 3 and PARP in Panc-1 cells (Fig. 4C). Similarly, treatment with the Pan-caspase inhibitor Z-VAD-FMK prevented reductions in cell viability in response to moscatilin (Fig. 4D). These results indicate that moscatilin inhibits viability and induces apoptosis via the mitochondrial apoptosis pathway.
Figure 4.
Moscatilin induced the mitochondrial apoptotic pathway. (A and B) Following treatment with moscatilin (0–25 µM) for 24 h, the protein expression levels of Bcl2, Bax, Bak and GAPDH were determined by western blotting in Panc-1 cells. (A) Representative immunoblot. (B) Ratio of Bax/Bcl2 expression. (C) Following treatment with moscatilin (0–25 µM) for 24 h, the protein expression levels of caspase 3, cleaved-caspase 3, PARP and GAPDH were determined by western blotting in Panc-1 cells. (D) Panc-1 cells were treated with moscatilin (25 µM) for 24 h following pretreatment with Z-VAD-FMK (50 µM) for 30 min. Cell viability was measured by MTT assay. Data are presented as the mean ± standard error of the mean (n=3). ***P<0.001, compared with the control group; ###P<0.001 compared with the moscatilin group. Bcl2, B-cell lymphoma 2; Bax, Bcl2-associated X protein; Bak, Bcl2 homologous antagonist killer; PARP, poly (ADP-ribose) polymerase.
ROS/JNK signaling pathway is involved in cell apoptosis in response to moscatilin
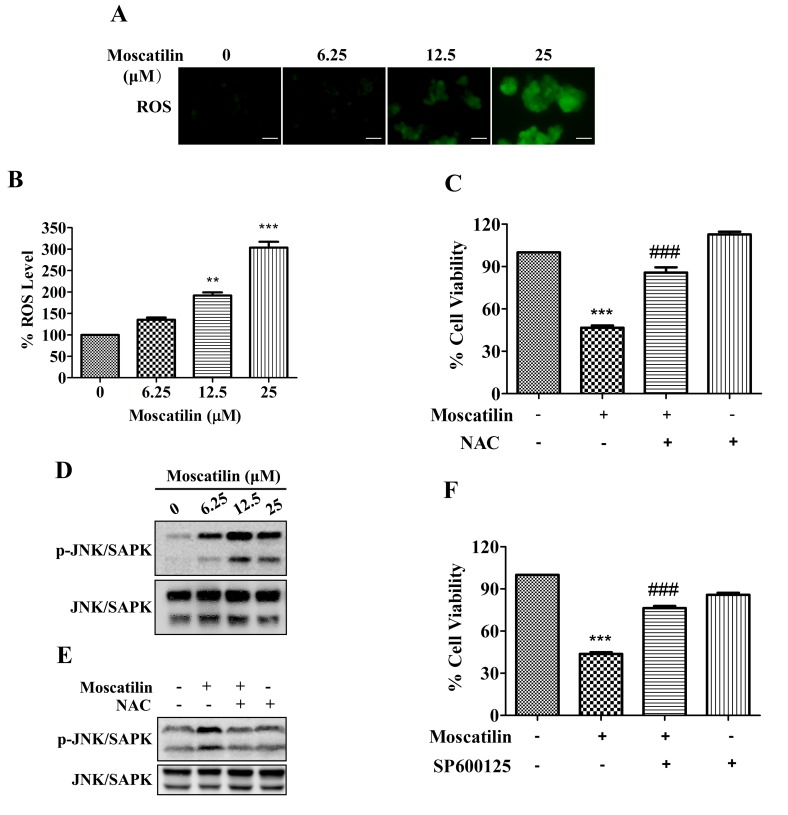
ROS exert a pivotal role in various processes associated with tumor progression. High ROS levels are required for the initiation of apoptotic responses induced by some anticancer agents (22,23). The present study demonstrated that treatment with moscatilin led to an increase in ROS generation. The ROS levels in Panc-1 cells were increased by moscatilin in a dose-dependent manner, with 1.2-, 1.9- and 3.0-fold increases detected following treatment with 6.25, 12.5 and 25 µΜ moscatilin, respectively (Fig. 5A and B). Similarly, treatment with the ROS scavenger NAC prevented the reductions in cell viability in response to moscatilin treatment (Fig. 5C).
Figure 5.
ROS/JNK signaling pathway was required for moscatilin-induced effects. (A and B) Following treatment of Panc-1 cells with moscatilin (0–25 µM) for 24 h, ROS were measured using the fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate. (A) Stained cells were observed under an inverted fluorescent microscope and (B) the fluorescence intensity units were measured at 488 nm (excitation wavelength) and 525 nm (emission wavelength). Scale bar, 100 µm. (C) Panc-1 cells were treated with moscatilin (25 µM) for 24 h following pretreatment with NAC (5 mM) for 30 min. Cell viability was measured by MTT assay. (D) Following treatment with moscatilin (0–25 µM) for 24 h, the protein expression levels of p-JNK/SAPK, JNK/SAPK and GAPDH were determined by western blotting in Panc-1 cells. (E) Panc-1 cells were treated with moscatilin (25 µM) for 24 h following pretreatment with NAC (5 mM) for 30 min. The protein expression levels of p-JNK/SAPK and JNK/SAPK were determined by western blotting. (F) Panc-1 cells were treated with moscatilin (25 µM) for 24 h following pretreatment with SP600125 (20 µM) for 30 min. Cell viability was measured by MTT assay. Data are presented as the mean ± standard error of the mean. (n=3). **P<0.01, ***P<0.001, compared with the control group; ###P<0.001 compared with the moscatilin group. ROS, reactive oxygen species; NAC, N-acetylcysteine; p-, phosphorylated; JNK, c-Jun N-terminal kinase; SAPK, stress-associated protein kinases.
The JNK/SAPK signaling pathway is downstream of the ROS signaling pathway, which is implicated in controlling cell proliferation, differentiation and apoptosis (24). As shown in Fig. 5D, phosphorylation of JNK/SAPK was observed following treatment with 6.25 µΜ moscatilin, and was maintained up to 25 µΜ. Conversely, moscatilin-induced activation of JNK/SAPK was attenuated in Panc-1 cells following pretreatment with the ROS scavenger NAC (Fig. 5E). Furthermore, the moscatilin-induced reduction in cell viability was attenuated by the JNK/SAPK-specific inhibitor SP600125 (Fig. 5F). These results indicate that the ROS/JNK signaling pathway may serve a pivotal role in apoptotic induction of Panc-1 cells by moscatilin.
Moscatilin inhibits pancreatic cancer growth in vivo
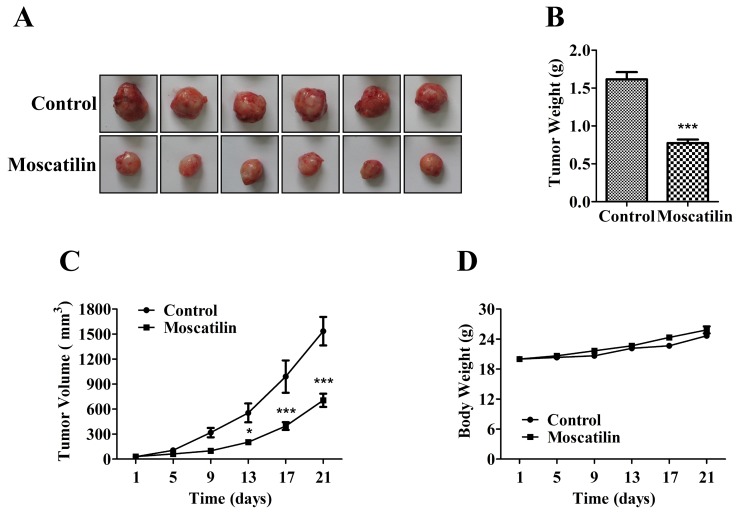
To determine the potential effects of moscatilin on pancreatic cancer growth and development, equal numbers of Panc-1 pancreatic cancer cells were subcutaneously injected into the left front leg of nude mice. Once the tumors reached ~30 mm3, the mice were treated intraperitoneally with control (0.5% DMSO and 0.5% Tween-80 in normal saline) or moscatilin (25 mg/kg) every day. Following treatment with moscatilin for 21 days, tumor weight was reduced by 52% compared with in the control group (Fig. 6A-C). There was no difference in body weight between the control and moscatilin-treated groups (Fig. 6D).
Figure 6.
Moscatilin inhibited pancreatic cancer growth and development in vivo. (A) Tumors were removed and images of the tumors were captured. (B) Tumors were weighed. (C) Tumor size was recorded every 4 days by Vemier caliper measurements, and was calculated as [(length × width2)/2]. (D) Body weight was recorded every 4 days. Data are presented as the mean ± standard error of the mean (n=6). *P<0.05, ***P<0.001, compared with the control group.
Discussion
Pancreatic cancer is the fourth leading cause of cancer-associated mortality worldwide, and is one of the most invasive and frequently diagnosed malignancies, due to its poor prognosis (1,4). The high mortality rate associated with pancreatic cancer indicates that the currently available treatments, such as chemotherapy, are ineffective (3,4). Therefore, there is an increasing need to identify novel proapoptotic agents that can be used to treat tumor growth in the advanced stages of pancreatic cancer.
Cell proliferation is considered to be an important feature of cell growth and development. The present study demonstrated that moscatilin markedly inhibited the viability of various cancer cell lines, and exhibited higher sensitivity in pancreatic cancer cell lines. Conversely, moscatilin failed to affect the cell viability of normal HUVECs. These results indicated the specific anti-tumor activity of moscatilin, and suggested it possessed a favorable therapeutic index. Furthermore, moscatilin was revealed to strongly suppress pancreatic tumor growth and development, without any apparent toxicity, in vivo. In previous studies, moscatilin has been demonstrated to possess proapoptotic effects in esophageal cancer, antimetastatic effects in lung and breast cancer, and anti-angiogenic activity against malignant tumors (16–19). These previous data indicated that moscatilin is worthy of being developed into a therapeutic agent for the prevention and treatment of pancreatic cancer.
Apoptosis serves a pivotal role in the development of cancer, including cancer initiation, progression and metastasis (25,26). It has previously been reported that apoptosis is characterized by certain hallmarks, such as phosphatidyl serine exposure on the plasma membrane, activation of caspase 3 and PARP, and DNA fragmentation (27). The results of the present study indicated that activation of caspase 3 and PARP contribute to moscatilin-induced apoptosis of Panc-1 cells. The key event in the intrinsic apoptotic pathway is permeabilization of the mitochondrial outer membrane, which occurs in response to various stimuli, and is regulated by several cytoplasmic proteins, including Bcl2 family members (28). Anti-apoptotic members, such as Bcl2, prevent apoptosis, whereas proapoptotic members, such as Bax and Bak, reside on the outer mitochondrial membrane or cytosol and oligomerize under stress to facilitate the release of factors from the mitochondria, which trigger apoptosis (29). Therefore, the Bax/Bcl2 ratio, as a candidate prognostic biomarker for cancer, indicates the degree of mitochondrial outer membrane permeabilization and hence the entrance to the execution phase of the apoptotic pathway. The present study indicated that an increase in Bak expression and an increase in the Bax/Bcl2 (proapoptotic/anti-apoptotic) ratio contribute to the involvement of the mitochondrial-mediated intrinsic pathway in moscatilin-induced apoptosis.
ROS are recognized as intracellular secondary messengers in various cell receptor signal transduction pathways. Although higher than normal ROS levels are observed in cancer cells, it has previously been suggested that cancer cells are more vulnerable to intracellular ROS introduction (30). Therefore, cancer treatment by means of enhancing intracellular ROS production may be considered an effective approach. Natural compounds, such as nimbolide and bufalin, are able to induce pancreatic cancer cell apoptosis by increasing ROS levels (31,32). The present study demonstrated that treatment with 25 µM moscatilin led to an increase in ROS generation; however, moscatilin-inhibited cell viability was abolished by NAC, thus suggesting that ROS is responsible for the proapoptotic effects of moscatilin on pancreatic cancer cells. Kowitdamrong et al (18) demonstrated that moscatilin (1 µM) suppressed ROS generation and FeSO4-mediated ROS generation, and the inhibition of ROS was critical for moscatilin-mediated suppression of cell motility and invasion, but not cell growth, in lung cancer. The different effects of moscatilin on ROS levels may be due to the various concentrations of moscatilin used, or may be attributed to different cancer cell functions (cancer cell growth and cancer cell metastasis), or the fact that Panc-1 pancreatic cancer cells and H23 lung cancer cells are two different cancer cell lines.
Mitogen-activated protein kinases, including extracellular signal-regulated kinase (ERK1/2), p38 and JNK/SAPK, are primarily activated by exposure to ROS and serve important roles in regulating cell proliferation, differentiation, mitosis, survival and apoptosis (33,34). Although the ERK1/2 pathway is considered a great contributor to oncogenesis, a previous study demonstrated that it serves a lesser role in mitogen-induced survival of pancreatic cancer (35). In addition, JNK/SAPK activation is considered an important apoptosis-inducing factor that exerts proapoptotic effects on apoptosis of cancer cells (36,37). Previous studies have indicated that JNK/SAPK activation results in an increase in the number of apoptotic cells in response to several anticancer agents in pancreatic cancer (38,39). The present study demonstrated that treatment with moscatilin induced sustained activation of JNK/SAPK, and the phosphorylation of JNK/SAPK was dependent on ROS generation, which was prevented by treatment with the ROS scavenger NAC. Furthermore, treatment with a JNK/SAPK inhibitor restored the viability of moscatilin-treated Panc-1 cells. These observations indicate that elevation of ROS generation and the subsequent activation of JNK/SAPK serves a crucial role in the induction of programmed cell death in pancreatic cancer in response to moscatilin.
In conclusion, the present study demonstrated that moscatilin increases ROS generation and subsequently activates the JNK/SAPK pathway, which modulates the Bax/Bcl2 ratio, thus leading to the caspase-dependent mitochondrial apoptotic pathway. The present study provided detailed mechanistic insights into the proapoptotic effects of moscatilin in cells; these data strongly support the application of moscatilin as a potential future treatment against pancreatic cancer.
Acknowledgements
The present study was supported financially by the Youth Foundation of Zhongshan Hospital Fudan University (grant no. 2014ZSQN39).
References
- 1.Rahib L, Smith BD, Aizenber R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulson AS, Cao Tran HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gasroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 5.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 7.Paduch R, Kandefer-Szerszeń M, Piersiak T. The important of release of proinflammatory cytokines, ROS, and NO in different stages of colon carcinoma growth and metastasis after treatment with cytotoxic drugs. Oncol Res. 2010;18:419–436. doi: 10.3727/096504010X12671222663593. [DOI] [PubMed] [Google Scholar]
- 8.Groninger E, Meeuwsen-De Boer GJ, De Graaf SS, Kamps WA, De Bont ES. Vincristine induced apoptosis in acute lymphoblastic leukaemia cells: A mitochondrial controlled pathway regulated by reactive oxygen species? Int J Oncol. 2002;21:1339–1345. doi: 10.3892/ijo.21.6.1339. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Xu JK, Wang J, Wang NL, Kuriihara H, Kitanaka S, Yao XS. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J Nat Prod. 2007;70:24–28. doi: 10.1021/np060449r. [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Wu LG, Ko FN, Teng CM. Antiplatelet aggregation principles of Dendrobium loddigesii. J Nat Prod. 1994;57:1271–1274. doi: 10.1021/np50111a014. [DOI] [PubMed] [Google Scholar]
- 11.Hu JM, Chen JJ, Yu H, Zhao YX, Zhou J. Two novel bibenzyls from Dendrobium trigonopus. J Asian Nat Prod Res. 2008;10:653–657. doi: 10.1080/10286020802133605. [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, Fan Y, Liu L, Wu D, Chang Z, Wang Z. Erianin induces a JNK/SAPK-dependent metabolic inhibition in human umbilical vein endothelial cells. In Vivo. 2004;18:223–228. [PubMed] [Google Scholar]
- 13.Gong YQ, Fan Y, Wu DZ, Yang H, Hu ZB, Wang ZT. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur J Cancer. 2004;40:1554–1565. doi: 10.1016/j.ejca.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Pengpaeng P, Sritularak B, Chanvorachote P. Dendrofalconerol A suppresses migrating cancer cells via EMT and integrin proteins. Anticancer Res. 2015;35:201–205. [PubMed] [Google Scholar]
- 15.Charoenrungruang S, Chanvorachote P, Sritularak B, Pongrakhananon V. Gigantl, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J Nat Prod. 2014;77:1359–1366. doi: 10.1021/np500015v. [DOI] [PubMed] [Google Scholar]
- 16.Chen CA, Chen CC, Shen CC, Chang HH, Chen YJ. Moscatilin induces apoptosis and mitotic catastrophe in human esophageal cancer cells. J Med Food. 2013;16:869–877. doi: 10.1089/jmf.2012.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai HC, Chang LH, Peng CY, Chang YL, Chen CC, Shen CC, Teng CM, Pan SL. Moscatilin inhibits migration and metastasis of human breast cancer MDA-MB-231 cells through inhibition of Akt and Twist signaling pathway. J Mol Med (Berl) 2013;91:347–356. doi: 10.1007/s00109-012-0945-5. [DOI] [PubMed] [Google Scholar]
- 18.Kowitdamrong A, Chanvorachote P, Sritularak B, Pongrakhananon V. Moscatilin inhibits lung cancer cell motility and invasion via suppression of endogenous reactive oxygen species. Biomed Res Int. 2013;2013:765894. doi: 10.1155/2013/765894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai AC, Pan SL, Liao CH, Guh JH, Wang SW, Sun HL, Liu YN, Chen CC, Shen CC, Chang YL, Teng CM. Moscatilin, a bibenzyl derivative from the India orchid Dendrobium loddigesii, suppresses tumor angiogenesis and growth in vitro and in vivo. Cancer Lett. 2010;292:163–170. doi: 10.1016/j.canlet.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Wang Y, Zhang G, Zhang F, Zhang Z, Wang Z, Xu L. Stimulaneous quantitative and qualitative analysis of bioactive phenols in Dendrobium aurantiacum var. Denneanum by high-performance liquid chromatography coupled with mass spectrometry and diode array detection. Biomed Chromatogr. 2007;21:687–694. doi: 10.1002/bmc.801. [DOI] [PubMed] [Google Scholar]
- 21.Marin V, Kaplanski G, Grès S, Farnarier C, Bongrand P. Endothelial cell culture: Protocol to obtain and cultivate human umbilical endothelial cells. J Immunol Methods. 2001;254:183–190. doi: 10.1016/S0022-1759(01)00408-2. [DOI] [PubMed] [Google Scholar]
- 22.Jo GH, Kim GY, Kim WJ, Park KY, Choi YH. Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: The involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway. Int J Oncol. 2014;45:1497–1506. doi: 10.3892/ijo.2014.2536. [DOI] [PubMed] [Google Scholar]
- 23.Kello M, Drutovic D, Chripkova M, Pilatova M, Budovska M, Kulikova L, Urdzik P, Mojzis J. ROS-dependent antiproliferative effect of brassinin derivative homobrassinin in human colorectal cancer Caco2 cells. Molecules. 2014;19:10877–10897. doi: 10.3390/molecules190810877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vriz S, Reiter S, Galliot B. Cell death: A program to regenerate. Curr Top Dev Biol. 2014;108:121–151. doi: 10.1016/B978-0-12-391498-9.00002-4. [DOI] [PubMed] [Google Scholar]
- 25.Li MX, Dewson G. Mitochondria and apoptosis: Emerging concepts. F1000Prime Rep. 2015;7:42. doi: 10.12703/P7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solary E, Dubrez L, Eymin B. The role of apoptosis in the pathogenesis and treatment of diseases. Eur Respir J. 1996;9:1293–1305. doi: 10.1183/09031936.96.09061293. [DOI] [PubMed] [Google Scholar]
- 27.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: Apoptosis through mitochondrial-dependent and independent-pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 28.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 29.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 30.Tong LY, Chuang CC, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367:18–25. doi: 10.1016/j.canlet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Surbramani R, Gonzalez E, Arumugam A, Nandy S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S, Narayan M, et al. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-mesenchymal transition. Sci Rep. 2016;6:19819. doi: 10.1038/srep19819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian X, Dai S, Sun J, Jiang S, Sui C, Meng F, Li Y, Fu L, Jiang T, Wang Y, et al. Bufalin induces mitochondrial-dependent apoptosis in pancreatic and oral cancer cells by downregulating hTERT expression via activation of the JNK/p38 pathway. Evid Based Complement Alternat Med. 2015;2015:546210. doi: 10.1155/2015/546210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei YY, Wang WJ, Mei JH, Wang CL. Mitogen-activated protein kinase signal transduction in solid tumors. Asian Pac J Cancer Prev. 2014;15:8539–8548. doi: 10.7314/APJCP.2014.15.20.8539. [DOI] [PubMed] [Google Scholar]
- 34.Pearson G, Robinson F, Gibson Beers T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- 35.Perugini RA, McDade TP, Vittimeberga FJ, Jr, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90:39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 36.Tournier C. The 2 faces of JNK signaling in cancer. Genes Cancer. 2013;4:397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi R, Hirata Y, Sakitani K, Nakata W, Kinoshita H, Hayakawa Y, Nakagawa H, Sakamoto K, Hikiba Y, Ijichi H, et al. Theraputic effect of c-Jun N-terminal kinase inhibition on pancreatic cancer. Cancer Sci. 2013;104:337–344. doi: 10.1111/cas.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia G, Kong R, Ma ZB, Han B, Wang YW, Pan SH, Li YH, Sun B. The activation of c-Jun NH2-terminal kinase is required for dihydroartemisinin-induced autophagy in pancreatic cancer cells. J Exp Clin Cancer Res. 2014;33:8. doi: 10.1186/1756-9966-33-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Liang X, Yang X. Ursolic acid inhibits growth and induces apoptosis in gemcitabine-resistant human pancreatic cancer via the JNK and PI3K/Akt/NF-kB pathways. Oncol Rep. 2012;28:501–510. doi: 10.3892/or.2012.1827. [DOI] [PubMed] [Google Scholar]