Abstract
Background
Serum YKL-40 is an inflammatory biomarker associated with disease activity and mortality in diseases characterized by inflammation such as coronary artery disease (CAD). Exercise has a positive effect on CAD, possibly mediated by a decreased inflammatory activity. This study aimed to compare serial measurements of serum YKL-40 before and after exercise in patients with stable CAD versus controls.
Materials and methods
Eleven patients with stable CAD verified by coronary angiography (>70% stenosis) and 11 patients with a computer tomography angiography with no stenosis or calcification (calcium score=0) (controls) performed a standard clinical maximal exercise test. Serum YKL-40 was measured before exercise, immediately after exercise, and every hour for 6 h.
Results
Cardiovascular risk factors were more prevalent among the CAD patients compared with the controls. CAD patients had higher serum concentration of YKL-40 at baseline compared with controls, median (interquartile range) 94 (52–151) versus 57 (45–79) μg/l. Serum YKL-40 decreased stepwise after exercise, with a median decrease of 16 (13–39) μg/l for the CAD patients and 13 (10–22) μg/l for the controls from baseline to the lowest value. Thereafter, values increased again toward baseline level. Time after exercise was a significant factor for decrease in serum YKL-40 (P<0.0001), but no difference in YKL-40 decrease over time could be demonstrated between the groups (P=0.12).
Conclusion
Serum YKL-40 is elevated in patients with documented CAD compared with controls, and it decreases stepwise after exercise in both groups, indicating an anti-inflammatory effect of exercise independent of the presence of coronary atherosclerosis.
Keywords: biomarkers, coronary artery disease, exercise test, inflammation, serum YKL-40, stable angina pectoris
Introduction
Coronary artery disease (CAD) is the most common cause of death in western countries 1. Many factors, including inflammation, are involved in the development of CAD 2. Inflammatory processes play a pivotal role in all stages of the development of both acute and chronic atherosclerosis – from the initial induction of endothelial dysfunction and plaque formation to plaque destabilization and disruption with superimposed thrombosis leading to acute myocardial infarction or death 3. Therefore, biomarkers with the ability to monitor inflammatory processes are of incremental importance in CAD.
YKL-40 (also named chitinase 3-like 1 protein, CHI3L1) is a highly conserved heparin-binding glycoprotein produced by several cell types of the immune system 4 and macrophages in atherosclerotic plaques 5,6. Circulating YKL-40 is regarded as a non-disease-specific biomarker of inflammation and tissue remodeling 7–9. YKL-40 increases with age 8,10–12 and is elevated in diseases characterized by inflammation, increased extracellular remodeling, and ongoing fibrosis 4, such as cancer 8, heart failure 13–15, and ischemic cerebrovascular disease 16. YKL-40 is increased in patients with acute myocardial infarction 17,18 and in patients with stable CAD 12,14,18–21 and furthermore related to prognosis 12.YKL-40 has also been found to be a strong predictor of death independent of diagnosis 22. A recent study demonstrated that plasma CHI3L1 (YKL-40) and muscle CHI3L1 mRNA increase after 1 h of intensive exercise in healthy participants 23.
Physical inactivity is an important risk factor for CAD, and increasing evidence suggests that the beneficial effects of physical activity are mediated partly by reducing vessel wall inflammation 24,25. The purpose of this study was to compare serial measurements of serum YKL-40 before and after exercise in patients with stable CAD and angiographic documented coronary artery stenosis versus controls referred for suspected CAD, but found to have no stenosis nor calcification of the coronary arteries. We hypothesized that serum YKL-40 would decrease after exercise with a potentially different effect depending on the degree of coronary artery stenosis.
Materials and methods
Patients referred to the Department of Cardiology, Hillerød Hospital, Denmark because of stable angina pectoris were screened for inclusion between March 2010 and March 2012 26. Patients with a moderate to high a-priori risk for CAD underwent direct invasive coronary angiography (CAG), and the degree of epicardial stenosis was determined by the invasive cardiologist performing the CAG. Patients with a low a-priori risk for CAD had a computer tomography (CT) angiography. The coronary artery calcium score was obtained from the CT angiography according to the method described by Agatston using a threshold of 130 Hounsfield Units (HU) 27. In the present study, patients with CAG-documented CAD (>70% stenosis in minimum 1 epicardial coronary artery) comprised the CAD patient group, whereas participants with no stenosis or coronary calcification (calcium score=0) served as the control group.
Data were collected via interview with trained health professionals. A transthoracic echocardiography (Vivid 7; General Electric, Horten, Norway) was performed before inclusion, and patients were excluded if the left ventricular ejection fraction was below 50% or in the case of disease of the heart valves. Furthermore, patients were excluded if the CAG revealed three-vessel disease or left main artery stenosis. Further exclusion criteria were as follows: unstable angina pectoris, previous coronary artery bypass graft surgery, history of arrhythmia, renal insufficiency, chronic obstructive pulmonary disease, inability to perform a bicycle exercise test, or inability to provide written informed consent.
Bicycle exercise test
A maximal exercise test was performed in both the CAD patients and the control group. The test was performed on a bicycle ergometer (eBike Basic; General Electric) applying a standard clinical protocol. Work load was gradually increased with 25 W every second minute while patients were monitored with continuous 12-lead ECG and noninvasive blood pressure measurement every minute. The exercise test was terminated when patients reached physical exhaustion or experienced severe chest pain. The following was registered for all participants: symptoms, ST-segment changes, maximal work load, metabolic equivalents, peak blood pressure, and heart rate.
Blood samples
Peripheral blood samples were drawn a total of eight times: at baseline right before the exercise test, immediately after test termination, and once every hour until 6 h after the exercise test had terminated. Serum was extracted and stored at −80°C until analysis was performed. YKL-40 is stable in blood stored at −80°C 10. Serum concentrations of YKL-40 were measured in November 2013. Samples were determined in duplicates by a commercial enzyme-linked immunosorbent assay (Quidel, Santa Clara, California, USA). The detection limit was 20 μg/l. The intra-assay coefficients of variation were 5% (at 40 μg/l), 4% (at 104 μg/l), and 4% (at 155 μg/l). The interassay variation coefficient of variation was less than 6%.
Ethics
The study was approved by the regional ethics committee with the reference number H-3-2010.013. All included patients gave written consent following oral and written information.
Statistics
Continuous data with a Gaussian distribution are presented as mean±SD. Continuous data with a non-Gaussian distribution are presented as median (interquartile range). Proportions are presented as number (n) and percentage (%). Serum YKL-40 was logarithmically transformed (log10) because of a non-Gaussian distribution.
An unpaired Student’s t-test was performed for within-group comparisons of continuous variables with a Gaussian distribution. A Wilcoxon signed-rank test was performed on the continuous data with a non-Gaussian distribution (YKL-40, pack-years, alcohol, and weekly exercise). Unpaired comparisons of categorical variables were performed with the χ2-test or Fisher’s exact test, if any expected value was below 5.
Differences in YKL-40 between groups were tested using a linear mixed model. Serum YKL-40 was logarithmically transformed (log10) to stabilize the variance.
Statistical analysis was performed using SAS Enterprise Guide 7.1 (SAS institute Inc., Cary, North Carolina, USA). A two-sided P-value below 0.05 was considered statistically significant.
Results
Baseline characteristics
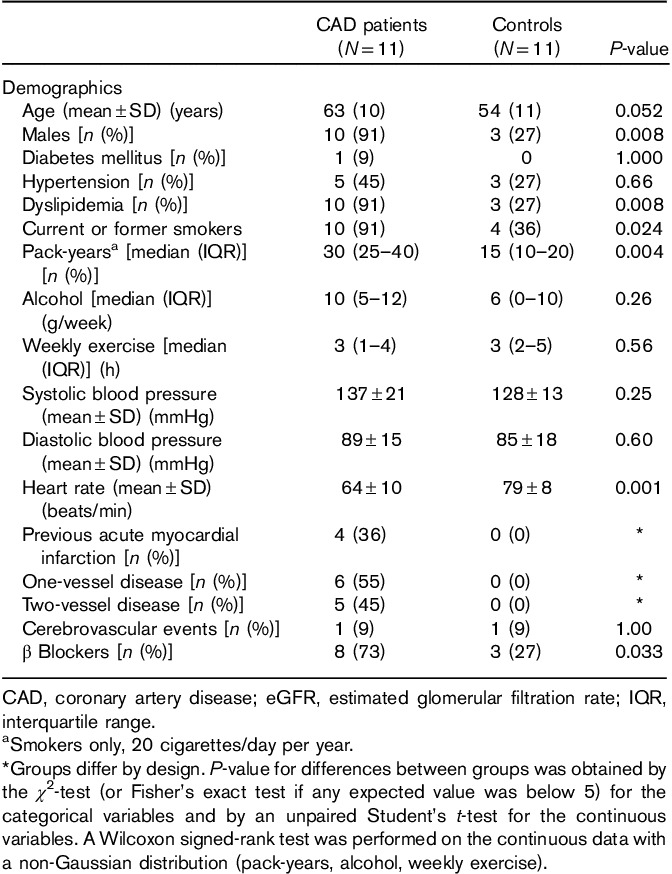
Twelve CAD patients and 12 controls were initially included. One patient with CAD was excluded because of missing serum YKL-40 values. One control was excluded because of very high serum YKL-40 concentrations (i.e. 1644 μg/l and >20 times above normal levels). Baseline characteristics for the 11 CAD patients and 11 controls are given in Table 1. Patients with CAD were slightly older than the controls (62.5 vs. 53.5 years), and a higher proportion of CAD patients were male (91 vs. 27% in the control group). The control group fulfilled the inclusion and exclusion criteria, but had a normal CT angiogram. CAD patients were more likely to have dyslipidemia and a history of smoking. Among the smokers in both groups, those with documented CAD had smoked more pack-years (i.e. 20 cigarettes/day per year). Furthermore, CAD patients were more often treated with β blockers and had a lower heart rate at baseline compared with controls. By design, the distribution of cardiovascular disease differed between the two groups. Four (36%) CAD patients had a previous myocardial infarction, six (55%) had one-vessel disease, and five (45%) had two-vessel disease. Angiographic stenosis of the right coronary artery was observed in eight patients (72%), left anterior descending artery in four patients (36%), and in the left circumflex artery in four (36%) patients.
Table 1.
Demographic data of the patients and controls
Bicycle exercise test
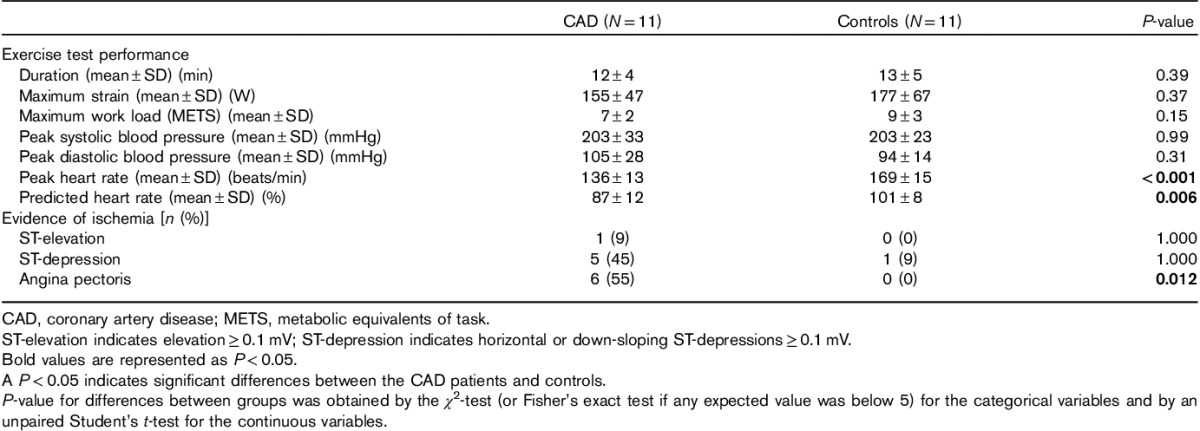
The exercise test variables were comparable in the two groups, with no difference in duration or work load. Peak heart rate was significantly lower for the CAD patients compared with the controls. The CAD patients reached a significantly lower percentage of predicted heart rate compared with the controls. ECG changes indicating ischemia were not significantly more frequently seen in the CAD patients, but angina pectoris was more frequently experienced by the CAD patients compared with the controls (Table 2). Angina pectoris was the limiting factor leading to termination of the exercise test in five CAD-positive patients and none of the controls (P=0.04).
Table 2.
Exercise test variables
Serum YKL-40
Table 3 and Fig. 1 illustrate serum YKL-40 at baseline right before exercise, immediately after exercise, and during the first 6 h after exercise, as well as the median change between each measurement. CAD patients had a higher level of serum YKL-40 at baseline compared with controls, with a median (interquartile range) of 94 (52–151) versus 57 (45–79) μg/l. Serum YKL-40 at baseline was above the 95th percentile for healthy individuals according to the mean age of the group in two (18%) of the CAD patients and in one (9%) of the controls. Overall serum YKL-40 was higher in CAD patients compared with the controls, but the difference was only borderline significant 6 h after exercise (P=0.063). Men had a higher, although not significant, serum YKL-40 at baseline compared with women [93 (52–97) vs. 76 (45–79) μg/l, P=0.48]. Furthermore, the proportion of patients who had CAD was also higher among the men compared with the women (77 vs. 11%).
Table 3.
Serum YKL-40 concentrations and changes before and after exercise test
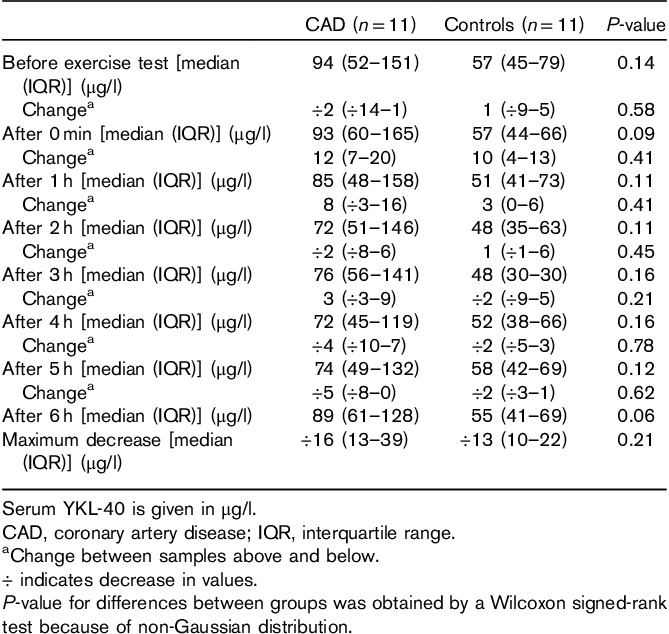
Fig. 1.
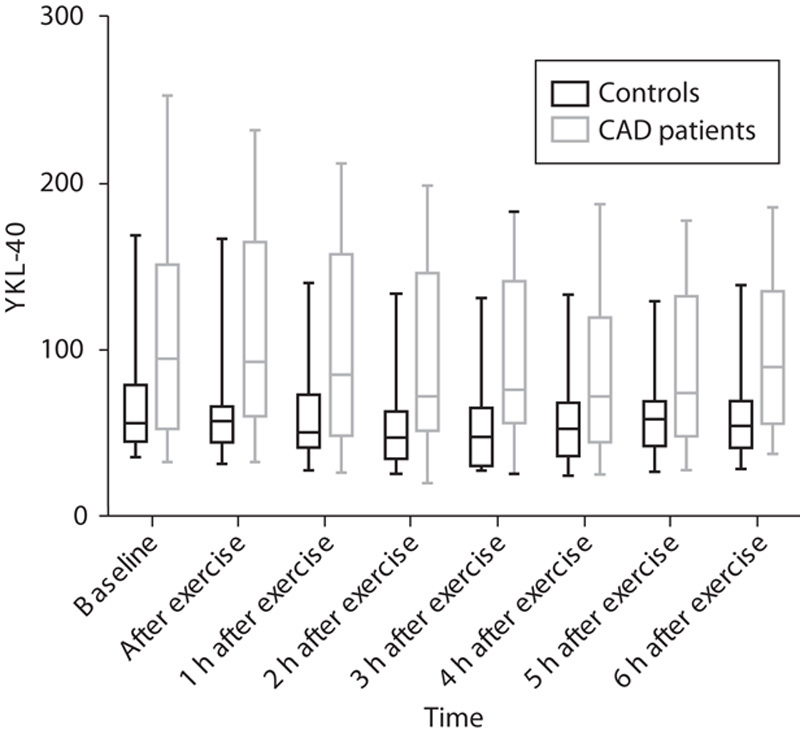
Concentrations of serum YKL-40 (μg/l). Serum YKL-40 was measured at baseline, immediately after exercise, and once every hour for 6 h in patients with documented CAD (gray) and controls (black). Data are presented as median (line), interquartile range (box), and minimum and maximum observations (whiskers).
As illustrated in Figs 1 and 2, serum YKL-40 decreased stepwise after exercise, reaching the lowest values 2 h after exercise for the controls [48 (35–63) μg/l] and 4 h after exercise for the CAD patients [72 (45–119) μg/l]. There was no significant difference between the two groups with regard to change in serum YKL-40 at any of the different time points (Table 3). CAD-positive patients had a median decrease in YKL-40 of 16 (13–39) μg/l, whereas the controls had a median decrease of 13 μg/l 8,10–21 from baseline to the lowest observed serum YKL-40 value (P=0.212). Thereafter, values increased again toward baseline serum YKL-40 level (Table 4).
Fig. 2.
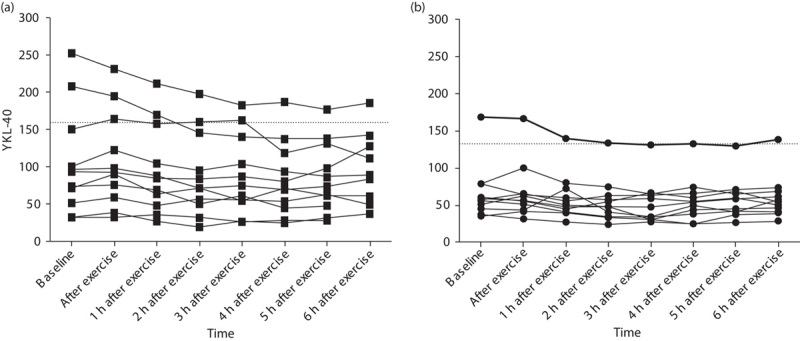
Individual changes in serum YKL-40 (μg/l) after exercise test. Serum YKL-40 was measured at baseline, immediately after exercise, and once every hour for 6 h after exercise. The lines represent each individual. (a) CAD patients; (b) controls. The horizontal dotted line represent 95th percentile (YKL-40 concentration) according to the mean age for each group 10. CAD, coronary artery disease.
Table 4.
Research on YKL-40 in cardiovascular disease
In the mixed model, time after exercise was a significant factor for the decrease in serum YKL-40 overall (P<0.0001), but no difference in YKL-40 decrease over time could be demonstrated between the two groups (P=0.12). There was no difference of the effect of time on YKL-40 between the groups (P=0.76), as illustrated in Fig. 3.
Fig. 3.
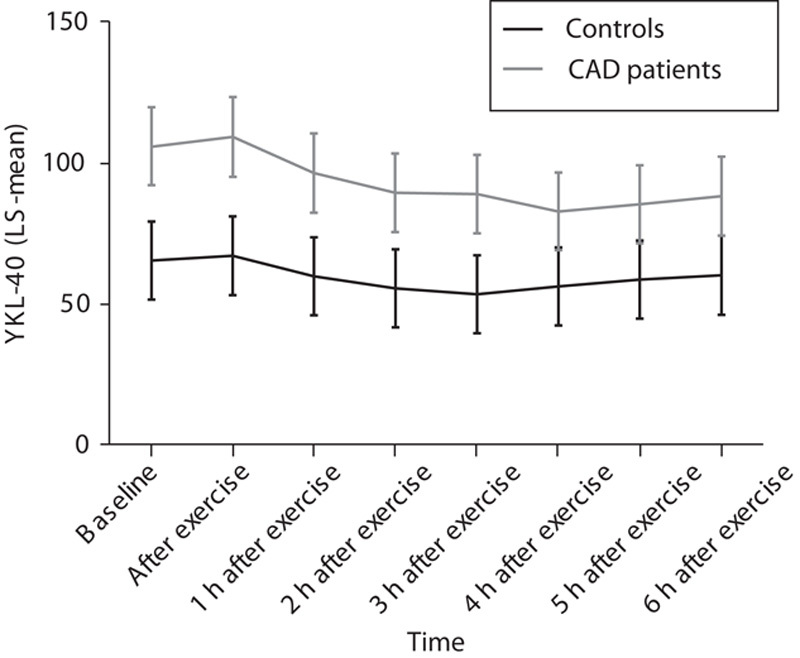
Mixed model for the interaction of time after exercise test and group (CAD patients vs. controls). y-axis: YKL-40 (LS-mean). x-axis: time after exercise test. CAD, coronary artery disease; LS, least square.
Discussion
In this study, we investigated the effect of exercise on the inflammatory biomarker serum YKL-40 in patients with CAD versus controls. We found that serum YKL-40 was higher at baseline for the CAD patients compared with the controls, and in both groups serum YKL-40 decreased after exercise with a slightly steeper decrease for the CAD patients than the controls. Time after exercise was a significant factor for decrease in serum YKL-40 overall, but we did not observe a difference in serum YKL-40 decrease over time between the two groups, indicating that exercise has a beneficial effect on the inflammatory level independent of atherosclerotic degree.
Serum YKL-40 is a new inflammatory biomarker, which potentially could be of interest in monitoring treatment efficacy, and as a prognostic factor in patients with CAD. Earlier studies suggest that serum YKL-40 could be a new biomarker of acute and chronic inflammation in patients with stable CAD. Circulating serum YKL-40 may reflect the total burden of coronary atherosclerosis or identify high-risk atherosclerosis with ongoing inflammation and atherosclerotic plaque formation.
In this study, it was interesting that we did not see a difference between CAD patients and controls with respect to YKL-40 decrease after exercise. It has already been established that the formation of fatty streaks and atherosclerosis begins early in life 28,29 and advanced atherosclerotic lesions may already appear in young adulthood 29,30. Our control group had a mean age of 53.5 years and were therefore likely to have developed atherosclerosis despite the fact that they did not have stenosis nor coronary artery calcification on CT angiography. Inflammation plays a central role in atherosclerosis 2, and this could explain why we observed a decrease in both groups and why exercise had a positive effect on inflammation in both CAD patients and controls.
A recent study demonstrated increasing plasma YKL-40 and muscle tissue YKL-40 mRNA values after 1 h of intensive exercise 23. Another recent study showed that marathon running increased the levels of circulating YKL-40 by 56% 31. However, yet another study observed no effect on serum YKL-40 after physical exercise 32. It is possible that a higher intensity and/or a longer duration are necessary for induction of increases in circulating YKL-40 levels in response to exercise. However, none of the mentioned studies considered the changes in YKL-40 after exercise as did the present study.
Inflammation is an important factor in the pathogenesis of atherosclerosis, and several markers of inflammation have been associated with an increased risk of cardiovascular events. Physical activity may lower the risk for CAD by decreasing inflammation. Other studies have shown that exercise decreases inflammation by decreasing inflammatory markers such as C-reactive protein and VCAM-1 33. In a study of 177 inactive patients and overweight patients, a single measurement of the inflammatory marker high-sensitivity C-reactive protein was inversely correlated to physical fitness independent of other cardiovascular risk factors. The authors conclude that this indicates the beneficial effects of physical activity on inflammation 34.
Strengths and limitations
To our knowledge, this is the first study to compare serial measurements of serum YKL-40 after exercise in a population of patients with documented CAD and a control group with suspected stable angina but with normal coronary arteries. The cohort was consecutively collected and was representative of CAD patients. Some important limitations in this study need to be addressed. First of all, there were a limited number of participants, which has an impact on the possible generalizability of the results. The borderline significant difference in age between the two groups represents a possible confounder, as age is known to be associated with serum concentrations of YKL-40. The choice of the control group can also be discussed, as these were referred because of stable angina pectoris and possible CAD, but were found to have no stenosis nor calcification on CT angiography. Ideally, the control group should be free from symptoms, and it is possible that the control group could represent a population with stable microvascular disease causing them to have angina pectoris. Furthermore, coronary artery pathology was assessed with different methods in the CAD patients and the controls, because of different a-priori risk for CAD. This, however, represents a daily clinical practice scenario, and a CT angiography has a high negative predictive value and therefore it is unlikely that the difference method has affected the results.
Conclusion
Serum YKL-40 was higher in CAD patients compared with controls, but both groups experienced declining values after exercise, indicating that exercise has an anti-inflammatory effect independent of the degree of the atherosclerotic burden.
Acknowledgements
The authors thank the Department of Cardiology at Hillerød Hospital Copenhagen University Hospital, Denmark where the examinations have taken place. They thank Ulla Kjærulff-Hansen, Dorthe Mogensen, and Marianne Sørensen, Department of Medicine, Herlev Hospital for excellent technical assistance with the serum YKL-40 analysis. Last but not least, they also thank the participants for their willingness to contribute to the research.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001; 104:2746–2753. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med 1999; 340:115–126. [DOI] [PubMed] [Google Scholar]
- 3.Biasillo G, Leo M, Della BR, Biasucci LM. Inflammatory biomarkers and coronary heart disease: from bench to bedside and back. Intern Emerg Med 2010; 5:225–233. [DOI] [PubMed] [Google Scholar]
- 4.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 2006; 53:172–209. [PubMed] [Google Scholar]
- 5.Boot RG, van Achterberg TA, van Aken BE, Renkema GH, Jacobs MJ, Aerts JM, de Vries CJ. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol 1999; 19:687–694. [DOI] [PubMed] [Google Scholar]
- 6.Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, Krause SW. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem 2003; 278:44058–44067. [DOI] [PubMed] [Google Scholar]
- 7.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol 2011; 73:479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz NA, Johansen JS. YKL-40-A protein in the field of translational medicine: a role as a biomarker in cancer patients? Cancers (Basel) 2010; 2:1453–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastrup J. Can YKL-40 be a new inflammatory biomarker in cardiovascular disease? Immunobiology 2012; 217:483–491. [DOI] [PubMed] [Google Scholar]
- 10.Bojesen SE, Johansen JS, Nordestgaard BG. Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta 2011; 412 (9–10):709–712. [DOI] [PubMed] [Google Scholar]
- 11.Johansen JS, Bojesen SE, Tybjaerg-Hansen A, Mylin AK, Price PA, Nordestgaard BG. Plasma YKL-40 and total and disease-specific mortality in the general population. Clin Chem 2010; 56:1580–1591. [DOI] [PubMed] [Google Scholar]
- 12.Kastrup J, Johansen JS, Winkel P, Hansen JF, Hildebrandt P, Jensen GB, et al. High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. Eur Heart J 2009; 30:1066–1072. [DOI] [PubMed] [Google Scholar]
- 13.Bilim O, Takeishi Y, Kitahara T, Ishino M, Sasaki T, Suzuki S, et al. Serum YKL-40 predicts adverse clinical outcomes in patients with chronic heart failure. J Card Fail 2010; 16:873–879. [DOI] [PubMed] [Google Scholar]
- 14.Harutyunyan M, Christiansen M, Johansen JS, Køber L, Torp-Petersen C, Kastrup J. The inflammatory biomarker YKL-40 as a new prognostic marker for all-cause mortality in patients with heart failure. Immunobiology 2012; 217:652–656. [DOI] [PubMed] [Google Scholar]
- 15.Rathcke CN, Kistorp C, Raymond I, Hildebrandt P, Gustafsson F, Lip GY, et al. Plasma YKL-40 levels are elevated in patients with chronic heart failure. Scand Cardiovasc J 2010; 44:92–99. [DOI] [PubMed] [Google Scholar]
- 16.Kjaergaard AD, Bojesen SE, Johansen JS, Nordestgaard BG. Elevated plasma YKL-40 levels and ischemic stroke in the general population. Ann Neurol 2010; 68:672–680. [DOI] [PubMed] [Google Scholar]
- 17.Hedegaard A, Ripa RS, Johansen JS, Jorgensen E, Kastrup J. Plasma YKL-40 and recovery of left ventricular function after acute myocardial infarction. Scand J Clin Lab Invest 2010; 70:80–86. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Ripa RS, Johansen JS, Gabrielsen A, Steinbruchel DA, Friis T, et al. YKL-40 a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand Cardiovasc J 2008; 42:295–302. [DOI] [PubMed] [Google Scholar]
- 19.Mathiasen AB, Harutyunyan MJ, Jorgensen E, Helqvist S, Ripa R, Gotze JP, et al. Plasma YKL-40 in relation to the degree of coronary artery disease in patients with stable ischemic heart disease. Scand J Clin Lab Invest 2011; 71:439–447. [DOI] [PubMed] [Google Scholar]
- 20.Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL-40 levels in patients with coronary artery disease. Coron Artery Dis 2007; 18:391–396. [DOI] [PubMed] [Google Scholar]
- 21.Zheng JL, Lu L, Hu J, Zhang RY, Zhang Q, Chen QJ, et al. Increased serum YKL-40 and C-reactive protein levels are associated with angiographic lesion progression in patients with coronary artery disease. Atherosclerosis 2010; 210:590–595. [DOI] [PubMed] [Google Scholar]
- 22.Mygind ND, Iversen K, Kober L, Goetze JP, Nielsen H, Boesgaard S, et al. The inflammatory biomarker YKL-40 at admission is a strong predictor of overall mortality. J Intern Med 2013; 273:205–216. [DOI] [PubMed] [Google Scholar]
- 23.Gorgens SW, Hjorth M, Eckardt K, Wichert S, Norheim F, Holen T, et al. The exercise-regulated myokine chitinase-3-like protein 1 stimulates human myocyte proliferation. Acta Physiol (Oxf) 2015. [doi: 10.1111/apha.12579; Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med 2002; 162:1286–1292. [DOI] [PubMed] [Google Scholar]
- 25.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol 2001; 153:242–250. [DOI] [PubMed] [Google Scholar]
- 26.Axelsson A, Ruwald MH, Dalsgaard M, Rossing K, Steffensen R, Iversen K. Serial measurements of high-sensitivity cardiac troponin T after exercise stress test in stable coronary artery disease. Biomarkers 2013; 18:304–309. [DOI] [PubMed] [Google Scholar]
- 27.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15:827–832. [DOI] [PubMed] [Google Scholar]
- 28.McGill HC, Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 2000; 72 (Suppl):1307S–1315S. [DOI] [PubMed] [Google Scholar]
- 29.McGill HC, Jr, Herderick EE, McMahan CA, Zieske AW, Malcolm GT, Tracy RE, Strong JP. Atherosclerosis in youth. Minerva Pediatr 2002; 54:437–447. [PubMed] [Google Scholar]
- 30.Holman RL, McGill HC, Jr, Strong JP, Geer JC. The natural history of atherosclerosis: the early aortic lesions as seen in New Orleans in the middle of the of the 20th century. Am J Pathol 1958; 34:209–235. [PMC free article] [PubMed] [Google Scholar]
- 31.Vuolteenaho K, Leppanen T, Kekkonen R, Korpela R, Moilanen E. Running a marathon induces changes in adipokine levels and in markers of cartilage degradation – novel role for resistin. PLoS One 2014; 9:e110481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansen JS, Lottenburger T, Nielsen HJ, Jensen JE, Svendsen MN, Kollerup G, Christensen IJ. Diurnal, weekly, and long-time variation in serum concentrations of YKL-40 in healthy subjects. Cancer Epidemiol Biomarkers Prev 2008; 17:2603–2608. [DOI] [PubMed] [Google Scholar]
- 33.Ranković G, Milicić B, Savić T, Dindić B, Mancev Z, Pesić G. Effects of physical exercise on inflammatory parameters and risk for repeated acute coronary syndrome in patients with ischemic heart disease. Vojnosanit Pregl 2009; 66:44–48. [DOI] [PubMed] [Google Scholar]
- 34.Hjelstuen A, Anderssen SA, Holme I, Seljeflot I, Klemsdal TO. Markers of inflammation are inversely related to physical activity and fitness in sedentary men with treated hypertension. Am J Hypertens 2006; 19:669–675. [DOI] [PubMed] [Google Scholar]


