Abstract
Purpose: To identify and describe the transgender population in the Medicare program using administrative data.
Methods: Using a combination of International Classification of Diseases ninth edition (ICD-9) codes relating to transsexualism and gender identity disorder, we analyzed 100% of the 2013 Centers for Medicare & Medicaid Services (CMS) Medicare Fee-For-Service (FFS) “final action” claims from both institutional and noninstitutional providers (∼1 billion claims) to identify individuals who may be transgender Medicare beneficiaries. To confirm, we developed and applied a multistage validation process.
Results: Four thousand ninety-eight transgender beneficiaries were identified, of which ∼90% had confirmatory diagnoses, billing codes, or evidence of a hormone prescription. In general, the racial, ethnic, and geographic distribution of the Medicare transgender population tends to reflect the broader Medicare population. However, age, original entitlement status, and disease burden of the transgender population appear substantially different.
Conclusions: Using a variety of claims information, ranging from claims history to additional diagnoses, billing modifiers, and hormone prescriptions, we demonstrate that administrative data provide a valuable resource for identifying a lower bound of the Medicare transgender population. In addition, we provide a baseline description of the diversity and disease burden of the population and a framework for future research.
Keywords: : administrative data, disease burden, intersectionality, Medicare, transgender
Introduction
Despite increased awareness and greater societal acceptance of people who are transgender, the inability to systematically identify and study the transgender population greatly hampers our capacity to conduct meaningful analysis of this group. Minimal representative national data exist,1 studies attempting to estimate the size and health needs of the transgender population have generally relied on nonprobability survey samples,2 and analyses utilizing more robust research designs have largely focused on single states.3 Furthermore, population-based studies of transgender individuals entitled to Medicare due to age (65 and older), disability, or end-stage renal disease are nonexistent, demonstrating the need for more and better research focused on sexual and gender minorities, including the transgender population. Toward this end, recent research conducted at the Department of Veterans Affairs suggests the potential utility of using healthcare administrative data to identify persons who are transgender.4 Expanding on this work, we explore the use of Medicare billing data from the Centers for Medicare & Medicaid Services (CMS), the federal agency that administers the Medicare and Medicaid programs, to identify and describe Medicare's transgender population.
The transgender population includes individuals whose gender identity, gender expression, or gender behavior does not typically conform to the sex they were assigned at birth.5 This community experiences a particularly high disease burden, including significantly higher rates of substance abuse,6–8 HIV/AIDS,9–11 and mental illness.10,12,13 Discrimination in the healthcare setting only exacerbates these adverse health outcomes. Twenty-eight percent of transgender persons report postponing medical care when sick due to discrimination, 19% report that doctors have refused to provide them care because of their transgender status, 28% report facing harassment in the medical setting, 2% report facing violence in a doctor's office, and >50% report that they had to teach their doctor about transgender healthcare.2 Taken together, transgender persons experience suboptimal health outcomes across a variety of areas while systematically lacking access to the institutions that have the ability to address these medical needs.
Even when transgender persons are able to receive care, insurers routinely deny treatment related to medical transitions. Transitioning is the process of living as the gender with which a transgender person identifies, rather than the gender assigned to them at birth.2 Medical transitions include any type of transgender-related surgery, such as sex-reassignment surgery or cosmetic procedures, and hormone therapy, such as taking prescriptions for cross-sex hormones. Medical transitions are particularly relevant for the Medicare program, which covers certain aspects of these medical treatments and includes this information in Medicare claims data.
Until 2015, providers treating patients enrolled in Medicare used the International Classification of Diseases ninth edition (ICD-9) to indicate a patient's specific medical diagnoses when submitting medical claims to CMS. ICD-9 contains multiple diagnosis codes that are transgender specific, including the following codes14: 302.50 (Transsexualism with unspecified sexual history), 302.51 (Transsexualism with asexual history), 302.52 (Transsexualism with homosexual history), 302.53 (Transsexualism with heterosexual history), 302.6 (Gender Identity Disorder [GID] in children), and 302.85 (GID in adolescents or adults).
CMS also advises providers to utilize two billing modifiers that apply to the transgender population, including the condition code 45 modifier and the KX modifier. Medicare billing modifiers are two-digit codes appended to procedure codes or Healthcare Common Procedure Coding System (HCPCS) codes that provide additional information about the billed procedures.15,16 Providers use billing modifiers to avoid rejection of claims with a gender/procedure conflict. For example, the CMS system will reject a claim where a physician provided a female pelvic examination for a male beneficiary, as female pelvic examinations are considered sex specific (i.e., only for females). Because transgender beneficiaries may have changed their sex on record, they are at a high risk for experiencing gender/procedure conflicts. In this instance, a transman (female to male transition) may have his claim for a medically necessary pelvic examination rejected inappropriately. Therefore, condition code 45 and the KX modifier are used to process claims with gender-specific editing that CMS would normally reject due to gender/procedure mismatches. A list of the gender-specific procedure codes related to condition code 45 and the KX modifier is included in Appendix Table 1.
Similar to diagnosis codes and billing modifiers, CMS also maintains a record of each Medicare beneficiary's prescriptions. The Medicare Part D prescription drug plan covers medically necessary hormones for transgender persons, such as cross-sex hormones. Records of these prescriptions are available in CMS's administrative files and include the generic and brand names of prescription drugs, as well as details about the prescription. A list of hormone therapy-related prescription drugs is included in Appendix Table 2.
As a result, it may be possible to identify transgender Medicare beneficiaries using one or a combination of these diagnosis codes, billing modifiers, and prescription drug events.
Methods
Utilizing the CMS Chronic Conditions Data Warehouse (CCW), which contains CMS data on Medicare and Medicaid beneficiaries and their claims, we analyzed 100% of the CMS Fee-For-Service (FFS) final action claims from both institutional and noninstitutional providers for calendar year 2013. These claims included inpatient and outpatient hospital claims, carrier claims (e.g., physicians, physician assistants, nurse practitioners), and claims from skilled nursing facilities, home health agencies, hospice care, and those relating to durable medical equipment. In total, this covered ∼1 billion claims.
In the first component of the analysis, we searched each claim for any occurrence in any position of diagnosis codes 302.50, 302.51, 302.52, 302.53, 302.6, or 302.85. Once we identified the universe of claims meeting our criteria, we used the unique Medicare beneficiary identifier present on each claim to identify unique observations. Following this identification process, we used the unique beneficiary identifier to link to the Medicare Enrollment and Medicare Part D Data in the CCW.
Because administrative records contain a degree of error and the billing modifiers are not unique to the transgender community, the data potentially contain a high probability of producing false positives, in which nontransgender beneficiaries are identified as transgender. To address this concern, we developed a supplementary method for validating the initial classification. The first validation step analyzes the repeated application of ICD-9 codes 302.50, 302.51, 302.52, 302.53, 302.6, and/or 302.85, with persons receiving more than one diagnosis in 2013 having a validated classification. The second and third validation steps analyze the relevant ICD-9 codes over time. If the beneficiary had one or more of these diagnoses in the preceding year (2012) or subsequent year (2014), indicating an ongoing trend of receiving the diagnosis, the classification was validated. The fourth validation step incorporated data on ICD-9 code 259.9 (Unspecified Endocrine Disorder), which is frequently used by the transgender community to combat the perceived stigma of a GID diagnosis. If a beneficiary received at least one diagnosis from the transgender-specific ICD-9 codes and also received a diagnosis of 259.9, the classification was validated. The fifth validation step incorporated prescriptions for sex hormones, with persons receiving a transgender-specific diagnosis code and a prescription for a sex hormone representing a validated classification. The sixth validation step examined the principal diagnosis code and, if the principal diagnosis code was from a transgender-specific ICD-9 code, that observation was validated. Finally, the seventh and eighth validation steps incorporated the billing claims modifiers to validate classifications. If a beneficiary received a relevant ICD-9 code and had at least one claim containing the condition code 45 modifier or the KX modifier, the classification was validated.
Given the limitations of using ICD-9 259.9, sex hormones, and claim modifiers to identify transgender Medicare beneficiaries, these aspects of medically transitioning were only included as validation steps, rather than unique identifiers. Although this conservative approach restricts the size of the cohort, it is the only mechanism for guaranteeing that nontransgender persons are not misclassified as transgender. To demonstrate, there were over 5000 Medicare beneficiaries in 2013 with a claim containing the KX modifier or condition code 45 and a gender/procedure conflict, with only 3.90% of these beneficiaries receiving a transgender-specific ICD-9 diagnosis code. Given our limited ability to determine if the remaining 96.10% of these beneficiaries are transgender or not, we recommend that researchers avoid utilizing these modifiers alone and incorporate additional data, such as ICD-9 codes, to classify beneficiaries as transgender.
Results
Enumerating Medicare's transgender population
Using this methodology, we identified 4098 persons as transgender Medicare beneficiaries. Table 1 demonstrates these findings along with results from the validation logic. This classification method was highly accurate, with 89.26%, or 3658 persons, having enough information in their claims history to validate their classification as transgender. This demonstrates that researchers interested in studying Medicare's transgender population can identify a meaningfully large and accurate population using ICD-9 codes in conjunction with supplementary claims data. This does not imply that the 10.74% of observations not validated are incorrectly classified or that this method identifies all transgender persons enrolled in Medicare, rather, it provides a conservative estimate (lower bound) of Medicare's transgender population and details a methodology for identifying and validating this population using administrative data. Consequently, these tools provide a replicable foundation for researchers interested in analyzing health outcomes in the transgender community.
Table 1.
Identification and Validation Logic
| Transgender Medicare Beneficiaries | |||
|---|---|---|---|
| No. identified | No. validated | % validated | |
| ICD-9 diagnosis codes 302.50, 302.51, 302.52, 302.53, 302.6, 302.85a | 4098 | 3658 | 89.26 |
| Validation method | |||
| ICD-9 302 series diagnosis code and 1 or more of the following: | |||
| No. validated | % validated | |
|---|---|---|
| More than 1 claim with an ICD-9 302 series diagnosis code in 2013 | 2706 | 66.03 |
| 1 or more claims with an ICD-9 302 series diagnosis code in 2012 | 1577 | 38.48 |
| 1 or more claims with an ICD-9 302 series diagnosis code in 2014 | 1937 | 47.26 |
| 1 or more claims with an ICD-9 259.9 diagnosis code in 2013 | 568 | 13.86 |
| 1 or more prescriptions for a sex hormone in 2013 | 2005 | 48.89 |
| Principal diagnosis code is from ICD-9 302 series | 1736 | 42.36 |
| 1 or more CC 45 modifier | 167 | 4.08 |
| 1 or more KX modifier | 26 | 0.6 |
Each validation step is calculated independently from all other validation steps, and “% validated” is calculated from the total number of transgender beneficiaries identified (N=4098).
Referred to as the 302 series for the purposes of this table.
ICD-9, International Classification of Diseases ninth edition.
Table 1 also demonstrates the validation results in greater detail. For individuals identified using only ICD-9 codes, the majority of beneficiaries (66.03%) had more than one claim with a transgender-specific ICD-9 code within the calendar year. Other validation methods, such as using claims from bordering calendar years and hormone prescriptions, had very similar results. Approximately, forty percent of the beneficiaries identified by transgender-specific ICD-9 codes had similar claims in 2012, 2014, filled a prescription for a sex hormone in 2013, or received a transgender-specific principal diagnosis code. A considerably smaller number of transgender beneficiaries had claims with ICD-9 code 259.9 or billing modifiers, although these validation methods did validate >700 observations. In total, the results indicate that our validation methodology supplements the initial classifications by incorporating additional detail and analyzing the validity of using administrative data to identify the transgender population.
Demographic variability in Medicare's transgender population
Using this foundation to identify transgender Medicare beneficiaries, analyzing their demographic characteristics also helps describe this population. Results demonstrate that Medicare's transgender population is racially and ethnically diverse, spans the entire United States, and experiences many chronic conditions. Analyses reported here utilize the entire cohort of 4098 individuals identified as transgender (3658 identified and validated through administrative data and 440 identified but not validated through administrative data). We conducted separate analyses (not shown), which excluded the 440 individuals for whom we have no additional claims-based validation information. However, there were no systematic or substantive differences in the results. Therefore, we report results on the entire cohort.
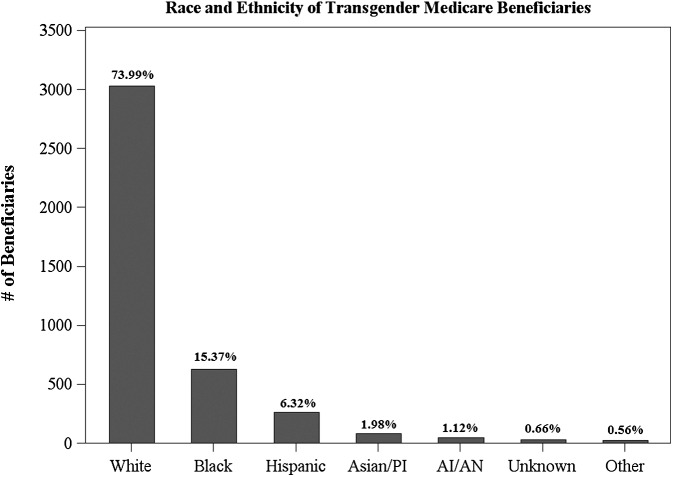
Beginning with race, the data demonstrate that the transgender Medicare population contains members from all racial and ethnic groups.‡ This population is racially and ethnically diverse, with substantial representation among Whites, Blacks/African Americans, and Hispanics. Figure 1 displays the distribution of racial and ethnic identity within the transgender population. In this population of transgender persons, Whites comprise 73.99% of the total population, Blacks/African Americans comprise the next largest group, representing 15.37% of the transgender Medicare population, and Hispanics, Asians/Pacific Islanders (APIs), American Indians/Alaska Natives (AIANs), Unknowns, and Others comprise relatively smaller proportions of the transgender Medicare population. This analysis of the racial and ethnic diversity of the transgender population is significant, as >85% of studies that examine sexual and gender minorities fail to report data on race.20 This lack of data on the racial and ethnic diversity of transgender persons inhibits our ability to understand the intersectionality of gender identity and racial/ethnic identity, which is expected to have important effects on health outcomes. Because research has consistently identified the prevalence of minority health disparities,21–26 these disparities may disproportionately affect the diverse transgender community. Therefore, understanding how race and ethnicity interact with transgender identity is an important component of studying transgender health and this analysis provides the foundation for future research on this topic.
FIG. 1.
Racial/ethnic identification of transgender beneficiaries.
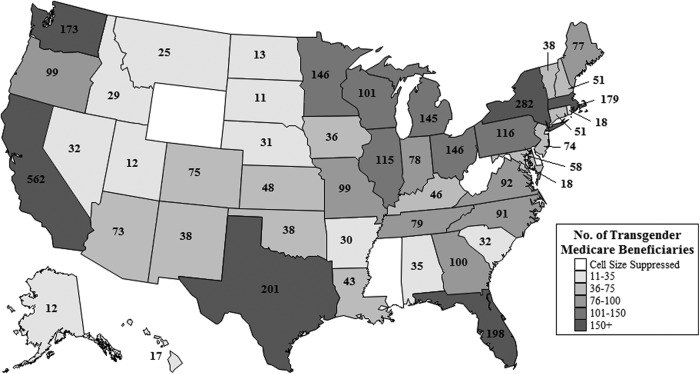
The transgender population enrolled in the Medicare program displays a high level of geographic diversity. Figure 2 demonstrates that transgender Medicare beneficiaries reside in every state, with many states containing large populations. California contains the largest number of transgender Medicare beneficiaries, with 562 beneficiaries. New York (282), Texas (201), Florida (198), Massachusetts (179), Washington (173), Ohio (146), Minnesota (146), Michigan (145), Pennsylvania (116), Illinois (115), Wisconsin (101), and Georgia (100) also contain large populations, with each state containing 100 or more transgender beneficiaries. This is an important finding, as it demonstrates that the transgender population spans the entire United States, making transgender health relevant to local providers across the entire country.
FIG. 2.
Geographic distribution of transgender beneficiaries.
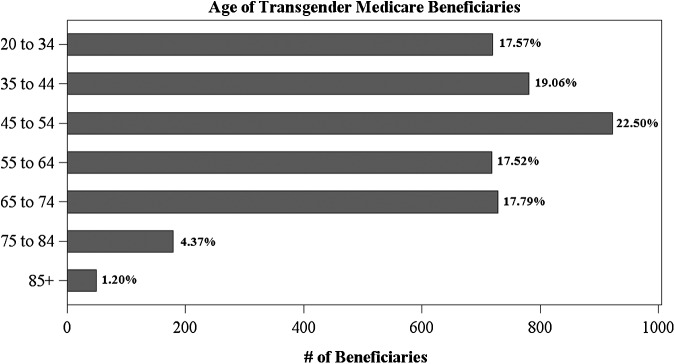
Unlike the racial, ethnic, and geographic distribution of the transgender population, which tends to reflect broader population distributions, the age, original entitlement status, and chronic condition burden of the transgender population appear substantially different. Figure 3 displays the age distribution of the transgender population, showing that the majority of transgender Medicare beneficiaries were under age 65 in 2013 (76.65%). This is a somewhat surprising result, as age is the primary mechanism through which most Americans qualify for Medicare. To demonstrate, 75.55% of the general Medicare population qualified for Medicare through Old Age and Survivors Insurance (OASI), indicating that the majority are age 65 or older. The transgender Medicare population, conversely, primarily qualified for Medicare through Disability Insurance (84.06%), implying that many transgender persons enrolled in the program are disabled. This trend reflects an almost exact reversal of the general population's Medicare eligibility. Thus, the transgender population may be disproportionately disabled relative to the general Medicare population, which suggests an avenue for future research that examines these differences.
FIG. 3.
Age distribution of transgender beneficiaries.
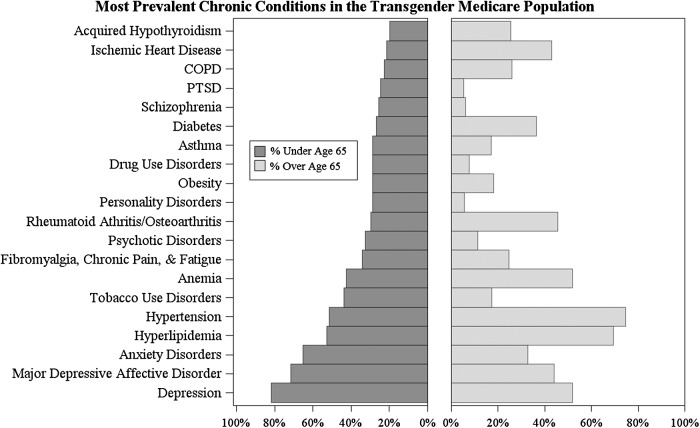
Using CMS's chronic condition categories, which analyze 60 chronic medical conditions and other chronic or potentially disabling conditions, Figure 4 highlights the chronic condition prevalence in the Medicare transgender population, demonstrating the significant burden placed on many beneficiaries. This is particularly relevant for depression, which has affected 81.79% of those under the age of 65. Because three-quarters of the transgender population has been diagnosed with depression at some point during their life, the data suggest that the community disproportionately suffers from depression. Other mental health issues, such as post-traumatic stress disorder, schizophrenia, psychotic disorders, anxiety disorders, and major depressive affective disorders, also affect a large proportion of the population, demonstrating the significant mental health burden facing transgender Medicare enrollees. This echoes findings from previous studies,13 which report that there is a high prevalence of depression in the transgender community and transgender persons are more likely to report depression if they have not begun a medical transition. This finding suggests an opportunity for future research that examines the role that receiving medically necessary treatment may play in reducing depression rates and improving the mental health of transgender beneficiaries.
FIG. 4.
Chronic conditions and the transgender Medicare population.
Hyperlipidemia and hypertension also affect the majority of transgender beneficiaries, with 58.49% of beneficiaries reporting either condition. This is especially relevant for those under the age of 65, with a majority of those in this age category reporting these chronic conditions, even though they are typically associated with advancing age.26,27 This is consistent with previous studies on the transgender population28 and suggests a need for additional research that analyzes the association between medical transitions and hyperlipidemia/hypertension, which appear to affect a statistically high proportion of the transgender population, relative to their age. Other conditions, such as tobacco and drug use disorders, fibromyalgia, and other forms of chronic pain or fatigue, obesity, anemia, rheumatoid arthritis/osteoarthritis, asthma, diabetes, and heart disease, affect more than one-quarter of the population and reflect the broader trend of the transgender community reporting a disproportionately high disease burden. Future research that examines the causes of these high prevalence rates would help inform the treatment of transgender Medicare beneficiaries and explain why these diseases are manifesting in transgender persons at early ages.
Discussion
Using CMS's administrative data, we were able to identify and validate nearly 3700 transgender beneficiaries enrolled in Medicare during the 2013 calendar year. Using a variety of claims information, ranging from claims history to additional diagnoses, billing modifiers, and hormone prescriptions, we demonstrate that administrative data provide a valuable resource for studying the transgender population. ICD-9 codes specific to medical transitions are especially useful, with 90% of those identified using this method being validated. Therefore, ICD-9 codes provide an excellent foundation for future research on the transgender population, and we encourage researchers interested in transgender health and health outcomes to utilize this methodology for future research.
The resulting cohort of transgender Medicare beneficiaries also demonstrates the significant racial, ethnic, and geographic diversity of the population. The results indicate that the transgender population is very diverse, containing members of every racial and ethnic group and residing in every U.S. state. Because fewer than 15% of studies on the health status of lesbian, gay, bisexual, and transgender (LGBT) persons include an analysis of race,20 this examination provides an important contribution to health services research. The geographic distribution of transgender Medicare beneficiaries also provides important implications for transgender-specific care. Given that >50% of transgender persons report having to teach their provider about transgender healthcare,2 these results suggest that providers across the nation should better prepare for providing care to Medicare's transgender population, as there is a high probability that providers may encounter transgender patients. This is particularly relevant, given the lack of LGBT outreach across the country, with few agencies providing LGBT-specific training or outreach.29 Because agencies that provide LGBT-specific services are more likely to address LGBT issues, receive LGBT assistance requests, and understand the unique needs facing the community,29 these results provide support for increasing education and training throughout the provider community. This geographic distribution may also help inform the areas that may benefit most from targeted interventions, such as California, New York, and Texas, which all have large transgender populations.
The data provide particularly valuable insight regarding the burden of chronic conditions in the community, given the incredibly high prevalence of disability and the very high rates of certain conditions. For example, nearly 80% of the transgender community has been diagnosed with depression during their lifetime. Not only does this signal the heightened level of medical need within the community but it also lays the foundation for future research that examines the prevalence and causes of chronic conditions in the transgender community. Future research could compare the chronic condition burden to the burden found in a matched cohort, helping to clarify the role that being transgender plays in affecting health outcomes. By identifying patterns of transgender health disparities, ranging from discrimination and stigma to the potential long-term effects of hormone therapy, health services researchers will be better able to address the care of transgender persons in the medical setting.
Limitations of Medicare's transgender-related data
Although CMS's administrative data contain numerous methods for identifying transgender Medicare beneficiaries, these identification methods are not without limitations. CMS data are limited in their ability to identify all transgender beneficiaries because (1) they only identify transgender persons who are medically transitioning and/or have been diagnosed with GID, (2) their administrative data sets contain unobservable error, and (3) billing modifiers, alternative diagnosis codes, and hormone therapy fail to uniquely identify transgender persons.
Because CMS data are based on medical claims for treatment, they only capture persons who are medically transitioning or who have been diagnosed with GID. Focusing on those who are medically transitioning is problematic, given that only 62% of transgender persons report using hormone therapy.2 Although an additional 23% hope to have hormone therapy in the future, only 62% to 85% of transgender persons want or utilize hormone therapy. Therefore, by focusing on medical transitions, this analysis may underestimate the size of the transgender population. Similarly, using ICD-9 codes related to GID may limit the sample, as GID diagnoses are highly controversial in the transgender community, with many transgender persons avoiding the diagnosis. The primary controversy surrounding the diagnosis is that it is considered a mental disorder, which carries the stigma of mental illness and potentially reinforces the gender binary that treats transgender persons as deviant.30 Because of this, some transgender persons will avoid the GID diagnosis, requesting other nontransgender-specific diagnoses. Among the most commonly used nontransgender-specific diagnosis codes is ICD-9 code 259.9 (Unspecified Endocrine Disorder).31,32 Because transgender Medicare beneficiaries may not medically transition and/or may actively resist the GID diagnosis, using CMS data to identify transgender Medicare beneficiaries is expected to represent a conservative estimate of Medicare's transgender community.
Errors inherent to administrative data also pose a methodological problem to using administrative data to identify transgender Medicare enrollees. Numerous studies document the limitations of using administrative data to identify diseases, given wide variation in coding accuracy across conditions and settings.33 In the Medicare program specifically, a systematic analysis of Medicare claims data compared to medical charts revealed that the percentage of agreement between ICD-9 diagnosis and medical records was, on average, between 73.2% and 78.2%, with accuracy of diagnosis varying substantially across conditions.34 Additional Medicare data validations demonstrate that conditions such as diabetes are highly accurate (100% claims accuracy), while conditions such as alcohol and drug abuse are highly inaccurate (20% claims accuracy).35,36 Therefore, one can assume that using ICD-9 codes to estimate the transgender Medicare population contains a degree of inherent coding error, which may distort the population estimates.
The final limitation of using CMS's administrative data is the inability of billing modifiers to uniquely identify transgender beneficiaries. While the ICD-9 codes are specific to the transgender community, the billing modifiers are not. Because condition code 45 applies to both the transgender and intersex community, classifying all persons with a condition code 45 modifier as transgender may falsely classify intersex persons as transgender. Intersex persons are different from transgender persons, as they are born with a reproductive or sexual anatomy that does not fit typical definitions of male or female,37 making them a distinct subgroup of gender minorities. This measurement problem also affects the KX modifier, which applies to multiple types of claims, rather than only those with a gender/procedure conflict. For example, even though the KX modifier might apply to a claim for a transman receiving a female pelvic examination, it might also apply to a female born and identified beneficiary receiving two pelvic examinations in the same calendar year. Because neither of these modifiers applies solely to the transgender community, they cannot be used as a standalone method for classifying beneficiaries as transgender.
Overall, our results demonstrate that administrative data are a valuable resource for identifying the medically transitioning Medicare transgender population and that using ICD-9 codes and billing modifiers are a valid and replicable method that is relevant to many data systems. Using this method, we have made a number of important contributions to the literature, as there are currently no other studies that use Medicare claims data to identify transgender persons. First, we have developed a framework for identifying transgender persons using administrative data, as well as providing a method for validating these results. By replicating the methods outlined in this analysis, researchers can estimate the size of the transgender population and use this data to further analyze health disparities and outcomes in the transgender community. Second, we have provided a baseline description of the diversity and disease burden of the population, laying the foundation for future research programs that expand on this data and statistically model these relationships. Finally, we have proposed numerous avenues of future work to build upon this analysis, including an examination of the intersection between race and gender identity, an examination of the chronic condition burden of transgender persons relative to a matched cohort, and an examination of the underlying causes of chronic conditions in transgender persons. In conclusion, this analysis helps fill the void regarding research on Medicare's transgender population with the goal of informing and encouraging future research on gender minorities.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Centers for Medicare and Medicaid Services, the U.S. Department of Health and Human Services, or NORC at the University of Chicago.
Abbreviations Used
- AIANs
American Indians/Alaska Natives
- APIs
Asians/Pacific Islanders
- CCW
Chronic Conditions Data Warehouse
- CMS
Centers for Medicare & Medicaid Services
- FFS
Fee-For-Service
- GID
Gender Identity Disorder
- HCPCS
Healthcare Common Procedure Coding System
- ICD-9
International Classification of Diseases ninth edition
- LGBT
lesbian, gay, bisexual, and transgender
- OASI
Old Age and Survivors Insurance
Appendix Table 1.
Gender-Specific Procedure Codes Related to Condition Code 45 and the KX Modifier
| HCPCS | Valid sex | Code description | HCPCS | Valid sex | Code description |
|---|---|---|---|---|---|
| 0071T | Female | U/s leiomyomata ablate <200 | 57545 | Female | Remove cervix/repair pelvis |
| 0072T | Female | U/s leiomyomata ablate >200 | 57550 | Female | Removal of residual cervix |
| 00842 | Female | Anesth amniocentesis | 57555 | Female | Remove cervix/repair vagina |
| 00846 | Female | Anesth hysterectomy | 57556 | Female | Remove cervix/repair bowel |
| 00851 | Female | Anesth tubal ligation | 57558 | Female | D and c of cervical stump |
| 00865 | Male | Anesth removal of prostate | 57700 | Female | Revision of cervix |
| 00906 | Female | Anesth removal of vulva | 57720 | Female | Revision of cervix |
| 00908 | Male | Anesth removal of prostate | 57800 | Female | Dilation of cervical canal |
| 00914 | Male | Anesth removal of prostate | 58100 | Female | Biopsy of uterus lining |
| 00920 | Male | Anesth genitalia surgery | 58110 | Female | Bx done w/colposcopy add‐on |
| 00921 | Male | Anesth vasectomy | 58120 | Female | Dilation and curettage |
| 00922 | Male | Anesth sperm duct surgery | 58140 | Female | Myomectomy abdominal method |
| 00924 | Male | Anesth testis exploration | 58145 | Female | Myomectomy vaginal method |
| 00926 | Male | Anesth removal of testis | 58146 | Female | Myomectomy abdominal complex |
| 00928 | Male | Anesth removal of testis | 58150 | Female | Total hysterectomy |
| 00930 | Male | Anesth testis suspension | 58152 | Female | Total hysterectomy |
| 00932 | Male | Anesth amputation of penis | 58180 | Female | Partial hysterectomy |
| 00934 | Male | Anesth penis nodes removal | 58200 | Female | Extensive hysterectomy |
| 00936 | Male | Anesth penis nodes removal | 58210 | Female | Extensive hysterectomy |
| 00938 | Male | Anesth insert penis device | 58240 | Female | Removal of pelvis contents |
| 00940 | Female | Anesth vaginal procedures | 58260 | Female | Vaginal hysterectomy |
| 00942 | Female | Anesth surgery on vaginal/urethral | 58262 | Female | Vaginal hysterectomy including t/o |
| 00944 | Female | Anesth vaginal hysterectomy | 58263 | Female | Vaginal hysterectomy w/t/o and vaginal repair |
| 00948 | Female | Anesth repair of cervix | 58267 | Female | Vaginal hysterectomy w/urinary repair |
| 00950 | Female | Anesth vaginal endoscopy | 58270 | Female | Vaginal hysterectomy w/enterocele repair |
| 00952 | Female | Anesth hysteroscope/graph | 58275 | Female | Hysterectomy/revise vagina |
| 01960 | Female | Anesth vaginal delivery | 58280 | Female | Hysterectomy/revise vagina |
| 01961 | Female | Anesth cs delivery | 58285 | Female | Extensive hysterectomy |
| 01962 | Female | Anesth emergency hysterectomy | 58290 | Female | Vaginal hysterectomy complex |
| 01963 | Female | Anesth cs hysterectomy | 58291 | Female | Vaginal hysterectomy including t/o complex |
| 01965 | Female | Anesth inc/missed ab procedure | 58292 | Female | Vaginal hysterectomy t/o and repair complex |
| 01966 | Female | Anesth induced ab procedure | 58293 | Female | Vaginal hysterectomy w/uro repair complex |
| 01967 | Female | Anesth/analg vaginal delivery | 58294 | Female | Vaginal hysterectomy w/enterocele complex |
| 01968 | Female | Anes/analg cs deliver add‐on | 58300 | Female | Insert intrauterine device |
| 01969 | Female | Anesth/analg cs hysterectomy add‐on | 58301 | Female | Remove intrauterine device |
| 0336T | Female | Lap ablat uterine fibroids | 58321 | Female | Artificial insemination |
| 0500F | Female | Initial prenatal care visit | 58322 | Female | Artificial insemination |
| 0501F | Female | Prenatal flow sheet | 58323 | Female | Sperm washing |
| 0502F | Female | Subsequent prenatal care | 58340 | Female | Catheter for hysterography |
| 0503F | Female | Postpartum care visit | 58345 | Female | Reopen fallopian tube |
| 11976 | Female | Remove contraceptive capsule | 58346 | Female | Insert heyman uteri capsule |
| 19300 | Male | Removal of breast tissue | 58350 | Female | Reopen fallopian tube |
| 3015F | Female | Cerv cancer screen docd | 58353 | Female | Endometrial ablate thermal |
| 36460 | Female | Transfusion service fetal | 58356 | Female | Endometrial cryoablation |
| 37788 | Male | Revascularization penis | 58400 | Female | Suspension of uterus |
| 46744 | Female | Repair of cloacal anomaly | 58410 | Female | Suspension of uterus |
| 46746 | Female | Repair of cloacal anomaly | 58520 | Female | Repair of ruptured uterus |
| 46748 | Female | Repair of cloacal anomaly | 58540 | Female | Revision of uterus |
| 50722 | Female | Release of ureter | 58541 | Female | Lsh uterus 250 g or less |
| 51845 | Female | Repair bladder neck | 58542 | Female | Lsh w/t/o ut 250 g or less |
| 51920 | Female | Close bladder–uterus fistula | 58544 | Female | Lsh w/t/o uterus above 250 g |
| 51925 | Female | Hysterectomy/bladder repair | 58545 | Female | Laparoscopic myomectomy |
| 52010 | Male | Cystoscopy and duct catheter | 58546 | Female | Laparomyomectomy complex |
| 52270 | Female | Cystoscopy and revise urethra | 58548 | Female | Lap radical hyst |
| 52275 | Male | Cystoscopy and revise urethra | 58550 | Female | Laparo‐asst vaginal hysterectomy |
| 52285 | Female | Cystoscopy and treatment | 58552 | Female | Laparovaginal hysterectomy including t/o |
| 52402 | Male | Cystourethro cut ejaculatory duct | 58553 | Female | Laparovaginal hysterectomy complex |
| 52450 | Male | Incision of prostate | 58554 | Female | Laparovaginal hysterectomy w/t/o complex |
| 52601 | Male | Prostatectomy (turp) | 58555 | Female | Hysteroscopy dx sep procedure |
| 52647 | Male | Laser surgery of prostate | 58558 | Female | Hysteroscopy biopsy |
| 52648 | Male | Laser surgery of prostate | 58559 | Female | Hysteroscopy lysis |
| 52649 | Male | Prostate laser enucleation | 58560 | Female | Hysteroscopy resect septum |
| 52700 | Male | Drainage of prostate abscess | 58561 | Female | Hysteroscopy remove myoma |
| 53210 | Female | Removal of urethra | 58562 | Female | Hysteroscopy remove fb |
| 53215 | Male | Removal of urethra | 58563 | Female | Hysteroscopy ablation |
| 53230 | Female | Removal of urethra lesion | 58565 | Female | Hysteroscopy sterilization |
| 53235 | Male | Removal of urethra lesion | 58570 | Female | Tlh uterus 250 g or less |
| 53410 | Male | Reconstruction of urethra | 58571 | Female | Tlh w/t/o 250 g or less |
| 53415 | Male | Reconstruction of urethra | 58572 | Female | Tlh uterus over 250 g |
| 53420 | Male | Reconstruct urethra stage 1 | 58573 | Female | Tlh w/t/o uterus over 250 g |
| 53425 | Male | Reconstruct urethra stage 2 | 58578 | Female | Laparo proc uterus |
| 53430 | Female | Reconstruction of urethra | 58579 | Female | Hysteroscope procedure |
| 53440 | Male | Male sling procedure | 58600 | Female | Division of fallopian tube |
| 53442 | Male | Remove/revise male sling | 58605 | Female | Division of fallopian tube |
| 53502 | Female | Repair of urethra injury | 58611 | Female | Ligate oviduct(s) add‐on |
| 53505 | Male | Repair of urethra injury | 58615 | Female | Occlude fallopian tube(s) |
| 53510 | Male | Repair of urethra injury | 58660 | Female | Laparoscopy lysis |
| 53515 | Male | Repair of urethra injury | 58661 | Female | Laparoscopy remove adnexa |
| 53520 | Male | Repair of urethra defect | 58662 | Female | Laparoscopy excise lesions |
| 53600 | Male | Dilate urethra stricture | 58670 | Female | Laparoscopy tubal cautery |
| 53601 | Male | Dilate urethra stricture | 58671 | Female | Laparoscopy tubal block |
| 53605 | Male | Dilate urethra stricture | 58672 | Female | Laparoscopy fimbrioplasty |
| 53620 | Male | Dilate urethra stricture | 58673 | Female | Laparoscopy salpingostomy |
| 53621 | Male | Dilate urethra stricture | 58679 | Female | Laparoscopy procedure oviduct–ovary |
| 53660 | Female | Dilation of urethra | 58700 | Female | Removal of fallopian tube |
| 53661 | Female | Dilation of urethra | 58720 | Female | Removal of ovary/tube(s) |
| 53665 | Female | Dilation of urethra | 58740 | Female | Adhesiolysis tube ovary |
| 53850 | Male | Prostatic microwave thermotx | 58750 | Female | Repair oviduct |
| 53852 | Male | Prostatic rf thermotx | 58752 | Female | Revise ovarian tube(s) |
| 53855 | Male | Insert prost urethral stent | 58760 | Female | Fimbrioplasty |
| 53860 | Female | Transurethral rf treatment | 58770 | Female | Create new tubal opening |
| 54000 | Male | Slitting of prepuce | 58800 | Female | Drainage of ovarian cyst(s) |
| 54001 | Male | Slitting of prepuce | 58805 | Female | Drainage of ovarian cyst(s) |
| 54015 | Male | Drain penis lesion | 58820 | Female | Drain ovary abscess open |
| 54050 | Male | Destruction penis lesion(s) | 58822 | Female | Drain ovary abscess percut |
| 54055 | Male | Destruction penis lesion(s) | 58825 | Female | Transposition ovary(s) |
| 54056 | Male | Cryosurgery penis lesion(s) | 58900 | Female | Biopsy of ovary(s) |
| 54057 | Male | Laser surgery penis lesion(s) | 58920 | Female | Partial removal of ovary(s) |
| 54060 | Male | Excision of penis lesion(s) | 58925 | Female | Removal of ovarian cyst(s) |
| 54065 | Male | Destruction penis lesion(s) | 58940 | Female | Removal of ovary(s) |
| 54100 | Male | Biopsy of penis | 58943 | Female | Removal of ovary(s) |
| 54110 | Male | Treatment of penis lesion | 58951 | Female | Resect ovarian malignancy |
| 54111 | Male | Treat penis lesion graft | 58952 | Female | Resect ovarian malignancy |
| 54112 | Male | Treat penis lesion graft | 58953 | Female | Tah rad dissect for debulk |
| 54115 | Male | Treatment of penis lesion | 58954 | Female | Tah rad debulk/lymph remove |
| 54120 | Male | Partial removal of penis | 58956 | Female | Bso omentectomy w/tah |
| 54125 | Male | Removal of penis | 58957 | Female | Resect recurrent gyn mal |
| 54130 | Male | Remove penis and nodes | 58958 | Female | Resect recur gyn mal w/lym |
| 54135 | Male | Remove penis and nodes | 58960 | Female | Exploration of abdomen |
| 54150 | Male | Circumcision w/regionl block | 58970 | Female | Retrieval of oocyte |
| 54160 | Male | Circumcision neonate | 58974 | Female | Transfer of embryo |
| 54161 | Male | Circum 28 days or older | 58976 | Female | Transfer of embryo |
| 54162 | Male | Lysis penil circumic lesion | 58999 | Female | Genital surgery procedure |
| 54163 | Male | Repair of circumcision | 59000 | Female | Amniocentesis diagnostic |
| 54164 | Male | Frenulotomy of penis | 59001 | Female | Amniocentesis therapeutic |
| 54200 | Male | Treatment of penis lesion | 59012 | Female | Fetal cord puncture prenatal |
| 54205 | Male | Treatment of penis lesion | 59015 | Female | Chorion biopsy |
| 54220 | Male | Treatment of penis lesion | 59020 | Female | Fetal contract stress test |
| 54230 | Male | Prepare penis study | 59025 | Female | Fetal nonstress test |
| 54231 | Male | Dynamic cavernosometry | 59030 | Female | Fetal scalp blood sample |
| 54235 | Male | Penile injection | 59050 | Female | Fetal monitor w/report |
| 54240 | Male | Penis study | 59051 | Female | Fetal monitor/interpret only |
| 54250 | Male | Penis study | 59070 | Female | Transabdom amnioinfus w/us |
| 54300 | Male | Revision of penis | 59072 | Female | Umbilical cord occlusion w/us |
| 54304 | Male | Revision of penis | 59074 | Female | Fetal fluid drainage w/us |
| 54308 | Male | Reconstruction of urethra | 59076 | Female | Fetal shunt placement w/us |
| 54312 | Male | Reconstruction of urethra | 59100 | Female | Remove uterus lesion |
| 54316 | Male | Reconstruction of urethra | 59120 | Female | Treat ectopic pregnancy |
| 54318 | Male | Reconstruction of urethra | 59121 | Female | Treat ectopic pregnancy |
| 54322 | Male | Reconstruction of urethra | 59130 | Female | Treat ectopic pregnancy |
| 54324 | Male | Reconstruction of urethra | 59135 | Female | Treat ectopic pregnancy |
| 54326 | Male | Reconstruction of urethra | 59136 | Female | Treat ectopic pregnancy |
| 54328 | Male | Revise penis/urethra | 59140 | Female | Treat ectopic pregnancy |
| 54332 | Male | Revise penis/urethra | 59150 | Female | Treat ectopic pregnancy |
| 54336 | Male | Revise penis/urethra | 59151 | Female | Treat ectopic pregnancy |
| 54340 | Male | Secondary urethral surgery | 59160 | Female | D and c after delivery |
| 54344 | Male | Secondary urethral surgery | 59200 | Female | Insert cervical dilator |
| 54348 | Male | Secondary urethral surgery | 59300 | Female | Episiotomy or vaginal repair |
| 54352 | Male | Reconstruct urethra/penis | 59320 | Female | Revision of cervix |
| 54360 | Male | Penis plastic surgery | 59325 | Female | Revision of cervix |
| 54380 | Male | Repair penis | 59350 | Female | Repair of uterus |
| 54385 | Male | Repair penis | 59400 | Female | Obstetrical care |
| 54390 | Male | Repair penis and bladder | 59409 | Female | Obstetrical care |
| 54400 | Male | Insert semirigid prosthesis | 59410 | Female | Obstetrical care |
| 54401 | Male | Insert self‐contd prosthesis | 59412 | Female | Antepartum manipulation |
| 54405 | Male | Insert multi‐comp penis prosthesis | 59414 | Female | Deliver placenta |
| 54406 | Male | Remove muti‐comp penis pros | 59425 | Female | Antepartum care only |
| 54408 | Male | Repair multi‐comp penis prosthesis | 59426 | Female | Antepartum care only |
| 54410 | Male | Remove/replace penis prosthesis | 59430 | Female | Care after delivery |
| 54411 | Male | Remov/replc penis pros comp | 59510 | Female | Cesarean delivery |
| 54415 | Male | Remove self‐contd penis pros | 59514 | Female | Cesarean delivery only |
| 54416 | Male | Remv/repl penis contain pros | 59515 | Female | Cesarean delivery |
| 54417 | Male | Remv/replc penis pros compl | 59525 | Female | Remove uterus after cesarean |
| 54420 | Male | Revision of penis | 59610 | Female | Vbac delivery |
| 54430 | Male | Revision of penis | 59612 | Female | Vbac delivery only |
| 54435 | Male | Revision of penis | 59614 | Female | Vbac care after delivery |
| 54440 | Male | Repair of penis | 59618 | Female | Attempted vbac delivery |
| 54450 | Male | Preputial stretching | 59620 | Female | Attempted vbac delivery only |
| 54500 | Male | Biopsy of testis | 59622 | Female | Attempted vbac after care |
| 54505 | Male | Biopsy of testis | 59812 | Female | Treatment of miscarriage |
| 54512 | Male | Excise lesion testis | 59820 | Female | Care of miscarriage |
| 54520 | Male | Removal of testis | 59821 | Female | Treatment of miscarriage |
| 54522 | Male | Orchiectomy partial | 59830 | Female | Treat uterus infection |
| 54530 | Male | Removal of testis | 59840 | Female | Abortion |
| 54535 | Male | Extensive testis surgery | 59841 | Female | Abortion |
| 54550 | Male | Exploration for testis | 59850 | Female | Abortion |
| 54560 | Male | Exploration for testis | 59851 | Female | Abortion |
| 54600 | Male | Reduce testis torsion | 59852 | Female | Abortion |
| 54620 | Male | Suspension of testis | 59855 | Female | Abortion |
| 54640 | Male | Suspension of testis | 59856 | Female | Abortion |
| 54650 | Male | Orchiopexy (fowler‐stephens) | 59857 | Female | Abortion |
| 54660 | Male | Revision of testis | 59866 | Female | Abortion (mpr) |
| 54670 | Male | Repair testis injury | 59870 | Female | Evacuate mole of uterus |
| 54680 | Male | Relocation of testis(es) | 59871 | Female | Remove cerclage suture |
| 54690 | Male | Laparoscopy orchiectomy | 59897 | Female | Fetal invas px w/us |
| 54692 | Male | Laparoscopy orchiopexy | 59898 | Female | Laparo proc ob care/deliver |
| 54699 | Male | Laparoscope proc testis | 59899 | Female | Maternity care procedure |
| 54700 | Male | Drainage of scrotum | 64435 | Female | N block inj paracervical |
| 54800 | Male | Biopsy of epididymis | 74440 | Male | X‐ray male genital tract |
| 54830 | Male | Remove epididymis lesion | 74445 | Male | X‐ray examination of penis |
| 54840 | Male | Remove epididymis lesion | 74710 | Female | X‐ray measurement of pelvis |
| 54860 | Male | Removal of epididymis | 74740 | Female | X‐ray female genital tract |
| 54861 | Male | Removal of epididymis | 74742 | Female | X‐ray fallopian tube |
| 54865 | Male | Explore epididymis | 74775 | Female | X‐ray examination of perineum |
| 54900 | Male | Fusion of spermatic ducts | 76801 | Female | Ob us <14 weeks single fetus |
| 54901 | Male | Fusion of spermatic ducts | 76802 | Female | Ob us <14 weeks addl fetus |
| 55000 | Male | Drainage of hydrocele | 76805 | Female | Ob us >/=14 weeks sngl fetus |
| 55040 | Male | Removal of hydrocele | 76810 | Female | Ob us >/=14 weeks addl fetus |
| 55041 | Male | Removal of hydroceles | 76811 | Female | Ob us detailed sngl fetus |
| 55060 | Male | Repair of hydrocele | 76812 | Female | Ob us detailed addl fetus |
| 55100 | Male | Drainage of scrotum abscess | 76813 | Female | Ob us nuchal meas 1 gest |
| 55110 | Male | Explore scrotum | 76814 | Female | Ob us nuchal meas add‐on |
| 55120 | Male | Removal of scrotum lesion | 76815 | Female | Ob us limited fetus(s) |
| 55150 | Male | Removal of scrotum | 76816 | Female | Ob us follow‐up per fetus |
| 55175 | Male | Revision of scrotum | 76817 | Female | Transvaginal us obstetric |
| 55180 | Male | Revision of scrotum | 76818 | Female | Fetal biophys profile w/nst |
| 55200 | Male | Incision of sperm duct | 76819 | Female | Fetal biophys profile w/o nst |
| 55250 | Male | Removal of sperm duct(s) | 76825 | Female | Echo examination of fetal heart |
| 55300 | Male | Prepare sperm duct x‐ray | 76826 | Female | Echo examination of fetal heart |
| 55400 | Male | Repair of sperm duct | 76827 | Female | Echo examination of fetal heart |
| 55450 | Male | Ligation of sperm duct | 76828 | Female | Echo examination of fetal heart |
| 55500 | Male | Removal of hydrocele | 76830 | Female | Transvaginal us non‐ob |
| 55520 | Male | Removal of sperm cord lesion | 76831 | Female | Echo examination uterus |
| 55540 | Male | Revise hernia and sperm veins | 76941 | Female | Echo guide for transfusion |
| 55550 | Male | Laparo ligate spermatic vein | 76945 | Female | Echo guide villus sampling |
| 55559 | Male | Laparo proc spermatic cord | 76946 | Female | Echo guide for amniocentesis |
| 55600 | Male | Incise sperm duct pouch | 76948 | Female | Echo guide ova aspiration |
| 55605 | Male | Incise sperm duct pouch | 77057 | Female | Mammogram screening |
| 55650 | Male | Remove sperm duct pouch | 78761 | Male | Testicular imaging w/flow |
| 55680 | Male | Remove sperm pouch lesion | 80055 | Female | Obstetric panel |
| 55700 | Male | Biopsy of prostate | 81025 | Female | Urine pregnancy test |
| 55705 | Male | Biopsy of prostate | 81500 | Female | Onco (ovar) two proteins |
| 55706 | Male | Prostate saturation sampling | 81503 | Female | Onco (ovar) five proteins |
| 55720 | Male | Drainage of prostate abscess | 81507 | Female | Fetal aneuploidy trisom risk |
| 55725 | Male | Drainage of prostate abscess | 81508 | Female | Ftl cgen abnor two proteins |
| 55801 | Male | Removal of prostate | 81509 | Female | Ftl cgen abnor three proteins |
| 55810 | Male | Extensive prostate surgery | 81510 | Female | Ftl cgen abnor three anal |
| 55812 | Male | Extensive prostate surgery | 81511 | Female | Ftl cgen abnor four anal |
| 55815 | Male | Extensive prostate surgery | 81512 | Female | Ftl cgen abnor five anal |
| 55821 | Male | Removal of prostate | 82120 | Female | Amines vaginal fluid qual |
| 55831 | Male | Removal of prostate | 82143 | Female | Amniotic fluid scan |
| 55840 | Male | Extensive prostate surgery | 82731 | Female | Assay of fetal fibronectin |
| 55842 | Male | Extensive prostate surgery | 84112 | Female | Eval amniotic fluid protein |
| 55845 | Male | Extensive prostate surgery | 84135 | Female | Assay of pregnanediol |
| 55860 | Male | Surgical exposure prostate | 84138 | Female | Assay of pregnanetriol |
| 55862 | Male | Extensive prostate surgery | 84152 | Male | Assay of psa complexed |
| 55865 | Male | Extensive prostate surgery | 84153 | Male | Assay of psa total |
| 55866 | Male | Laparo radical prostatectomy | 84154 | Male | Assay of psa free |
| 55870 | Male | Electroejaculation | 84163 | Female | Pappa serum |
| 55873 | Male | Cryoablate prostate | 84830 | Female | Ovulation tests |
| 55875 | Male | Transperi needle place pros | 85460 | Female | Hemoglobin fetal |
| 55876 | Male | Place rt device/marker pros | 85461 | Female | Hemoglobin fetal |
| 55899 | Male | Genital surgery procedure | 88141 | Female | Cytopath c/v interpret |
| 55970 | Male | Sex transformation m to f | 88142 | Female | Cytopath c/v thin layer |
| 55980 | Female | Sex transformation f to m | 88143 | Female | Cytopath c/v thin layer redo |
| 56405 | Female | I and d of vulva/perineum | 88147 | Female | Cytopath c/v automated |
| 56420 | Female | Drainage of gland abscess | 88148 | Female | Cytopath c/v auto rescreen |
| 56440 | Female | Surgery for vulva lesion | 88150 | Female | Cytopath c/v manual |
| 56441 | Female | Lysis of labial lesion(s) | 88153 | Female | Cytopath c/v redo |
| 56442 | Female | Hymenotomy | 88154 | Female | Cytopath c/v select |
| 56501 | Female | Destroy vulva lesions sim | 88155 | Female | Cytopath c/v index add‐on |
| 56515 | Female | Destroy vulva lesion/s compl | 88164 | Female | Cytopath tbs c/v manual |
| 56605 | Female | Biopsy of vulva/perineum | 88165 | Female | Cytopath tbs c/v redo |
| 56606 | Female | Biopsy of vulva/perineum | 88166 | Female | Cytopath tbs c/v auto redo |
| 56620 | Female | Partial removal of vulva | 88167 | Female | Cytopath tbs c/v select |
| 56625 | Female | Complete removal of vulva | 88174 | Female | Cytopath c/v auto in fluid |
| 56630 | Female | Extensive vulva surgery | 88175 | Female | Cytopath c/v auto fluid redo |
| 56631 | Female | Extensive vulva surgery | 88267 | Female | Chromosome analysis placenta |
| 56632 | Female | Extensive vulva surgery | 88269 | Female | Chromosome analysis amniotic |
| 56633 | Female | Extensive vulva surgery | 89264 | Male | Identify sperm tissue |
| 56634 | Female | Extensive vulva surgery | 89300 | Female | Semen analysis w/huhner |
| 56637 | Female | Extensive vulva surgery | 89310 | Male | Semen analysis w/count |
| 56640 | Female | Extensive vulva surgery | 89320 | Male | Semen anal vol/count/mot |
| 56700 | Female | Partial removal of hymen | 89321 | Male | Semen anal sperm detection |
| 56805 | Female | Repair clitoris | 89329 | Male | Sperm evaluation test |
| 56810 | Female | Repair of perineum | 89330 | Male | Evaluation cervical mucus |
| 56820 | Female | Examination of vulva w/scope | 89331 | Male | Retrograde ejaculation anal |
| 56821 | Female | Examination/biopsy of vulva w/scope | 99500 | Female | Home visit prenatal |
| 57000 | Female | Exploration of vagina | 99501 | Female | Home visit postnatal |
| 57010 | Female | Drainage of pelvic abscess | A4261 | Female | Cervical cap contraceptive |
| 57020 | Female | Drainage of pelvic fluid | A4264 | Female | Intratubal occlusion device |
| 57022 | Female | I and d vaginal hematoma pp | A4266 | Female | Diaphragm |
| 57023 | Female | I and d vaginal hematoma non‐ob | A4267 | Male | Male condom |
| 57061 | Female | Destroy vaginal lesions simple | A4268 | Female | Female condom |
| 57065 | Female | Destroy vaginal lesions complex | A4269 | Female | Spermicide |
| 57100 | Female | Biopsy of vagina | A4281 | Female | Replacement breast pump tube |
| 57105 | Female | Biopsy of vagina | A4282 | Female | Replacement breast pump adpt |
| 57106 | Female | Remove vagina wall partial | A4283 | Female | Replacement breast pump cap |
| 57107 | Female | Remove vagina tissue part | A4284 | Female | Replacement breast pump shield |
| 57109 | Female | Vaginectomy partial w/nodes | A4285 | Female | Replacement breast pump bottle |
| 57110 | Female | Remove vagina wall complete | A4286 | Female | Replacement breastpump lok ring |
| 57111 | Female | Remove vagina tissue compl | A4326 | Male | Male external catheter |
| 57112 | Female | Vaginectomy w/nodes compl | A4327 | Female | Female urinary collect dev cup |
| 57120 | Female | Closure of vagina | A4328 | Female | Female urinary collect pouch |
| 57130 | Female | Remove vagina lesion | C9739 | Male | Cystoscopy prostatic imp 1‐3 |
| 57135 | Female | Remove vagina lesion | C9740 | Male | Cysto impl 4 or more |
| 57150 | Female | Treat vagina infection | E0325 | Male | Urinal male jug type |
| 57155 | Female | Insert uteri tandem/ovoids | E0326 | Female | Urinal female jug type |
| 57156 | Female | Ins vaginal brachytx device | E0602 | Female | Manual breast pump |
| 57160 | Female | Insert pessary/other device | E0603 | Female | Electric breast pump |
| 57170 | Female | Fitting of diaphragm/cap | E0604 | Female | Hosp grade elec breast pump |
| 57180 | Female | Treat vaginal bleeding | G0027 | Male | Semen analysis |
| 57200 | Female | Repair of vagina | G0101 | Female | Ca screen; pelvic/breast exam |
| 57210 | Female | Repair vagina/perineum | G0102 | Male | Prostate ca screening; dre |
| 57220 | Female | Revision of urethra | G0103 | Male | Psa screening |
| 57230 | Female | Repair of urethral lesion | G0123 | Female | Screen cerv/vaginal thin layer |
| 57240 | Female | Repair bladder and vagina | G0124 | Female | Screen c/v thin layer by md |
| 57250 | Female | Repair rectum and vagina | G0141 | Female | Scr c/v cyto, autosys and md |
| 57260 | Female | Repair of vagina | G0143 | Female | Scr c/v cyto, thin layer, rescr |
| 57265 | Female | Extensive repair of vagina | G0144 | Female | Scr c/v cyto, thin layer, rescr |
| 57267 | Female | Insert mesh/pelvic flr addon | G0145 | Female | Scr c/v cyto, thin layer, rescr |
| 57268 | Female | Repair of bowel bulge | G0147 | Female | Scr c/v cyto, automated sys |
| 57270 | Female | Repair of bowel pouch | G0148 | Female | Scr c/v cyto, autosys, rescr |
| 57280 | Female | Suspension of vagina | G0202 | Female | Screeningmammographydigital |
| 57282 | Female | Colpopexy extraperitoneal | G0416 | Male | Biopsy prostate 10–20 |
| 57283 | Female | Colpopexy intraperitoneal | G0417 | Male | Biopsy prostate 21–40 |
| 57284 | Female | Repair paravag defect open | G0418 | Male | Biopsy prostate 41–60 |
| 57285 | Female | Repair paravag defect vaginal | G0419 | Male | Biopsy prostate: >60 |
| 57287 | Female | Revise/remove sling repair | G0458 | Male | Ldr prostate brachy comp rat |
| 57288 | Female | Repair bladder defect | G8806 | Female | Transab or transvag us |
| 57289 | Female | Repair bladder and vagina | G8807 | Female | Doc reas no us |
| 57291 | Female | Construction of vagina | G8808 | Female | No transab or transvag us |
| 57292 | Female | Construct vagina with graft | G8809 | Female | Rh‐immunoglobulin order |
| 57295 | Female | Revise vaginal graft through vagina | G8810 | Female | Doc reas no rh‐immuno |
| 57296 | Female | Revise vaginal graft open abd | G8811 | Female | No rh‐immunoglobulin order |
| 57307 | Female | Fistula repair and colostomy | P3000 | Female | Screen pap by tech w md supv |
| 57308 | Female | Fistula repair transperine | P3001 | Female | Screening pap smear by phys |
| 57310 | Female | Repair urethrovaginal lesion | Q0091 | Female | Obtaining screen pap smear |
| 57311 | Female | Repair urethrovaginal lesion | S0610 | Female | Annual gynecological examina |
| 57320 | Female | Repair bladder‐vagina lesion | S0612 | Female | Annual gynecological examina |
| 57330 | Female | Repair bladder‐vagina lesion | S4005 | Female | Interim labor facility globa |
| 57335 | Female | Repair vagina | S4011 | Female | IVF package |
| 57400 | Female | Dilation of vagina | S4013 | Female | Complete GIFT case rate |
| 57410 | Female | Pelvic examination | S4014 | Female | Complete ZIFT case rate |
| 57415 | Female | Remove vaginal foreign body | S4015 | Female | Complete IVF nos case rate |
| 57420 | Female | Examination of vagina w/scope | S4016 | Female | Frozen IVF case rate |
| 57421 | Female | Examination/biopsy of vaginal w/scope | S4017 | Female | IVF canc a stim case rate |
| 57423 | Female | Repair paravag defect lap | S4018 | Female | F EMB trns canc case rate |
| 57425 | Female | Laparoscopy surg colpopexy | S4020 | Female | IVF canc a aspir case rate |
| 57426 | Female | Revise prosth vaginal graft lap | S4021 | Female | IVF canc p aspir case rate |
| 57452 | Female | Examination of cervix w/scope | S4022 | Female | Asst oocyte fert case rate |
| 57454 | Female | Bx/curett of cervix w/scope | S4023 | Female | Incomplete donor egg case rate |
| 57455 | Female | Biopsy of cervix w/scope | S4025 | Female | Donor serv IVF case rate |
| 57456 | Female | Endocerv curettage w/scope | S4026 | Male | Procure donor sperm |
| 57460 | Female | Bx of cervix w/scope leep | S4027 | Female | Store prev frozen embryos |
| 57461 | Female | Conz of cervix w/scope leep | S4028 | Male | Microsurg epi sperm asp |
| 57500 | Female | Biopsy of cervix | S4030 | Male | Sperm procure init visit |
| 57505 | Female | Endocervical curettage | S4031 | Male | Sperm procure subs visit |
| 57510 | Female | Cauterization of cervix | S4035 | Female | Stimulated IUI case rate |
| 57511 | Female | Cryocautery of cervix | S4037 | Female | Cryo embryo transf case rate |
| 57513 | Female | Laser surgery of cervix | S4040 | Female | Monit store cryo embryo 30 d |
| 57520 | Female | Conization of cervix | S4989 | Female | Contracept IUD |
| 57522 | Female | Conization of cervix | S4993 | Female | Contraceptive pills for bc |
| 57530 | Female | Removal of cervix | S9001 | Female | Home uterine monitor with or |
| 57531 | Female | Removal of cervix radical | S9436 | Female | Lamaze class |
| 57540 | Female | Removal of residual cervix | S9437 | Female | Childbirth refresher class |
| S9438 | Female | Cesarean birth class | |||
| S9439 | Female | VBAC class |
HCPCS, Healthcare Common Procedure Coding System.
Appendix Table 2.
Sex Hormones
| Avodart |
| Briellyn |
| Cenestin |
| Climara |
| CombiPatch |
| Delestrogen |
| Depo-Estradiol |
| Depo-Provera |
| Depo-Testosterone |
| Dutasteride |
| Estrace |
| Estradiol |
| Estradiol Cypionate |
| Estradiol Valerate |
| Estradiol Valerate |
| Estradiol/Norethindrone Acetate |
| Estrogen, Conjugated/M-Progesterone Acetate |
| Estrogens, Conjugated |
| Estrogens, Conjugated, Synthetic A |
| Estrogens, Esterified |
| Estropipate |
| Estropipate |
| Ethinyl Estradiol/Drospirenone |
| Ethynodiol d-Ethinyl Estradiol |
| Finasteride |
| Fluoxymesterone |
| Fortesta |
| Gianvi |
| Gildess Fe |
| Junel |
| Junel Fe |
| Ketoconazole |
| Ketoconazole |
| Leuprolide Acetate |
| Loryna |
| Lupron Depot |
| Medroxyprogesterone Acetate |
| Menest |
| Microgestin |
| Microgestin Fe |
| Mononessa |
| Necon |
| Norelgestromin/Ethinyl Estradiol |
| Norethindrone A-Ethinyl Estradiol/Ferrous Fumarate |
| Norethindrone A–E Estradiol |
| Norethindrone-Ethinyl Estradiol |
| Norethindrone-Mestranol |
| Norgestimate-Ethinyl Estradiol |
| Norgestimate-Ethinyl Estradiol |
| Norgestrel-Ethinyl Estradiol |
| Nortrel |
| Ocella |
| Ogestrel |
| Ortho Evra |
| Ortho Tri-Cyclen |
| Ortho Tri-Cyclen Lo |
| Ortho-Cyclen |
| Philith |
| Premarin |
| Prempro |
| Progesterone |
| Progesterone |
| Progesterone, Micronized |
| Spironolactone |
| Spironolactone |
| Sprintec |
| Syeda |
| Testim |
| Testosterone |
| Testosterone Cypionate |
| Testosterone Cypionate |
| Testosterone Enanthate |
| Testosterone Enanthate |
| Tri-Linyah |
| TriNessa |
| Tri-Previfem |
| Tri-Sprintec |
| Xolegel |
| Zovia 1-35E |
Author Disclosure Statement
No competing financial interests exist.
Due to the high degree of error in Centers for Medicare & Medicaid Services (CMS's) race/ethnicity data this analysis uses CMS's RTI race code to identify a beneficiary's race.17–19
References
- 1.Graham R, Berkowitz B, Blum R, et al. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: Institute of Medicine, 2011 [PubMed] [Google Scholar]
- 2.Grant JM, Mottet L, Tanis JE, et al. Injustice at every turn: a report of the national transgender discrimination survey: National Center for Transgender Equality; 2011. [August 18, 2015]. Available from: www.thetaskforce.org/static_html/downloads/reports/reports/ntds_full.pdf (accessed November22, 2016)
- 3.Fredriksen-Goldsen KI, Kim H-J, Emlet CA, et al. The aging and health report: disparities and resilience among lesbian, gay, bisexual, and transgender older adults. Seattle, WA: Institute for Multigenerational Health, 2011 [Google Scholar]
- 4.Blosnich JR, Brown GR, Shipherd P, et al. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103:e27–e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychological Association Committee on Lesbian G, Bisexual, and Transgender Concerns. Answers to your Questions about Transgender People, Gender Identity, and Gender Expression 2014. [August 18, 2015]. Available from: www.apa.org/topics/lgbt/transgender.aspx (accessed November22, 2016)
- 6.Hughes TL, Eliason M. Substance use and abuse in lesbian, gay, bisexual and transgender populations. J Prim Prev. 2002;22:263–298 [Google Scholar]
- 7.Jordan KM. Substance abuse among gay, lesbian, bisexual, transgender, and questioning adolescents. School Psychol Rev. 2000;29:201–206 [Google Scholar]
- 8.Lombardi EL, van Servellen G. Building culturally sensitive substance use prevention and treatment programs for transgendered populations. J Subst Abuse Treat. 2000;19:291–296 [DOI] [PubMed] [Google Scholar]
- 9.Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1–17 [DOI] [PubMed] [Google Scholar]
- 10.Clements-Nolle K, Marx R, Katz M. Attempted suicide among transgender persons: the influence of gender-based discrimination and victimization. J Homosex. 2006;51:53–69 [DOI] [PubMed] [Google Scholar]
- 11.Nemoto T, Operario D, Keatley J, Villegas D. Social context of HIV risk behaviours among male-to-female transgenders of colour. AIDS Care. 2004;16:724–735 [DOI] [PubMed] [Google Scholar]
- 12.Mustanski BS, Garofalo R, Emerson EM. Mental health disorders, psychological distress, and suicidality in a diverse sample of lesbian, gay, bisexual, and transgender youths. Am J Public Health. 2010;100:2426–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotondi NK, Bauer GR, Scanlon K, et al. Prevalence of and risk and protective factors for depression in female-to-male transgender Ontarians: trans PULSE Project. Can J Commun Ment Health. 2012;30:135–155 [Google Scholar]
- 14.Centers for Medicare & Medicaid Services. ICD-9 Code Lookup 2015. [August 18, 2015]. Available from: https://www.cms.gov/Medicare/Coding/ICD9ProviderDiagnosticCodes/codes.html (accessed November22, 2016)
- 15.Centers for Medicare & Medicaid Services—CMS Manual System. Pub 100-04: Transmittal 1877-Instructions Regarding Processing Claims Rejecting for Gender/Procedure Conflict 2009a. [August 18, 2015]. Available from: www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R1877CP.pdf (accessed November22, 2016)
- 16.Centers for Medicare & Medicaid Services—MLM Matters. MM6638: Instructions Regarding Processing Claims Rejecting for Gender/Procedure Conflict 2009b. Available from: www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6638.pdf (accessed November22, 2016)
- 17.Arday SL, Arday DR, Monroe S, Zhang J. HCFA's racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116 [PMC free article] [PubMed] [Google Scholar]
- 18.Waldo DR. Accuracy and bias of race/ethnicity codes in the Medicare enrollment database. Health Care Financ Rev. 2004;26:61–72 [PMC free article] [PubMed] [Google Scholar]
- 19.Zaslavsky AM, Ayanian JZ, Zaborski LB. The validity of race and ethnicity in enrollment data for Medicare beneficiaries. Health Serv Res. 2012;47(3 pt 2):1300–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehmer U. Twenty years of public health research: inclusion of lesbian, gay, bisexual, and transgender populations. Am J Public Health. 2002;92:1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335:791–799 [DOI] [PubMed] [Google Scholar]
- 22.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press, 2002 [PubMed] [Google Scholar]
- 23.Virnig BA, Lurie N, Huang Z, et al. Racial variation in quality of care among Medicare+Choice enrollees. Health Aff. 2002;21:224–230 [DOI] [PubMed] [Google Scholar]
- 24.Weech‐Maldonado R, Morales LS, Elliott M, et al. Race/ethnicity, language, and patients' assessments of care in Medicaid managed care. Health Serv Res. 2003;38:789–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sequist TD, Schneider EC. Addressing racial and ethnic disparities in health care: using federal data to support local programs to eliminate disparities. Health Serv Res. 2006;41(4 pt 1):1451–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7:8–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freid VM, Bernstein AB, Bush MA. Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. Women. 2012;45:6–4. [PubMed] [Google Scholar]
- 28.Shipherd JC, Mizock L, Maguen S, Green KE. Male-to-female transgender veterans and VA health care utilization. Int J Sex Health. 2012;24:78–87 [Google Scholar]
- 29.Knochel KA, Croghan CF, Moone RP, Quam JK. Training, geography, and provision of aging services to lesbian, gay, bisexual, and transgender older adults. J Gerontol Soc Work. 2012;55:426–443 [DOI] [PubMed] [Google Scholar]
- 30.Lev AI. Disordering gender identity: gender identity disorder in the DSM-IV-TR. J Psychol Human Sex. 2006;17:35–69 [Google Scholar]
- 31.Mayer G. Providing Cross-Gender Hormone Therapy for Transgender Patients Boston, MA: The Fenway Institute, 2013. [August 18, 2015]. Available from: www.lgbthealtheducation.org/wp-content/uploads/Providing-Cross-Gender-Hormone-Therapy-to-Transgender-Patients.pdf (accessed November22, 2016)
- 32.Callen-Lorde Community Health Center. Protocols for the Provision of Cross Gender Hormone Therapy 2012. [August 18, 2015]. Available from: www.tmeltzer.com/assets/callen-lorde-revised-protocols.pdf (accessed November22, 2016)
- 33.Yasmeen S, Romano PS, Schembri ME, et al. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol. 2006;194:992–1001 [DOI] [PubMed] [Google Scholar]
- 34.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992;82:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fowles JB, Fowler EJ, Craft C. Validation of claims diagnoses and self-reported conditions compared with medical records for selected chronic diseases. J Ambul Care Manage. 1998;21:24–34 [DOI] [PubMed] [Google Scholar]
- 36.Fowles JB, Lawthers AG, Weiner JP, Garnick DW. Agreement between physicians' office records and Medicare Part B claims data. Health Care Financ Rev. 1995;16:189–199 [PMC free article] [PubMed] [Google Scholar]
- 37.Intersex Society of North America. What is Intersex? 2015. [August 18, 2015]. Available from: www.isna.org/faq/what_is_intersex (accessed November22, 2016)
References
Cite this article as: Proctor K, Haffer SC, Ewald E, Hodge C, James CV (2016) Identifying the transgender population in the Medicare program, Transgender Health 1:1, 250–265, DOI: 10.1089/trgh.2016.0031.