Abstract
Background:
Transbronchial lung cryobiopsy (TBLC) has been used to establish the diagnosis of interstitial lung disease (ILD) in recent years. The technique and diagnostic yield vary among institutions. We report a new 2-scope technique and the results of TBLC in our institution.
Methods:
This is a retrospective chart review of patients who underwent TBLC for evaluation of ILD. Bronchoscopy with TBLC was performed by a board-certified interventional pulmonologist with a 2-scope technique under general anesthesia.
Results:
A total of 74 patients underwent TBLC with a 2-scope technique. Their mean age was 54±14 years. The mean tissue surface area was 63.54±6.76 mm2. The average anesthesia time was 80.66 minutes. The diagnostic yield was 87.84%. The most common diagnosis was sarcoidosis pneumothorax, which occurred in 5 cases (7%). There was 1 case with bronchoscopic-related respiratory failure associated with significant bleeding. Death occurred in 3 cases (4%), which is comparable to recent mortality data for “elective” surgical lung biopsy for ILD (1.7% to 4.2%).
Conclusion:
TBLC with a 2-scope technique could be an alternative method for diagnosing various types of ILD in patients unfit for surgical lung biopsy. Further prospective studies should clarify its role in the diagnostic armamentarium for undiagnosed ILDs.
Key Words: interstitial lung disease, diagnosis, cryobiopsy, transbronchial biopsy, new technique
BACKGROUND
To establish the diagnosis of interstitial lung disease (ILD), tissue diagnosis is often required. Conventional transbronchial biopsy through a flexible bronchoscope has low diagnostic yield. In a large review of 801 patients who underwent transbronchial biopsy, diagnosis was achieved in <30%.1 Therefore, surgical lung biopsy (SLB) is considered the gold standard for pathologic diagnosis of ILD.2 However, it requires endotracheal intubation, good performance status to tolerate general anesthesia, chest tube placement, and hospitalization with a postoperative mortality rate of 1.7% to 4.2% in “elective” cases and 16% to 17.5% in “urgent” cases.3–7
Transbronchial lung cryobiopsy (TBLC) has been introduced in recent years to establish the diagnosis of ILD. The size of the biopsy specimen obtained from TBLC is significantly larger than that obtained through conventional transbronchial biopsy with preserved histopathology.8,9 The procedural technique varies among institutions, ranging from using the flexible bronchoscope with conscious sedation to using the rigid bronchoscope with Fogarty balloon under general anesthesia.8–11 The diagnostic yield of TBLC was >70%.9–13 The complication rates vary among studies with reported pneumothorax rates of 0% to 29%.10–13 Data on TBLC are limited to only 1 retrospective study in the United States that included 25 patients.10
We report a new technique of TBLC using 2 bronchoscopes and our initial experience with this new technique, including diagnostic yield, safety, and complications.
METHODS
This is a retrospective study of 74 patients referred from the University of Cincinnati Interstitial Lung Disease Center or inpatients from the University of Cincinnati Medical Center to the Interventional Pulmonology Service for tissue diagnosis of ILD between October 2013 and June 2015. A total of 239 patients were evaluated during this time period: 59 patients underwent conventional transbronchial biopsies when the suspected diagnosis was either sarcoidosis, hypersensitivity pneumonitis, or cryptogenic organizing pneumonia; 77 patients with undifferentiated ILD underwent SLB, whereas 74 patients underwent TBLC (they either declined SLB or their functional status was prohibitive for SLB); the remainder 29 patients did not require tissue diagnosis as laboratory and imaging data were deemed sufficient for diagnosis. The study was approved by our Institutional Review Board (Study 2015-2392). All patients underwent flexible bronchoscopy with TBLC, providing written informed consent for the procedure.
We reviewed the following data from the patients’ charts: age, sex, past medical history, functional status, pulmonary function tests, echocardiograms, laboratory data, radiographic data, procedure, anesthesia and pathology reports, and complications. All patients were classified by the American Society of Anesthesiologists (ASA) scoring system before the procedure.13
Bronchoscopies were performed by board-certified interventional pulmonologists. The patients underwent general anesthesia with spontaneous breathing. A laryngeal mask airway or endotracheal tube was used to secure the airway. Two bronchoscopes (Olympus XT 180; Olympus, Tokyo, Japan) were utilized. The first bronchoscope was advanced into the airway. An Erbe (Tübingen, Germany) 1.9- or 2.4-mm cryoprobe was advanced through the working channel to the distal lung parenchyma under fluoroscopic guidance. The probe was withdrawn approximately 1 cm from the point of resistance. After this, cryofreezing was activated with freeze times of 5 to 8 seconds. The bronchoscope and cryoprobe were then withdrawn en bloc and handed to an assistant. Immediately, another bronchoscope was advanced into the airway and wedged at the bronchus to control bleeding. An assistant then submerged the probe and frozen specimen into formalin. Once thawing was adequate, the specimen was removed from the probe. At least 2 biopsies from different segments of both the upper and lower lobes were performed, and 1 biopsy was also obtained from the lingular or right middle lobe. All patients underwent a chest x-ray immediately after the procedure. Patients were monitored for an additional 2 hours before discharge in the endoscopy area.
Complications associated with the bronchoscopic procedure included acute respiratory failure, acute coronary syndromes, new cardiac arrhythmias requiring short-term or long-term therapy, pneumonia, lobar atelectasis, pneumothorax, bleeding, thromboembolic disease, and death, regardless of cause, within 30 days of the procedure.
Bleeding at the time of bronchoscopy was classified as follows: grade 1: no bleeding or minimal bleeding without the need to use bronchoscopic suction; grade 2: bleeding that was controlled with bronchoscopic suction; grade 3: bleeding that required intrabronchial epinephrine or cold saline; or grade 4: bleeding that required other interventions such as intubation, blood transfusion, or laser ablation.
Anesthesia time was calculated from the anesthesiology reports in the electronic medical record (anesthesia start and end times).
Hematoxylin and eosin slides were prepared from the formalin-fixed samples and reviewed by a pulmonary pathologist. The histopathologic results were integrated with the patient’s clinical history, physical examination, work history, relevant blood testing, pulmonary function testing, and radiographic imaging including high-resolution computer tomography images. The diagnosis was established by agreement by a multidisciplinary team including pulmonologists, pulmonary radiologists, and a pulmonary pathologist in a clinical-radiologic-pathologic consensus conference.
Statistics
Results were expressed as mean with SD and median with value range as appropriate.
RESULTS
Overall, 74 patients with a mean age of 54±14 years were analyzed. Of them, 36/74 (49%) were male patients. The average body mass index was 29.4±8.5. All patients underwent high-resolution computer tomography for initial evaluation. Table 1 shows the demographic and baseline data.
TABLE 1.
Demographic and Baseline Data
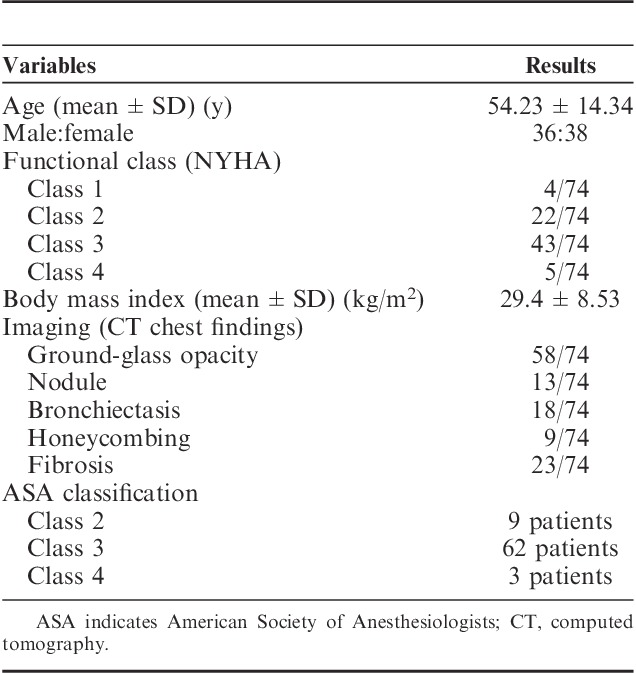
Table 2 summarizes the biopsy features. The median number of samples from each area is indicated, with the total size of the specimen indicated as mean surface area. All biopsies contained alveolar parenchyma.
TABLE 2.
Biopsy Features

All patients but 1 underwent general anesthesia. The average anesthesia time was 80.66 minutes. In our institution, the average anesthesia time for an SLB ranged between 45 and 60 minutes.
Table 3 summarizes the pathologic diagnoses. The overall rate with a specific diagnosis was 87.84% (65/74). The most common diagnosis was noncaseating granuloma in 18 cases. In 17 of these, the final diagnosis was sarcoidosis. In 1 case, an alternative cause for the granuloma was identified (Mycobacterium abscessus based on culture). Two patients received a diagnosis of extrapulmonary sarcoidosis with normal lung tissue on transbronchial cryobiopsy, and the diagnosis was confirmed by SLB. These 2 patients were highly suspicious for neurosarcoidosis and lung biopsies were needed. One patient received a diagnosis of adenocarcinoma on the basis of biopsy, another had chronic lymphocytic leukemia, whereas another received a diagnosis of acute inflammation from biopsy and was found to have bacterial pneumonia on the basis of culture.
TABLE 3.
Pathologic Diagnosis Made by Transbronchial Cryobiopsy
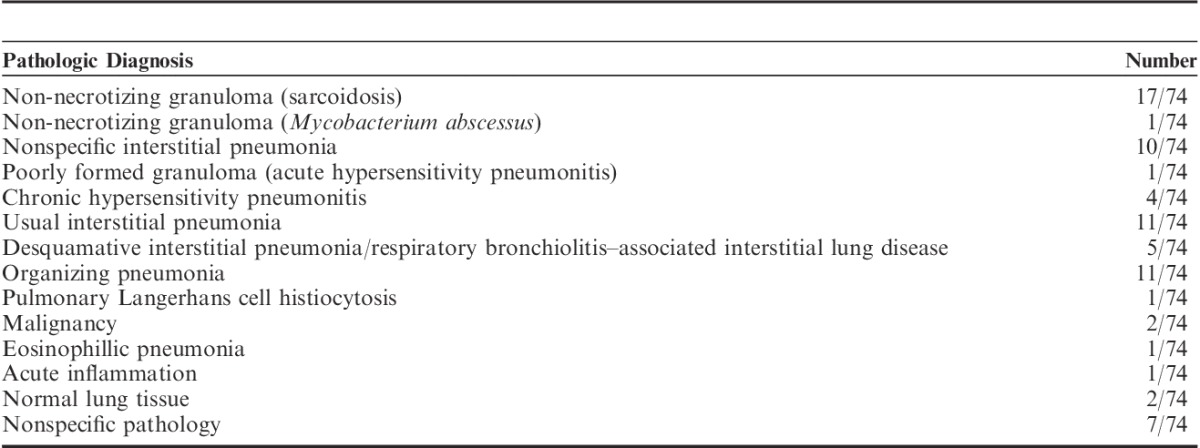
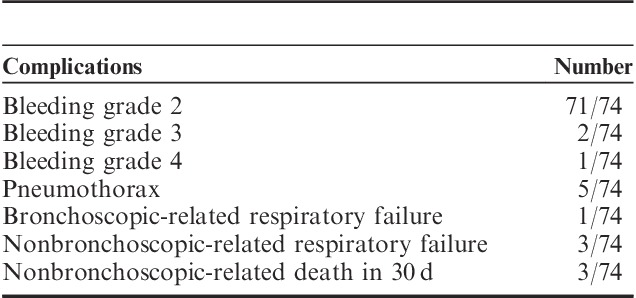
Table 4 lists the complications encountered. Most patients had grade ≥2 bleeding, although only 1 case had severe (grade 4) bleeding requiring intubation. Of the 5 cases with pneumothoraces, all of them required chest tube placement for 3 to 5 days. One patient had respiratory failure due to bleeding but survived. Two patients had respiratory failure due to pulmonary edema, one of whom died. Overall, 3 patients died within 30 days of the procedure (1 from pulmonary edema from newly diagnosed severe aortic stenosis, 1 from organizing pneumonia who underwent comfort care, and 1 from pulmonary embolism). No acute coronary syndromes, new cardiac arrhythmias, pneumonia, or lobar atelectasis occurred.
TABLE 4.
Complications From Transbronchial Cryobiopsies
DISCUSSION
TBLC has been recently described in patients with ILD. The procedural yield and safety vary among published studies. We describe a technique utilizing 2 bronchoscopes, which is associated with minimal complications from the procedure. Despite the relatively low risk, adequate sampling to obtain a specific diagnosis was obtained in over 80% of cases.
Previous reports using the cryoprobe have demonstrated a high yield but variable complication rates. Table 5 summarizes the results of some of the reports.
TABLE 5.
Comparison of Yield and Pneumothorax of Cryobiopsy Sampling
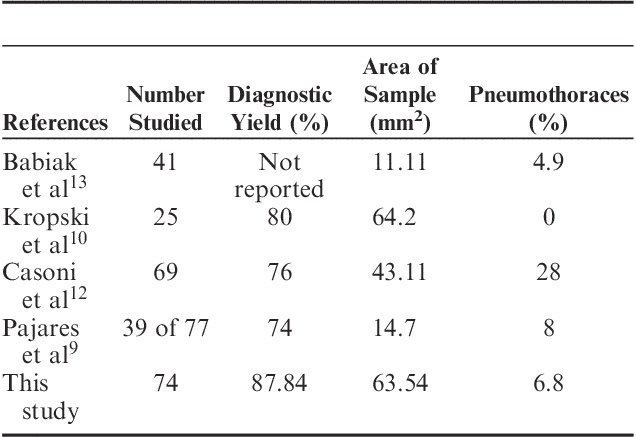
Two of the prior studies directly compared cryoprobe versus conventional transbronchial biopsy. In the study by Babiak et al,13 all patients underwent both procedures. They found the sample size to be significantly larger when using the cryoprobe than that observed in specimens retrieved from transbronchial forceps biopsy (15.11 vs. 5.82 mm2, P<0.01). Moreover, there was no crush artifact for the cryoprobe-obtained sample. Pajares et al9 reported a randomized controlled trial comparing transbronchial cryobiopsy and conventional forceps by sampling 77 ILD patients. The mean area of tissue retrieved from cryoprobe biopsies was significantly larger than that from conventional forceps biopsies (14.7 vs. 3.3 mm2, P<0.001). Diagnostic yield in the cryoprobe group was also higher than that in the conventional forceps group (74.4% vs. 34.1%, P<0.001). A higher percentage of grade 2 bleeding (56.4% vs. 34.2%) and pneumothoraces (7.7% vs. 5.2%) was observed in the cryoprobe group, but these differences were not statistically significant.
Although almost all of our patients had some bleeding after biopsy, only 3 had moderate to severe bleeding. This is in line with other reports.9,12
Given the retrospective nature of our study, the calculated average anesthesia time for all cases was 100.41 minutes. This included other sampling techniques such as bronchioalveolar lavage, transbronchial lung biopsies, endobronchial ultrasound fine needle aspiration, and combined surgical procedures (in 1 patient the anesthesia time was 386 min, for TBLC and robotic-assisted total laparoscopic hysterectomy). When considering the cases with TBLC only, the average anesthesia time was 80.66 minutes. This also reflects the novelty of the procedure. Currently in our institution, the average time for TBLC is 40 to 45 minutes (Sadia Benzaquen, Alejandro Aragaki, Thitiwat Sriprasart, unpublished data , 2015-2016).
We describe a new technique of TBLC using 2 bronchoscopes. Our patients were symptomatic with functional impairment and demonstrated both physiological and radiographic pulmonary abnormalities. The majority of our cohort patients who underwent general anesthesia were ASA 3. TBLC was performed with an average of 2 biopsies obtained from both the upper lobe and lower lobe. One biopsy was carried out in the middle lobe or lingular lobe. We achieved 87% diagnostic yield with low pneumothorax and bleeding rates. The mean area of the specimen was larger than that in other studies. We believe that the 2-scope technique may have improved bleeding control and increased the number of biopsies taken on each patient.
Our TBLC technique’s novelty is that we included patients otherwise deemed not surgical candidates or who refused SLB. Recent publications7,14 have described in-hospital mortality for SLB ranging from 1.7% to 4.2% in “elective” cases and from 16% to 17.5% in “urgent” cases, which had various combinations of respiratory failure, immunosuppression, multiple organ failure, and underlying solid or hematologic malignancies. Two out of 3 patients who died in the 30-day follow-up were “urgent” cases: both had chronic respiratory failure with elevated oxygen requirements, one of them was not a surgical candidate for SLB, and one had a fairly recently diagnosed aortic stenosis, upon admission to the medical intensive care unit.
TBLC may be sufficient for diagnosing various types of ILDs. The most frequent histopathologic finding in this study was non-necrotizing granuloma (sarcoidosis) followed by usual interstitial pneumonia and non specific interstitial pneumonia. As patients with sufficient clinical and radiographic features13 to be diagnosed with a certain ILD were not referred for biopsy, we believe the diagnosis by histopathology from our study was slightly different from previous reports.
Although about a fourth of our cases were finally diagnosed with entities that could have been sampled by less-invasive standard transbronchial biopsies (sarcoidosis and chronic lymphocytic pneumonia), we would like to point out that these cases did not have the typical time course or laboratory and imaging data that would have suggested these diagnoses (low pretest probability) before they underwent TBLC.
CONCLUSIONS
TBLC with a 2-scope technique could be an alternative method for diagnosing various types of ILD in patients unfit for SLB. Further prospective studies should clarify its role in the diagnostic armamentarium for undiagnosed ILD.
Footnotes
Disclosure: There is no conflict of interest or other disclosures.
REFERENCES
- 1.Poletti V, Patelli M, Poggi S, et al. Transbronchial lung biopsy and bronchoalveolar lavage in diagnosis of diffuse infiltrative lung diseases. Respiration. 1998;54:66–72. [DOI] [PubMed] [Google Scholar]
- 2.Deconinck B, Verschakelen J, Coolen J, et al. Diagnostic workup for diffuse parenchymal lung disease: schematic flowchart, literature review and pitfalls. Lung. 2013;191:19–25. [DOI] [PubMed] [Google Scholar]
- 3.Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83:1120–1145. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Kim DK, Kim DS, et al. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardiothorac Surg. 2007;31:1115–1119. [DOI] [PubMed] [Google Scholar]
- 5.Lettieri CJ, Veerappan GR, Helman DL, et al. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest. 2005;127:1600–1605. [DOI] [PubMed] [Google Scholar]
- 6.Kayatta MO, Ahmed S, Hammel JA, et al. Surgical biopsy of suspected interstitial lung disease is superior to radiographic diagnosis. Ann Thorac Surg. 2013;96:399–402. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson JP, Fogarty AW, McKeever TM, et al. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States 2000 to 2011. Am J Respir Crit Care Med. 2016;193:1161–1167. [DOI] [PubMed] [Google Scholar]
- 8.Griff S, Ammenwerth W, Schonfeld N, et al. Morphometrical analysis of transbronchial cryobiopsies. Diagn Pathol. 2011;6:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of tranbronchial cryobiopsy in interstitial lung disease: a randomize trial. Respirology. 2014;19:900–906. [DOI] [PubMed] [Google Scholar]
- 10.Kropski J, Pritchett J, Mason W, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLOS ONE. 2014;8:e78674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griff S, Schonfeld N, Ammenwerth W, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: a retrospective case series. BMC Pulm Med. 2014;3:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babiak A, Hetzel J, Krisna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration. 2009;78:203–208. [DOI] [PubMed] [Google Scholar]
- 14.Raj R, Brown KK. Mortality related to surgical lung biopsy in patients with interstitial lung disease. The devil is in the denominator. Am J Respir Crit Care Med. 2016;193:1082–1084. [DOI] [PubMed] [Google Scholar]


