Abstract
Progesterone (P) binding to the intracellular progesterone receptors (PRs) plays a key role in epilepsy via modulation of GABA-A receptor plasticity in the brain. This is thought to occur via conversion of P to neurosteroids such as allopregnanolone, an allosteric modulator of GABA-A receptors. In the female brain, the composition of GABA-A receptors is not static and undergoes dynamic spatial changes in response to fluctuations in P and neurosteroid levels. Synaptic α2-containing GABA-A receptors contribute to phasic neuronal excitability and seizure susceptibility. However, the mechanisms underlying α2-subunit remain unclear. Here we utilized the neurosteroid synthesis inhibitor finasteride and PR knockout mice to investigate the role of PRs in α2-subunit in the hippocampus. α2-subunit expression was significantly upregulated during the high-P state of diestrous stage and with P treatment in wildtype and PR knockout mice. In contrast, there was no change in α2-subunit expression when metabolism of P into neurosteroids was blocked by finasteride in both genotypes. These findings suggest that ovarian cycle-related P and neurosteroids regulate α2-GABA-A receptor in the hippocampus via a non-PR pathway, which may be relevant to menstrual-cycle related brain conditions.
Keywords: Progesterone, GABA receptor, α2-subunit, perimenstrual, neurosteroid, epilepsy
1. Introduction
Progesterone (P) is an anticonvulsant hormone (Backstrom et al., 1984; Herzog, 1995; 1999; Frye et al., 2000; Reddy, 2004) which plays an important role in women with catamenial epilepsy, a menstrual cycle-related seizure disorder (Reddy, 2016). Low P levels during the perimenstrual and ovulatory period are linked to higher seizures compared to luteal phase with high P levels (Herzog et al., 1997; Reddy, 2016). P modulates seizure susceptibility by binding to the genomic progesterone receptors (PR) and non-genomic targets by converting into allopregnanolone (AP) and related neurosteroids, which are positive modulators of GABA-A receptors (Reddy et al., 2010; Reddy, 2013; Wu et al., 2013; Carver et al., 2014; Reddy and Mohan, 2011). Consequently, P and neurosteroids play a significant role in the pathophysiology of epilepsy, anxiety, stress, and postpartum depression (Purdy et al., 1991; Smith et al., 1998a; Reddy, 2010; Shen et al., 2010; Carver and Reddy, 2013; Reddy, 2013). P and neurosteroids may exert disease-modifying effects on epileptogenesis (Reddy et al., 2010; Reddy and Mohan, 2011). Therefore, there is emergent interest in the translational potential of neurosteroid regulation of GABA-A receptors in the brain.
Neuronal GABA-A receptors are composed of five subunits from several classes (α1–6, β1–4, γ1–3, δ, ε, θ, ρ1–3) (Korpi et al., 2002). The major pentameric forms include 2α, 2β, and 1γ or δ subunits. The subunit expression of these receptors varies with development stage, neuronal cell type, and brain region. Such heterogeneity in receptor composition confers distinct physiological and pharmacological properties, including different roles for synaptic and extrasynaptic GABA-A receptors. Neurosteroids modulate most receptor isoforms (Belelli et al., 2002; Carver and Reddy, 2013; 2016). They can modulate both synaptic (γ2-containing) and extrasynaptic (δ-containing) receptor isoforms (Carver and Reddy, 2013; Reddy and Estes, 2016). Neurosteroids have been shown to play a critical role in the pathogenesis of catamenial epilepsy and post-partum depression (Reddy et al., 2001; Reddy, 2013; Maguire and Mody, 2008). Alterations in the subunit expression and function of GABA-A receptors in the hippocampus have been reported during estrous cycle, pregnancy, and after delivery (Smith et al., 1998a; Smith et al., 1998b; Follesa et al., 2000; Biggio et al., 2001; Pierson et al., 2005; Gangisetty and Reddy, 2010; Wu et al., 2013; Carver et al., 2014). We have discovered that neurosteroids regulate the expression of α4 and δ-subunit plasticity in catamenial epilepsy models (Gangisetty and Reddy, 2010; Wu et al., 2013; Carver et al., 2014; Reddy, 2016). Although α1 and α2-subunits are highly expressed in limbic regions, where they mediate phasic inhibition, there is limited information on the contribution of α2-containing receptors. GABA-A receptor α2 knockout mice displayed anxiety/depression-like effects (Fritschy and Mohler, 1995; Low et al., 2000; Vollenweider et al., 2011; Durkin et al., 2011), which underscores the integral physiological role of the subunit in the brain. In human temporal lobe epilepsy, prominent upregulation of mainly α2-subunit was seen on somata and apical dendrites, with reduced labeling on basal dendrites in hippocampal granule cells (Loup et al., 2000). However, the neuroendocrine mechanisms underlying α2-subunit plasticity in the hippocampus remain unclear.
In this study, we sought to determine the molecular pathways of neurosteroid and progesterone receptor (PR) regulation of α2-subunit GABA-A receptor in the hippocampus using the PR knockout mouse model. Our results show that a high P (diestrous) stage and exogenous P treatment upregulates α2-subunit mRNA expression via a PR-independent neurosteroid mechanism.
2. Results
2.1. Upregulation of GABA-A receptor α2-subunit mRNA expression in the hippocampus subfields in diestrus phase
To model the menstrual cycle-related changes in ovarian hormones, we investigated the changes in receptor subunit expression at two distinct phases of the mouse estrous cycle. Different stages of the cycle were determined by microscopic examination of vaginal smears and by estimating plasma P levels (Wu et al., 2013). Plasma P levels were found to be nearly 4-fold higher in the diestrus (10± 2 ng/ml) than estrus stage (2.5± 0.3 ng/ml) (Wu et al., 2013). These two stages of the estrous cycle were selected to study α2-subunit mRNA expression. To determine the cyclical changes in GABA-A receptor α2-subunit mRNA in the hippocampus, we carried out TaqMan real-time PCR assay of α2-subunit mRNA expression in the hippocampus subfields of the CA1, CA3, and dentate gyrus (DG) regions. The expression of GABA-A receptor α2-subunit mRNA in the CA3 and DG significantly increased in the diestrus as compared to estrus stage (Fig. 1). The increased α2-subunit expression in diestrus was about 2-fold in CA3 and 4-fold in DG region. There was no significant change of α2-subunit mRNA expression in the CA1 region. These results suggest that ovarian cycling is closely associated with regulation of α2-subunit mRNA expression.
Fig. 1. Changes in GABA-A receptor α2-subunit mRNA expression in the hippocampus subfields CA1, CA3, and dentate gyrus (DG) during various phases of the estrous cycle.
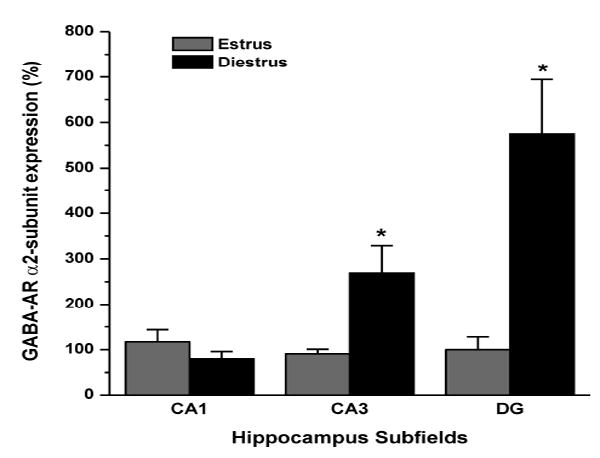
Total RNA was extracted from the microdisected hippocampus tissue and cDNA was prepared for TaqMan PCR analysis. The TaqMan PCR data was normalized in every assay using GAPDH and expressed as percent over the control. The data represents the mean ±SEM (n=8 mice per group). *p<0.05 vs. estrus group.
2.2. Progesterone upregulates GABA-A receptor α2-subunit mRNA expression via a PR-independent pathway
To determine whether the PR pathway is involved in regulating GABA-A receptor α2-subunit mRNA expression in response to P/neurosteroid treatment, we utilized female homozygous progesterone receptor knockout (PRKO) mice, which lack PR-A and PR–B receptor subtypes in the brain (Reddy et al., 2004). Twenty-four hours after 7-day P exposure, the abundance of α2-subunit mRNA in the hippocampus was increased by 204% in wild-type mice as compared with vehicle control (Fig. 2A). Such an upregulation was undiminished in PRKO mice (Fig. 2B). In PRKO mice, there was a 523% increase in α2-subunit mRNA at 24 h following P exposure. The α2-expression response was higher in PRKO than in wildtype (WT) mice (Fig.2B). These results indicate a role of P-derived AP and related neurosteroids, independent of PRs, in regulating α2-subunit mRNA expression in the hippocampus.
Fig.2. Changes in GABA-A receptor α2-subunit mRNA expression in the hippocampus during neurosteroid exposure in wildtype (A) and PRKO (B) mice.
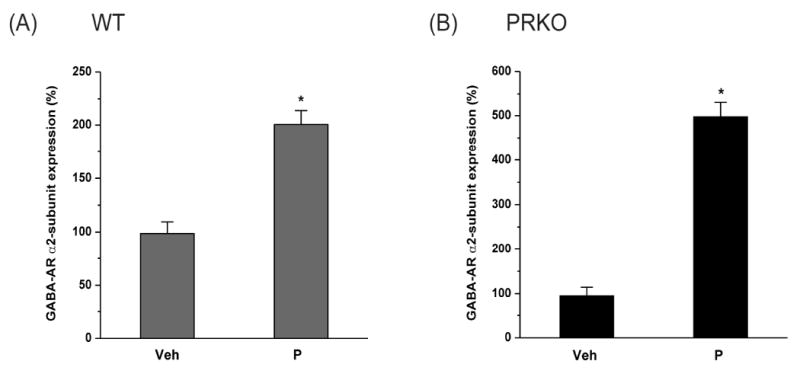
The α2-subunit mRNA expression was quantified in the hippocampus samples collected from female mice following 7-day treatment with vehicle, or neurosteroid exposure by progesterone (P) treatment. The α2-subunit mRNA expression was normalized with GAPDH and expressed as percent over the control. The data represents the mean ±SEM (n=8 mice per group). *p<0.05 vs. vehicle group.
2.3. Neurosteroid inhibition prevents the P regulation of GABA-A receptor α2-subunit mRNA expression
To test whether P-derived neurosteroids are involved in the upregulation of α2-subunit mRNA expression, we utilized the neurosteroid synthesis inhibitor finasteride (Gangisetty and Reddy, 2010). We treated mice with P and finasteride to block 5α-reductase activity for inhibiting P conversion to AP and related neurosteroids. After finasteride, α2-subunit mRNA expression was analyzed in the hippocampus. The P-induced upregulation of α2-subunit mRNA expression was significantly reduced after finasteride in WT (Fig.3A) and PRKO mice (Fig.3B). Overall, these results suggest that progesterone and neurosteroids play a key role in regulation of α2-subunit expression in the hippocampus.
Fig. 3. Changes in GABA-A receptor α2-subunit mRNA expression in the hippocampus during neurosteroid inhibition in WT (A) and PRKO (B) mice.
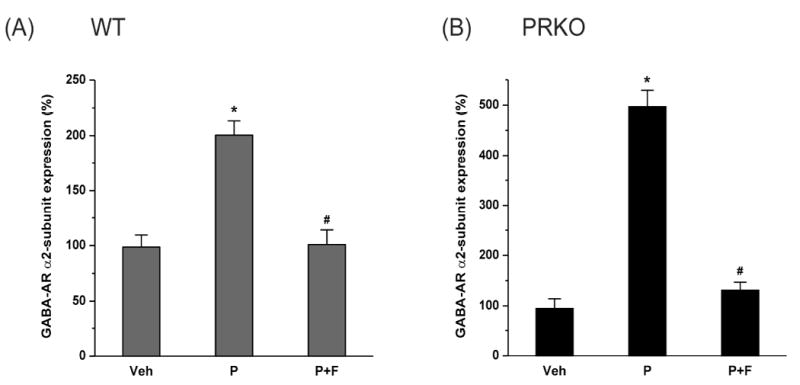
The GABA-AR α2-subunit mRNA expression was quantified in the hippocampus samples collected from wild-type (WT) mice following 7-day treatment with vehicle, progesterone (P) or progesterone and finasteride (P+F). The α2-subunit mRNA expression was normalized with GAPDH and expressed as percent over the control. The data represents the mean ±SEM (n=8 mice per group). *p<0.05 vs. vehicle group; #p<0.05 vs. P treatment group.
3. Discussion
The principal outcome of this study shows that cyclical elevations in P levels during the diestrus phase and neurosteroid exposure are accompanied by subfield-specific, increased α2-subunit GABA-A receptor expression in the hippocampus. The α2-subunit GABA-A receptor contributes to phasic inhibition in the hippocampus and thereby plays a contributory role, especially in response to changes in P levels in female brain. We have reported similar outcomes on the α4 and δ-subunits, which contribute to tonic inhibition in the brain (Gangisetty and Reddy, 2010; Wu et al., 2013; Carver et al., 2014). The α2-subunit is known to play a physiological role in cellular and behavioral responses to neurosteroids (Durkin et al., 2011). Collectively, these novel findings provide further evidence for the dynamic neurosteroid-mediated regulation of neuronal excitability with therapeutic implications in menstrual conditions such as catamenial epilepsy, premenstrual syndrome, menstrual migraine, and post-partum depression.
In perimenstrual catamenial epilepsy, there is emerging evidence to suggest that P withdrawal plays a key role in seizure exacerbations occurring around the time of menstruation (Backstrom et al., 1984; Bonuccelli et al., 1989; Scharfman and MacLusky, 2006). The mechanisms underlying ovarian cycle-related α2-subunit plasticity remain unclear. However, there is evidence that cyclical fluctuations in steroid hormones across the estrous cycle regulate several GABA-A receptor subunits at the diestrus phase (Maguire et al., 2005; Gangisetty and Reddy, 2010; Reddy et al., 2012; Wu et al., 2013; Carver et al., 2014). Previous studies reported increased α4 and δ-subunits at diestrus relative to estrus phase (Maguire et al., 2005; Wu et al., 2013). Other studies demonstrated upregulation of α4 and δ-subunits in response to P treatment and neurosteroid withdrawal (Reddy and Kulkarni, 1999; Mostallino et al., 2006; Gangisetty and Reddy, 2010; Wu et al., 2013; Carver et al., 2014). Similarly, there is evidence for changes in GABA-A receptor subunit plasticity on proestrus when α4 and δ-subunit expression increases significantly in hippocampus CA1 (Sabaliauskas et al., 2015). Moreover, P-induced increases in α4 and δ-subunit are increased by the addition of estradiol, which is elevated on proestrus (Shen et al., 2005).
A number of studies shown that the α2-subunit is linked to behavior conditions such as anxiety and depression (Low et al., 2000; Vollenweider et al., 2011). Altered expression of α1 and α2-subunits in different estrous cycle stages have been reported previously (Byrnes et al., 2007). There are some discrepancies in expression of these subunits during estrous cycle stages. It is possible that these discrepancies occurred due to species variation, selected brain region, and technique used. The overall significance of changes in the α2 and other subunits during the estrous cycle need to be reconciled for physiological significance. Aside from α2, there are other synaptic subunits such as γ2 that are also regulated during estrous cycle. For example, there was significant reduction in γ2 expression at the diestrous phase (Maguire and Mody, 2005). In accordance with the suggested rules of GABA-A receptor subunit assembly, γ2-containing receptors are expressed at synaptic sites. Moreover, the loss of one subunit can result in compensatory upregulation of partner subunits. Therefore, it is likely that the alterations in α2-subunit during the estrous cycle are reflected by changes in partnering subunits. The net contribution of these changes are consistent with functional alterations in GABAergic currents (Maguire and Mody, 2005; Wu et al., 2013).
The present study utilizes endogenous fluctuations in P and neurosteroids across the estrous cycle to examine expressional plasticity of the α2-subunit in the hippocampal subfields. We used highly sensitive, optimized assays for subunit mRNA expression (Gangisetty and Reddy, 2009). We found regulation of α2-subunit expression to be subfield-specific, with greater α2-subunit expression in the CA3 and DG during the diestrus compared to estrus phase (Fig. 1). Emerging evidence suggests that the α2-subunit is involved in synaptic targeting, but pentameric channels made of α2β3δ receptors have very low affinity to GABA (Wu et al., 2012). Receptors with α2 and δ-subunit are abundantly present in the molecular layer of the DG in the rat brain (Sperk et al., 1997). Therefore, it is still not clear whether α2-containing receptors assemble extrasynaptically with the δ-subunit or respond to low micromolar extracellular GABA to regulate tonic inhibition. Nevertheless, substantial increases in synaptic α2 levels could affect overall neuronal excitability. For example, the α2βγ2 receptor has faster activation kinetics, prolonged open duration and bursting activity, and slower deactivation/decay time than α1βγ2 (Lavoie et al., 1997). A large switch from α1 to α2-containing receptors in the hypothalamus has been previously shown to be coupled with slower phasic current decay (Brussaard et al., 1997; Brussaard and Herbison, 2000). Therefore, a relative increase in α2-subunit proportion could result in greater net inhibitory current in neuronal networks. Durkin and colleagues (2011) have created a glutamine to methionine knock-in mouse line in which the neurosteroid potentiation site on the α2-subunit has been disrupted. This mutation reduced the effect of neurosteroids on the decays of phasic currents. Homozygous mice displayed a tendency toward increased anxiety-like behavior, suggesting a role for α2-receptor in anxiety behavior.
There are additional implications of α2-subunit alterations in the hippocampus. P has anticonvulsant activity (Herzog, 1995; Herzog et al., 1997; Reddy et al., 2001; Reddy, 2010; Herzog et al., 2012). These actions result from the metabolic conversion of P to GABA-A receptor-modulating, antiseizure neurosteroids (Frye et al., 2002; Reddy et al., 2004). During the menstrual cycle, circulating P levels are low in the follicular phase, but rise in the mid-luteal phase before declining around the premenstrual phase. Circulating AP levels increase in parallel to those of its parent P (Tuveri et al., 2008). Neurosteroid exposure and withdrawal, a disease model for perimenstrual catamenial epilepsy, has profound influence on GABA-A receptor subunit plasticity (Reddy, 2016). In the present study, we found that there is a sustained rise in α2-subunit expression during neurosteroid exposure with P treatment. This phenomenon is similar as in the diestrous phase with increased P level. This type of increased expression of α2 subunit with P treatment for 2 and 7 days was also observed in NTERA-2 neuronal cells (Pierson et al., 2005).
We utilized two distinct models to simulate the hormonal milieu of the luteal phase: natural estrous cycle and exogenous P administration. In both models, our results are consistent with a critical mediating role for neurosteroids in α2 mRNA expression. We didn’t utilize approaches to block natural P cycling because it may not reflect the local synthesis or accumulation of neurosteroids within the brain (Reddy, 2010). In contrast to α4-subunit plasticity, the expression of α2-subunit remains unaffected with neurosteroid withdrawal. These findings are consistent with previous report of increased α2 expression after pregnancy in rats (Brussaard et al., 1999). Neurosteroid withdrawal-related regulation of α4-subunit expression has been extensively studied in cerebellar granule cells (Follesa et al., 2000) and hippocampus neurons (Smith et al., 1998a; Gangisetty and Reddy, 2010; Wu et al., 2013). The potential role of neurosteroids in regulating α2-subunit expression is not well understood. P-related upregulation of α2-subunit was retained in PRKO mice (Fig. 2), suggesting that PRs are not responsible for the P regulation of α2-subunit expression. These findings are consistent with other GABA-A receptor subunits, specifically the PR-independent changes in α4 and δ-subunit expression following neurosteroid withdrawal (Gangisetty and Reddy, 2010; Wu et al., 2013) and ovarian cycle (Maguire and Mody, 2007). Nevertheless, based on the outcomes from finasteride experiments, we conclude that the regulation of α2-subunit plasticity is neurosteroid-dependent.
4. Conclusions
In conclusion, we have found estrous cycle-related, neurosteroid-dependent cyclical alterations in α2-containing GABA-A receptor expression in the hippocampus. Neurosteroid regulation of α2-containing GABA-A receptor expression may represent yet another mechanism for menstrual cycle-induced alterations in neuronal excitability, especially in hormone-sensitive conditions such as catamenial epilepsy and migraine. These mechanisms are also relevant to premenstrual syndrome, post-partum depression and other hormone-related brain conditions.
5. Materials and Methods
5.1. Animals
Female WT and PRKO mice aged 3-4 months were used in the study. The development of the PRKO mouse strain and genotyping procedures have been described previously (Lydon et al., 1995; Reddy et al., 2004; Reddy and Mohan, 2011; Wu et al., 2013; Carver et al., 2014). These mice were maintained on a hybrid C57BL6/129SV background. Basal levels of P in serum were reported to be similar in the WT and PRKO groups (Chappell et al., 1997). The mice were housed in an environmentally-controlled animal facility under a 12 h/12 h light/dark cycle and allowed free access to food and water, except during the experimental sessions. Genotype was confirmed by PCR using mouse tail genomic DNA (Reddy et al., 2005). All procedures were performed in strict compliance with the guidelines of National Institutes of Health Guide for the Care and Use of Laboratory Animals under a protocol approved by the university’s Institutional Animal Care and Use Committee.
5.2. Determination of estrous cycle and plasma progesterone levels
Mice normally exhibit a 6-day ovarian cycle/estrous cycle, which is subdivided into four stages that are associated with a distinct hormonal milieu. The estrous cycle stage was determined by microscopic examination of vaginal smears with eosin staining (Maguire et al., 2005; Wu et al., 2013; Carver et al., 2014). The diestrous stage was characterized by the presence of many leukocytes; the proestrus stage was determined by mostly small, round, nucleated epithelial cells; the estrus stage was confirmed by large, anucleated, cornified, squamous epithelial cells; and the metestrus stage was identified by both leukocytes and epithelial cells. Plasma concentrations of P were confirmed by an immunoassay for estrous and diestrous stages (Wu et al., 2013). In order to simulate the luteal (high P) and follicular (low P) phases, we focused on diestrous (high P) and estrus (low P) stages that exhibit comparable hormonal levels to the human menstrual cycle. For the purposes of this study, the diestrus phase was selected because of its relatively longer duration in mice with high P levels (10±2 ng/ml), which is thought to more robustly modulate the expression levels of GABA-A receptor subunits than the proestrus phase, which is more transient with slightly lower P levels (8.7±2 ng/ml) (Wu et al., 2013).
5.3. Exogenous P treatment protocol
P was administered to animals as described previously (Gangisetty and Reddy, 2010; Carver et al., 2014). Mice were given P (25 mg/kg, s.c.) twice-daily for seven days. In a subgroup, P was given along with finasteride (50 mg/kg, i.p.) to block 5α-reductase activity for inhibiting P conversion to AP and related neurosteroids. P is readily converted to neurosteroids in the brain regions that express neurosteroid synthesizing enzymes (Mellon et al., 2001; Agis-Balboa et al., 2006). The P administration protocol results in a high physiological concentration of AP in plasma that can be significantly reduced after finasteride administration (Gangisetty and Reddy, 2010; Carver et al., 2014). Control mice were administered 15% β-cyclodextrin vehicle with the same frequency for the seven-day injection period. Brain tissue samples were collected from a group of 6–10 mice for each treatment condition.
5.4. TaqMan Real-time PCR assay
The GABA-A receptor subunit mRNA expression was determined by the TaqMan real-time PCR assay from our laboratory as described previously (Gangisetty and Reddy, 2009). Mice were anesthetized with isoflurane, brains were excised rapidly, and the hippocampus was dissected for RNA isolation. The total RNA was extracted from the hippocampus using a Trizol reagent and cDNA was prepared using the Superscript II first-strand cDNA synthesis kit (Invitrogen Inc., Carlsbad, CA). The PCR primers and TaqMan probe specific for GABA-AR α2-subunit and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes were designed using the Primer Express software (Applied Biosystems Inc. Foster City, CA. USA). The primer set and probe for the α2-subunit were composed of the following sequences: forward, 5’-GCT ACG CTT ACA CAA CCT CAG A -3’; reverse, 5’-GAC TGG CCC AGC AAA TCA TAC T-3’; and sequence specific TaqMan probe: 5’-6-FAM-ACC TGG AGC CAT CGG GAG CAA CCT -TAMRA-3’. TaqMan PCR reactions were carried out in an AB 7500 fast real–time system (Applied Biosystems). Real-time PCR was performed with TaqMan Universal PCR Master Mix (Applied Biosystems), which contained AmpliTaq Gold DNA Polymerase, AmpErase, UNG, dNTPs with dUTP, and optimized buffer components. Each sample was run in triplicate design and each 25-μl reaction mixture consisted of 12.5 μl TaqMan Universal PCR master mix, 400 nM primers, and 300 nM TaqMan probe for the target gene as described previously (Gangisetty and Reddy, 2009). The real-time PCR run consisted of first one cycle of 50 °C for 2 min, then one cycle of 95 °C for 10 min, and 50 cycles of 95 °C for 15 sec plus 60 °C for 1 min. The GABA-AR subunit mRNA expression was normalized to GAPDH expression as a percent change in the same samples.
5.5. Drugs and treatments
Stock solutions of finasteride and P (Steraloids Inc., Newport, RI) and other drugs for injection were made in 15% β-cyclodextrin in saline, and additional dilutions were made using sterile saline. Drug solutions were administered by subcutaneous (s.c.) or intraperitoneal (i.p.) injection in a volume equaling 1% of the animal’s body weight. β-Cyclodextrin and other reagents were procured from Sigma-Aldrich unless otherwise noted.
5.6. Data analysis
Group data were expressed as mean ± S.E.M. The α2-subunit mRNA expression was analyzed based on the relative quantification approach as described previously (Gangisetty and Reddy, 2010). Statistical comparisons of the percent change in α2-subunit expression were performed with the non-parametric Wilcoxon signed ranks test. Differences were considered statistically significant at p< 0.05. A general caveat of using percent-change or fold-change is that genes with low basal expression show large fold changes in response to a small absolute change. Thus, a fold change may be large but biological impact may be minuscule. However, the α2-subunit is abundantly expressed in the brain and changes in its expression are associated with functional changes in GABA currents (Brussard et al., 1997; 1999; Follesa et al., 2000; Wu et al., 2013).
HIGHLIGHTS.
Neuronal GABA-A receptors are the major targets for neurosteroids in the brain.
However, the GABA-A receptor α2-subunit plasticity remains unclear.
We show that high P (diestrous) stage and P treatment upregulates α2-mRNA via a PR-independent neurosteroid mechanism.
These findings are relevant to menstrual-cycle related brain conditions.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Neurologic Disorders and Stroke Grant R01 NS051398 (to D.S.R.).
Footnotes
Conflicts of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Nat Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom T, Zetterlund B, Blom S, Romano M. Effects of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand. 1984;69:240–248. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA-A receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Biggio G, Follesa P, Sanna E, Purdy RH, Concas A. GABA-A receptor plasticity during long-term exposure to and withdrawal from progesterone. Int Rev Neurobiol. 2001;46:207–241. doi: 10.1016/s0074-7742(01)46064-8. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–106. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol. 1999;516(Pt 2):513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABA-A receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA-A receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Lee JO, Bridges RS. Alterations in GABA-A receptor α2 subunit mRNA expression following reproductive experience in rats. Neuroendocrinology. 2007;85:148–156. doi: 10.1159/000102535. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Wu X, Gangisetty O, Reddy DS. Perimenstrual-like hormonal regulation of extrasynaptic delta-containing GABA-A receptors mediating tonic inhibition and neurosteroid sensitivity. J Neurosci. 2014;34(1):4181–14197. doi: 10.1523/JNEUROSCI.0596-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid structure-activity relationships for functional activation of extrasynaptic δGABA-A receptors in the hippocampus. J Pharmacol Exp Therap. 2016;357:188–204. doi: 10.1124/jpet.115.229302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PE, Lydon JP, Conneely OM, O’Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Durkin EJ, Lumb MJ, Stanford SC, Moss SJ, Smart TG. Definign the role of the GABA-A alpha-2 subunit in cellular and behavioral respones to neurosteroids. Soc Neurosci. 2011 Abstr # 42.12/G1. [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA-A receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABA-A receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Frye CA, Manjarrez J, Camacho-Arroyo I. Infusion of 3α,5α-THP to the pontine reticular formation attenuates PTZ-induced seizures. Brain Res. 2000;881:98–102. doi: 10.1016/s0006-8993(00)02897-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Walf A, Harney J. Progesterone reduces pentylenetetrazol-induced ictal activity of wild-type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;43(Suppl 5):14–17. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. The optimization of TaqMan real-time RT-PCR assay for transcriptional profiling of GABA-A receptor subunit plasticity. J Neurosci Methods. 2009;181:58–66. doi: 10.1016/j.jneumeth.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–1918. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Smithson SD, Kalayjian LA, Heck CN, Sperling MR, Liporace JD, Harden CL, Dworetzky BA, Pennell PB, Massaro JM. Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurology. 2012;78:1959–1966. doi: 10.1212/WNL.0b013e318259e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABA-A receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABA-A receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABA-A receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20:5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA-A receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA-A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA-A receptor plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–13. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the delta subunit of the GABA-A receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99:321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- Pierson RC, Lyons AM, Greenfield LJ., Jr Gonadal steroids regulate GABA-A receptor subunit mRNA expression in NT2-N neurons. Brain Res Mol Brain Res. 2005;138:105–115. doi: 10.1016/j.molbrainres.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. The clinical potentials of endogenous neurosteroids. Drugs Today. 2002;38:465–485. doi: 10.1358/dot.2002.38.7.820115. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3alpha-androstanediol. Neuroreport. 2004;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Progr Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Horm Behav. 2013;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Catamenial epilepsy: discovery of an extrasynaptic molecular mechanism for targeted therapy. Front Cell Neurosci. 2016;10(101):1–14. doi: 10.3389/fncel.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes W. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol Sci. 2016;37(7):543–561. doi: 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59:573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. A Mouse Kindling Model of Perimenstrual Catamenial Epilepsy. J Pharmacol Exp Therap. 2012;341:784–793. doi: 10.1124/jpet.112.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacol Biochem Behav. 1999;62:53–60. doi: 10.1016/s0091-3057(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Molla J, Gong QH, Kuver A, Aoki C, Smith SS. Neurosteroid effects at α4βδ GABA-A receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse. Brain Res. 2014;1621:170–186. doi: 10.1016/j.brainres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49(5):573–86. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA-A receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABA-A-gated current and increases the GABA-A receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABA-A receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orru M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of alpha2-containing GABA-A receptors. Behav Brain Res. 2011;217:77–80. doi: 10.1016/j.bbr.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic delta-containing GABA-A receptor plasticity and tonic inhibition in the hippocampus subfields. J Pharmacol Exp Therap. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, De Blas AL, Luscher B, Chen G. GABA-A receptor α-subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem. 2012;287(2):7417–27430. doi: 10.1074/jbc.M112.360461. [DOI] [PMC free article] [PubMed] [Google Scholar]


