Abstract
Background and Purpose:
We have developed a 12-week balance training program for older adults shown to improve fall-related concerns, gait speed, balance performance, and physical function. We hypothesized that this balance training would also contribute to higher habitual physical activity (PA) levels and improved health-related quality of life (HRQoL). The primary aim was to evaluate short- and long-term effects of the balance training program on objectively measured habitual PA in older adults with osteoporosis. Secondary aims were to assess the effects of the balance training on HRQoL, and to study whether any effects on PA were associated with changes in HRQoL, gait speed, balance performance, fall-related concerns, and physical function.
Methods:
A randomized controlled trial with follow-up at 3, 9, and 15 months, including 91 participants with osteoporosis (75.6 ± 5.4 years), compared a balance training group (n = 61) with a control group (n = 30). The primary outcome was effect on habitual PA measured as steps/day, dichotomized into less than 5000 or 5000 or more steps/day. Physical activity was assessed with pedometers (Yamax) and accelerometers (Actigraph), HRQoL with the Short Form-36 (SF-36), gait with a GAITRite walkway, balance performance with Modified-Figure-Eight test and one-leg stance, fall-related concerns with Falls Efficacy Scale International, and physical function with the advanced lower extremity subscale of the questionnaire Late Life Function and Disability Instrument. Statistical methods used were multivariate logistic regression and logistic generalized estimating equation.
Results:
Sixty-eight participants completed the short-term follow-up at 3 months, and 53 participants completed the long-term follow-up at 15 months. Per-protocol analysis (n = 68) showed that the odds ratio for having a daily step count of 5000 or more at 3 months was 6.17 (95% confidence interval, 1.23-30.91), P = .027, for the intervention group compared with the control group. The longitudinal analysis (n = 91) showed that the odds ratio for having a daily step count of 5000 or more at 15 months was 2.02 (95% confidence interval, 0.88-4.64), P = .096, for the intervention group compared with the control group. The mental component sum of the SF-36 improved significantly from baseline to 3 months in the intervention group, and the physical component sum improved in both groups, but no statistically significant differences were found between groups. No associations were found between PA and changes in covariates.
Discussion and Conclusions:
The short-term evaluation showed that balance training increased habitual PA in community-dwelling older adults with osteoporosis. A significantly higher proportion of participants in the intervention group reached a level of 5000 or more steps/day, which is important for overall health. This effect was not associated with improvements in HRQoL, gait speed, balance performance, or fall-related concerns, and did not persist through the long-term follow-up. To accomplish a sustained PA change, a prolonged intervention or more support regarding habitual PA may be required, such as reinforcement with personalized behavior change counseling or PA on prescription.
Keywords: accelerometer, exercise, pedometer, quality of life, steps/day
INTRODUCTION
Physical activity (PA) is essential for healthy ageing, and it has been established that a physically active life contributes to health-related quality of life (HRQoL) and helps to retain a necessary balance function.1 Older adults are advised to be active daily and to accumulate at least 150 minutes per week of moderate to vigorous intensity PA (MVPA), performed in bouts of at least 10 minutes.1 Recommendations are that individuals with osteoporosis should engage in weight-bearing PA, such as walking, to retain bone mineral density, strength, and balance, thereby reducing the risk of falls and fractures.2,3 However, fall-related concerns and impaired balance may lead to avoidance of activities and result in a low habitual PA level and a sedentary behavior.4
A habitual PA level of less than 5000 steps/day is generally considered to be very low, and several studies have reported that this PA level is associated with a higher prevalence of cardiovascular risk factors, obesity and depression, and lower HRQoL.5,6 We have previously reported that older adults with osteoporosis taking less than 5000 steps/day spent more time sedentary, had slower gait speed, lower HRQoL, and poorer balance performance than those with a habitual PA level of 5000 or more steps/day.7
Systematic reviews have shown that PA interventions can increase daily step count8,9 and reduce fall-related concerns in community-dwelling older adults,10 but the effects on habitual PA after a balance training intervention remain unclear. Our group has developed a 12-week specific, progressive balance training program focusing on dual- and multitask exercises for older adults.11 This balance training has previously been evaluated in a study by Halvarsson et al,12 showing that older adults with osteoporosis participating in the balance training intervention improved their fall-related concerns, gait speed, balance performance, and physical function compared with controls.12 We hypothesized that this balance training would also contribute to higher habitual PA levels and improved HRQoL. The primary aim of this study was to evaluate short- and long-term effects of this balance training program on objectively measured habitual PA in older adults with osteoporosis. Secondary aims were to assess the effects of the balance training on HRQoL, and to assess whether any effects on habitual PA were associated with changes in HRQoL, gait speed, balance performance, fall-related concerns, and physical function.
METHODS
Study Design and Participants
This study was a part of a randomized controlled trial (BETA study; NCT01417598, ClinicalTrials.gov) with follow-up at 3, 9, and 15 months, comparing a balance training intervention, with or without supplementary PA, with a control group. The primary outcome measure in this study was effect on habitual PA measured as steps/day. Eligible participants were community-dwelling women and men aged 65 years or more, living in Stockholm County, Sweden, with osteoporosis objectively diagnosed via bone densitometry, and with impaired balance and fall-related concerns, determined by interviews and tests at baseline assessments. Exclusion criteria were fall-related fractures during the last year, Mini-Mental State Examination score of less than 24,13 other diseases (such as severe cardiovascular, pulmonary, or neurological disease) with symptoms that might influence participating in the training program, or inability to walk indoors without aid.
Sample size was calculated on the primary outcome in the BETA study, Falls Efficacy Scale-International, resulting in 21 participants in each of 3 groups to provide a power of 80% at the 0.05 significance level (2-sided). Additional sample size analysis was conducted for this study using steps/day as primary outcome, on the basis of previous studies on older populations, which resulted in similar estimated group sizes (n = 19). To allow for postrandomization dropouts 96 participants were recruited. Recruitment was achieved by advertisement in local newspapers, through the Osteoporosis Association in Stockholm and from the Endocrinology Clinic at Karolinska University Hospital. Ethical approval was obtained from the Regional Board of Ethics in Stockholm (Dnr: 2006/151-31, 2009/819-32, and 2012/1829-32).
Procedures
Assessments of PA, HRQoL, gait, balance performance, and fall-related concerns were performed at baseline and at 3, 9, and 15 months. All assessments were conducted at a movement laboratory at Karolinska Institutet, Stockholm, Sweden, by experienced physiotherapists after a predesigned protocol. At baseline, participants were first informed about the study protocol, and individual informed written consent was obtained. The subsequent test procedure included an initial trial session of the physical tests, followed by administration of the questionnaires and the gait and balance tests. Finally, instructions were given on how to wear the movement sensors during the following week (before the training) for assessing PA. Follow-up assessments included the same procedure, except for the trial session. Data were collected between November 2009 and December 2012.
Randomization
An external research assistant used web-based software to administer the randomization, in blocks of 9 into 3 different groups: 2 intervention groups, Training and Training+Nordic Walking (NW), or Control group. Participants picked a sealed envelope after finishing the baseline testing for allocation to groups. Researchers were blinded to group allocation at baseline assessments, but not at follow-up assessments.
Intervention
Both intervention groups participated in a 12-week balance training program with three 45-minute sessions per week. The balance training intervention has been described in a previous publication providing both a detailed description of the program and how the different components in the program relate to exercise physiology and balance control theories.11
In short, the exercises in the balance training program were progressive and specific to functional balance and incorporated dual- and multitask exercises, for example counting, carrying a tray, or having to avoid obstacles. Even though the training was performed as group sessions, the exercises were individually adjusted for each participant, with the aim to challenge their balance control. All exercises could be performed in 3 degrees of difficulty: basic, moderate, and advanced. Every session included exercises while sitting on a large balance ball, while standing, and while walking. The exercises differed across sessions to achieve variety, but every exercise was repeated later on in the program, often in a more challenging form. The groups consisted of 6 to 10 participants, with 2 or 3 physical therapists present at each session to ensure participant safety and allow individual progression of exercises.
Participants randomized to the Training+NW were also provided with walking poles and an activity diary. They were instructed to perform NW for 30 minutes at least 3 times per week, on their own, in addition to the balance training. No instructions about continued balance training or PA were given at the end of the intervention.
Control Group
Participants randomized to the control group were asked to continue with their usual activities, and had no trial contacts other than for data collection at baseline, 3, 9, and 12 months. Control group participants were offered to participate in balance training when the study period was completed.
Outcome Measures
Habitual physical activity
Habitual PA was objectively assessed with 2 types of movement sensors: a pedometer (Yamax LS2000, Yamax Corporation, Japan) and an accelerometer (Actigraph GT1M or GT3X+, ActiGraph). Pedometers measure ambulatory activity and provide information on the number of steps taken and the Yamax LS2000 has been shown to be reliable and valid in several studies.14,15 Accelerometers record changes in movement over time (accelerations) expressed as activity counts, from which frequency, duration, and intensity of PA can be calculated, and also provide data on the number of steps taken. Both the GT1M and the GT3X+ are reliable and valid instruments, with a strong correlation between the 2 models.16
Participants were asked to continue with their usual PA while wearing the sensors for 7 consecutive days during all waking hours (excluding showering and swimming), and to record pedometer steps and time when they put on and removed the sensors each day on a log sheet. The accelerometer was worn at the side of the waist attached to an elastic belt, and the pedometer was worn attached either to the same belt or at the waistband of clothing, in the midline of the thigh. At baseline assessment, 69 participants were equipped with both a pedometer and an accelerometer, whereas 22 participants wore only a pedometer (because of limited access to the accelerometers). Background characteristics did not differ between those who wore 2 sensors and those who wore only the pedometer. At all follow-up assessments, all participants wore both sensors. The primary outcome variable for PA was steps/day. Additional outcome variables for PA were number of sedentary bouts/day, mean and maximum length of sedentary bouts in minutes/day, total time in MVPA/day, or 150 or more minutes/week in 10-minute or more bouts of MVPA.
ActiGraph data were reduced using ActiLife software (v6.11.4). The 15-second epoch and activity counts from the vertical axis of the GT3X+ were used. More than 90 consecutive minutes of zero counts was considered nonwear time and 600 minutes or more daily wear time was considered a valid day.17 Accelerometer data were compared with the log sheet and participants with at least 3 valid days were included.18 When valid accelerometer data were provided, accelerometer-derived steps/day were used; otherwise, pedometer-derived steps/day were used for analysis.19
For statistical analyses, daily step count was dichotomized into 2 categories: less than 5000 steps/day and 5000 or more steps/day.5,6 By choosing 5000 steps/day as a cutoff point for the outcome measure in this study, we focused on investigating the effect of the balance training on the least active participants. To identify PA pattern, counts from the vertical axis were analyzed using Copeland cut points for older adults: 0 to 99 counts per minute (cpm) for sedentary time and 1041 or more cpm for MVPA.20
Health-related quality of life
Health-related quality of life was assessed using the questionnaire Short Form-36 Health Survey (SF-36).21 The SF-36 has been found to be valid and reliable for the general Swedish population.21,22 The outcome variables used were values from the 2 sum scores: Physical Component Sum (PCS) regarding physical health, and Mental Component Sum (MCS) for mental health. An improvement of 5 or more was considered the minimal clinically important difference (MCID).
Covariates
Gait speed was assessed using a walkway system (GAITRite Walkway, GAITRite, CIR Systems Inc) with embedded pressure sensors that provide information about spatial and temporal gait parameters.23 Participants were asked to walk back and forth 3 times first at a self-selected comfortable speed, and then as fast as possible, without tripping or falling. Outcome variables used were mean gait speed (m/s), at normal (self-selected) speed and fast speed. Gait speed is a highly valid functional measurement, both at self-selected and maximum speed,23,24 and a 0.10 m/s difference was considered MCID.25
Balance performance was assessed with the Modified-Figure-Eight test (MFE) and one-leg stance.26,27 For the MFE, participants were asked to walk 2 complete rounds as fast as possible on a figure of 8 marked on the floor, with a 4-cm wide line and an inner diameter of 163 cm for each circle. In addition to time, numbers of oversteps (no part of the shoe touched the line) were noted. For one-leg stance, each participant was asked to stand first on the right and then on the left leg for as long as possible, arms hanging down. Timing started when the foot was raised and stopped if the lifted foot touched the floor, if the participant was unable to maintain position, or when time reached 30 seconds. Both tests were performed 3 times. The validity and reliability of both tests for older adults has been established.26,27 The outcome variables were mean time in seconds and number of oversteps for MFE, and mean time in seconds for one-leg stance. A difference of 2 seconds or more in one-leg stance was considered MCID.
Fall-related concerns were assessed using the questionnaire Falls Efficacy Scale-International,28 previously shown to be reliable and valid for older adults.29 The sum score was used as the outcome variable, and a difference of 3 or more was considered MCID.29
Physical function was assessed by self-report using the advanced lower extremity subscale of the questionnaire Late Life Function and Disability Instrument. The validity and reliability of the questionnaire has been established and an improvement of 8 or more was considered MCID.30
Changes From Planned Analysis in Protocol
The original study protocol included 2 interventions groups: Training and Training+NW. However, analyses of the 2 interventions groups showed that some of the participants in the Training+NW did not adhere to the study protocol regarding NW, and many of the participants in the Training group had walking or NW as a regular activity in addition to the balance training. Because there was no difference between the 2 groups regarding PA level in addition to the balance training,12 we decided to perform the analyses and present the results on 2 groups: one intervention group, merged by Training and Training+NW, and one control group.
Statistical Analysis
Statistics were computed in Stata, version 11.2 (StataCorp LP). Data are presented as mean ± standard deviations for continuous variables and median and interquartile range for ordinal variables. Two sample t tests or the Wilcoxon rank-sum tests were used for continuous variables and the chi-squared test for categorical variables, to identify differences between groups. Odds ratios (OR) were determined with 95% confidence intervals (CI), and P values ≤.05 were considered statistically significant.
Participants who attended at least 24 training sessions were included in the analyses. For evaluation of the short-term effects of the intervention, we computed a per-protocol analysis including all participants who completed the 3-month follow-up (n = 68). Baseline covariates were examined as potential confounding factors, and a multivariate logistic regression model was fitted on the basis of this selection. Tests of model performance, Hosmer-Lemeshow goodness-of-fit test and ROC curves analysis, showed a good model fit. The final model assessed the association between step/day level (<5000 or ≥5000 steps/day) and treatment group, adjusting for mean steps/day at baseline, age, body mass index, and use of walking-aid outdoors.
To assess whether the 23 participants lost to the 3-month follow-up might have introduced bias, a sensitivity analysis was computed using the multiple imputation procedures31 in Stata to impute missing values for steps/day level. Twenty datasets were generated and the imputation model included all variables in the regression model and season of measurement. Estimates from the 20 datasets were pooled, and a logistic regression including all 91 participants was computed using the same multivariate model as in the per-protocol analysis.
For evaluation of the long-term effects of the intervention, we computed a longitudinal analysis using logistic generalized estimating equation with an unstructured correlation structure. The same variables as in the short-term analysis were included, and we used robust sandwich estimator for standard errors. The generalized estimating equation analysis takes into account the within-subject correlation of the 4 repeated measurements and does not require a balanced design (ie, observations at all measurements for each participant), which allows for maximum utilization of data from all 91 participants.
For evaluation of associations between habitual PA and changes in covariates, we dichotomized all covariates (except MFE) into 2 categories: no change or improved. To be classified as improved, the difference between baseline results and results at 3-month follow-up had to be at least the preestablished MCID. For evaluation of changes in MFE, time and number of oversteps were analyzed as continuous variables.
RESULTS
Participants
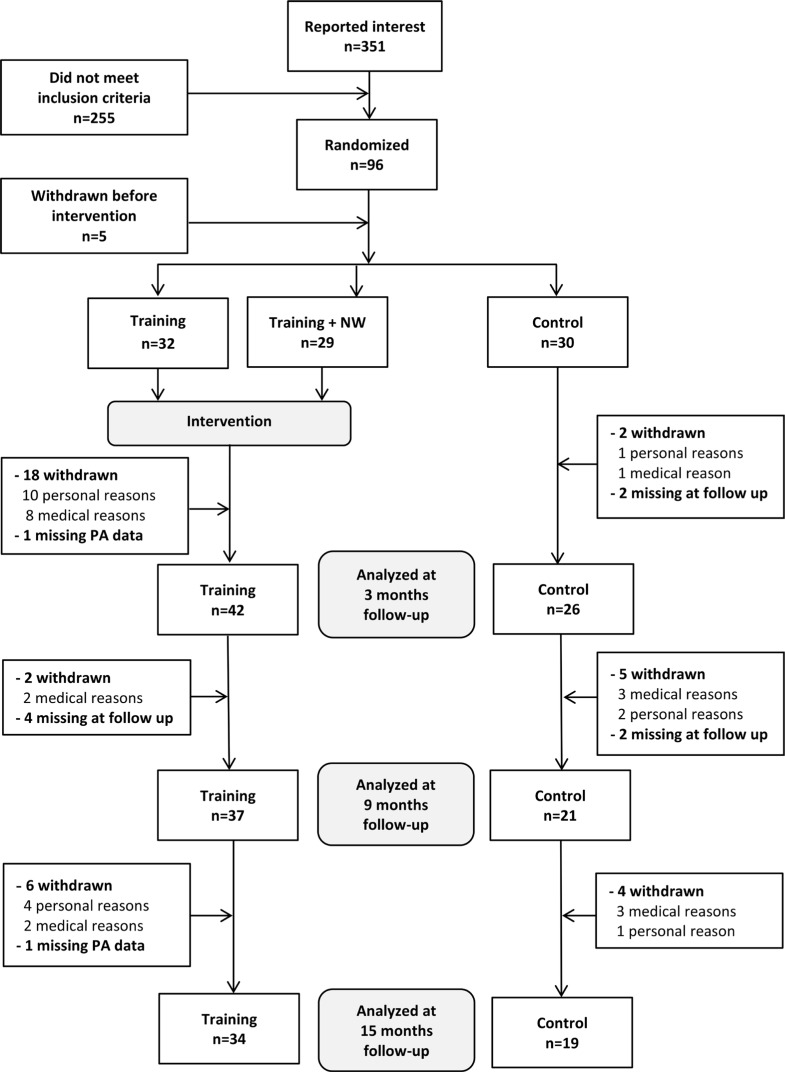
Of 351 individuals who reported interest to take part in the study, 96 participants (27%) met the inclusion criteria and were randomized (Figure 1). Two participants did not complete the PA assessment at baseline and 3 participants withdrew before the intervention started; thus, 91 individuals (89 women and 2 men), aged 66 to 86 years (mean: 75.6 ± 5.4), were included. Baseline characteristics were similar between randomized groups at baseline, with the exception of the SF-36 sum score for mental health, where the control group had a higher (better) score (Table 1).
Figure 1.
Flow chart of study participant enrollment, randomization, follow-up, and data analysis on the basis of CONSORT guidelines.
Table 1. Baseline Characteristics of Included Participants (n = 91).
| Variables | Intervention Group n = 61 | Control Group n = 30 |
|---|---|---|
| Women/men, n (%) | 60 (98)/1 (2) | 29 (97)/1 (3) |
| Age, y, mean (SD) | 75.7 (5.8) | 75.2 (4.6) |
| Body mass index, kg/m2, mean (SD) | 24.5 (4.0) | 25.4 (4.2) |
| University education, n (%) | 28 (46) | 14 (47) |
| Living alone, n (%) | 36 (59) | 19 (63) |
| Living in apartment, n (%) | 47 (77) | 25 (83) |
| Never or ex-smoker, n (%) | 59 (97) | 29 (97) |
| Experienced a fracture, last 10 y, n (%) | 40 (66) | 14 (47) |
| Experienced a fall, last year, n (%) | 26 (43) | 15 (50) |
| Use walking-aid outdoors, n (%) | 22 (36) | 15 (50) |
| Gait speed, normal, m/s, mean (SD) | 1.15 (0.21) | 1.20 (0.25) |
| Geriatric depression score (0-20), median (IQR) | 3 (2-6) | 2 (1-4) |
| Chronic diseases, n (%) | ||
| Cardiovascular diseasea | 33 (54) | 14 (47) |
| Diabetes | 3 (5) | 0 |
| COPD or asthma | 8 (13) | 5 (17) |
| Stroke | 5 (8) | 1 (3) |
| Health-related quality of life, SF-36, median (IQR) | ||
| Physical component sum | 39.7 (33.0-45.3) | 41.4 (33.0-47.4) |
| Mental component sum | 49.1 (36.2-56.4) | 53.6 (47.2-60.1) |
| Fall-related concerns, median (IQR) | ||
| Falls Efficacy Scale International (16-64) | 26 (24-34) | 27.5 (23-30) |
| LLFDI, mean (SD) | ||
| Advanced lower extremity function | 50.1 (11.5) | 49.7 (13.0) |
| Season of measure, n (%) | ||
| Fall | 30 (49) | 17 (57) |
| Spring | 31 (51) | 13 (43) |
| Physical activity | ||
| Steps, steps/day, mean (SD) | 6209 (2842) | 6313 (3734) |
| <5000 steps/day, n (%) | 23 (38) | 13 (43) |
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LLFDI, Late Life Function and Disability Instrument; SD, standard deviation; SF-36, Short Form-36 health survey.
aCardiovascular disease = hypertension, coronary artery disease, myocardial infarction, heart failure.
Sixty-eight participants (75%) completed the postintervention follow-up at 3 months, 58 participants (64%) completed the 9-month follow-up, and 53 participants (58%) completed the 15-month follow-up (Figure 1). There were no significant differences on any baseline characteristics between those who completed and those who were lost to the 3-month follow-up. A higher proportion of the participants in the intervention group, 31% compared with 13% in the control group, were lost to the 3-month follow-up; though, this difference was nonsignificant (P = .066).
All of the participants in the control group who were lost to the 9-month follow-up had a daily step level of less than 5000 (mean, 3683; range, 1742-4306 steps/day), which biased the results (P = .008); therefore, we decided not to further analyze the results at 9 months.
There were no differences in proportion of participants who were lost to the 15-month follow-up between treatment groups (P = .49), and there were no differences in PA level or PA pattern between participants who had withdrawn and those who completed the whole study. Analysis of all 38 participants who had withdrawn at the end of the study showed that they had more health problems (cardiovascular disease, stroke, and vertebral compression fractures) and lower physical function (slower fast gait speed and lower physical function score in the SF-36) at baseline than participants who completed the whole study period.
Short-Term Effects
Per-protocol analysis (n = 68) showed that the OR for having a daily step count of 5000 or more at 3 months was 6.17 (95% CI, 1.23-30.91), P = .027, for the intervention group compared with the control group (Table 2). Intention-to-treat analysis (n = 91), assuming participants lost to follow-up were missing at random, made little difference: OR = 7.14 (95% CI, 1.41-36.19), P = .018. Per-protocol analysis of difference in mean steps/day between the intervention group and the control group at 3 months was 1144 (95% CI, 2446 to −159), P = .084 (Table 2). There was a wide range of daily step count both in the intervention group, from 1372 to 12 102, and in the control group, from 377 to 13 470.
Table 2. Short- and Long-Term Values for Habitual Physical Activity (Steps/Day).
| Steps | Intervention Group | Control Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline n = 61 | 3 mos n = 42 | 9 mos n = 37 | 15 mos n = 34 | Baseline n = 30 | 3 mos n = 26 | 9 mos n = 21 | 15 mos n = 19 | |
| Daily count, mean (SD) | 6209 (2842) | 6064 (2430) | 5917 (2062) | 6013 (2241) | 6313 (3734) | 4921 (2890) | 7084 (3186) | 5698 (2835) |
| ≥5000 daily, n (%) | 38 (62) | 30 (71) | 25 (68) | 24 (71) | 17 (57) | 11 (42) | 14 (67) | 11 (58) |
Abbreviation: SD, standard deviation.
Long-Term Effects
The longitudinal analysis (n = 91) showed that the OR for having a daily step count of 5000 or more at 15 months was 2.02 (95% CI, 0.88-4.64), P = .096, for the intervention group compared with the control group. We also computed a longitudinal analysis excluding the results at 9-month follow-up, due to the bias in PA level of the participants lost to follow-up in the control group. The result was similar to the analysis including all follow-up data, but now significant: OR = 2.91 (95% CI, 1.15-7.36) (P = .024). Per-protocol analysis (n = 53) of difference in mean steps/day between the intervention group and the control group at 15 months was 315 (95% CI, 1734 to −1103), P = .658 (Table 2). There was a wide range of daily step count also at the long-term follow-up in both groups, in the intervention group 1501 to 10 728, and in the control group 228 to 10 096.
Subgroup Analyses of Physical Activity
Accelerometer data were obtained from 50 participants at 3 months, 30 in the intervention group and 20 in the control group. None of the variables examined: number of sedentary bouts, length of sedentary bouts, time in MVPA, or 150 minutes or more per week in 10-minute or more bouts of MVPA, showed statistically significant differences between treatment groups.
Secondary Outcome Analyses
The SF-36 sum score MCS improved (P = .01) from baseline to 3-month follow-up in the intervention group, and PCS improved significantly in both intervention (P < .001) and control groups (P = .02), but no statistically significant differences were found between groups in either PCS or MCS (per-protocol analysis, n = 68). No associations were found between habitual PA (steps/day, steps/day level, changes in steps/day) and changes in covariates (HRQoL, gait speed, balance performance, fall-related concerns, lower extremity physical function) from baseline to 3-month follow-up (per-protocol analysis, n = 68).
DISCUSSION
This study demonstrates that a specific, progressive balance training program, focusing on dual- and multitask exercises, can have an impact on objectively measured PA levels in older adults with osteoporosis. We found that participants who had taken part in a balance training program had a statistically significantly higher OR for having a habitual PA level of 5000 or more steps/day compared with controls after the 12-week trial. Given the evidence suggesting that PA is important for people with osteoporosis, a level of 5000 or more steps/day would be expected to contribute to maintained bone mass and reduced risk of many lifestyle-related diseases.1,3
The effect on PA was not maintained at 15-month follow-up, 1 year after cessation of the intervention. The absence of sustained PA benefits at long-term follow-up is disappointing, but not surprising, and consistent with other studies.32,33 Physical activity is a complex behavior, and to accomplish a sustained change, a prolonged intervention or more support regarding habitual PA may be required, such as reinforcement with personalized behavior change counseling or PA on prescription. Interventions aiming to increase PA in older adults targeting specific activities, such as walking, and those involving personalized step-count goals or feedback of PA intensity from accelerometers are found to be effective, with long-term improvements in PA after 12 months.9,34
The primary aim of the BETA study was to reduce fall-related concerns, and not specifically to increase habitual PA; consequently, a low PA level was not an inclusion criterion. Baseline measures showed a wide range of daily steps, and many of the participants had a relatively high PA level with 11 participants (6 in intervention, 5 in control) recording over 10 000 steps/day (data not shown). This is consistent with previous research reporting that exercise trials might attract the more active part of the population.35 Even so, 40% of our sample had a PA level of less than 5000 steps/day. The dose-response relationship between PA and health shows that individuals with the lowest PA levels are the ones who benefit the most of increasing their PA1; accordingly, we wanted to focus on the least active participants in this evaluation. Health outcome-referenced values of steps/day differ in older adults depending upon which health-related outcome is desired, and translations of minimal recommendations including recommended amounts of MVPA have been proposed to 7100 steps/day if averaged over a week for healthy adults, and 4600 steps/day for the most sedentary older adults and individuals living with disability and chronic illness.6 We chose a minimum level of 5000 steps/day as a cutoff because this is suggested to be associated with health benefits,5 and has been used in several studies, which facilitates comparisons among studies and population groups.
Even though previous cross-sectional data have found associations between steps/day and sedentary time and time spent in MVPA in older adults with osteoporosis,7 we did not find any statistically significant differences in our subgroup analyses of accelerometer data. One possible explanation for this is that the intervention did not include PA promotion, such as setting specific goals for achieving recommended MVPA or emphasizing the importance of reducing or breaking up sedentary time. Another explanation is lack of power; the standard deviation for steps/day found in this study was larger compared with the data used in our sample size calculation, obtained from similar studies. Furthermore, we only had the possibility to collect accelerometer data at baseline from a subgroup of our sample, and in addition to the relatively high number of dropouts, that might not be sufficient to detect differences between groups.
Although previous evaluation of this balance training program12 found improvements in fall-related concerns, gait, balance, and physical function, expected associations between these positive changes and improvements in PA were not observed, which might also be explained by lack of power. Liu-Ambrose et al36 have previously reported similar results in a study including older women with low bone mass, which demonstrates the complexity of these associations. The fact that many participants, both in the training group and in the control group, already had walking or NW as a regular PA habit corroborates the findings from this study and our previous cross-sectional analyses,7 that fall-related concerns might not be as strongly associated with habitual PA in older adults with osteoporosis, as previously suggested.4 Qualitative research could provide a deeper understanding of these associations by exploring experiences and perceptions of PA in this population, which cannot be elicited through quantitative methods.
A Cochrane review evaluating the benefits of physical therapy interventions for improving HRQoL in adults with osteoporotic vertebral fractures found inconsistent results and stated that the quality of evidence was very low.37 This indicates that even though cross-sectional associations between daily steps and HRQoL have been found,5,7 treatment effects might be harder to verify and need larger samples,37 which is consistent with our findings. It is also possible that a prolonged intervention is needed to detect improvements in HRQoL. Madureira et al38 found that a 40-week long intervention, including physiotherapist-supervised balance training once a week and home-based exercises 3 times a week, improved HRQoL in a group of older women with osteoporosis.
Among the strengths of this research is the use of a theory-based, well-defined intervention. We designed this balance training program on the basis of well-established principles of exercise, and on the knowledge that balance control relies on the interaction of several physiological systems, as well as interaction with environmental factors, and the performed task.11 Another strength is the longitudinal design of the study. It is critical to know whether the effects of interventions are sustained in the long-term, both from a physical therapy perspective and from a public health perspective, and the evidence of the long-term effect on PA in older adults is limited with only a few studies with assessments beyond 12 months.9 A further strength is the use of objective measurements of PA. Self-reported PA is afflicted by recall bias, and the accuracy of subjective assessments of PA compared with objective assessments is weak; self-reported PA has been shown both to overestimate and underestimate actual PA level.39 We also had a high compliance in wearing the movement sensors and, accordingly, we believe that our PA data are reliable.
It is a limitation that the design of the study, offering individuals in the control group the opportunity to participate in balance training after the long-term follow-up rather than offering a “sham” intervention, did not allow participants to be blinded to allocation. Still, this is a problem shared with all exercise intervention trials. The researchers assessing outcomes at follow-up occasions could not remain blinded for pragmatic reasons alone; the funding allowed only enough researchers to carry out recruitment, intervention, and follow-up simultaneously. However, the fact that the main outcome, habitual PA, was objectively assessed attenuated the problems with researchers not blinded to group status.
Another limitation of the present study is that our sample may not represent the average population of older adults with osteoporosis. The participants were recruited by advertisement, a method that may attract individuals more interested in training and more physically active than average. Although we did address both sexes when recruiting participants, only 2 men were included, which implies that the results might only be true for women. The small proportion of men in this study is shared with other osteoporosis research,37 and there are several explanations for this. Low bone mineral density is more prevalent in older women, and more women than men are diagnosed with osteoporosis.40 Women perceive more fall-related concerns41 and may, therefore, be more conscious about their balance and more interested in taking part in a balance training intervention. It has been suggested that older men may not be willing to admit being afraid of falling or having balance problems,41 and future studies should consider this when recruiting participants. However, we do not believe that our study would have shown a different result if more men were included. The balance training program was developed for older individuals, disregarding sex, and the results from a previous evaluation of the program including healthy older women and men did not show any sex differences.42
Despite efforts made, retention of participants in the study was difficult; of the 91 included participants, 53 (58%) remained in the trial the whole study period. This is a problem our study shares with other research including older adults37,43 and may be an unavoidable consequence of targeting an older population with chronic disease; 50% of those who withdrew cited medical problems (not related to the training) as their reason for discontinuing with the study. A problem with a relatively large proportion of dropout is that it may introduce bias. We did an extensive exploration of the characteristics of the participants who withdrew from the study, and found that missing outcome data could be assumed to be “missing at random.” By including baseline steps/day as a covariate in the regression models, we also effectively measured change in step count over the 3 months, irrespective of the PA level of missing participants.
CONCLUSIONS
Our hypothesis, that a balance training intervention would increase habitual PA in community-dwelling older adults with osteoporosis, was confirmed in the short-term evaluation of this study. A significantly higher proportion of older adults reached a level of 5000 or more steps/day, which is important for overall health. This effect was not associated with improvements in HRQoL, gait speed, balance performance, or fall-related concerns, and did not persist through the long-term follow-up, 12 months after cessation of training.
ACKNOWLEDGMENTS
The authors thank all the participants who made this study possible. We also acknowledge Lisbet Broman for her guidance and help with data collection and processing, Robert Szulkin for statistical advice, and the staff at Karolinska Institutet and Karolinska University Hospital who have been engaged in the project.
Footnotes
The main results of the study has been presented as a poster at the International 22nd Puijo Symposium in Kuopio, Finland, June 2014, and at the World Confederation for Physical Therapy 2015 Congress in Singapore, May 2015.
The study was supported by grants through the Regional Agreement on Medical Training and Clinical Research between Stockholm County council and Karolinska Institutet (ALF), and from the Swedish Research Council (521-2010-2483, 521-2013-255).
Kevin Chui was the Decision Editor.
The authors declare no conflicts of interest.
REFERENCES
- 1.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–1105. [DOI] [PubMed] [Google Scholar]
- 2.Bonaiuti D, Shea B, Iovine R, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;3:CD000333. [DOI] [PubMed] [Google Scholar]
- 3.Boyer KA, Kiratli BJ, Andriacchi TP, Beaupre GS. Maintaining femoral bone density in adults: how many steps per day are enough? Osteoporos Int. 2011;22(12):2981–2988. [DOI] [PubMed] [Google Scholar]
- 4.Delbaere K, Crombez G, Vanderstraeten G, Willems T, Cambier D. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing. 2004;33(4):368–373. [DOI] [PubMed] [Google Scholar]
- 5.Tudor-Locke C, Craig CL, Thyfault JP, Spence JC. A step-defined sedentary lifestyle index: <5000 steps/day. Appl Physiol Nutr Metab. 2013;38(2):100–114. [DOI] [PubMed] [Google Scholar]
- 6.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohrn IM, Hagstromer M, Hellenius ML, Stahle A. Gait speed, quality of life, and sedentary time are associated with steps per day in community-dwelling older adults with osteoporosis. J Aging Phys Act. 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. [DOI] [PubMed] [Google Scholar]
- 9.Hobbs N, Godfrey A, Lara J, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendrick D, Kumar A, Carpenter H, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev. 2014;11:CD009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halvarsson A, Dohrn IM, Stahle A. Taking balance training for older adults one step further: the rationale for and a description of a proven balance training programme. Clin Rehabil. 2015;29(5):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halvarsson A, Franzen E, Stahle A. Balance training with multi-task exercises improves fall-related self-efficacy, gait, balance performance and physical function in older adults with osteoporosis: a randomized controlled trial. Clin Rehabil. 2015;29(4):365–375. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 14.Corder K, Brage S, Ekelund U. Accelerometers and pedometers: methodology and clinical application. Curr Opin Clin Nutr Metab Care. 2007;10(5):597–603. [DOI] [PubMed] [Google Scholar]
- 15.Schneider PL, Crouter S, Bassett DR. Pedometer measures of free-living physical activity: comparison of 13 models. Med Sci Sports Exerc. 2004;36(2):331–335. [DOI] [PubMed] [Google Scholar]
- 16.Kaminsky LA, Ozemek C. A comparison of the Actigraph GT1M and GT3X accelerometers under standardized and free-living conditions. Physiol Meas. 2012;33(11):1869–1876. [DOI] [PubMed] [Google Scholar]
- 17.Keadle SK, Shiroma EJ, Freedson PS, Lee IM. Impact of accelerometer data processing decisions on the sample size, wear time and physical activity level of a large cohort study. BMC Public Health. 2014;14:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benka Wallén MDI, Ståhle A, Franzén E, Hagströmer M. Comparison of pedometer and accelerometer derived steps in elderly individuals with Parkinson's disease or osteoporosis under free-living conditions. J Aging Phys Act. 2014;22(4):550–556. [DOI] [PubMed] [Google Scholar]
- 20.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17(1):17–30. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan M, Karlsson J, Ware JE., Jr The Swedish SF-36 Health Survey–I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41(10):1349–1358. [DOI] [PubMed] [Google Scholar]
- 22.Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10(5):395–404. [DOI] [PubMed] [Google Scholar]
- 23.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20(1):20–25. [DOI] [PubMed] [Google Scholar]
- 24.Rydwik E, Bergland A, Forsen L, Frandin K. Investigation into the reliability and validity of the measurement of elderly people's clinical walking speed: a systematic review. Physiother Theory Pract. 2012;28(3):238–256. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract. 2014;20(4):295–300. [DOI] [PubMed] [Google Scholar]
- 26.Jarnlo GB, Nordell E. Reliability of the modified figure of eight—a balance performance test for elderly women. Physiother Theory Pract. 2003;19(1):35–43. [Google Scholar]
- 27.Padgett PK, Jacobs JV, Kasser SL. Is the BESTest at its best? A suggested brief version based on interrater reliability, validity, internal consistency, and theoretical construct. Phys Ther. 2012;92(9):1197–1207. [DOI] [PubMed] [Google Scholar]
- 28.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34(6):614–619. [DOI] [PubMed] [Google Scholar]
- 29.Halvarsson A, Franzen E, Stahle A. Assessing the relative and absolute reliability of the Falls Efficacy Scale-International questionnaire in elderly individuals with increased fall risk and the questionnaire's convergent validity in elderly women with osteoporosis. Osteoporos Int. 2013;24(6):1853–1858. [DOI] [PubMed] [Google Scholar]
- 30.Roaldsen KS, Halvarsson A, Sarlija B, Franzen E, Stahle A. Self-reported function and disability in late life—cross-cultural adaptation and validation of the Swedish version of the late-life function and disability instrument. Disabil Rehabil. 2014;36(10):813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata, Third Edition. Volume 1: Continuous Responses. 3rd ed: Stata Press; 2012. [Google Scholar]
- 32.Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity in adults with fibromyalgia: results at follow-up. J Clin Rheumatol. 2011;17(2):64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson JL, Vos CM, Fernandez D. Interventions to increase physical activity in people with COPD: systematic review. Annu Rev Nurs Res. 2013;31:297–326. [DOI] [PubMed] [Google Scholar]
- 34.Harris T, Kerry SM, Victor CR, et al. A primary care nurse-delivered walking intervention in older adults: PACE (pedometer accelerometer consultation evaluation)-Lift cluster randomised controlled trial. PLoS Med. 2015;12(2):e1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005;1:CD003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu-Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Balance confidence improves with resistance or agility training. Increase is not correlated with objective changes in fall risk and physical abilities. Gerontology. 2004;50(6):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giangregorio LM, Macintyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev. 2013;1:CD008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madureira MM, Bonfa E, Takayama L, Pereira RM. A 12-month randomized controlled trial of balance training in elderly women with osteoporosis: improvement of quality of life. Maturitas. 2010;66(2):206–211. [DOI] [PubMed] [Google Scholar]
- 39.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1–2):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legters K. Fear of falling. Phys Ther. 2002;82(3):264–272. [PubMed] [Google Scholar]
- 42.Halvarsson A, Franzen E, Faren E, Olsson E, Oddsson L, Stahle A. Long-term effects of new progressive group balance training for elderly people with increased risk of falling—a randomized controlled trial. Clin Rehabil. 2013;27(5):450–458. [DOI] [PubMed] [Google Scholar]
- 43.Iliffe S, Kendrick D, Morris R, et al. Multicentrecluster randomised trial comparing a community group exercise programme and home-based exercise with usual care for people aged 65 years and over in primary care. Health Technol Assess. 2014;18(49):vii–xxvii, 1–105. [DOI] [PMC free article] [PubMed] [Google Scholar]