Abstract
Previous studies have suggested that oral zinc supplementation can help reduce the duration of the common cold; however, the use of intranasal (IN) zinc is strongly associated with anosmia, or the loss of the sense of smell, in humans. Prior studies from this lab showed that upregulation of metallothioneins (MT) is a rapid and robust response to zinc gluconate (ZG). Therefore, we assessed the role of MT in the recovery of nasal epithelial damage resulting from IN zinc administration. The main studies in this investigation used a high dose of ZG (170 mM) to ensure ablation of the olfactory mucosa, so that the progression of histological and functional recovery could be assessed. In vivo studies using wild-type, MT1/2 knockout mice (MT KO), and heterozygotes administered ZG by IN instillation showed profound loss of the olfactory mucosa in the nasal cavity. Recovery was monitored, and a lower percentage of the MT KO mice were able to smell 28 d after treatment; however, no significant difference was observed in the rate of cell proliferation in the basal layer of the olfactory epithelium between MT KO and wild-type mice. A lower concentration of ZG (33 mM), equivalent to that found in homeopathic IN ZG preparations, also caused olfactory epithelial toxicity in mice. These studies suggest that the use of zinc in drug formulations intended for IN administration in humans must be carefully evaluated for their potential to cause olfactory functional deficits.
Keywords: Metallothionein, zinc, olfactory, intranasal, olfactory behavior
Introduction
Zinc is an essential metal, but also capable of causing toxicity when exposure far exceeds the physiological need. Zinc deficiency has many consequences, including impaired immune function, learning deficiencies, growth deficiencies, complications during pregnancy and delivery, and anorexia (Bonaventura et al., 2015; Golub et al., 1995; Favier 1992a, 1992b; Katz et al., 1987). A recent meta analysis showed that zinc acetate lozenges shortened the duration of various respiratory tract symptoms, as well as muscle aches, with no effect on the duration of headache and fever when started within 24 hr of appearance of symptoms (Hemilä and Chalker, 2015). Despite the reported benefits of oral zinc supplementation, intranasal (IN) administration of zinc gluconate (ZG) for treating symptoms of the common cold causes olfactory damage in humans (DeCook and Hirsch 2000; Jafek et al., 2004; Alexander and Davidson 2006). These recent findings were not unprecedented; based on evidence that IN administration of zinc protected monkeys from contracting polio (Schultz and Gebhardt 1936), a small cohort of medical students and several thousand school children were treated with intranasal zinc as a prophylaxis against polio. The medical students reported a temporary loss of the sense of smell, which recovered after a few weeks (Peet et al., 1937). Not only did the therapy fail to protect the children from polio, but approximately 25% of the treated children reported persistent disturbance of smell and taste (Tisdall et al., 1938). More recently, due to the proliferation of over-the-counter intranasal zinc gluconate nasal sprays and gels, the US Food and Drug Administration warned against the use of these products, largely due to consumer complaints of olfactory dysfunction after using these products (NY Times, 2009).
Metallothioneins (MT) are small, cysteine-rich proteins that bind zinc, copper, cadmium, and other heavy metals with high affinity, and can reduce heavy metal toxicity (Coyle et al., 2002). Recent reports suggest that MT have biological importance beyond their well-known role in metal ion sequestration. MT proteins clearly also have a role in wound healing and neuroprotection (Pedersen et al., 2009; Morellini et al., 2008; Chung and West, 2004; Chung et al., 2003; Penkowa et al., 2003). Prior work demonstrated MT1 and MT2 in olfactory supporting cells, and in proliferating basal cells following olfactory epithelial damage (Skabo et al., 1997). Mice have four MT isoforms, with broad tissue expression of MT1 and MT2; MT3 is found predominantly in the nervous system, while MT4 is expressed in stratified squamous epithelia associated with oral epithelia, esophagus, upper stomach, tail, footpads, and neonatal skin (Quaife et al., 1994; Tio et al., 2004). The goal of this study was to evaluate olfactory function and olfactory epithelial recovery in wild-type vs. mice lacking MT1 and MT2 isoforms (MT KO). After assessment of basal olfactory function, wild-type, heterozygous, and MT KO mice were administered ZG (170 mM) by intranasal instillation. Olfactory function was measured using the buried food pellet assay at multiple time points after ZG treatment, and olfactory recovery was assessed in histological sections. Olfactory cell proliferation was assessed in wild-type and MT KO mice using proliferating cell nuclear antigen (PCNA) immunohistochemistry in untreated mice of each genotype. In addition, we studied the very early time course of ZG-induced olfactory degeneration (2-24 hr), as the earliest time point previously examined in the literature after IN zinc administration was 24 hr after treatment. Finally, we examined the olfactory epithelial damage induced by a clinically-relevant concentration of ZG (33 mM; Jafek et al., 2004).
Materials & Methods
Chemicals and Reagents
Zinc gluconate ((ZG) MP Biomedicals LLC) solution was prepared using distilled water and sterilized by syringe-filtering through a 0.2 μm filter disc. Cap’n’Crunch cereal (Quaker Oats Co.), was used for olfactory behavioral assessment. Direct PCR Lysis Reagent (Ear) and Proteinase K solution from Viagen Biotech (Los Angeles, CA) were used for genotyping. Taq PCR Master Mix Kit was purchased from Qiagen (Valencia, CA) for genotyping reactions. Proliferating cell nuclear antigen primary antibody (NeoMarkers, Fremont, CA; 1:100 dilution) and Vectastain Universal and DAB reagent kits (Vector, Burlingame, CA) were used to assess olfactory basal cell proliferation in nasal cavity sections.
Mouse husbandry
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati (Cincinnati, OH). In brief, heterozygous metallothionein 1/2 mice were generated by crossing metallothionein 1/2 null mice with wild-type 129S1 mice (Jackson Laboratory, Bar Harbor, ME) (Masters et al., 1994). The heterozygotes were crossed to generate wild-type, heterozygous, and MT KO mice for behavioral assessment and histological recovery studies. Mice were weaned at 3 wk of age and were group-housed with littermates based upon sex in a temperature and humidity controlled facility under 12 hr light / 12 hr dark cycle with free access to food (Harlan 7922 (irradiated)) and water except for the night before a buried food pellet test. Water supplied to the barrier facility underwent filtration (5 micron filters), followed by reverse osmosis and UV irradiation.
Because MT1 and MT2 genes are adjacent in a single locus on chromosome 8, genotyping was accomplished with a single PCR reaction. A standard ear punch (1.5 mm diameter) was lysed overnight at 55°C in 100 μL Direct PCR Lysis Reagent containing proteinase K, following by 60 min protease inactivation at 85°C. The resulting lysate was used in a PCR reaction with the following primers:
5′-TCACCAGATCTCGGAATGG-3′ (sense)
5′-AAGAACCGGAATGAATCGC-3′ (antisense)
The PCR reaction proceeded under the following conditions: 2 min at 94°C; 30 sec at 94°C, 30 sec at 54°C, 30 sec at 72°C, repeat for 35 cycles; 2 min at 72°C. PCR products were separated on 3% agarose gel containing ethidium bromide and visualized using High Performance UV Transilluminator (UVP, USA). Wild-type mice had a single band at 161 bp, heterozygotes had 2 bands at 161 and 176 bp, and MT KO mice had a single band at 176 bp.
Intranasal zinc gluconate exposure
One-day time course study
For the initial time course (0-24 hr) all mice used were heterozygotes. Mice were dosed IN with 10 μL of 170 mM ZG per nostril at 8 wk of age under light isoflurane anesthesia This concentration of ZG was previously reported to induce robust damage to the olfactory epithelium, with subsequent recovery (Harding et al., 1978), and the volume was based on previous experience in our lab and recommendations of Dhuria et al., 2010. Mice were held at an approximately 45-degree angle from the horizontal position, with the head pointing to the floor. Each nostril was treated with a clean 10 μL pipet tip (10 μL/nostril). Upon regaining consciousness, the mice were placed in a holding cage, where they were allowed to awaken completely before returning to the home cage. Mice were euthanized for histological evaluation 1, 2, 4, 8, 12, and 24 hr post-dosing, with 2 - 3 mice euthanized at each time point. Sterile filtered water was used as the vehicle control.
28 d time course study
Mice of all genotypes (n=20 wild-type, 28 heterozygotes, 21 MT KO) were administered 170 mM ZG as described above. Olfactory function (buried food pellet test, described below), followed by histopathological evaluation, was assessed 1, 7, 14, and 28 d post-dosing, with 15-20 mice used at each time point.
Low-dose ZG study
Wild-type mice (n=4) were administered 33 mM ZG as described above and evaluated for olfactory epithelial damage 24 hr later by histological evaluation
Tissue Processing and Staining
After euthanasia with carbon dioxide (medical grade, in a euthanasia chamber), mice were decapitated and the lower jaw was removed. The nasal cavity was flushed retrogradely via the nasopharynx with approximately 1 ml of 10% neutral buffered formalin, and the heads were then fixed overnight in the same fixative. Nasal cavities were de-calcified in 10% formic acid for approximately 1 wk, and washed overnight in tap water prior to sectioning according to Young (1981). Nasal cavity sections at Level 2 (at the incisive papilla, containing respiratory, olfactory, and squamous epithelia) and Level 3 (taken at the second palatal ridge and lined almost exclusively by olfactory epithelium) from each mouse were submitted for routine paraffin embedding. Noses were dehydrated, paraffin embedded, sectioned (5 μm), and stained with hematoxylin and eosin (H&E) for light microscopic evaluation.
Proliferating cell nuclear antigen (PCNA) immunohistochemistry
In order to ascertain whether MT KO had a lower intrinsic rate of cell proliferation in the olfactory epithelium, PCNA immunohistochemistry was performed on untreated wild-type and MT KO mice. Paraffin embedded sections from untreated wild-type (n=4) and MT KO mice (n=3) were deparaffinized, rehydrated and microwaved in citric acid (10 mM, pH 5.2) for 10 min for antigen retrieval. After washing with 0.01M phosphate buffered saline, sections were blocked with 10% horse serum and then incubated with biotinylated anti-PCNA antibody (NeoMarkers, Fremont, CA; 1:100 dilution) at 4°C overnight in a humidified chamber. Vectastain Universal and DAB reagent kits (Vector, Burlingame, CA) were used to localize antibody binding. PCNA positive cells were counted in the basal layer of the olfactory epithelium of the dorsal meatus of the nasal cavity, and basement membrane lengths were measured using ImageJ software (http://imagej.nih.gov/ij/). Labeling indices were calculated as the number of PCNA positive basal cells divided by the length of the basement membrane.
Buried Food Pellet test
Olfactory function was assessed using the buried food pellet assay as described by Yang and Crawley (2009) with several modifications, described herein. Mice were fasted overnight for 12-14 hr before testing with the exception of day 1 testing, where the mice were not fasted. Testing was carried out in a polycarbonate mouse cage (12×7.75×6.25 in) which had been filled with 2.5 cm of bedding (Bed-o-Cobs, The Andersons Lab Bedding Products, Maumee, OH). Mice were allowed to acclimate to the test cage for 10 min before being moved to an empty cage, while the Cap’n’Crunch cereal was hidden (approximately 1cm below the surface) in a randomly selected corner. Mice were then returned to the test cage and it was recorded whether the mice were able to find the food within the 5 min time limit.
Statistical analysis
Buried food pellet data were analyzed using Fisher's exact text, given that data were quantal in nature (i.e. mice either found or did not find the buried pellet with the allotted 5-min period) (Gad 2008). Statistical significance was set at p < 0.05.
Results
As seen in Table 1, none of the mice were able to find the buried food 1 d after IN ZG dosing, thereby confirming that the dosing had been properly administered and all the mice were anosmic. At 2 wk post-IN dosing, 27% of the WT mice recovered olfactory function, while 42% of the heterozygotes and KO mice found the food. At the 4 wk time point, all of the WT mice found the food within 5 min, while only 78% of the heterozygotes and 43% of the KO mice were able to find the food.
Table 1. Buried Food Pellet Results.
| Wild type | Heterozygote | MT1/2 KO | |
|---|---|---|---|
| Day 1 | 0% (0/20) | 0% (0/28) | 0% (0/21) |
| 1 week | 22% (4/18) | 21% (6/28) | 5% (1/19) |
| 2 weeks | 27% (3/11) | 42% (8/19) | 42% (5/12) |
| 4 weeks | 100% (8/8) | 78% (7/9) | 43% (3/7)* |
Percentage (successful/total) of mice who found the buried food pellet within 5 min 1d, or 1, 2, or 4 wk after IN ZG administration. Fisher's exact test revealed a significant difference between the recovery of wild-type mice and MT KO mice at the 4 wk time point
exact statistical value = 0.004.
The time course of olfactory epithelial damage and recovery of the olfactory epithelium following ZG administration was assessed by evaluation of H&E-stained histological sections. All mice, regardless of genotype or gender, showed identical degrees of histological injury and repair at the time points evaluated after ZG treatment. The normal vehicle-treated olfactory epithelium (Figure 1A) first showed histological changes in appearance 2 hr after ZG treatment (Figure 1B), with the olfactory epithelium taking on a ruffled appearance, followed by sloughing of the entire epithelial layer from the basement membrane (Figure 1C, 1D) 4h after ZG treatment. As shown in Figure 1D, the entire epithelium appears to lift off from the basement membrane, with no discernable toxicity to the olfactory neuron or supporting cell populations in the epithelium. By 12 hr after treatment (Figure 1E), epithelial debris was readily visible in the nasal airways. By 24 hr after ZG treatment, all mice (WT, heterozygotes, and MT KO) exhibited complete degeneration of the olfactory epithelium, as shown in Figure 1F. Note the complete absence of epithelium. Some recovery was evident 7d after ZG treatment (Figure 1G), with further recovery, including regeneration of Bowman's glands (Figure 1H) 14 d after treatment. By 28 d, most of the mice had recovered full-thickness, properly differentiated olfactory epithelium in the level III nasal cavity sections (Figure 1I). However, foci of respiratory metaplasia were present in all mice in the Level 2 (more anterior) nasal cavity sections at the 28 d time point, with approximately half of these mice also showing foci of metaplasia in the more posterior (Level 3) nasal cavity section (Figure 1J). IN administration of ZG at 33 mM, which is the concentration found in a ZG over-the-counter preparation used by humans (Jafek et al., 2004), was also found to cause olfactory epithelial degeneration in wild-type mice (Figure 2).
Figure 1.
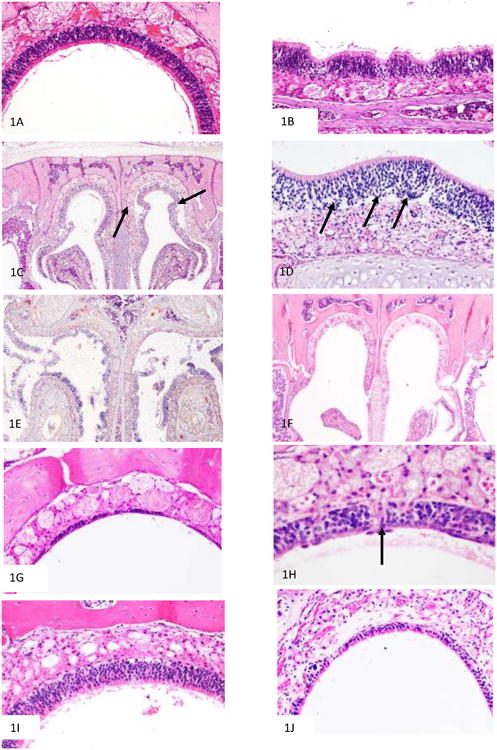
H&E stained sections showing the time course of olfactory epithelial degeneration and regneration after intranasal zinc gluconate exposure. Mice were treated by IN instillation with 170 mM ZG dissolved in sterile distilled water (10 μL/nostril) and examined 1 hr through 28 d after treatment. A: Normal olfactory epithelium from dorsal medial region of nasal cavity of a vehicle-treated wild-type mouse (200× magnification); B: Heterozygous mouse 2 hr after IN ZG administration (400× magnification). Note that the olfactory epithelium is beginning to take on a ruffling appearance; C: Heterozygous mouse 4 hr after IN ZG administration. Note areas of epithelial detachment from the basement membrane (arrows) (20× magnification); D: Higher power (400× magnification) photomicrograph showing olfactory epithelium detaching from basement membrane (heterozygous mouse) (arrow tips are at the level of the basement membrane) with no apparent toxicity to epithelial cell populations, 4h after IN ZG administration; E: Heterozygous mouse 12 hr after IN ZG administration (20× magnification). Note fragmented epithelial debris in the nasal airways; F: Wild-type mouse 24 hr after IN ZG administration (20× magnification). Note the complete absence of olfactory epithelium (20× magnification); G: KO mouse, 7 d after IN ZG administration. Note patchy epithelial regeneration (200× magnification); H: Heterozygous mouse, 14 d after ZG treatment. Note regenerating Bowman's gland duct in center of figure (arrows; 400× magnification). I. Heterozygous mouse 28 d after IN ZG administration (200× magnification). Note the complete regeneration of olfactory epithelium. J. Complete recovery was not always observed 28 d after treatment; in this case respiratory metaplasia has occurred (knockout mouse) (200× magnification).
Figure 2.
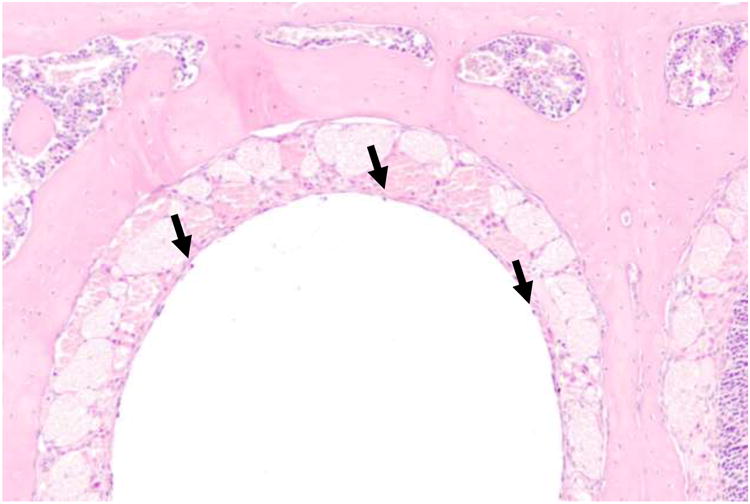
Dorsal medial nasal cavity of a wild-type mouse, 24 hr after instillation of 10 μ/nostril of zinc gluconate (33 mM, which is equivalent to the concentration in an over-the-counter zinc gluconate cold remedy). Note the complete absence of olfactory epithelium, except for some spared basal cells (arrows). H&E, 40× magnification.
PCNA immunohistochemistry was used to test the hypothesis that the delay in recovery of the MT KO mice was due to a slower rate of cell proliferation in the basal layer of the olfactory epithelium, compared to wild type mice. Cell proliferation was assessed in untreated 8 wk old mice (wild-type and MT KO). However, immunohistochemistry found no difference in the basal level of cell proliferation between wild-type and MT KO mice (mean and ± SD: 13.6. ± 4.4 PCNA positive cells/mm basement membrane for wild-type mice and 13.6 ± 3.8 PCNA positive cells/mm basement membrane for MT KO mice); representative photomicrograph is shown in Figure 3.
Figure 3.
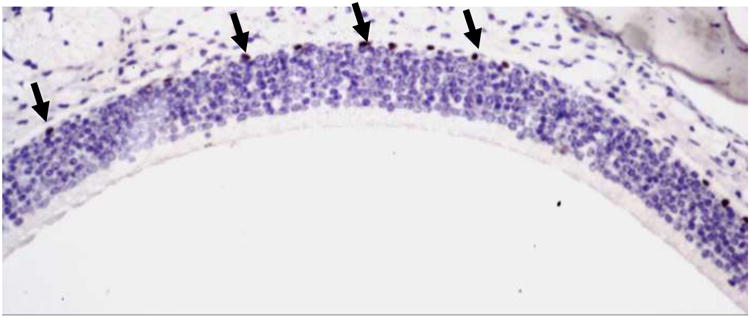
Representative photomicrograph of the epithelium of the dorsal medial nasal cavity of a vehicle-treated wild-type mouse, showing PCNA-positive cells with black nuclei in the basal cell layer of the olfactory epithlium (examples shown with arrows). Sections were counterstained with hematoxylin. (200× magnification).
Discussion
MT are integral to controlling the levels of free zinc, and its expression is highly up-regulated in response to excess zinc (Ohly and Gleichmann, 1995; Ohly et al., 2000; Hsieh et al., 2016). However, it is clear that MT are also important in responses to other insults. Hydrogen peroxide causes an up-regulation of Mt gene expression, while MT protein can scavenge peroxide and hydroxyl radicals to prevent lipid membrane damage (Dalton et al., 1994; Miura et al., 1997; Suntres and Lui, 2006). In humans, MT polymorphisms are associated with increased sensitivity to cadmium-induced nephrotoxicity in Chinese populations, and elevated blood cadmium and lead levels in a Turkish population (Lei et al., 2012; Kayaalti et al., 2011). These observations suggest that MT are not only important in regulating free metal ion concentration, but also responses to other cellular stressors, such as oxidative stress.
Polymorphisms in human MT1 and MT2 have been investigated with regard to their relationship to respiratory tract cancers. The association of single nucleotide polymorphisms (SNPs) at loci -5 A/G (rs28366003) and -209 A/G (rs1610216), as well as +838 C/G (rs10636) in the 3′ untranslated region of the MT2 gene were investigated in sinonasal inverted papillomas, which are locally aggressive benign epithelial neoplasms in the cavity and paranasal sinuses (Starska et al., 2015a). MT2 mRNA expression and Cd and Cu concentrations were negatively correlated with the -5 A/G SNP (Starska et al., 2015b). This same research group also examined the association between the same three SNPs and laryngeal cancer risk. In this study, the G variant of the -5 A/G locus was associated with squamous cell laryngeal cancer (odds ratio (OR) 2.9, 95% confidence interval (CI): 1.53-5.21). The minor allele (G) carriers also had a higher stage of laryngeal cancer, including increased aggressiveness and more diffuse tumor growth (Starska et al., 2014). MT1 polymorphisms were investigated for their association with oral squamous cell carcinoma (OSSC). Three loci, rs11076161 A, rs964372 C, and rs719179 C were protective against OSSC, whereas rs805239 A alleles were associated with increased risk (Zavras et al., 2011). A modest increased risk of lung cancer was associated with a polymorphism (rs7196890) in the MT1 gene, but the effect size decreased as the amount of smoking increased (Nakane et al., 2015). Thus, MT polymorphisms, particularly those in the non-coding regions of the gene, may have a role in the functionality of MT in responses to environmental agents associated with respiratory tract injuries and cancer.
Zinc gluconate (170 mM) was chosen for our analysis of olfactory epithelial damage and recovery studies, based upon the use of 170 mM zinc sulfate to induce anosmia in mice in previous studies (Harding et al., 1978). Previously, large volumes (up to 100 μL) of zinc sulfate solution were administered; however, with such a large volume, mice experienced respiratory distress and death (Duncan-Lewis et al., 2011). Our group determined that 10 μL of solution/nostril is sufficient to reach and cover the olfactory epithelium in mice, and this volume is similar to that recommended previously (total volume of 24 μl divided between both nostrils (Dhuria et al., 2010)). In accordance with others, we demonstrated that after IN ZG exposure, mice became anosmic and started recovering their sense of smell by day 7 with almost all WT mice recovering their sense of smell 4 wk after treatment (Harding et al., 1978; Duncan-Lewis et al., 2011; Ducray et al., 2002). The rate of recovery in this study was more rapid than that described by Harding and colleagues. This difference may be due to the fact that Harding et al. used larger volumes (50 to 100 μL of zinc sulfate solution), a different mouse strain (CD-1), and much older mice (retired female breeders). Induction of MT was demonstrated to vary among different mouse strains (Farr and Hunt, 1989), thus it is not surprising that different mouse strains respond differently to IN zinc administration. Additionally, all of the WT mice could smell by the end of the study, while only 40% of the MT KO mice could smell, which suggests that MT are important for recovery of the sense of smell, or for epithelial repair in general.
MT appear to be critical during wound repair; MT KO mice showed impaired microglia function and debris clearance in response to cryolesions in the brain, which resulted in apoptosis in surrounding tissue and decreased neuron survival (Penkowa et al., 1999). Additionally, exogenously-applied MT resulted in increased rate of cell proliferation and keratinocyte migration in vitro, while in vivo it promoted faster re-epithelization and wound healing compared to saline control (Morellini et al., 2008). Thus, MT may be critical in the regeneration of olfactory neurons and explain why MT KO mice showed a delay in recovery of olfactory function. PCNA immunohistochemistry revealed that there were no genotype-related differences in basal cell proliferation between wild-type and MT KO mice in this study.
It is now clear that intranasal administration of zinc salts is not safe for the olfactory epithelium in most instances. This was originally demonstrated in this country and in Canada in the 1930s, and again in recent times with the use of zinc gluconate intranasal cold products. However, zinc is not necessarily completely gone from intranasally-administered products. Zinc is an integral part of insulin formulations, as it promotes the proper hexameric structure of insulin and prevents agglomeration (Li, 2014). According to Chapman et al. (2013), intranasal administration of insulin is “a promising therapeutic strategy for the treatment of diseases with CNS involvement, such as obesity, Alzheimer's disease, Parkinson's disease, Huntington's disease, depression, anxiety, autism spectrum disorders, seizures, drug addiction, eating disorders, and stroke”. In light of this, investigators have evaluated the potential risks associated with the concentration of zinc in insulin formulations (Hansen et al., 1994; Hamidovic 2015). Based on observations that zinc toxicity occurs at concentrations between 0.01 and 0.05% zinc, current insulin products appear to be safe, as the concentration in these formulations ranges between 0.007 and 0.0065% (Hansen et al., 1994; Hamidovic 2015). However, intranasal insulin was found to reduce sensitivity to the odorant n-butanol (i.e. increased threshold concentration), but not olfactory discrimination, in normosmic subjects receiving 40 IU of insulin intranasally (zinc concentration not specified; Brünner et al., 2013).
By examining the olfactory epithelium at very early time points after IN administration of ZG, we noted that the loss of the epithelium appears to arise as detachment of the entire epithelial layer, not due to death of a specific cell population. This prompted the question as to what molecules are responsible for adhesion of the olfactory epithelium to the olfactory basement membrane. Carter et al. demonstrated the expression ICAM-1, as well as α1-, α3-, α6-, β1- and β4 integrins adjacent to the olfactory basement membrane in mice. After loss of olfactory neurons due to olfactory bulbectomy, adhesion molecule expression in the olfactory epithelium declined, with concomitant basal cell proliferation to replace the damaged epithelium (Carter et al., 2004).
Integrin molecules are composed of two non-covalently linked subunits, with ligand binding requiring extracellular divalent cations, typically Ca2+ or Mg2+ (Alberts et al., 2002). We hypothesize that zinc ions may dysregulate integrin-ligand binding, and previous studies showed that zinc also down-regulates a-actinin expression (Hsieh et al., 2016), which is one of many ligands that interact with integrins for cell adhesion (Alberts et al., 2002). In support of this hypothesis, Lymburner et al (2013) demonstrated that zinc (10-50 micromolar concentrations) inhibited magnesium-dependent adhesion of MDA-MB-231 cells on fibronectin. Efforts to confirm loss of the adhesion molecule actinin (based on previous RNAseq results (Hsieh et al., 2016)) and integrin β4 (based on Carter et al., 2004) were unsuccessful due to the failure of the respective antibodies to work, likely due to the decalcification procedure performed on the tissues.
The present studies demonstrate that MT1 and MT2 deficiency is associated with delayed recovery of the olfactory epithelium after a toxic insult, adding to the growing body of knowledge of the importance of MT in epithelial homeostasis and repair. If zinc salts are required components of intranasally-administered drugs, such as the insulin example described herein, care must be exercised to assure that levels of zinc are below the known levels to cause damage in humans.
Highlights.
Zinc gluconate (ZG) causes olfactory damage in wild-type and MT KO mice.
Clinically-relevant concentration of ZG (33 mM) causes olfactory damage in mice.
MT appears to be important in recovery of olfactory function after IN ZG treatment.
Olfactory basal cell proliferation is not different between wild-type and MT KO mice.
Acknowledgments
These studies were funded in part by, NIEHS P30-ES06096, NIH AI106269, NIH T32 GM063483, and the University of Cincinnati Graduate Student Governance Association. We thank Dr. Sarah Pixley and Tracy Hopkins for technical assistance. We also thank Dr. George S. Deepe, Jr. for providing the Mt1/2 heterozygous breeding pairs, and both Dr. Deepe and Dr. Kavitha Subramanian Vignesh (University of Cincinnati Department of Internal Medicine) for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th. New York: Garland Science; 2000. Viewed at http://www.ncbi.nlm.nih.gov/books/NBK26867/ 4/11/2016. [Google Scholar]
- Alexander TH, Davidson TM. Intranasal zinc and anosmia: the zinc-induced anosmia syndrome. Laryngoscope. 2006;116:217–220. doi: 10.1097/01.mlg.0000191549.17796.13. [DOI] [PubMed] [Google Scholar]
- Bonaventura P, Benedetti G, Albarede F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmunity Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Brünner YF, Benedict C, Freiherr J. Intranasal insulin reduces olfactory sensitivity in normosmic humans. J Clin Endocrinol Metab. 2013;98:E1626–30. doi: 10.1210/jc.2013-2061. [DOI] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CD, Frey WH, 2nd, Craft S, Danielyan L, Hallschmid M, Schiöth HB, Benedict C. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30(10):2475–84. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RS, West AK. A role for extracellular metallothioneins in CNS injury and repair. Neuroscience. 2004;123(3):595–599. doi: 10.1016/j.neuroscience.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Chung RS, Vickers JC, Chuah MI, West AK. Metallothionein-IIA promotes initial neurite elongation and postinjury reactiveneurite growth and facilitates healing after focal cortical brain injury. J Neurosci. 2003;23(8):3336–3342. doi: 10.1523/JNEUROSCI.23-08-03336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P, Philcox JC, Carey LC, Rofe AM. Metalliothein: the multipurpose protein. Cell Mol Life Sci. 2002;59(4):627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T, Palmiter RD, Andrews GK. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994;22:5016–5023. doi: 10.1093/nar/22.23.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCook C, Hirsch A. Anosmia due to inhalational zinc: a case report. Chem Senses. 2000;25:593–659. [Google Scholar]
- Ducray A, Bondier JR, Michel G, Bon K, Millot JL, Propper A, Kastner A. Recovery following peripheral destruction of olfactory neurons in young and adult mice. Eur J Neurosci. 2002;15:1907–1917. doi: 10.1046/j.1460-9568.2002.02044.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Lewis CA, Lukman RL, Banks RK. Effects of zinc gluconate and 2 other divalent cationic compounds on olfactory function in mice. Comp Med. 2011;61:361–365. [PMC free article] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- Farr C, Hunt DM. Genetic differences in zinc and copper induction of liver metallothionein in ingred strains of the mouse. Biochem Genet. 1989;27(3-4):199–217. doi: 10.1007/BF02401801. [DOI] [PubMed] [Google Scholar]
- Favier AE. The role of zinc in reproduction. Hormonal mechanisms. Biol Trace Elem Res. 1992a;32:363–382. doi: 10.1007/BF02784623. [DOI] [PubMed] [Google Scholar]
- Favier AE. Hormonal effects of zinc on growth in children. Biol Trace Elem Res. 1992b;32:383–398. doi: 10.1007/BF02784624. [DOI] [PubMed] [Google Scholar]
- Gad SC. Statistics for Toxicologists. In: Hayes AW, editor. Principles and Methods of Toxicolgy. 5th. CRC Press; Boca Raton, FL: 2008. pp. 369–452. [Google Scholar]
- Golub MS, Keen CL, Gershwin ME, Hendrickx AG. Developmental zinc deficiency and behavior. J Nutr. 1995;125:2263S–2271S. doi: 10.1093/jn/125.suppl_8.2263S. [DOI] [PubMed] [Google Scholar]
- Hamidovic A. Position on zinc delivery to olfactory nerves in intranasal insulin phase I–III clinical trials. [Accessed 4/10/2016];Contemporary Clinical Trials. 2015 doi: 10.1016/j.cct.2015.08.011. doi: http://dx.doi.org/10.1016/j.cct.2015.08.011. [DOI] [PubMed]
- Hansen LF, Hammer M, Petersen SH, Nielsen GD. Effects of intranasal ZNSO4 irrigation on olfactoryand trigeminal cues. Physiol Behav. 1994;55(4):699–704. doi: 10.1016/0031-9384(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Harding JW, Getchell TV, Margolis FL. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978;140:271–285. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- Hemilä H ChalkerE. The effectiveness of high dose zinc acetate lozenges on various common cold symptoms: a meta-analysis. BMC Fam Pract. 2015;16:24. doi: 10.1186/s12875-015-0237-6. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Vignesh KS, Deepe GS, Jr, Choubey D, Shertzer HG, Genter MB. Mechanistic studies of the toxicity of zinc gluconate in the olfactory neuronal cell line Odora. Toxicol in Vitro. 2016 doi: 10.1016/j.tiv.2016.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafek BW, Linschoten MR, Murrow BW. Anosmia after intranasal zinc gluconate use. Am J Rhinol. 2004;18:137–141. [PubMed] [Google Scholar]
- Katz RL, Keen CL, Litt IF, Hurley LS, Kellams-Harrison KM, Glader LJ. Zinc deficiency in anorexia nervosa. J Adolesc Health Care. 1987;8(5):400–406. doi: 10.1016/0197-0070(87)90227-0. [DOI] [PubMed] [Google Scholar]
- Kayaaltı Z, Aliyev V, Söylemezoğl T. The potential effect of metallothionein 2A - 5 A/G single nucleotide polymorphism on blood cadmium, lead, zinc and copper levels. Toxicol Appl Pharmacol. 2011;256:1–7. doi: 10.1016/j.taap.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Lei L, Chang X, Rentschler G, Tian L, Zhu G, Chen X, Jin T, Broberg K. A polymorphism in metallothionein 1A (MT1A) is associated with cadmium-related excretion of urinary beta 2-microglobulin. Toxicol Appl Pharmacol. 2012;265:373–379. doi: 10.1016/j.taap.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Li YV. Zinc and insulin in pancreatic beta cells. Endocrine. 2014;45(2):178–189. doi: 10.1007/s12020-013-0032-x. [DOI] [PubMed] [Google Scholar]
- Lymburner S, McLeod S, Purtzki M, Roskelley C, Xu Z. Zinc inhibits magnesium-dependent migration of human breast cancer MDA-MB-231 cells on fibronectin. J Nutr Biochem. 2013;24(6):1034–1040. doi: 10.1016/j.jnutbio.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Muraoka S, Ogiso T. Antioxidant activity of metallothionein compared with reduced glutathione. Life Sci. 1997;60:301–309. doi: 10.1016/s0024-3205(97)00156-2. [DOI] [PubMed] [Google Scholar]
- Morellini NM, Giles NL, Rea S, Adcroft KF, Falder S, King CE, Dunlop SA, Beazley LD, West AK, Wood FM, Fear MW. Exogenous metallothionein-IIA promotes accelerated healing after a burn wound. Wound Repair Regen. 2008;16(5):682–90. doi: 10.1111/j.1524-475X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- Nakane H, Hirano M, Ito H, Hosono S, Oze I, Matsuda F, Tanaka H, Matsuo K. Impact of metallothionein gene polymorphisms on the risk of lung cancer in a Japanese population. Mol Carcinog. 2015;54(1):E122–8. doi: 10.1002/mc.22198. Erratum in Mol Carcinog. 2015 54(9):935. [DOI] [PubMed] [Google Scholar]
- New York Times. F.D.A Warns Against Use of Popular Cold Remedy. [accessed 8/12/2016];2009 Jun 16; http://www.nytimes.com/2009/06/17/health/policy/17nasal.html.
- Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43:1020–1030. doi: 10.1007/s001250050009. [DOI] [PubMed] [Google Scholar]
- Ohly P, Gleichmann H. Metallothionein: in vitro induction with zinc and streptozotocin in pancreatic islets of mice. Exp Clin Endocrinol Diabetes. 1995;103(2):79–82. doi: 10.1055/s-0029-1211399. [DOI] [PubMed] [Google Scholar]
- Pedersen MØ, Jensen R, Pedersen DS, Skjolding AD, Hempel C, Maretty L, Penkowa M. Metallothionein-I+II in neuroprotection. Biofactors. 2009;35(4):315–325. doi: 10.1002/biof.44. [DOI] [PubMed] [Google Scholar]
- Peet MM, Echols DH, Richter JJ. The chemical prophylaxis for poliomyelitis. JAMA. 1937;108(26):2184–2187. [Google Scholar]
- Penkowa M, Carrasco J, Giralt M, Moos T, Hidalgo J. CNS wound healing is severely depressed in metallothionein I- and II-deficient mice. J Neurosci. 1999;19:2535–2545. doi: 10.1523/JNEUROSCI.19-07-02535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Camats J, Giralt M, Molinero A, Hernández J, Carrasco J, Campbell IL, Hidalgo J. Metallothionein-I overexpression alters brain inflammation and stimulates brain repair in transgenic mice with astrocyte-targeted interleukin-6 expression. Glia. 2003;42(3):287–306. doi: 10.1002/glia.10208. [DOI] [PubMed] [Google Scholar]
- Quaife CJ, Findley SD, Erickson JC, Froelick GJ, Kelly EJ, Zambrowicz BP, Palmiter RD. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33(23):7250–9. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- Schultz EW, Gebhardt LP. Chemoprophylaxis of poliomyelitis. Cal West Med. 1936;45(2):138–140. [PMC free article] [PubMed] [Google Scholar]
- Skabo SJ, Holloway AF, West AK, Chuah MI. Metallothioneins 1 and 2 are expressed in the olfactory mucosa of mice in untreated animals and during the regeneration of the epithelial layer. Biochem Biophys Res Commun. 1997;232(1):136–42. doi: 10.1006/bbrc.1997.6243. [DOI] [PubMed] [Google Scholar]
- Starska K, Krześlak A, Forma E, Olszewski J, Morawiec-Sztandera A, Aleksandrowicz P, Lewy-Trenda I, Bryś M. The -5 A/G single-nucleotide polymorphism in the core promoter region of MT2A and its effect on allele-specific gene expression and Cd, Zn and Cu levels in laryngeal cancer. Toxicol Appl Pharmacol. 2014;280(2):256–63. doi: 10.1016/j.taap.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Starska K, Bryś M, Forma E, Olszewski J, Pietkiewicz P, Lewy-Trenda I, Stasikowska-Kanicka O, Danilewicz M, Krześlak A. Metallothionein 2A core promoter region genetic polymorphism and its impact on the risk, tumor behavior, and recurrences of sinonasal inverted papilloma (Schneiderian papilloma) Tumour Biol. 2015a;36(11):8559–71. doi: 10.1007/s13277-015-3616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starska K, Bryś M, Forma E, Olszewski J, Pietkiewicz P, Lewy-Trenda I, Danilewicz M, Krześlak A. The effect of metallothionein 2A core promoter region single-nucleotide polymorphism on accumulation of toxic metals in sinonasal inverted papilloma tissues. Toxicol Appl Pharmacol. 2015b;285(3):187–97. doi: 10.1016/j.taap.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Suntres ZE, Lui EMK. Antioxidant effect of zinc and zinc-metallothionein in the acute cytotoxicity of hydrogen peroxide in Ehrlich ascites tumour cells. Chem Biol Interact. 2006;162:11–23. doi: 10.1016/j.cbi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Tío L, Villarreal L, Atrian S, Capdevila M. Functional differentiation in the mammalian metallothionein gene family: metal binding features of mouse MT4 and comparison with its paralog MT1. J Biol Chem. 2004;279(23):24403–13. doi: 10.1074/jbc.M401346200. [DOI] [PubMed] [Google Scholar]
- Tisdall FF, Brown A, Defries RD. Persistent anosmia following zinc sulfate nasal spraying. J Pediatr. 1938;13(1):60–62. [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1(4):309–12. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]
- Zavras AI, Yoon AJ, Chen MK, Lin CW, Yang SF. Metallothionein-1 genotypes in the risk of oral squamous cell carcinoma. Ann Surg Oncol. 2011;18(5):1478–83. doi: 10.1245/s10434-010-1431-3. [DOI] [PubMed] [Google Scholar]


