Abstract
The incidence rate for scarlet fever in South Korea is rising. During 2008–2015, we collected group A Streptococcus isolates and performed emm and exotoxin genotyping and disk-diffusion antimicrobial tests. Scarlet fever in South Korea was most closely associated with emm types emm4, emm28, emm1, and emm3. In 2015, tetracycline resistance started increasing.
Keywords: scarlet fever, emm, exotoxin, disk diffusion antimicrobial tests, South Korea, group A strep, bacteria, streptococci, virulence factors, antimicrobial resistance, Streptococcus pyogenes
Scarlet fever is a common disease caused by group A Streptococcus (GAS; also known as Streptococcus pyogenes). In the Far East and the United Kingdom, the incidence of scarlet fever has been increasing since 2008 (1–3), and according to the Infectious Disease Statistics System of Korea, the incidence rate for scarlet fever in South Korea increased from 0.3 cases/100,000 persons in 2008 to 13.7 cases/100,000 persons in 2015 (https://is.cdc.go.kr/dstat/index.jsp).
Several antimicrobial drugs, including β-lactams and tetracyclines, effectively treat scarlet fever, and macrolides and lincosamides can be used in patients with penicillin (β-lactam) allergy (4,5). However, resistance to erythromycin and clindamycin has been reported for GAS isolates in mainland China and Hong Kong, China (2,3). The streptococcal M protein and exotoxins are 2 of several virulence factors in GAS (6). The streptococcal M protein is a long fimbrial adhesion protein encoded by >220 M protein gene sequence types (emm types). Because of the high genetic variability of emm, which varies by geographic region, molecular emm genotyping is mandatory for epidemiologic investigations of GAS infections (7). The incidence of these infections is closely related to variations in the predominance of certain emm types (7). speA and speC, which are 2 of 11 genes encoding for superantigens found in GAS, are often associated with scarlet fever (8). Our objective was to identify the overall trend in the annual incidence and characteristics of scarlet fever in South Korea by studying its upsurge in Gwangju, South Korea, because incidence during the past 8 years was highest for this city (https://is.cdc.go.kr/dstat/index.jsp).
The Study
The incidence of scarlet fever in the Gwangju metropolitan area is the highest among all South Korea cities (61.5 cases/100,000 persons); according to the Korean Disease Web Statistics System, the national incidence from 2008 through 2015 was 36.9 cases/100,000 persons (https://is.cdc.go.kr/dstat/index.jsp). Incidence of scarlet fever in South Korea began to increase in 2011 (Figure 1, panel A), coinciding with an outbreak of scarlet fever in China and Hong Kong. Scarlet fever mainly occurs during the late fall, winter, and early spring. Our study results indicate that the incidence of scarlet fever in South Korea peaks in the winter; however, it also peaked in the summers of 2011 and 2015 (Figure 1, panel B).
Figure 1.
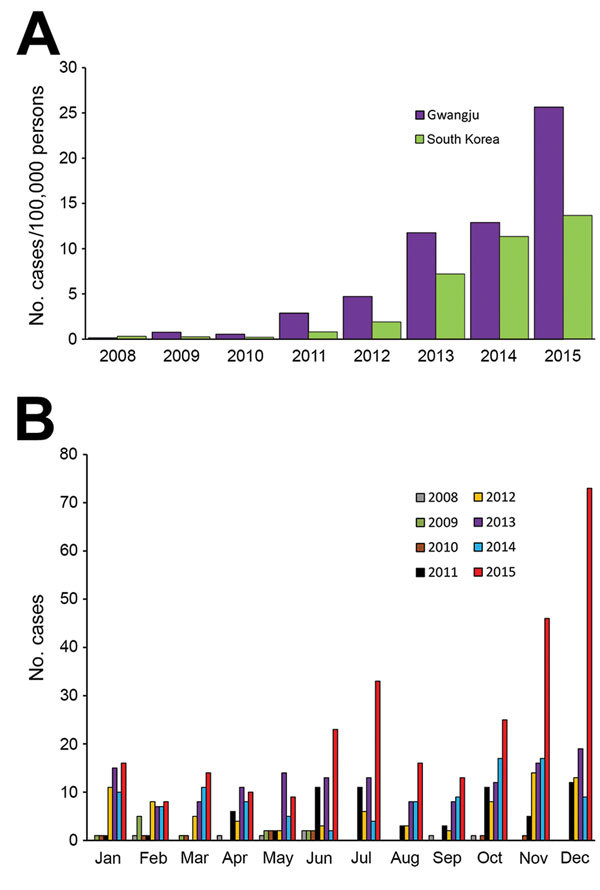
Incidence of scarlet fever in Gwangju, South Korea, 2008–2015. A) The number of cases per 100,000 persons in Gwangju and South Korea. B) Distribution of cases by month of each year.
During 2008–2015, we collected 1,460 pharyngeal swab samples from patients suspected of having scarlet fever from 8 major hospitals in the Gwangju metropolitan area. We tested the β-hemolytic isolates for susceptibility to bacitracin (0.04 U) and for streptococcal grouping; a total of 705 samples were positive for GAS.
Because variation in the circulating emm types could contribute to the incidence of disease and changes in the epidemiology of scarlet fever, we determined the emm sequence type for the 705 samples by using a standard protocol (https://www.cdc.gov/streplab/protocol-emm-type.html) (2). A total of 11 different emm sequence types were identified (Figure 2, panels A and B). emm4 (35.6%, 251/705) was the most predominant. The other 3 predominant emm types were emm28 (14.8%, 104/705), emm1 (14.5%, 102/705), and emm3 (11.6%, 82/705), and these 4 emm types accounted for ≈76.5% (539/705) of all isolates. These results differed from those of previous studies in mainland China and Hong Kong, where the outbreaks of scarlet fever were caused mainly by emm12 (2,3). Our results showed that emm3 in 2011 (the year incidence began increasing in South Korea) and emm1 and emm28 in 2015 (the year of a sharp increase in incidence) played major roles in the epidemics in the Gwangju metropolitan area (Figure 2, panel A). To our knowledge, emm3 has not been reported to be prevalent in other Asian countries but has been associated with scarlet fever in the United Kingdom (9).
Figure 2.
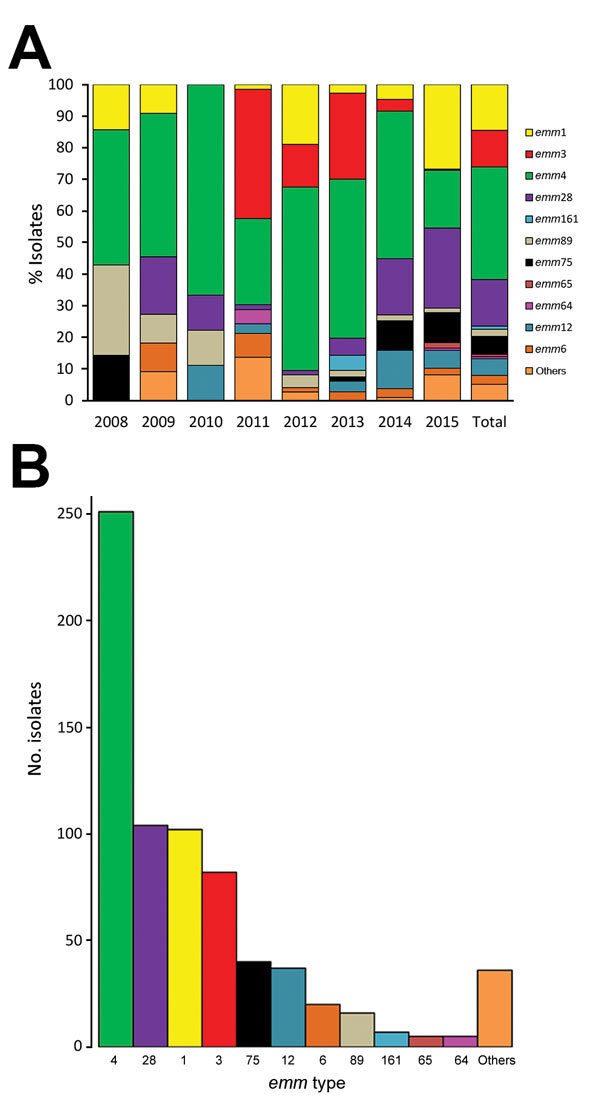
emm type characterization of group A Streptococcus isolates from patients with scarlet fever, Gwangju, South Korea, 2008–2015. A) Annual fluctuations of emm types. Number of isolates by year: 7 in 2008, 11 in 2009, 9 in 2010, 66 in 2011, 74 in 2012, 147 in 2013, 107 in 2014, and 284 in 2015. B) Total number of isolates by emm type. Others refers to rarely found emm types (emm11, emm13, emm17, emm23, emm26, emm30, emm31, emm43, emm49, emm59, emm81, emm82, emm87, emm101, emm107, emm131, emm135, emm161, emm163, emm174, emm183, emm196, emm203, emm204, emm227, emm236, and emm241).
Scarlet fever is a toxin-mediated disease (10). Therefore, we tested all isolates for the presence of the speA and speC genes by using PCR and previously reported primer pairs and reaction conditions (8). We found isolates that harbored speA (57/705, 8.1%), speC (249/705, 35.3%), and both (3/705, 0.4%). The exotoxin gene detection rate differed by emm gene type. The isolates positive for speA were predominantly emm1 (56.1%, 32/57) and emm28 (28.1%, 16/57); the other emm types made up only 15.8% (9/57). When we examined the reverse association, 31.4% (32/102) of emm1 isolates and 15.4% (16/104) of emm28 isolates were positive for speA; in contrast, only 1.8% (9/499) of the other emm types were positive for speA. The main emm types identified in the speC-positive isolates were emm4 (68.7%, 171/249), emm75 (10.8%, 27/249), and emm28 (6.4%, 16/249); 68.1% (171/251) of emm4, 67.5% (27/40) of emm75, and 15.4% (16/104) of emm28 isolates were positive for speC. Only 11.3% (35/310) of the other emm types were positive for speC. Therefore, speA and speC exotoxin genes were more prevalent in bacteria of certain emm types (p<0.01).
GAS remains universally susceptible to β-lactams and glycopeptides. However, the rates of resistance against the macrolides and lincosamides used in penicillin-allergic patients have increased (11). We performed susceptibility tests by using the disk-diffusion method as recommended by the Clinical and Laboratory Standards Institute (12). For all samples collected 2008–2015, the antimicrobial agents chloramphenicol, tetracycline, erythromycin, and clindamycin were tested. Resistance to antimicrobial drugs was detected in 9.1% (64/705) of isolates: 0.3% of the isolates (2/705) showed resistance to chloramphenicol, 7.0% (49/705) to tetracycline, 3.0% (21/705) to erythromycin, and 2.8% (20/705) to clindamycin (Table).
Table. Characterization of antimicrobial drug resistance according to emm types in Gwangju, South Korea, 2008–2015*.
| Year, antimicrobial drug |
emm type |
|||||
|---|---|---|---|---|---|---|
| emm1 | emm4 | emm12 | emm28 | Others | Total | |
| 2008 | ||||||
| Tetracycline |
– |
1† |
– |
– |
– |
1† |
| 2009 | ||||||
| Erythromycin | – | – | – | 2 | – | 2 |
| Clindamycin | – | – | – | 2 | – | 2 |
| Tetracycline |
– |
– |
– |
1 |
– |
1 |
| 2010 | ||||||
| Erythromycin | – | – | – | 1 | – | 1 |
| Clindamycin |
– |
– |
– |
1 |
– |
1 |
| 2011 | ||||||
| Erythromycin | – | 1 | – | 1 | 1† | 1/3† |
| Clindamycin | – | 1 | – | 1 | – | 2 |
| Tetracycline |
– |
– |
– |
1 |
– |
1 |
| 2012 | ||||||
| Erythromycin | – | – | – | 1 | – | 1 |
| Clindamycin | – | 1 | – | 1 | – | 2 |
| Tetracycline |
– |
1 |
– |
– |
– |
1 |
| 2013 | ||||||
| Chloramphenicol | 1 | – | – | – | – | 1 |
| Erythromycin | 1 | – | – | 2 | 1† | 1/4† |
| Clindamycin | – | 1 | – | 2 | – | 3 |
| Tetracycline |
– |
1 |
– |
1 |
– |
2 |
| 2014 | ||||||
| Chloramphenicol | – | – | 1† | – | – | 1† |
| Erythromycin | – | – | – | 4 | – | 4 |
| Clindamycin | – | – | – | 4 | – | 4 |
| Tetracycline |
– |
1† |
– |
4 |
– |
1/5† |
| 2015 | ||||||
| Erythromycin | – | – | 1 | 5 | – | 6 |
| Clindamycin | – | – | 1 | 5 | – | 6 |
| Tetracycline |
22/23† |
– |
1 |
2/7† |
5/7† |
29/38† |
| Isolates, % (no./total) | 24.5% (25/102) | 3.2% (8/251) | 10.8% (4/37) | 17.3% (18/104)‡ | 4.3% (9/211) | 9.1% (64/705) |
| p value | <0.01 | <0.01 | <0.01 | |||
*Dashes indicate no isolates were drug resistant. †Intermediate resistance. With fractions, the numerator indicates the number of isolates with intermediate resistance, and the denominator indicates the total number of resistant isolates. ‡Numbers in column do not add up to 18 (the number of isolates) because of multidrug resistance.
In some isolates, antimicrobial drug resistance is tightly correlated with specific emm types (13). Resistance to erythromycin, clindamycin, and tetracycline is common in bacteria with the emm28 gene. In our study, 18/104 (17.3%) isolates that harbored emm28 were resistant to erythromycin, clindamycin, or tetracycline (p<0.01). Of all emm types, emm28 accounted for 71.0% (44/62) of all cases of resistance to these 3 antimicrobial drugs. In our study, 16/44 isolates harboring emm28 showed resistance to >2 antimicrobials. emm28 isolates in France were also found to be associated with multidrug resistance (13,14). Furthermore, in 2015, we found sharp increases in intermediate tetracycline resistance mainly in isolates harboring emm1 (57.9%, 22/38). Tetracycline resistance associated with emm12 and emm1 isolates was also found in scarlet fever patients in Hong Kong and China (3,15).
This study has a limitation. We collected samples from only 1 city in South Korea, the Gwangju metropolitan area. Because of the genetic diversity of GAS, our results should not be applied to other countries, even those nearby. However, we do believe that our data are representative of South Korea.
Conclusions
In 2011, rapid increases in the incidence of scarlet fever in South Korea, as well as China and Hong Kong, reflected the beginning of a pandemic in Asia. However, the emm types contributing to disease differed from country to country. emm4, emm28, emm1, and emm3 were the most common emm types associated with scarlet fever in South Korea. Antimicrobial drug resistance in GAS in South Korea is closely associated with emm28, and resistance to tetracycline (observed emerging in 2015) is associated with type emm1. However, further studies are necessary to characterize the circulating strains and to control and prevent the further spread of scarlet fever.
Acknowledgments
This study was supported by the infectious disease surveillance project from the Health and Environment Research Institute of Gwangju and the Korea National Institute of Health, South Korea.
Biography
Mr. Park works as a researcher in the Health and Environment Research Institute of Gwangju, Gwangju, South Korea. He is a doctoral candidate studying medicine at Chonnam National University Medical School, Gwangju, South Korea. His primary research interests are human infectious diseases, especially respiratory diseases.
Footnotes
Suggested citation for this article: Park DW, Kim S, Park JW, Kim M-J, Cho SJ, Park HJ, et al. Incidence and characteristics of scarlet fever, South Korea, 2008–2015. Emerg Infect Dis. 2017 Apr [date cited]. http://dx.doi.org/10.3201/eid2304.160773
These authors contributed equally to this article.
References
- 1.Guy R, Williams C, Irvine N, Reynolds A, Coelho J, Saliba V, et al. Increase in scarlet fever notifications in the United Kingdom, 2013/2014. Euro Surveill. 2014;19:20749. 10.2807/1560-7917.ES2014.19.12.20749 [DOI] [PubMed] [Google Scholar]
- 2.Lau EH, Nishiura H, Cowling BJ, Ip DKM, Wu JT. Scarlet fever outbreak, Hong Kong, 2011. Emerg Infect Dis. 2012;18:1700–2. 10.3201/eid1810.120062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang P, Peng X, Zhang D, Wu S, Liu Y, Cui S, et al. Characteristics of group A Streptococcus strains circulating during scarlet fever epidemic, Beijing, China, 2011. Emerg Infect Dis. 2013;19:909–15. 10.3201/eid1906.121020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. 10.1128/CMR.13.3.470-511.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JH, Yang NR, Lee WJ, Lee H, Choi EH, Lee HJ. Distribution of emm types among group A Streptococcus isolates from children in Korea. Diagn Microbiol Infect Dis. 2015;82:26–31. 10.1016/j.diagmicrobio.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3:191–200. 10.1016/S1473-3099(03)00576-0 [DOI] [PubMed] [Google Scholar]
- 7.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611–6. 10.1016/S1473-3099(09)70178-1 [DOI] [PubMed] [Google Scholar]
- 8.Yamaoka J, Nakamura E, Takeda Y, Imamura S, Minato N. Mutational analysis of superantigen activity responsible for the induction of skin erythema by streptococcal pyrogenic exotoxin C. Infect Immun. 1998;66:5020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner CE, Pyzio M, Song B, Lamagni T, Meltzer M, Chow JY, et al. Scarlet fever upsurge in England and molecular-genetic analysis in North-West London, 2014. Emerg Infect Dis. 2016;22:1075–8. 10.3201/eid2206.151726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva-Costa C, Carriço JA, Ramirez M, Melo-Cristino J. Scarlet fever is caused by a limited number of Streptococcus pyogenes lineages and is associated with the exotoxin genes ssa, speA and speC. Pediatr Infect Dis J. 2014;33:306–10. 10.1097/INF.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 11.Plainvert C, Doloy A, Loubinoux J, Lepoutre A, Collobert G, Touak G, et al. ; CNR-Strep network. Invasive group A streptococcal infections in adults, France (2006-2010). Clin Microbiol Infect. 2012;18:702–10. 10.1111/j.1469-0691.2011.03624.x [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement (M100-S25). Wayne (PA): The Institute; 2015. [Google Scholar]
- 13.Metzgar D, Zampolli A. The M protein of group A Streptococcus is a key virulence factor and a clinically relevant strain identification marker. Virulence. 2011;2:402–12. 10.4161/viru.2.5.16342 [DOI] [PubMed] [Google Scholar]
- 14.Mihaila-Amrouche L, Bouvet A, Loubinoux J. Clonal spread of emm type 28 isolates of Streptococcus pyogenes that are multiresistant to antibiotics. J Clin Microbiol. 2004;42:3844–6. 10.1128/JCM.42.8.3844-3846.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies MR, Holden MT, Coupland P, Chen JH, Venturini C, Barnett TC, et al. Emergence of scarlet fever Streptococcus pyogenes emm12 clones in Hong Kong is associated with toxin acquisition and multidrug resistance. Nat Genet. 2015;47:84–7. 10.1038/ng.3147 [DOI] [PubMed] [Google Scholar]


