Published ahead of print December 19, 2016.
Abstract
BACKGROUND:
Both pharmacologic and genetic approaches have been used to study the involvement of the muscarinic acetylcholine system in the regulation of chronic pain. Previous studies suggest that the M2 and M4 subtypes of muscarinic acetylcholine receptors (mAChRs) are important targets for the development of chronic pain. (5R,6R)6-(3-Propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1] octane (PTAC) has agonist effects on muscarinic M2 and M4 receptors and antagonist effects on muscarinic M1, M3, and M5 receptors. However, its analgesic effects have been less studied.
METHODS:
Male C57B L/6 mice were anesthetized, and left common peroneal nerve (CPN) ligation was performed to induce neuropathic pain. Before and after the application of PTAC systemically or specifically to the anterior cingulate cortex (ACC), the withdrawal thresholds to mechanical stimulation and static weight balance were measured, and the effects of PTAC on the conditioned place preference (CPP) were further evaluated. Western blotting was used to examine the expression of M1 and M2 in the striatum, ACC, and ventral tegmental area.
RESULTS:
The application of PTAC ([i.p.] intraperitoneal injection) increased the paw withdraw threshold in both the early (0.05 mg/kg, mean difference [95% confidence interval, CI]: 0.19 [0.05–0.32]; 0.10 mg/kg: mean difference [95% CI]: 0.34 [0.22–0.46]) and the late phases (0.05 mg/kg: mean difference [95% CI]: 0.45 [0.39–0.50]; 0.1 mg/kg: mean difference [95% CI]: 0.44 [0.37–0.51]) after nerve injury and rebalanced the weight distribution on the hind paws of mice (L/R ratio: before, 0.56 ± 0.03. 0.05 mg/kg, 1.00 ± 0.04, 0.10 mg/kg, 0.99 ± 0.03); however, it failed to induce place preference in the CPP (0.05 mg/kg, 2-way analysis of variance, P > .05; 0.2 mg/kg, 2-way analysis of variance, P > .05,). At the same doses, the analgesic effects at D3–5 lasted longer than the effects at D14–16. This may be due to the down-regulation of the M2 and M1 in tested brain regions.
CONCLUSIONS:
These observations suggested that PTAC has analgesic effects on the neuropathic pain induced by nerve injury.
The muscarinic acetylcholine receptors (mAChRs) are involved in the regulation of chronic pain.1 Chronic pain refers to pain that lasts over 1 month, which is caused by different factors.2 Chronic pain decreases life quality, increases life costs, and also leads to emotional changes.3 Different subtypes of mAChRs are expressed in the sensory pathway, including at the terminal of the primary afferent nerve,4,5 the spinal cord,6 and the superspinal cord brain regions.7 Both genetic and pharmacologic approaches have been used to study the involvement of mAChRs in the modulation of nociception.8 Significant antinociceptive effects were observed after the administration of cholinesterase inhibitors or agonists of mAChRs in basic research studies.8,9 Detailed information was obtained using genetic knock-out approaches on the subtypes of mAChRs, such as M2 and M4.8 Therefore, the mAChRs have been proposed as promising targets for developing novel analgesic agents.
The G(i/o)-coupled subtypes of mAChRs are important for the development of new analgesic agents. Different subtypes of mAChRs are distributed in both the peripheral and the central nervous systems. The M2 and M4 receptors couple with the Gi and Go α subunits, which inhibit cAMP signaling. The M1, M3, and M5 receptors are related to the Gq/11 signaling pathway.1 It was found that inhibiting the pertussis toxin–sensitive G(i/o)-protein function induced long-lasting thermal allodynia.10 Using genetic approaches, Duttaroy et al11 showed that the antinociception induced by muscarinic agonists was totally abolished in M(2)/M(4) double-knockout mice. Recent studies showed that the cAMP signaling pathway was involved in the maintenance of neuropathic pain.12,13 Therefore, the M2 and M4 receptors may contribute to the development and maintenance of chronic pain.9 Vedaclidine and (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1] octane (PTAC) are partial muscarinic receptors ligands that have agonist effects at muscarinic M2 and M4 receptors and antagonist effects at muscarinic M1, M3, and M5 receptors.14 The analgesic effects of vedaclidine were evaluated using different pain models.10,15,16 Previous studies showed that PTAC inhibited the conditioned avoidance response and decreased self-administration in rats.17 However, its analgesic effect on neuropathic pain have not been evaluated. In the current study, PTAC was applied to a neuropathic pain mouse model to activate the mAChRs directly, and the analgesic effects on both evoked pain and spontaneous pain were evaluated using von Frey filaments, static weight balance, and conditioned place preference (CPP) behavioral assay.
The mAChRs are involved in the regulation of chronic pain. We hypothesized that the PTAC, an agonist of M2 and M4 but antagonist of M1, M3, and M5, has good analgesic effects on the neuropathic pain. To test this hypothesis, we employed the common peroneal nerve (CPN) ligation-induced neuropathic pain mice model and applied PTAC systemically or specifically to the anterior cingulate cortex (ACC). We assessed the paw withdraw thresholds (PWTs), weight distribution and CPP, rota-rod and forced swimming test, and the protein expression level of M1 and M2 in different brain regions.
MATERIALS AND METHODS
Animals
C57B L/6 mice aged 8 to 10 weeks were used in this study. The animals (20–25 g) were housed 4 or 5 per cage at a constant room temperature (25°C ± 1°C) and stable relative humidity (60% ± 5%) under a 12-hour light/dark schedule (light from 7.00 am to 7.00 pm); food and water were available ad libitum. For the behavioral tests, the mice were allowed to adapt to laboratory conditions for about 1 week and to habituate to the testing situation for at least 15 minutes before the experiments. The animal care and use committee of Zhejiang University approved all the mouse protocols.
CPN Model
The CPN ligation mouse model of neuropathic pain has been described previously.13,18,19 In brief, mice were anesthetized with isoflurane (1%–3%, as needed). The left CPN between the anterior and posterior groups of muscles were slowly ligated with a chromic gut suture 5-0 (Ethicon, Blue Ash) until the digits began to twitch. The skin was sutured using a 5-0 silk suture and cleaned with povidone iodine. Sham surgery was conducted in the same manner but the nerve was not ligated. All animals were kept in a normal living chamber after surgery. The mice were given the behavioral test on postsurgical days 3 to 16.
Mechanical Allodynia Test
On the first day of the experiment, the von Frey behavioral test was performed according to the up-down algorithm described by Dixon.20 To determine the evoked reflex responses to mechanical stimuli, animals were placed on a raised mesh grid and covered with a clear plastic box for containment. Calibrated von Frey filaments were applied to the middle of the plantar surface of each paw until the filament bent. Brisk withdrawal or paw flinching were considered as positive responses. Lifting of the paw due to normal locomotor behavior was ignored. In the absence of a response, a filament of the next greater force was applied. Following a response, the filament of the next lower force was applied. The tactile stimulus that produced a 50% likelihood of a withdrawal response was calculated and treated as the PWT. The PWTs of the mice were normalized by the results of the PWT tests conducted before the sham or nerve injury operations.
CPP Test
The CPP test was adapted from the behavioral paradigm established by King et al for adult rats.19,21,22 In brief, mice were preconditioned for 3 days, starting day 1 post-CPN ligation, and the chamber preference was evaluated on preconditioned day 3. The following day (day 4 post-CPN), mice received the appropriate control (ie, vehicle) paired with a randomly chosen chamber in the morning, and the appropriate drug treatment paired with the other chamber 4 hours later (afternoon). The chamber pairings were counterbalanced. Twenty hours after the afternoon pairing, the mice were placed in the CPP box with access to all the chambers for 15 minutes and their behavior was analyzed for chamber preference. The preference time was calculated as the time spent in the drug-paired chamber subtracted from the time spent in the saline-paired chamber.
Cannulation and Microinjection
The cannula surgery and microinjection were performed as described previously.13,19 In brief, mice were anesthetized with isoflurane (1%–3%, as needed) inhalation of 100% oxygen with a flow of 0.5 L/min delivered by facemask. The scalp was shaved and then cleaned with iodine (Triadine) and alcohol. The head of each mouse was fixed into a stereotaxic adapter mounted on a stereotaxic frame (Kopf model 962) and lubricant (Artificial Tears) was applied to the eyes. An incision was made over the skull and the surface was exposed. Two small holes were drilled above the ACC, and the dura was gently reflected. Guide cannulas were placed 0.7 mm anterior to the bregma, 0.3 mm lateral to the midline, and 1.75 mm ventral to the surface of the skull. For the microinjection, the mice were restrained in a plastic cone (Braintree Scientific) and a small hole was cut in the plastic overlying the microinjection guides. The dummy cannulas were removed, and the microinjection cannula was inserted into the guide. A 30-gauge injection cannula was placed 0.7 mm lower than the guide. PTAC (0.5 μL, 0.5 ng/μL) was bilaterally delivered at 0.5 μL/min using a syringe driven by an infusion pump (Harvard Apparatus, Inc, South Natick, MA). The volume delivered was confirmed by watching the movement of the meniscus down a length of calibrated polyethylene (PE10) tubing. After delivery to each side of the brain, the injection cannula was left in place for 1 minute to prevent any solution from flowing back up the guide. The cannula was then retracted and inserted into the opposite side of the brain. Ten minutes after microinjection, the mechanical allodynia test was administered.
Static Weight Bearing
Static weight bearing was performed as described in a previous study.23 In brief, the incapacitance meter (IITC Life Science, Woodland Hills, CA) was used. The animals were allowed to explore the instruments freely for 2 days before the examination; during the test, the animals were positioned in such a way as to set their hind paws individually on load cells (also known as force plates). The recorded data represented the average weight borne by each cell over a 5-second period that was manually initiated when the animal stabilized its position. The measurement was repeated 3 times and the results were averaged for each mouse. Acclimatization to the apparatus occurred for 2 consecutive days before the measurements were performed.
Forced Swim Test
The forced swim test (FST) was performed as described in a previous study.24 During the pretest, PTAC was not given and behavior was not recorded. In the pretest, both the sham and CPN mice were individually placed into a clear polypropylene, cylindrical water tank (diameter 30 cm; height 60 cm; water depth >40 cm; water temperature between 23°C and 26°C) for 15 minutes, to establish immobility for the subsequent test. The FST test session occurred during the second swim session, which took place 24 hours after the pretest. The mice were placed in the water tank after a 10-minute injection of PTAC intraperitoneally (i.p.) and immobility was scored from minute 1 to minute 6 (time: 1:00–6:00, 5 minutes total) of the 10-minute test session. FST was recorded by video camera and immobility was defined as an interruption of swimming behavior, when the mice showed a lack of hind paw paddling. Thus, scoring of immobility time started when a mouse assumed a passive floating position, using only the minimal movements required to keep their heads above water. For the FST analysis, each test session was quantified by stopwatch by an experimenter blind to the treatment condition. At the end of the test session, the mice were dried with a clean towel and monitored for 30 minutes in their home cage.
Measurement of Motor Performance
To evaluate the possible nonspecific effects of PTAC on motor coordination, the mice were tested on the rota-rod apparatus.25 The apparatus consists of a bar, 3.0 cm in diameter, subdivided into 5 compartments by disks of 30-cm diameter (Lihua, Xuzhou, China). The bar rotated between 5 and 40 rpm over the course of 2 minutes. The animals were selected 6 hours before by eliminating those mice that did not remain on the bar for 2 consecutive periods of 110 to 120 seconds. After the selection, animals were treated with 0.05 mg/kg PTAC or received the same volume of saline 10 minutes before the test. The results were expressed as the time the animals remained on the rota-rod. The cutoff time used was 300 seconds.
Western Blot Analysis
Western blot was performed essentially as described previously.25 The mice were lightly anesthetized with isoflurane and then decapitated. The regions of the spinal cord, ACC, ventral tegmental area (VTA), prefrontal cortex (PFC), and striatum (caudate putamen [CPU]) were dissected and then homogenized in an RIPA buffer (50 mM pH 7.6 Tris-Cl, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 1 mM dithiothreitol, 0.5% sodium deoxycholate) containing a protease inhibitor cocktail. After centrifugation, the supernatants were used for protein quantification by the Bradford assay. Electrophoresis of equal amounts of total protein was performed on sodium dodecyl sulfate-polyacrylamide gels. The separated proteins were transferred onto polyvinylidene membranes at 4°C. The membranes were blocked for 2 hours with 5% milk in TBST (Tris-buffered saline with Tween 20, room temperature) and incubated with a primary antibody (M1 1:500, Ruiying; M2 1:1000, Ruiying) at 4°C overnight. After being washed, the membranes were incubated with the appropriate horseradish peroxidase–coupled secondary antibody (Beyotime, China) diluted 1:1000 for 1 hour, followed by enhanced chemiluminescence detection of the proteins with Western lightning Chemiluminescence Reagent Plus, according to the manufacturer’s instructions. To verify equal loading, we also probed the membranes with an antibody against tubulin (1:10,000, Sigma). The density of the immunoblots was measured with the NIH ImageJ program.
Chemicals
The PTAC oxalate was purchased from Tocris Bioscience (Cat.No.4533, UK) and the atropine (Cat.ab145582, UK) was purchased from Abcam. All drugs were dissolved in 0.9% saline.
Data Analysis
Sigmaplot 11.0 was used to plot and fit the data. The sample size of each group was chosen based on our experimental experience that allows to detect significant differences with minimal animal numbers, and a power analysis was performed and 80% power was required to determine the sample size. Therefore, n = 5–10 was chosen; for the Western blotting experiments, n = 3–5 was chosen.
Statistical comparisons on the changing of analgesic effects of PTAC on the sham and nerve injury mice following time were made by two-way repeated-measures analysis of variance (ANOVA), followed by a Student-Newman-Keuls (SNK) for post hoc comparison. We formally tested normality and homogeneity of variance by applying Shapiro-Wilk test and Levene test, respectively, with α = .05. If any of these 2 assumptions was violated, we performed a Tukey test for post hoc comparison, we also examined the interaction between treatments and groups, and P < .05 was considered as significant. For the PWT data, the raw data of baseline were used without adjusting in the analyses.
For the CPP experiments, the effects of surgery treatments and injected chemicals were examined by 2-way repeated-measures ANOVA, followed by a SNK test for post hoc comparison if the normality and homogeneity of variance test passed, otherwise a Tukey test for post hoc comparison, and the interaction between treatments and groups was also examined.
The summarized data in the figures were presented as the mean ± SE, to clearly show the effects of PTAC, and the estimated treatment effects were presented as mean difference with 95% confidence intervals in the context. In all cases, P < .05 was considered statistically significant. All statistical tests are 2-tailed; significance was established at P < .05.
RESULTS
PTAC Alleviated the Mechanical Allodynia When Applied Systemically
The mouse model of neuropathic pain was used to evaluate the analgesic effects of PTAC oxalate, which is an agonist of M2 and M4 but an antagonist of M1, M3, and M5. As shown in Figure 1A, the PWTs were significantly decreased on day 3 after the CPN ligation (mean difference [95% CI]: −0.22 [−0.31 to −0.13], n = 8). The PTAC was systemically applied (i.p.) at different doses, and the effects were evaluated at 0.5 hour after injection. No effect was detected on the PWTs at 0.01 mg/kg (mean difference [95% CI]: −0.01 [−0.03 to 0.01], n = 8, Figure 1A), whereas increased PWTs was observed at 0.05 mg/kg (mean difference [95% CI]: 0.18 [0.06–0. 30], Figure 1B) and 0.1 mg/kg (mean difference [95% CI]: 0.35 [0.23–0.47], n = 8, Figure 1C), suggesting that the mechanical allodynia was alleviated. Interestingly, the PWTs of the mice with CPN ligation were at the same level as those of the sham group 2 hours after the PTAC injection (0.05 mg/kg: mean difference [95% CI]: −0.08 [−0.19 to 0.02], Figure 1B; 0.10 mg/kg; mean difference [95% CI]: −0.07 [−0.18 to 0.05], Figure 1C), suggesting that the analgesic effects of PTAC could last for over 2 hours.
Figure 1.
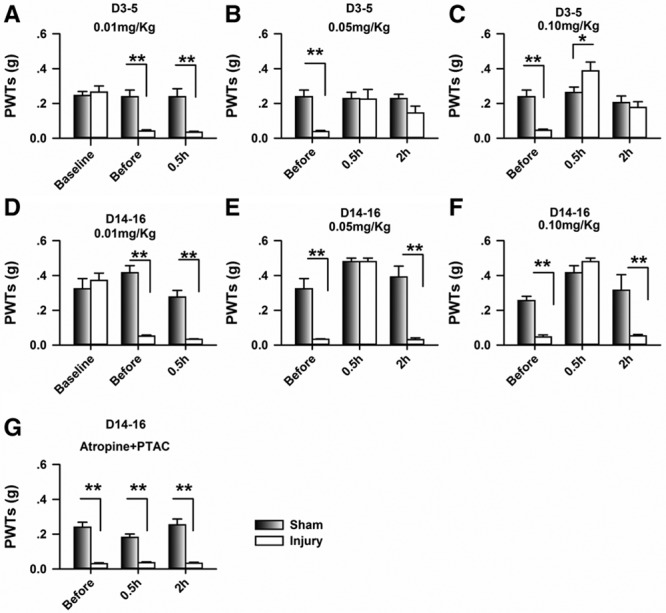
Application of (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1] octane (PTAC) systemically raised the paw withdrawal threshold (PWT) in nerve-injured mice. A, Nerve injury decreased the PWT, which was not changed by the application of PTAC at 0.01 mg/kg at day 3 after nerve injury (2-way RM ANOVA, sham versus injury: F1;44 = 17.46, P < .01; treatments: F2;44 = 16.78, P < .01, interaction: F2;44 = 14.98, P < .01, n = 7 for sham, n = 8 for injury group, **P < .01 under SNK test). B, PTAC at 0.05 mg/kg increased the PWTs of the nerve-injury groups injected at day 4 after nerve injury (2-way RM ANOVA, sham versus injury: F1;44 = 9.32, P < .01; treatments: F2;44 = 2.98, P = .07, interaction: F2;44 = 3.79, P < .05, n = 7 for sham, n = 8 for injury group, **P < .01 under SNK test). C, PTAC at 0.05 mg/kg increased the PWTs of the nerve-injury groups injected at day 5 after nerve injury (2-way RM ANOVA, sham versus injury: F1; 44 = 0.98, P = .34; treatments: F2;44 = 16.63, P < .01, interaction: F2; 44 = 11.76, P < .01, n = 7 for sham, n = 8 for injury group, * P < .05, **P < .01 under SNK test). D, PTAC at 0.01 mg/kg had no effect on the PWTs in the sham and nerve-injury groups injected at day 14 after nerve injury (2-way RM ANOVA, sham versus injury: F1;29 = 32.95, P < .01; treatments: F2;29 = 14.71, P < .01, interaction: F2;44 = 17.49, P < .01, n = 5 for each group, **P < .01 under SNK test). E, PTAC at 0.05 mg/kg increased the PWTs of the nerve-injury groups injected at day 15 after nerve injury (2-way RM ANOVA, sham versus injury: F1;29 = 69.17, P < .01; treatments: F2;29 = 35.73, P < .01, interaction: F2;29 = 11.94, P < .01, n = 5 for each group, **P < .01 under SNK test). F, PTAC at 0.10 mg/kg increased the PWTs in both the sham and nerve-injury group injected at day 16 after nerve injury (2-way RM ANOVA, sham versus injury: F1;29 = 12.54, P < .01; treatments: F2;29 = 33.56, P < .01, interaction: F2;29 = 9.76, P < .01, n = 5 for each group, *P < .05, **P < .01 under SNK test). G, The application of atropine (i.p., 0.1 mg/kg) blocked the analgesic effects of PTAC (0.10 mg/kg) (2-way RM ANOVA, sham versus injury: F1;56 = 260.17, P < .01; treatments: F2;56 = 1.52, P = .23, interaction: F2;56 = 1.93, P = .16; n = 10 for sham, n = 9 for injury group, **P < .01 under SNK test). ANOVA indicates analysis of variance; RM, repeated measures; SNK, Student-Newman-Keuls.
Both basic and clinical research has shown that neuronal changes in the early phases of chronic pain differ from those in the late phase of chronic pain. We, therefore, applied the PTAC and evaluated the analgesic effects from day 14 to day 16. As shown in Figure 1D, the PTAC at 0.01 mg/kg had no effects on the PWTs for either the sham or nerve injury group (mean difference [95% CI]: −0.02 [−0.03 to 0.00], n = 5 for each group), whereas it raised the PWTs of the nerve injury group at 0.05 mg/kg (mean difference [95% CI]: 0.45 [0.39–0.50], Figure 1E) and 0.1 mg/kg (mean difference [95% CI]: 0.44 [0.37–0.51], Figure 1F), but not in the sham group. However, the PWTs decreased 2 hours after injection (0.05 mg/kg: mean difference [95% CI]: −0.01 [−0.02 to 0.01]; 0.10 mg/kg; mean difference [95% CI]: 0.01 [−0.04 to 0.05], Figure 1F), suggesting that the analgesic effects disappeared by that time. Compared with the results from day 3 to day 5, the analgesic effects of PTAC lasted a shorter time from day 14 to day 16.
Furthermore, when atropine (0.1 mg/kg, i.p.) was used to block the activities of mAChRs, it abolished the effects of PTAC (0.05 mg/kg, i.p.) on the PWTs (mean difference [95% CI]: 0.01 [−0.01 to 0.02], n = 9 for injury, Figure 1G), suggesting that the mAChRs were involved in the analgesic effects of PTAC.
Microinfusion of PTAC to the ACC Increased the PWTs
The ACC is an important brain area for the regulation of chronic pain. To further confirm the analgesic effects of PTAC, we microinfused the PTAC to the ACC of the mice with CPN ligation. As shown in Figure 2, A and B, the PTAC significantly increased the PWTs on both the left (mean difference [95% CI]: 0.20 [0.03–0.37], n = 5 for injury) and right hind paws (mean difference [95% CI]: 0.39 [0.28–0.49], n = 5 for injury), and this effect could last for over 3 hours, whereas the PWTs of the hind paws decreased in 24 hours after the injection.
Figure 2.
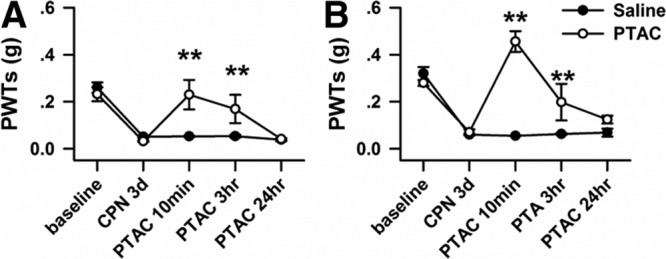
Micro-infusion of (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1] octane (PTAC) to the anterior cingulate cortex (ACC) raised the paw withdrawal threshold (PWT) in nerve-injured mice. A, Microinfusion of PTAC into ACC increased the PWTs of the left hind paw of mice with nerve injuries (2-way RM ANOVA, saline versus PTAC: F1;54 = 15.30, P < .01; treatments: F4;54 =17.37, P < .01, interaction: F4;54 = 5.05, P < .01; n = 5 for PTAC and n = 6 for saline, **P < .01 under Tukey test). B, Microinfusion of PTAC into ACC increased the PWTs of the right hind paw of mice with nerve injuries (2-way RM ANOVA, saline versus PTAC: F1;4 = 26.92, P < .01; treatments: F4;54 = 29.35, P < .01, interaction: F4;54 = 20.96, P < .01; n = 5 for PTAC and n = 6 for saline, **P < .01 under Tukey test). ANOVA indicates analysis of variance; RM, repeated measures.
Application of PTAC Did Not Induce CPP
We further evaluated the possible effects of PTAC on place preference using the CPP behavioral paradigm. As shown in Figure 3A, the mice with sham treatments spent equal time in the chambers during the preconditioning and testing period, and the application of PTAC at 0.05 mg/kg did not induce a preference for the paired chamber (Figure 3B); consistently, no difference was detected in the preference time (Figure 3C), indicating that the sham treatment failed to induce place preference. To further confirm this point, a higher dose of PTAC (0.2 mg/kg) was systemically applied, and the same behavior was observed (Figure 3, D–F). Therefore, neither dose of PTAC induced place preference in the CPP.
Figure 3.
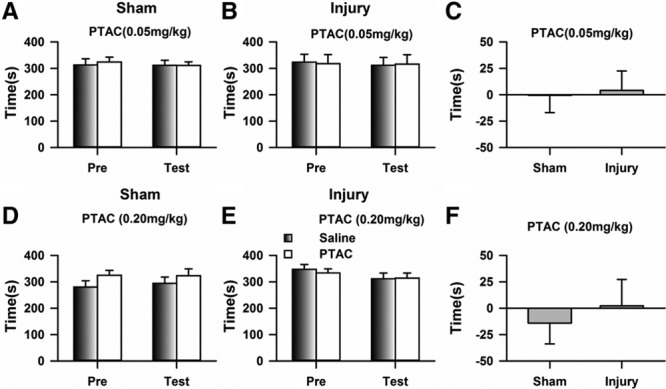
Systematic application of (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1] octane (PTAC) did not induce a place preference for the chemical-paired chamber. A, Mice with sham treatments spent equal times in the chambers during the preconditioning and testing periods (2-way RM ANOVA, pre versus test: F1;39 = 0.97, P = .35; drug versus saline: F1;39 = 0.07, P = .80, interaction: F1;39 = 0.09, P = .77; n = 10). B, No place preference was detected after the application of PTAC (0.05 mg/kg) to the mice with nerve injuries (2-way RM ANOVA, pre versus test: F1;35 = 0.02, P = .90; drug versus saline: F1;35 = 0.01, P = .91, interaction: F1;35 = 0.18, P = .68; n = 9). C, No difference was detected in the preference times of the sham and injury groups (t test, P > .05). D, Application of PTAC (0.20 mg/kg) did not induce a place preference in mice with sham treatments (2-way RM ANOVA, pre versus test: F1;39 = 9.54, P < .05; drug versus saline: F1;39 = 0.02, P = .99, interaction: F1;39 = 0.37, P = .56; n = 10). E, No place preference was detected after the application of PTAC (0.20 mg/kg) to the mice with nerve injuries (2-way RM ANOVA, pre versus test: F1;39 = 0.29, P = .60; drug versus saline: F1;39 = 1.01, P = .34, interaction: F1;39 = 0.17, P = .69; n = 10). F, Preference time of mice with sham treatments was similar to that of the injury group during the test period (t test, P > .05). ANOVA indicates analysis of variance; RM, repeated measures.
Application of PTAC Rebalanced the Weight Distribution on the Hind Paws
The difference in weight distribution between the normal and pain-suffering subjects has been considered as an index of the level of discomfort. As shown in Figure 4A, the weight distribution on the left hind paw was significantly lower than that on the right hind paw at day 3 after CPN, and the left/right ratio (L/R ratio) changed from 1.05 ± 0.03 to 0.56 ± 0.03 (mean difference [95% CI]: −0.49 [−0.40 to −0.58], n = 6, Figure 4B), suggesting that the weight distribution was changed by the nerve injury. The application of PTAC at 0.01 mg/kg had no effect on weight distribution (mean difference [95% CI]: 0.01 [−0.05 to 0.06]), whereas it increased weight distribution on the left hind paw at 0.05 mg/kg (mean difference [95% CI]: 0.44 [0.30–0.58]) and 0.10 mg/kg (mean difference [95% CI]: 0.43 [0.36–0.50]); therefore, the L/R ratio changed to about 1.00, suggesting that the unbalanced weight distribution induced by nerve ligation was changed by the PTAC.
Figure 4.
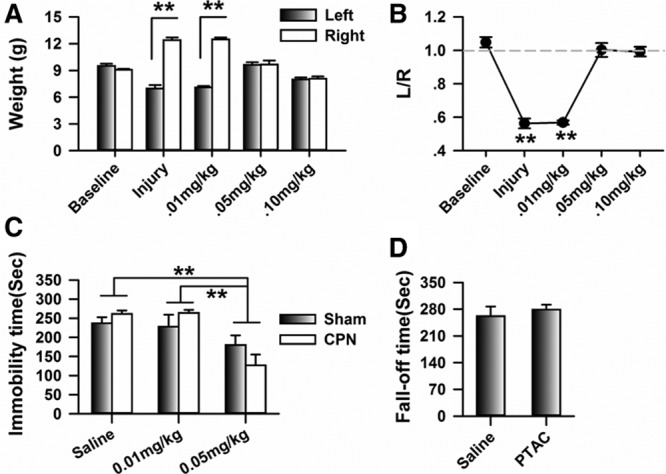
Systemic application of (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1] octane (PTAC) rebalanced the weight distribution on the left and right hind paws of mice with nerve injuries. A, Application of the PTAC at 0.05 mg/kg and 0.10 mg/kg rebalanced the weight distribution on the left and right hind paws of mice with common peroneal nerve (CPN) ligation (2-way RM ANOVA, sham versus injury: F1;59 = 204.12, P < .01; treatments: F5;59 = 9.29, P < .01, interaction: F4;59 = 95.75, P < .01; n = 6 for CPN, **P < .01 under SNK test). B, PTAC at 0.05 mg/kg and 0.10 mg/kg changed the ratio of the weight distribution on the left and right hind paws (1-way RM ANOVA, F4;29 = 65.44, P < .01, **P < .01 under SNK test). C, PTAC decreased the immobility time of mice from both the sham and nerve injury groups at the dose of 0.05 mg/kg, but not at the dose of 0.01 mg/kg (2-way ANOVA, sham versus injury: F1;39 = 3.45, P = .07, treatments: F2;39 = 53.11, P < .01, interaction: F2;39 = 1.94, P = .16; n = 10 for each group with saline injection, n = 5 for each group with PTAC injection). D, 0.05 mg/kg PTAC did not affect the motor responses of the animals on the rota-rod (t test, P > .05, n = 5 for each group). ANOVA indicates analysis of variance; RM, repeated measures; SNK, Student-Newman-Keuls.
Antidepressant Effects of PTAC
To test whether PTAC exposure can reduce initial depression in a pain model, the FST was applied after 14 days on the CPN model. As in previous reports, the application of PTAC at 0.05 mg/kg decreased the immobility time of both the sham and nerve injured mice, simultaneously (2-way ANOVA, sham versus injury: F1;39 = 3.45, P = .07, treatments: F2;39 = 53.12, P < .001, interaction: F2;39 = 1.94, P = .16, Figure 4C). These results confirmed the antidepressant-like effects of PTAC. No difference was detected between the sham and nerve injury group, suggesting that PTAC had similar antidepression effects on the sham and nerve injury groups.
To confirm that the analgesic effects of PTAC were not due to changes in motor function, we further evaluated the effects of PTAC on the rota-rod performance. As shown in Figure 4D, the application of PTAC (0.05 mg/kg) did not change the stay time of the normal mice (before: 261.4 ± 25.53 seconds, after 278.8 ± 13.04 seconds, n = 5, Figure 4D). Therefore, the application of PTAC to the normal mice has no effect on the motor function.
Expression Level of M2 Receptors in the Spinal Cord and ACC of Mice With Peripheral Nerve Injury
The analgesic effects of PTAC on the D3–5 lasted longer than the effects on D14–16, possibility because the expression levels of M2 and M4 in the pain-related brain areas are different. We, therefore, examined the expression of M2 subtypes of mAChRs in the spinal cord and ACC at D3, D7, and D14 after the CPN ligation. As shown in Figure 5A, the protein level of M2 in the spinal cord decreased to 0.38 ± 0.12 times (n = 3) and 0.38 ± 0.19 times than that of the sham group at D7 (mean difference [95% CI]: −0.62 [−0.29 to −0.95]) and D14 (mean difference [95% CI]: −0.01 [−0.08 to −1.17]), respectively. No decrease was observed on D3 (0.96 ± 0.34). Similarly, the M2 in the ACC decreased to 0.57 ± 0.08 and 0.19 ± 0.06 times (n = 5, Figure 5B) than that of sham group at day 7 (mean difference [95% CI]: −0.43 [−0.24 to −0.62]) and day 14 (mean difference [95% CI]: −0.81 [−0.67 to −0.96]), respectively. Therefore, the M2 in both the spinal cord and ACC decreased after the nerve injury.
Figure 5.
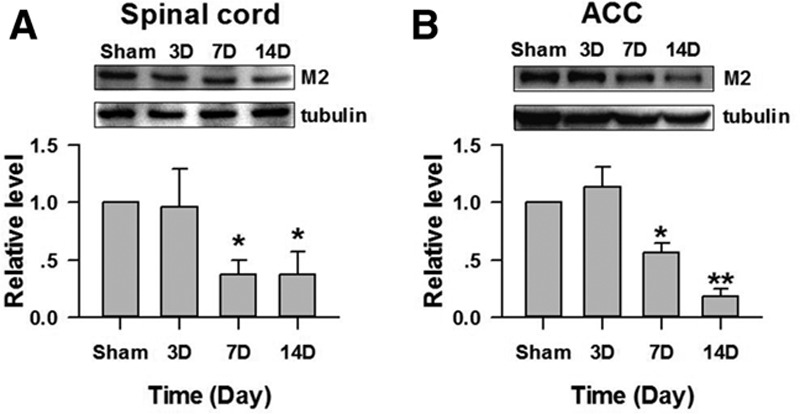
Change in the expression of M2 in the spinal cord and anterior cingulate cortex (ACC) after peripheral nerve injury. A, Expression levels of M2 in the spinal cord were decreased at D7 and D14 after nerve injury (*P < .01 compared to sham group, n = 3). B, Expression levels of M2 in the ACC were decreased at D7 and D14 after nerve injury (1-way ANOVA, F3;19 = 17.13, P < .01; n = 5 for each group *P < .05, **P < .01 compared with other groups under SNK test). SNK indicates Student-Newman-Keuls.
Expression Level of M1 and M2 Receptors in the PFC, CPU, and VTA of Mice With Peripheral Nerve Injuries
The CPP is dependent on the reward system. We, therefore, examined the expression of both M1 and M2 subtypes of mAChRs in the PFC, CPU, and VTA at D14 after the CPN ligation. As shown in Figure 6, A–C, the protein levels of M2 in the PFC (mean difference [95% CI]: −0.85 [−0.54 to −1.16], n = 4 for sham, n = 5 for injury), CPU (mean difference [95% CI]: −0.74 [−0.54 to −1.16]), and VTA (mean difference [95% CI]: −0.33 [−0.14 to −0.52]) decreased when compared with those of the sham group at D14, respectively. Unexpectedly, at day 14 the M1 in the PFC (mean difference [95% CI]: −0.58 [−0.17 to −0.99], n = 4 for sham, n = 5 for injury), CPU (mean difference [95% CI]: −0.66 [−0.16 to −1.16]), and VTA (mean difference [95% CI]: −0.33 [−0.18 to −0.47]) also decreased when compared with the sham group, respectively (n = 4, Figure 6, D–F). These results show that both the M1 and M2 in PFC, CPU, and VTA were decreased after nerve injury.
Figure 6.
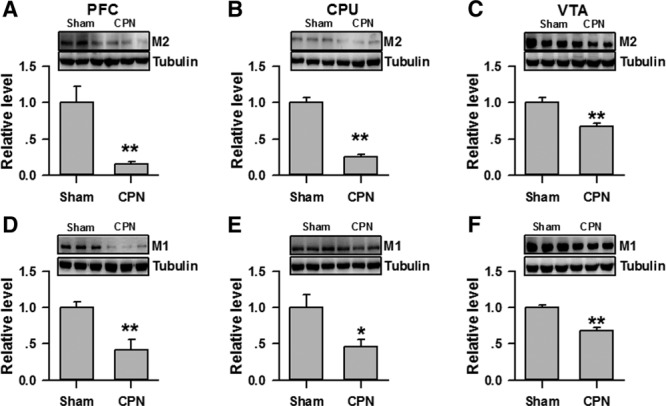
Expression level of M1 and M2 receptors in the prefrontal cortex (PFC), caudate putamen (CPU), and ventral tegmental area (VTA) of mice with peripheral nerve injuries. A, Expression levels of M2 in the PFC were decreased at D14 after nerve injury. (**P < .01, n = 4 for sham, n = 5 for injury). B, Expression levels of M2 in the CPU were decreased at D14 after nerve injury (**P < .01). C, Expression levels of M2 in the VTA were decreased at D14 after nerve injury (**P < .01). D, Expression levels of M1 in the PFC were decreased at D14 after nerve injury (**P < .01). E, Expression levels of M1 in the CPU were decreased at D14 after nerve injury (*P < .05). F, Expression levels of M1 in the VTA were decreased at D14 after nerve injury (**P < .01).
DISCUSSION
In this study, the analgesic effects of PTAC on a mouse model of neuropathic pain were evaluated for the first time. The application of PTAC alleviated the mechanical allodynia in a dose-dependent manner in the early and late phases after nerve injury. It rebalanced the weight distribution, but failed to induce a place preference in the CPP behavioral test. At the same doses, the analgesic effects at D3–5 lasted for a longer time than at D14–16, perhaps due to the down-regulation of the M2 in both the spinal cord and ACC. Our data suggest that the PTAC had potent analgesic effects on the mechanical allodynia, and the rebalancing of weight distributions suggests that the amount of discomfort changed.
Partial Agonists of mAChRs as Analgesic Agents
The analgesic effects of the partial agonists of mAChRs were evaluated using different animal models. Recently, 2 chemicals, including vedaclidine and PTAC were found to have partial agonists effects on mAChRs.14,15 The analgesic effects of vedaclidine (0.3–10 mg/kg s.c.) were evaluated with the formalin test, capsaicin-induced mechanical hyperalgesia, and the carrageenan test in rats.16 Subsequently, it was found that vedaclidine reversed pertussis toxin–induced thermal allodynia.10 In this study, the analgesic effects of PTAC were investigated using a neuropathic pain mouse model in which the motor function was unaffected.18 We found that PTAC increased the PWTs of mice with nerve injuries at 0.05 and 0.10 mg/kg, but it did not have an effect on mice that had undergone sham treatments. The dose was similar to that used in previous studies that found that PTAC (0.03, 0.10, and 0.30 mg/kg) affected the spontaneous locomotor activities of rats and apomorphine-induced climbing in mice.14 Our data, therefore, provided more information about the potential use of the partial agonists of mAChRs for pain management.
Analgesic Effects of PTAC on Spontaneous Pain
In this study, both CPP and static weight bearing were used to test the effects of PTAC on spontaneous pain.19 We found that PTAC at 0.05 mg/kg and 0.20 mg/kg did not induce CPP (Figure 3). CPP is dependent on the reward system. The enhanced dopamine (DA) released in the nucleus accumbens (NAc) shell was related to CPP in rats with tumor27 or spinal nerve ligation (SNL).28 Pharmacologic activation of the rACC opioid receptors of injured animals was sufficient to stimulate dopamine release in the NAc and produce CPP.29 Therefore, CPP is dependent on the engagement of the reward system in pain relief.30 It was reported that PTAC inhibits the firing of dopamine neurons,14,17 and the activities of M4 control the D1 dopamine receptors negatively,31 which inhibits the reward system associated with pain relief. This effect may disrupt the place preference for the chemical-paired chamber. Therefore, the negative effect on the CPP is not enough to suggest that PTAC did not alleviate spontaneous pain.
The mechanical allodynia assessment is different from the weight-bearing assessment. Changes in weight bearing on the hind paws in the chronic pain model have been reported in previous studies.23,32 In this study, we showed that the CPN ligation induced unbalanced weight distribution on the hind paws, which were rebalanced by the application of PTAC (0.05 mg/kg). These data suggest that the discomfort induced by nerve injury was changed by PTAC. Hypersensitivity to the mechanical stimulations was observed on both hind paws (Figure 2, A and B)18; therefore, the unbalanced weight distributions may simply come from discomfort rather than the allodynia, and the change in weight distribution may indicate changes in spontaneous pain.
Downregulation of mAChRs Under the Chronic Pain Condition
The change in the mAChRs system in the ACC was related to the pathological change. Previous studies found that the sciatic nerve denervation reduced both the mRNA level and protein level of M2 in the cg1 of rats at day 8 after surgery.33 Here, we found that the protein levels of M2 were downregulated at D7 and D14 after nerve injury; this provides more detailed information about the dynamic changes of M2 in the ACC that are induced by nerve injury. Our pervious study showed that the expression level and the activities of the AChEs in the ACC increased after nerve injury,19 indicating that the breakdown of acetylcholine in the ACC of mice with nerve injuries is faster than under normal conditions. Consequently, the level of acetylcholine (ACh) may be lower in the pain-related brain regions, which was observed in the poster insular cortex of oxaliplatin-treated rats.7 The basal release of ACh was significantly lower in rats with partial ligation of the sciatic nerve.34 Our data further showed that the expression levels of both M1 and M2 were decreased in the VTA, PFC, and striatum, suggesting that the regulation of the mAChRs system by the reward system was downregulated under the chronic pain condition. Therefore, our data provide more information about the dysfunction ACh system under the chronic pain condition.
ACKNOWLEDGMENTS
This study was supported by the National Program on Key Basic Research Project (973 Program) (No. 2014CB548200). The authors declare no competing financial interests.
DISCLOSURES
Name: Yong-Jie Wang, MS.
Contribution: This author helped conduct the behavioral and biochemical experiments, collect data, analyze the data, and prepare the manuscript.
Name: Zhen-Xing Zuo, PhD.
Contribution: This author helped to conduct the study, analyze the data, and prepare the manuscript.
Name: Mei Zhang, BS.
Contribution: This author helped collected the behavioral data and prepare the manuscript.
Name: Zhi-Hui Feng, PhD.
Contribution: This author helped to design the study, analyze the data, and prepare the manuscript.
Name: Min Yan, MD.
Contribution: This author helped to design the study, analyze the data, and prepare the manuscript.
Name: Xiang-Yao LI, PhD.
Contribution: This author helped to design the study, analyze the data, and prepare the manuscript.
This manuscript was handled by: Jianren Mao, MD, PhD.
Footnotes
Published ahead of print December 19, 2016.
Funding: None.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
REFERENCES
- 1.Wess J, Eglen RM, Gautam D.Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Saad Huijer H.Chronic pain: a review. J Med Liban. 2010;58:21–27. [PubMed] [Google Scholar]
- 3.Bushnell MC, Ceko M, Low LA.Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan IM, Wennerholm M, Singletary E, et al. Ablation of primary afferent terminals reduces nicotinic receptor expression and the nociceptive responses to nicotinic agonists in the spinal cord. J Neurocytol. 2004;33:543–556. [DOI] [PubMed] [Google Scholar]
- 5.Jeong SG, Choi IS, Cho JH, Jang IS.Cholinergic modulation of primary afferent glutamatergic transmission in rat medullary dorsal horn neurons. Neuropharmacology. 2013;75:295–303. [DOI] [PubMed] [Google Scholar]
- 6.Chen SR, Pan HL.Activation of muscarinic receptors inhibits spinal dorsal horn projection neurons: role of GABAB receptors. Neuroscience. 2004;125:141–148. [DOI] [PubMed] [Google Scholar]
- 7.Ferrier J, Bayet-Robert M, Dalmann R, et al. Cholinergic neurotransmission in the posterior insular cortex is altered in preclinical models of neuropathic pain: key role of muscarinic M2 receptors in donepezil-induced antinociception. J Neurosci. 2015;35:16418–16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorino DF, Garcia-Guzman M.Muscarinic pain pharmacology: realizing the promise of novel analgesics by overcoming old challenges. Handb Exp Pharmacol. 2012:191–221. [DOI] [PubMed] [Google Scholar]
- 9.Eisenach JC.Muscarinic-mediated analgesia. Life Sci. 1999;64:549–554. [DOI] [PubMed] [Google Scholar]
- 10.Womer DE, Shannon HE.Reversal of pertussis toxin-induced thermal allodynia by muscarinic cholinergic agonists in mice. Neuropharmacology. 2000;39:2499–2504. [DOI] [PubMed] [Google Scholar]
- 11.Duttaroy A, Gomeza J, Gan JW, et al. Evaluation of muscarinic agonist-induced analgesia in muscarinic acetylcholine receptor knockout mice. Mol Pharmacol. 2002;62:1084–1093. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Xu H, Wu LJ, et al. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med. 2011;3:65ra3. [DOI] [PubMed] [Google Scholar]
- 13.Li XY, Ko HG, Chen T, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–1404. [DOI] [PubMed] [Google Scholar]
- 14.Bymaster FP, Shannon HE, Rasmussen K, et al. Unexpected antipsychotic-like activity with the muscarinic receptor ligand (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1]octane. Eur J Pharmacol. 1998;356:109–119. [DOI] [PubMed] [Google Scholar]
- 15.Shannon HE, Sheardown MJ, Bymaster FP, et al. Pharmacology of butylthio[2.2.2] (LY297802/NNC11-1053): a novel analgesic with mixed muscarinic receptor agonist and antagonist activity. J Pharmacol Exp Ther. 1997;281:884–894. [PubMed] [Google Scholar]
- 16.Shannon HE, Jones CK, Li DL, Peters SC, Simmons RM, Iyengar S.Antihyperalgesic effects of the muscarinic receptor ligand vedaclidine in models involving central sensitization in rats. Pain. 2001;93:221–227. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen T, Sauerberg P, Nielsen EB, et al. Muscarinic receptor agonists decrease cocaine self-administration rates in drug-naive mice. Eur J Pharmacol. 2000;402:241–246. [DOI] [PubMed] [Google Scholar]
- 18.Vadakkan KI, Jia YH, Zhuo M.A behavioral model of neuropathic pain induced by ligation of the common peroneal nerve in mice. J Pain. 2005;6:747–756. [DOI] [PubMed] [Google Scholar]
- 19.Zuo ZX, Wang YJ, Liu L, et al. Huperzine A alleviates mechanical allodynia but not spontaneous pain via muscarinic acetylcholine receptors in mice. Neural Plast. 2015;2015:453170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL.Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 21.King T, Vera-Portocarrero L, Gutierrez T, et al. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu C, King T, Okun A, Lai J, Fields HL, Porreca F.Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tétreault P, Dansereau MA, Doré-Savard L, Beaudet N, Sarret P.Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol Behav. 2011;104:495–502. [DOI] [PubMed] [Google Scholar]
- 24.Small KM, Nunes E, Hughley S, Addy NA.Ventral tegmental area muscarinic receptors modulate depression and anxiety-related behaviors in rats. Neuroscience Letters. 2016;616:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunham NW, Miya TS.A note on a simple apparatus for detecting neurobiological deficit in rats and mice. J Am Pharmacol Assoc. 1957;46:208–209. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Wu LJ, Kim SS, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59:634–647. [DOI] [PubMed] [Google Scholar]
- 27.Remeniuk B, Sukhtankar D, Okun A, et al. Behavioral and neurochemical analysis of ongoing bone cancer pain in rats. Pain. 2015;156:1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie JY, Qu C, Patwardhan A, et al. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain. 2014;155:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navratilova E, Xie JY, Meske D, et al. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci. 2015;35:7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navratilova E, Xie JY, King T, Porreca F.Evaluation of reward from pain relief. Ann N Y Acad Sci. 2013;1282:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomeza J, Zhang L, Kostenis E, et al. Generation and pharmacological analysis of M2 and M4 muscarinic receptor knockout mice. Life Sci. 2001;68:2457–2466. [DOI] [PubMed] [Google Scholar]
- 32.Tappe-Theodor A, Kuner R.Studying ongoing and spontaneous pain in rodents–challenges and opportunities. Eur J Neurosci. 2014;39:1881–1890. [DOI] [PubMed] [Google Scholar]
- 33.Ortega-Legaspi JM, León-Olea M, de Gortari P, et al. Expression of muscarinic M1 and M2 receptors in the anterior cingulate cortex associated with neuropathic pain. Eur J Pain. 2010;14:901–910. [DOI] [PubMed] [Google Scholar]
- 34.Schechtmann G, Song Z, Ultenius C, Meyerson BA, Linderoth B.Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139:136–145. [DOI] [PubMed] [Google Scholar]


