Abstract
Background
Smoking acutely relieves negative affect (NA) due to smoking abstinence but may not relieve NA from other sources, such as stressors.
Methods
Dependent smokers (N=104) randomly assigned to one of three smoking conditions (nicotine or denic cigarettes, or no smoking) completed four negative mood induction procedures (one per session): 1) overnight smoking abstinence, 2) challenging computer task, 3) public speech preparation, and 4) watching negative mood slides. A fifth session involved a neutral mood control. The two smoking groups took 4 puffs on their assigned cigarette, and then smoked those same cigarettes ad libitum during continued mood induction. All subjects rated their level of NA and positive affect (PA) on several measures (Mood Form, PANAS, Stress-Arousal Checklist, and STAI-state). They also rated craving and withdrawal.
Results
NA relief from smoking depended on the NA source (i.e. mood induction procedure) and the affect measure. Smoking robustly relieved NA due to abstinence on all 4 measures, but only modestly relieved NA due to the other sources and typically on only some measures. Smoking’s effects on PA and withdrawal were similar to effects on NA, but relief of craving depended less on NA source. Smoking reinforcement only partly matched the pattern of NA relief. Few responses differed between the nicotine and denic smoking groups.
Conclusions
Acute NA relief from smoking depends on the situation and the affect measure used but may not depend on nicotine intake. These results challenge the common assumption that smoking, and nicotine in particular, broadly alleviates NA.
Keywords: smoking, negative affect, negative reinforcement, nicotine, nicotine dependence, withdrawal, stress
Smokers and clinicians, as well as researchers, assume that a critical reinforcing effect of smoking in general, and nicotine in particular, is relief of negative affect (1–3). Clinical research clearly shows that negative affect promotes smoking relapse (3,4), and smoking reliably relieves negative affect due to smoking abstinence (i.e. withdrawal; 5). However, controlled studies rarely show that smoking relieves negative affect due to other sources, such as stressors (2,6). Thus, smoking may actually relieve negative affect only under very limited conditions. Knowing under what conditions smoking does or does not relieve negative affect is important to guide research into the mechanisms of nicotine’s actions and smoking reinforcement.
Several explanations of when smoking may relieve negative affect have been proposed, with the range of these explanations perhaps best captured by two competing models. In one, the Nicotine Resource Model, smoking relieves negative affect from virtually all sources (see 1), even in the absence of withdrawal. The other view, the Deprivation Reversal Model, posits that smoking only relieves negative affect that arises from withdrawal and not from other sources (7,8). Intermediate views suggest that smoking may robustly relieve negative affect only in some vulnerable smokers, or only during some challenges depending on characteristics such as their immediacy, vagueness, demand for active coping, and opportunity for distraction (1,2,9).
Determining from the literature the conditions under which smoking relieves negative affect is complicated by procedural differences across studies. With very few exceptions (10), prior laboratory research examined smoking’s influence on affect during just one task, and different studies used different tasks (2). Moreover, different studies often used different measures of negative affect (2). Watson et al. (11) define negative affect as a “general dimension of subjective distress and unpleasurable engagement that subsumes a variety of aversive mood states, including anger, contempt, disgust, guilt, fear, and nervousness” (p. 1063). Such a broad concept cannot be easily captured with one measure, and no single measure of affect is considered the gold standard. Relief of negative affect due to smoking may depend on the affect measure, as well as its source, as suggested by rodent studies of nicotine’s effects on antinociception (12) and anxiety (13). As a result, these procedural differences may prevent clear interpretation of findings aggregated across studies.
This study examined the effects of smoking on negative affect relief as functions of the affect source and measure. Tasks varying in response demands were administered in separate sessions, for comparison with smoking effects after overnight abstinence. Subjects were randomized to nicotine versus denicotinized cigarette smoking versus no smoking, to determine the influence of nicotine per se, and smoking in general, on negative affect relief (14). Multiple common affect measures were employed to determine the consistency of findings across measures. We also assessed craving, withdrawal, cigarette reward (i.e. “liking”), and smoking behavior in response to these situations. A pattern of increase in smoking reinforcement coinciding with the pattern of negative affect relief could support a negative reinforcement explanation of greater smoking due to negative mood.
Methods and Materials
Study Design
Participants were randomly assigned to one of three groups varying in the smoking condition for all sessions: nicotine cigarettes, denicotinized cigarettes, and no smoking control. All participated in 5 experimental sessions, one per mood induction procedure (4 negative, 1 neutral). Thus, this study involved one between-subjects factor, smoking condition (3 levels), and one main within-subjects factor, mood induction procedure (5 levels). This study was approved by the University of Pittsburgh Institutional Review Board.
Participants
Participants (n=104; 62 male, 42 female) were adult smokers (> 10 cigs/day) recruited via ads posted in the surrounding community. Excluded were those admitting to treatment for psychiatric problems in the last year or who scored above 13 on the Beck Depression Inventory-II (15). Most were Caucasian (84.8%), with 14.2% African-American, and 1% Asian. Mean ± SE sample characteristics were: age of 27.2 ± 0.9 yrs, nicotine yield of preferred brand of 1.04 ± 0.02 mg, daily smoking rate of 19.3 ± 0.5 cigarettes/day for 11.0±0.9 years, and Fagerstrom Test of Nicotine Dependence (FTND; 16) score of 4.7 ± 0.2, indicating moderate dependence.
Smoking Conditions
Subjects in the smoking conditions received either nicotine (Quest 1; 0.6 mg nicotine, 9 mg tar) (n=37) or denicotinized cigarettes (Quest 3; 0.05 mg nicotine, 9 mg tar) (n=34) widely used in smoking research (e.g. 17,18) and obtained commercially from Vector Group, Ltd. (Miami, FL). All were blind to brand. The no smoking controls (n=33) were not given any cigarettes. These three groups did not differ in age, ethnicity, or smoking characteristics.
Mood Induction Procedures
Subjects were presented with five different procedures to induce negative (four) or neutral (one) mood, with one procedure per session. Neutral mood was a control. Overnight abstinence provided an assessment of the well-established effects of smoking on negative affect due to abstinence. The remaining three were non-abstinence negative mood induction procedures designed to induce a level of negative affect comparable to that due to overnight abstinence. These varied in active versus passive coping (i.e. explicit behavior that subjects believed could reduce the degree of challenge posed by the task) and between immediate and clear versus anticipatory and vague response demands (1).
1. Neutral Mood Control
Subjects watched slides from the International Affective Picture System (IAPS; 19) judged neutral in emotional content. Slides were shown in a dark room for 12 sec each, one after the other, to sustain the intended mood. A similar procedure produces no increase in either positive or negative affect (6). This condition was intended as a control, to determine effects of smoking and nicotine in the absence of elevated negative affect.
2. Tobacco Abstinence
Subjects abstained overnight from smoking for >12 hrs prior to this session, verified by expired-air CO ≤ 10 ppm (20). A half day of abstinence reliably produces increased negative affect due to withdrawal (5). To maintain subject attention during the session, they engaged in the control task (above), in the Neutral Mood condition, although different neutral slides were presented to eliminate repetition of stimuli. Abstinence-induced negative affect, which is widely shown to be alleviated by smoking and nicotine, provided a reference for the magnitude of negative affect relief due to smoking during the other mood procedures.
3. Computer Challenge
Subjects were presented with a sequence of the digits 1–4 in random order on a computer monitor and were instructed to repeat the sequence in either the same or the reverse order on command using a computer keypad (similar to the commercial game “Simon”). Responding required the use of just one hand, allowing the other hand for smoking. To standardize the degree of challenge, task difficulty (i.e. number of digits to remember) was continuously adjusted by the computer to ensure comparable success (40% correct) for all subjects, regardless of ability. Correct responses earned $.50 and errors resulted in a loss of $.25. This task, which required active coping and presented a clear and immediate challenge, increases self-reported negative affect (21).
4. Negative Mood Slides
This procedure altered an existing technique (22) involving high arousal negative affect pictorial slides from the IAPS (19) and was similar to the Neutral Mood procedure described above except for the mood valence of the slides. This task allowed no active coping (i.e. only passive viewing was possible) and presented a clear and immediate challenge. A similar procedure has been used to increase negative affect in prior smoking studies (6,17).
Subjects correctly identified 86.1% of the content of the slides shown them during the negative and neutral mood slide procedures, with no differences between mood valence, verifying that they attended to these procedures that lacked explicit response requirements.
5. Speech Preparation
Subjects were instructed to prepare and then give two 3-min speeches, one at a time, on what they like and dislike (in that order) about their body. Subjects were unaware they would have to give a second speech (dislike) when they received instructions for the first (like). Requiring two speeches allowed for extended periods of active speech preparation, to match the duration of the other mood induction procedures. A staff member dressed in a white coat was introduced and described as a professional observer who would rate the speech on psychological characteristics. Subjects also were shown a video camera and told it would be used to record the speech for later evaluation, although speeches were not actually recorded. Affect ratings were obtained prior to each speech (see below), so that affect during speech preparation was assessed. This task, which required active coping and presented a vague and anticipatory challenge, elicits robust negative affect in similar research (e.g. 23,24).
Self-report Measures
Affect
The four measures of negative affect were selected for their diversity of items and frequency of use in acute mood induction studies. These measures were: the Diener and Emmons (25) Mood Form (converted from 0–6 to a 0–100 visual analog scale, or VAS), the Positive and Negative Affect Scale (PANAS; 11), the “stress” items of the Stress-Arousal Checklist (SACL; 26), and an abbreviated version of the State-Trait Anxiety Inventory-state (27). This STAI version (STAI-brief) contains 6 of the 20 items, 3 negative affect items (upset, worried, frightened) and 3 reverse-scored items (calm, secure, self-confident), and has been shown to be sensitive to acute laboratory challenges (24).
Craving and Withdrawal
The brief 10-item version of the Questionnaire on Smoking Urges (QSU-brief; 28) provides two separate craving factors, one reflecting a strong intention and desire to smoke (Factor 1) and the other reflecting anticipation of relief from negative affect (Factor 2). Withdrawal was assessed by the Minnesota Nicotine Withdrawal Scale (MNWS; 29), with items rated on a 0 (“not at all”) to 100 (“extremely”) VAS and averaged for a total score.
Smoking Reward and Perception
Two items from the Cigarette Evaluation Scale (30), “How much do you like this cigarette?” and “How strong is this cigarette?”, assessed smoking reward (liking; 31) and perception, respectively. Each was rated on a 0 to 100 VAS.
Session Procedures
Subjects participated in 5 sessions, one per mood induction procedure, over an average of 15.9 days (mean of 2.8±0.1 days between sessions). The session timeline is shown in Figure 1. Except for the smoking abstinence session, participants smoked ad libitum prior to each session and smoked one cigarette of their own brand upon arrival. We wanted to ensure that negative affect later in those sessions was due to the specific mood induction procedure assigned for that day and not to withdrawal. They then rested quietly to obtain baseline (BL) measures, were introduced to the mood procedure for that session, and engaged in it for 5 mins to induce mood (followed by T1 measures). The nicotine and denic smoking groups were given their designated cigarette, took 4 standard puffs (see below), and rated it for reward and perception, while the no smoking control group simply rested. Four puffs was selected to provide sufficient basis to rate the cigarette but not enough to induce satiation, which would interfere with the subsequent assessment of ad libitum smoking. The mood induction procedure resumed for another 3 mins (T2 measures). Subsequently, mood induction continued, and the two smoking groups were allowed to smoke their assigned cigarettes ad libitum over the last 10 mins (T3 measures). Affect, craving, and withdrawal were assessed in all 3 groups at each time point (BL, T1, T2, T3). The order of mood induction procedures across sessions was randomized between subjects, but the same sequence of orders was used for each smoking group, stratified by sex.
Figure 1.
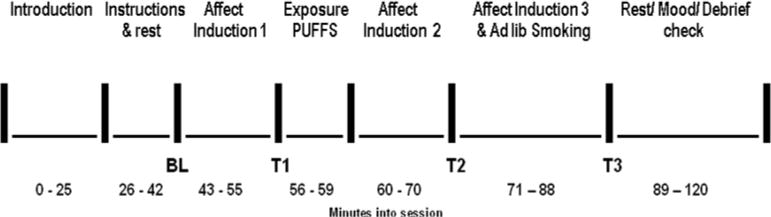
Timeline of each session. BL (baseline), T1, T2, and T3 indicate the points at which affect, craving, and withdrawal measures were obtained. Cigarette ratings were obtained at the unlabeled point just after the exposure puffs. The time intervals between points indicate the time between the start of the completion of the measures and not necessarily the duration of mood induction procedures (see text for details of mood induction timing).
All smoking was done via the Clinical Research Support System (CReSS; Borgwaldt KC, Inc., Richmond VA; www.plowshare.com), which assesses puff volume. Smoke inhalation during the 4 standard puffs was successfully controlled by computer instructions guiding the timing and amount of puff inhalation (17,21), as shown by the lack of differences in total volume from the 4 puffs across the 5 mood procedures (ranging from 235±8 to 248±9 ml) and between the nicotine and denic smoking groups (238±11 vs 243±11 ml, respectively). Smoking during the ad libitum period was not controlled by instructions.
Data Analyses
Order of mood procedures across sessions was not analyzed because of the large number of orders. No interactions involving sex were found in analyses, and so results were collapsed across men and women. For each set of affect analyses, the four negative affect measures and the two positive affect measures were first analyzed by multivariate analyses of variance (MANOVAs; Hotelling’s Trace), with follow-up ANOVAs and LSD t-tests (32) where significant. The validity of the negative mood induction procedures was determined by comparing affect at T1, after induction but prior to any smoking, between the four negative mood procedures versus neutral control.
Affect in response to smoking was determined by the change across time from T1 to T2 and T3. Only interactions involving time were of interest. We hypothesized that negative affect over time would be influenced by the interaction of smoking group by mood procedure, such that smoking would relieve negative affect due to abstinence but not during neutral mood and some of the other negative mood procedures. Affect relief during all four negative mood procedures would support the Nicotine Resource Model, while affect relief during abstinence but not the other procedures would support the Deprivation Reversal Model (1,7). Differences between the nicotine and denicotinized smoking groups tested the effects of nicotine per se, while differences between the denic smoking and no smoking groups indicated effects of the act of smoking, in the absence of significant nicotine. In follow-ups, the change from T1 (pre-smoking) to T2 examined the influence of a fixed amount of smoke intake (4 controlled puffs), while the change from T1 to T3 examined the influence of a greater (but variable, i.e. ad lib) amount of smoke intake. Because of the large number of these comparisons, significance was set at p<.01. Similar analyses were conducted for craving and withdrawal, except no initial MANOVAs were conducted since each involved a single measure.
Smoking reward and perception, rated after the four standard puffs (between T1 and T2), were analyzed by ANOVA, with task and nicotine/denic smoking groups as factors. Smoking reinforcement in the two smoking groups, determined by total puff volume during the ad libitum smoking period (between T2 and T3), was analyzed in the same manner.
RESULTS
Validation of Mood Induction
Negative affect upon initial mood induction (T1) was similarly elevated during each of the four negative mood induction procedures, compared with neutral control, as intended. The main effect of procedure, but no other effects, was highly significant both in the MANOVA, F(16,1598) = 17.89, p<.001, and in the individual ANOVAs for each of the four negative affect measures, F(4,404)’s of 21.79 to 46.24, all p<.001. Similar results were seen for the reduction in positive affect due to the negative mood induction procedures, as only the main effect of procedure was significant in the MANOVA, F(8,804)=31.23, p<.001, and the ANOVAs for the Mood Form and PANAS, F(4,404)’s of 38.72 and 20.38, respectively, both p<.001.
Affect, Craving, and Withdrawal in Response to Smoking
Negative Affect
Effects of smoking were determined by the change in affect across time (T1, T2, T3), by smoking group and mood procedure. As shown in Table 1, MANOVA results, and most of the follow-up ANOVAs for each affect measure, revealed significant interaction effects for time by smoking group, time by mood procedure, and time by smoking group by mood procedure.
Table 1.
MANOVA and follow-up ANOVA results (F ratios) for negative and positive affect, craving, and withdrawal across time (T1, T2, T3) as functions of mood induction procedure, smoking group, and, for affect, affect measure.
| Measure | Time × Smoking Group | Time × Mood Induction Procedure | Time × Smoking Group × Mood Induction Procedure |
|---|---|---|---|
| Negative Affect | |||
| MANOVA | F(16, 782)= 3.15** | F(32, 3182)= 3.48*** | F(64, 3182)= 1.24** |
| Univariate ANOVAs | F(4, 200)’s of: | F(8, 800)’s of: | F(16, 800)’s of: |
| Mood Form | 10.02** | 5.82*** | 1.87* |
| PANAS | 9.21*** | 6.08*** | 1.40 |
| SACL-stress | 6.16*** | 8.41*** | 2.29* |
| STAI | 2.35 | 5.80*** | 2.27** |
| Positive Affect | |||
| MANOVA | F(16, 796)= 1.14, ns | F(16, 1596)= 3.29*** | F(32, 1596)= 1.64* |
| Univariate ANOVAs | — | F(8, 800)’s of: | F(16, 800)’s of: |
| Mood Form | — | 5.07*** | 1.78* |
| PANAS | — | 2.20* | 1.30 |
| Craving and Withdrawal | F(4,200)’s of: | F(8, 800)’s of: | F(16, 800)’s of: |
| QSU-Factor 1 | 53.83*** | 10.95*** | 1.30 |
| QSU-Factor 2 | 34.24*** | 17.75*** | 2.38** |
| MNWS withdrawal | 13.48*** | 11.26*** | 2.44*** |
p<.05,
p<.01,
p<.001
The magnitude of negative affect relief from smoking varied strongly by mood procedure. Smoking robustly relieved negative affect after abstinence regardless of the affect measure. As shown in Figure 2, this relief was observed after the 4 standard puffs (T2), and only after abstinence did ad libitum smoking (T3) fully relieve negative affect to the level observed in the neutral mood procedure. However, the effects of smoking during the other sources of negative affect were modest and depended on the affect measure. Smoking modestly attenuated negative affect on the Mood form and SACL during the computer challenge, on all four measures during the negative mood slides, and on only the PANAS during speech preparation (Figure 2). Negative affect varied little between the nicotine and denic smoking groups, although negative affect on the SACL and STAI was attenuated during negative mood slides by smoking denic cigarettes, not nicotine cigarettes.
Figure 2.
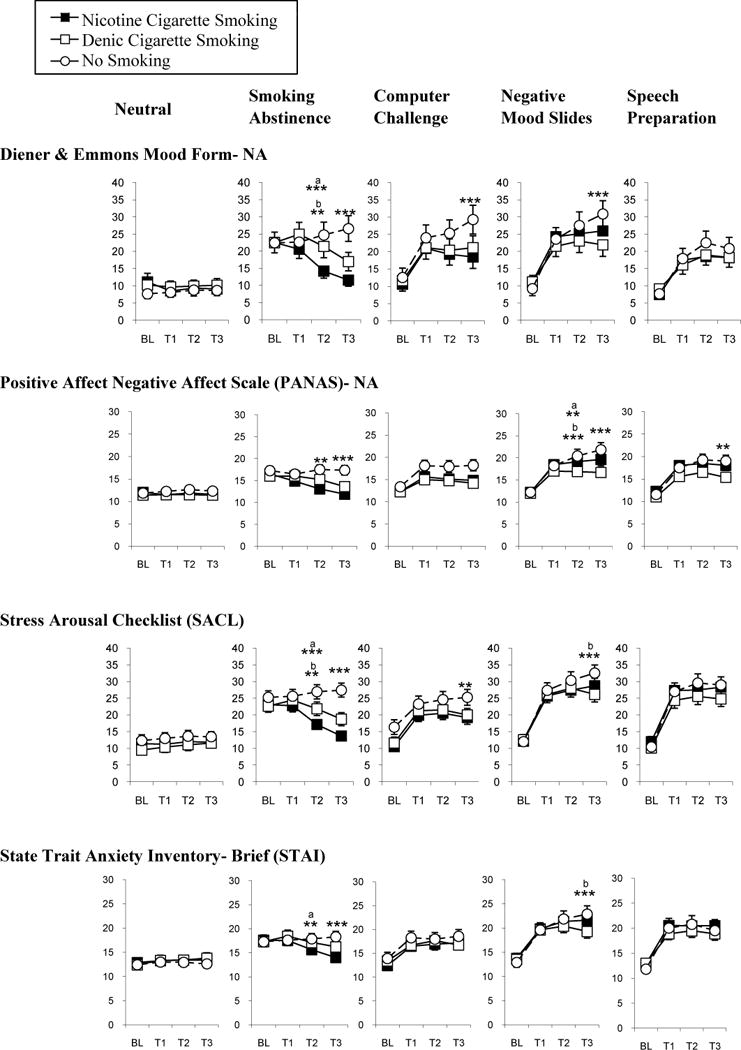
Negative affect across time points as functions of mood induction procedure, smoking condition, and affect measure. The five mood conditions are arrayed across the top in different columns, while results for the specific measures are presented in different rows. Results for neutral mood are presented in the first column, followed by abstinence, to show the effects of smoking after abstinence versus neutral mood. Effects of smoking under the three non-abstinence negative mood procedures are then presented, to facilitate comparison with smoking effects after abstinence. ** p<.01; *** p<.001 for comparisons between the no smoking versus the nicotine or denic cigarette smoking groups in the change from T1 to T2 or from T1 to T3. Asterisks apply to both comparisons unless otherwise specified by superscripts (a, b).
Positive Affect
Significant effects in the MANOVA and most of the follow-up ANOVAs were the interactions of time by mood procedure and of time by smoking group by mood procedure (Table 1). Consistent with the results for negative affect, smoking nicotine or denic cigarettes enhanced both measures of positive affect during abstinence but had minimal effects during the other mood induction procedures (see Figure S1 in Supplement 1).
Craving and Withdrawal
ANOVA results for QSU Factors 1 and 2 and MNWS withdrawal were similar to those for affect (Table 1). As with negative affect, smoking relieved withdrawal more robustly after abstinence than during the other procedures, although as expected withdrawal was elevated only after abstinence. However, relief of craving by smoking was substantial and generally similar across mood induction procedures (see Figure S2 in Supplement 1). The nicotine content of cigarettes again had little influence on these responses to smoking.
Smoking Reward, Perception, and Reinforcement
Reward and Perception
Cigarette liking was influenced by the main effects of procedure, F(4,188) = 3.33, p<.02, and smoking group, F(1,47) = 5.97, p<.02, but not by the interaction of smoking group by procedure, F(4,188) = 1.14. As shown in Figure 3, liking was greater for the nicotine versus denic cigarette, and after abstinence versus neutral mood; liking did not differ between the other negative mood procedures and neutral mood. “How strong” was influenced only by the main effect of smoking group, F(1,47) = 13.61, p<.001, as ratings were higher for the nicotine cigarette (not shown).
Figure 3.
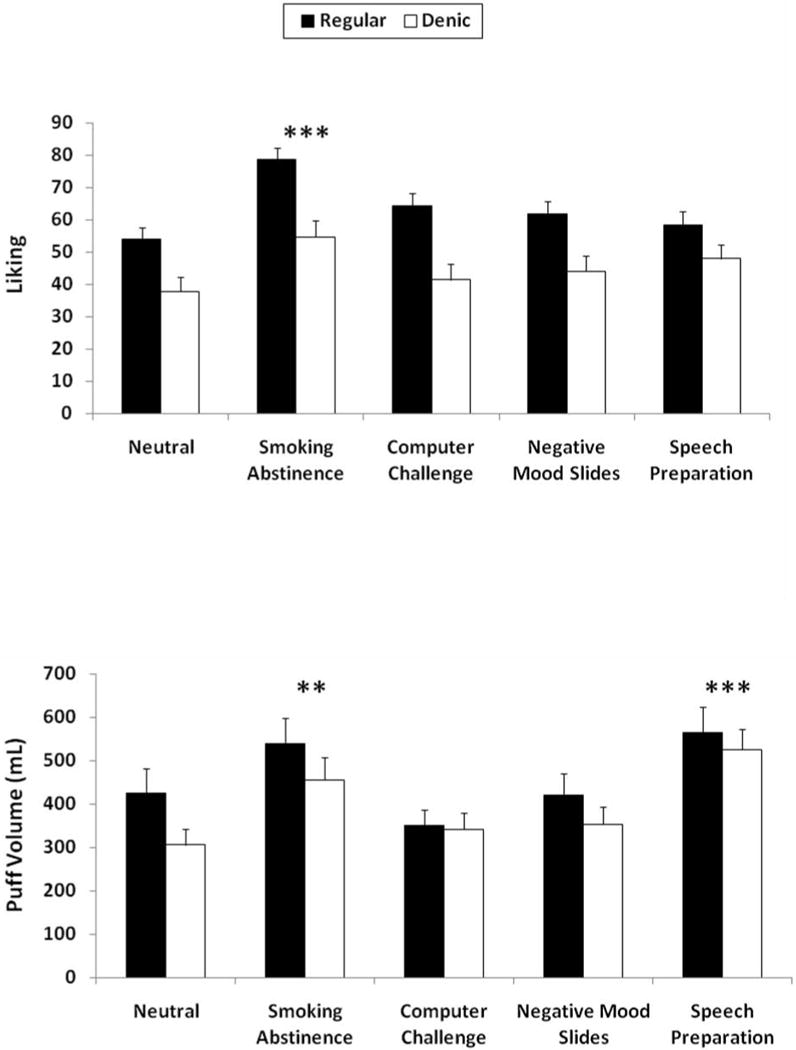
Smoking reward (top) and puff volume during the ad libitum smoking period (bottom) as functions of mood induction procedure and nicotine versus denic smoking group. The main effect of nicotine was significant for reward but not for puff volume. ** p<.01; *** p<.001 for the difference from neutral mood control procedure.
Reinforcement
Total puff volume during the ad libitum smoking period was significantly influenced only by mood procedure, F(4,256) = 14.21, p<.001. As also shown in Figure 3, smoke intake was greater than neutral control during the speech preparation and smoking abstinence procedures, but not during the computer challenge and negative mood slides. Effects of smoking group, F(1,64) = 2.44, p>.10, and the interaction of mood procedure by smoking group, F(4,256) < 1, were not significant, as no differences in smoking behavior were seen between the nicotine and denic cigarettes.
DISCUSSION
Rather consistent with the Deprivation Reversal Model (7,8), negative affect relief by cigarette smoking was robust after smoking abstinence but much more modest or absent during other negative mood procedures. Only with abstinence was negative affect relief reliably seen after the 4 standard puffs and virtually complete after the ad libitum smoking period (Figure 2). The influence of situation was also apparent for enhancement of positive affect, although the effects of smoking on craving were far less influenced by situation. We know of no other human study clearly demonstrating the situation-specificity of smoking’s influence on relieving negative affect.
A second novel finding was that even the limited relief of negative affect during some sources other than abstinence depended on the affect measure. During the computer challenge task, smoking attenuated negative affect measured by the Mood form and SACL but not the PANAS and STAI-brief. During the public speech, smoking attenuated negative affect assessed only by the PANAS. We are not aware of any other controlled study clearly demonstrating that negative affect relief from smoking may be specific to the affect measure used, in addition to the source of the negative affect. Future studies of acute negative affect relief due to smoking should employ multiple affect measures or risk the likelihood that their results may be specific only to the single measure used.
Another important, if less novel, finding is that the acute influences of smoking on affect, craving, and withdrawal were largely unrelated to nicotine intake, consistent with prior studies by us (17) and others (14,18,33–35). These results further emphasize that nonpharmacological, perhaps conditioned, influences are critical to these acute effects of smoking. Alternatively, constituents in tobacco other than nicotine may be psychoactive in ways that relieve negative affect, or even the tiny amounts of nicotine in denicotinized cigarettes may be sufficient to alleviate many effects of abstinence (36).
The increase in smoking behavior (reinforcement) did not completely parallel the influence of smoking on relieving negative affect across the five procedures. Smoking was increased by abstinence, the source of negative affect robustly relieved by smoking, but smoking was increased similarly by speech preparation, where smoking did not clearly relieve negative affect. Smoking was not increased during the other negative mood procedures, where negative affect was a bit more consistently relieved by smoking, depending on the measure. These observations question any straightforward negative reinforcement explanation for the relationship between negative mood and smoking behavior (2,6,17). Yet, the lack of influence of smoking on enhancing positive affect, other than during abstinence, may also be contrary to the notion that negative mood situations, or “stressors”, increase smoking behavior by enhancing smoking’s positive reinforcing effects.
The situational-specificity of negative affect relief by smoking makes it important to understand mediators of negative affect in these situations. The emergence of negative affect during abstinence may be due to resensitization of upregulated nicotine receptors, following the end of chronic nicotine effects in desensitizing the receptors (37,38). Nicotine intake would then desensitize receptors, providing a clear mechanism for smoking’s relief of negative affect due to abstinence that is likely not relevant to the origin and relief of negative affect due to situational challenges. Ventral striatal dopamine release may also be important (39). However, the conditioning of nicotine effects via smoking also likely plays a substantial role. Withdrawal symptoms, including negative affect, may be behavioral as well as pharmacological in nature (40), accounting for why smoking either nicotine or denic cigarettes fully relieved negative affect during abstinence but not the other conditions of this study. Other study findings do not appear to help clarify the situations in which negative affect is relieved by smoking. Although the non-abstinence negative mood procedures varied in terms of active coping, immediacy, and vagueness of the challenge, these characteristics appeared to have little impact in moderating relief of negative affect due to smoking since such relief was minimal.
Our results also may have implications for clinical research. Several times per day, smokers may experience mild withdrawal and, upon smoking, subsequent relief of negative affect related to withdrawal. Over time, the association of frequent complete negative affect relief from smoking during withdrawal could foster conditioning of smoking behavior in response to any experience of negative affect (4,7). Moreover, broad expectations of negative affect relief by smoking may also be promoted when smokers experience this relief during situational challenges that occur concomitant with brief smoking abstinence; the resulting relief of abstinence-induced negative affect may be interpreted as relief of negative affect arising from the situational challenges. Our findings may also be relevant to clinical research on the influence of other drug use, such as alcohol, on negative affect relief (41).
Study limitations include the uncertain generalizability of the mood procedures to common causes of negative affect in the natural environment, exclusion of smokers with current depression, use of cigarettes unfamiliar to subjects, the relatively brief period of mood induction and observation of smoking behavior, and the absence of non-self report measures of negative affect. Our results should be replicated in studies involving other sources of negative affect, the smokers’ preferred brand of cigarettes, with longer periods of mood induction and smoking access, and with psychophysiological or other measures of negative affect (42,43). It is possible that measures capturing other dimensions of negative affect not assessed here may reveal relief due to smoking during non-abstinence challenges. Other moderating or mediating factors of the situation not manipulated here, such as distraction, may also show that smoking relieves negative affect during such challenges (23). Individual differences in magnitude of negative affect relief may also be important (1,4,45). If our findings are replicated, smoking’s influence on negative affect may be far more modest and narrow than typically assumed, guiding future research on mechanisms of smoking reinforcement.
Supplementary Material
Acknowledgments
This research was supported by NIDA Grants DA16483, DA19478, and DA027449. The authors thank Amy Grottenthaler, Melissa Mercincavage, Jessica Briski, Roy Chengappa, and Carolyn Fonte for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilbert DG. Smoking: Individual differences, psychopathology, and emotion. London: Taylor and Francis; 1995. [Google Scholar]
- 2.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 3.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. PsycholRev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- 5.Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol. 1996;11:391–400. [Google Scholar]
- 6.Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. J Abnormal Psychol. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- 7.Parrott AC. Nesbitt’s Paradox resolved? Stress and arousal modulation during cigarette smoking. Addict. 1998;93:27–39. doi: 10.1046/j.1360-0443.1998.931274.x. [DOI] [PubMed] [Google Scholar]
- 8.Parrott AC, Kaye FJ. Daily uplifts, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behav Pharmacol. 1999;10:639–646. doi: 10.1097/00008877-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hasenfratz M, Battig K. Psychophysiological interactions between smoking and stress coping? Psychopharmacol. 1993;113:37–44. doi: 10.1007/BF02244331. [DOI] [PubMed] [Google Scholar]
- 10.Jarvik ME, Caskey NH, Rose JE, Herskovic JE, Sadeghpour M. Anxiolytic effects of smoking associated with four stressors. Addict Behav. 1989;14:379–386. doi: 10.1016/0306-4603(89)90025-7. [DOI] [PubMed] [Google Scholar]
- 11.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 12.Caggiula AR, Epstein LH, Perkins KA, Saylor S. Different methods of assessing nicotine-induced antinociception may engage in different neural mechanisms. Psychopharmacol. 1995;122:301–306. doi: 10.1007/BF02246552. [DOI] [PubMed] [Google Scholar]
- 13.Tian S, Gao J, Han L, Fu J, Li C, Li Z. Prior chronic nicotine impairs cued fear extinction but enhances contextual fear conditioning in rats. Neurosci. 2008;153:935–943. doi: 10.1016/j.neuroscience.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacol. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory - Second Edition. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 16.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Perkins KA, Ciccocioppo M, Conklin C, Milanak M, Grottenthaler A, Sayette M. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. J Abnormal Psychol. 2008;117:79–93. doi: 10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- 18.Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addict. 2006;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 19.Lang PJ, Ohman A, Vaitl D. The International Affective Picture System [Photographic Slides] Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1988. [Google Scholar]
- 20.SRNT subcommittee. Biochemical verification of tobacco use and cessation. Nic Tobacco Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 21.Perkins KA, Grobe JE, Fonte C, Breus M. ‘Paradoxical’ effects of smoking on subjective stress versus cardiovascular arousal in males and females. Pharmacol Biochem Behav. 1992;42:301–311. doi: 10.1016/0091-3057(92)90531-j. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin AH, Sher KJ. Effects of induced mood on diagnostic interviewing: Evidence for a mood and memory effect. Physiol Assess. 1993;5:197–202. [Google Scholar]
- 23.Kassel JD, Shiffman S. Attentional mediation of cigarette smoking’s effect on anxiety. Health Psychol. 1997;16:359–368. doi: 10.1037//0278-6133.16.4.359. [DOI] [PubMed] [Google Scholar]
- 24.Sayette MA, Martin CS, Perrott MA, Wertz JM, Hufford M. A test of the appraisal-disruption model of alcohol on stress. J Stud Alcohol. 2001;62:247–256. doi: 10.15288/jsa.2001.62.247. [DOI] [PubMed] [Google Scholar]
- 25.Diener E, Emmons RA. The independence of positive and negative affect. J Person Soc Psychol. 1984;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- 26.Mackey CJ. The measurement of mood and psychophysiological activity using self-report techniques. In: Martin I, Venables PH, editors. Techniques in psychophysiology. New York: Wiley; 1980. pp. 501–562. [Google Scholar]
- 27.Spielberger CD, Gorsuch RL, Lushene RE. The state-trait anxiety inventory (test manual) Palo Alto, Calif: Consulting Psychologists Press; 1970. [Google Scholar]
- 28.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nic Tobacco Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 29.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. Arch Gen Psychiatr. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- 30.Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 31.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 32.Huitema BE. Analysis of covariance and alternatives. New York: John Wiley & Sons; 1980. [Google Scholar]
- 33.Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacol Biochem Behav. 1995;50:91–96. doi: 10.1016/0091-3057(94)00269-o. [DOI] [PubMed] [Google Scholar]
- 34.Dallery J, Houtsmuller EJ, Pickworth WB, Stitzer ML. Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacol. 2003;165:172–180. doi: 10.1007/s00213-002-1242-8. [DOI] [PubMed] [Google Scholar]
- 35.Eid NC, Fant RV, Moolchan ET, Pickworth WB. Placebo cigarettes in a spaced smoking paradigm. Pharmacol Biochem Behav. 2005;81:158–164. doi: 10.1016/j.pbb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha-4 beta-2 nicotinic acetylcholine receptors. Arch Gen Psychiatr. 2009;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED. Ventral striatal dopamine release in response to smoking a regular vs denicotinized cigarette. Neuropsychopharmacol. 2009;34:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker TB, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmacologic and behavioral withdrawal from addictive drugs. Curr Dir Psychol Sci. 2006;15:232–236. [Google Scholar]
- 41.Wietkiewitz K, Villarroel NA. Dynamic association between negative affect and alcohol lapses following alcohol treatment. J Consult Clin Psychol. 2009;77:633–644. doi: 10.1037/a0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang PJ. The emotion probe: studies of motivation and attention. Amer Psychol. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- 43.Childs E, deWit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacol. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perkins KA, Lerman C, Grottenthaler A, Ciccocioppo M, Milanak M, Conklin CA, Bergen AW, Benowitz NL. Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement due to negative mood. Behav Pharmacol. 2008;19:641–649. doi: 10.1097/FBP.0b013e32830c367c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doran N, McChargue D, Spring B, VanderVeen J, Cook JW, Richmond M. Effect of nicotine on negative affect among more impulsive smokers. Exp Clin Psychopharmacol. 2006;14:287–295. doi: 10.1037/1064-1297.14.3.287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


