Abstract
Background:
Nonspecific shoulder pain has a high prevalence in older adults and causes functional alterations. Furthermore, there are difficulties in establishing a clinical diagnosis, effective treatments are lacking, and little evidence has been found regarding the use of invasive physical therapy techniques in this age group.
Purpose:
To determine the efficacy of a single physical therapy intervention with deep dry needling (DDN) on latent and active myofascial trigger points (MTrPs) in older adults with nonspecific shoulder pain.
Methods:
This pilot study is a single-blind, randomized, controlled clinical trial that included 20 participants, aged 65 years and older, who were diagnosed with nonspecific shoulder pain. The study was approved by the Clinical Research Ethics Committee of the area. Participants were recruited at their homes or at a care center and were randomly assigned into either an experimental group (n = 10), which received a session of DDN on 1 active and 1 latent MTrP of the infraspinatus muscle, or a control group (n = 10), which received a session of DDN on only 1 active MTrP. A blind examiner assessed the pain intensity, pain pressure threshold on the anterior deltoid, and extensor carpi radialis brevis muscles and grip strength before, immediately after, and 1 week after the intervention.
Results:
Statistically significant differences (P < .05) in the pressure pain thresholds (PPTs) of the extensor carpi radialis brevis were found in the experimental group in both posttreatment assessments. Moreover, the effect size values (d Cohen) varied from small for grip strength (0.017-0.36) to moderate for the pain intensity (0.46-0.78) and PPT in the anterior deltoid (0.49-0.66) and to large for the PPT in the extensor carpi radialis brevis (1.06-1.58).
Conclusions:
A single physical therapy intervention with DDN on 1 latent MTrP, in conjunction with 1 active MTrP, in the infraspinatus muscle may increase the PPT of the extensor carpi radialis brevis muscle area immediately following and 1 week after the intervention in older adults with nonspecific shoulder pain.
Keywords: aged, musculoskeletal pain, myofascial pain syndromes, shoulder pain, trigger point
INTRODUCTION
One of the most relevant aspects of society worldwide is the global aging of the population and its impact on healthspan and on chronic disease.1 Spain is one of the European countries that requires a higher rate of geriatric care.2 The aging process is associated with the deterioration of physiological capacity, which could cause an increased prevalence of chronic illnesses and alterations in the locomotor system, thereby leading to functional limitation and the loss of personal autonomy. In the Survey of Older Adults performed by IMSERSO3 in 2010, 54% of women and 38% of men older than the age of 60 years suffered from musculoskeletal pain, especially cervical, lumbar, and nonspecific shoulder pain.4
In their systematic review, Luime et al5 concluded that the prevalence (13.2%-26%) and annual incidence (1.6%) of nonspecific shoulder pain reported in older adults were highly variable. Up to 66.7% of the population is estimated to suffer from shoulder pain at least once. The prevalence of annual consultations for shoulder pain (1%) is similar in men and women, and the most frequent diagnoses are impingement and rotator cuff syndromes.6 Moreover, nonspecific shoulder pain in older adults may stem from their previous occupations having involved repetitively moving a physical load, experiencing vibrations, lifting loads, and working in unnatural positions.7
Currently, carrying out clinical tests to accurately diagnose nonspecific shoulder pain is often difficult,8 and there is a lack of effective treatments for subacromial impingement syndrome and rotator cuff tear.9 Existing treatments fail to remedy the functional limitation, which has led some authors to associate shoulder pain with myofascial pain syndrome (MPS).10–14 According to these authors, myofascial trigger points (MTrPs) are the main cause of pain, functional limitation, lack of coordination, and alterations in movement quality that quite often precede a tendinopathy.10–14 Myofascial pain syndrome is defined as the group of sensorial, motor, and autonomic signs and symptoms that originate from MTrPs.15 From a hypothetical etiological point of view, the key factors of MTrPs are local ischemia, low pH, and the release of inflammatory mediators.16 Regarding clinical activity, MTrPs may be active or latent, and both generate dysfunction. However, the symptoms differ because active MTrPs may cause spontaneous pain, whereas latent MTrPs only produce pain related to previous stimulation. In addition, latent and active MTrPs have electromyographic and biochemical differences.16–18 Bron et al12–14 determined that infraspinatus muscle MTrPs are the most prevalent (77%) MTrPs in nonspecific shoulder pain. Moreover, such points are the most responsive to palpation and the most effective in reducing symptoms and improving functionality in chronic shoulder pain.
A wide range of pharmacological or nonpharmacological treatments for MPS is available.19 Physical therapy interventions for MTrPs can be either conservative, using manual therapy techniques such as deep pressure massage, spray and stretch, surface heat, and myofascial release, or invasive, using deep dry needling (DDN) techniques, which seem to show greater efficacy than a placebo.20,21 According to previous studies, short-term segmental antinociceptive effects in the musculature at the same level of innervation are produced by DDN through segmental modulation mechanisms. DDN deactivates key MTrPs and inhibits the activity of satellite MTrPs in the area of the referred pain.22,23 Among all DDN techniques the Hong's fast-in and fast-out technique is used in several previous works.10,20–24 The local twitch responses (LTRs) attained are directly correlated with the speed of needle insertion, the clinical efficacy of DDN, and the sensitivity of the MTrPs.15,20,25
Recently, research into latent MTrPs has increased in the physical therapy field. Ge et al24,26 determined the presence of latent MTrPs in the central sensitization process in the infraspinatus muscle in participants with shoulder pain. Moreover, these studies suggest that DDN is a sensitive technique for the localization and treatment of such points, improving pain and motor function and preventing their activation in MPS. Celik and Yeldan27 indicated that muscular strength is lower in subjects with latent MTrPs compared with healthy subjects.
Considering the high prevalence and the functional alterations that occur in older adults with nonspecific shoulder pain, little evidence is found regarding invasive physical therapy techniques in this population.
The aim of this pilot study was to assess the immediate and short-term efficacy of a single physical therapy intervention with DDN on latent active MTrPs in conjunction with active MTrPs in the infraspinatus muscle in individuals older than 65 years old who are diagnosed with nonspecific shoulder pain.
METHODS
Study Design
A single-blind, randomized clinical trial (RCT) pilot study was performed with parallel groups between July 2012 and March 2013.
Participants
This RCT pilot study was developed to calculate the sample size for a larger RCT. Therefore, 20 participants, divided into an experimental group (EG; n = 10) and a control group (CG; n = 10), were recruited from their homes or a care center.
The inclusion criteria were as follows: people aged 65 years and older with uni- or bilateral nonspecific shoulder pain and at least 1 active and 1 latent MTrP in the infraspinatus muscle ipsilateral to the painful shoulder. Nonspecific shoulder pain was considered in cases in which the main source of symptoms was located in the space between the acromion, the insertion of the deltoid muscle, and the lateral region of the scapula without a prior diagnosis in medical record, according to the International Association for the Study of Pain criteria.28 Moreover, the diagnosis of active and/or latent MTrPs followed the essential and confirmatory criteria described by Simons et al.15 On the one hand, essential criteria included palpable tense bands, extreme local pain from pressure upon a nodule of the taut band, the patients' recognition of their pain upon pressing the sensitive nodule to identify an active MTrP, and painful limited range of movement at full stretch. On the other hand, confirmatory criteria included visual or tactile identification of an LTR, image of an LTR caused by inserting a needle into the sensitive knot and pain, or alteration of the sensitivity upon compressing the sensitive knot in the muscle. According to Tough et al,29 these criteria are widely used in research studies, and their interexaminer reliability was described by Lucas et al,30 although some of these criteria remain questionable. Active MTrPs produce spontaneous and recognizable pain under stimulation, whereas latent MTrPs generate localized pain or unrecognizable referred pain upon stimulation.12,22–24,29,30 Recent studies have determined that mechanosensitivity in the medial fibers of the infraspinatus muscle is higher depending on the topographical localization.24
According to the medical records, subjects who had prior myopathy or neuropathy diagnoses, cognitive deficits, cervical spine, rotator cuff tendons or glenohumeral joint problems, corticoid infiltration or local anesthetic use during the previous year or during follow-up, surgical procedures affecting the upper limb or preceding cervical joint, antiaggregant ingestion, or anticoagulant, analgesic or anti-inflammatory medication or abusive substance use in the week prior to treatment and during follow-up were excluded from the study. The inclusion and exclusion criteria were based in previous studies.22,23
After signing informed consent forms, the participants were randomly allocated into 2 groups by opaque closed envelopes, which included either the CG or the EG, associated each patient with a code (01-20). The study was approved by the area's Clinical Research Ethics Committee.
Outcome Measurement
Sociodemographic data such as age and sex were collected at baseline (A0), before the intervention.
The primary outcomes pain intensity, pressure pain threshold (PPT), and grip strength were measured at baseline (A0), immediately after the intervention (A1), and a week after the intervention (A2).
Pain intensity was measured with the Numerical Rating Scale (NRS) of 11 points (interval from 0 to 10), where 0 corresponds to no pain, and 10 corresponds to the worst pain imaginable. A graphical representation of 11 spaces was used to indicate the patient's own evaluation of his or her pain. The subjects were asked to assess the subjective pain intensity of the painful shoulder by pointing with 1 of their fingers to mark the level of pain on the scale. The NRS is valid and reliable for use in elderly people,31,32 and its correlation with the Visual Analogue Scale shows a high convergent validity (0.79-0.95).33 Moreover, a reduction of 30% to 33% in pain suggests a better response than to any other treatment.31
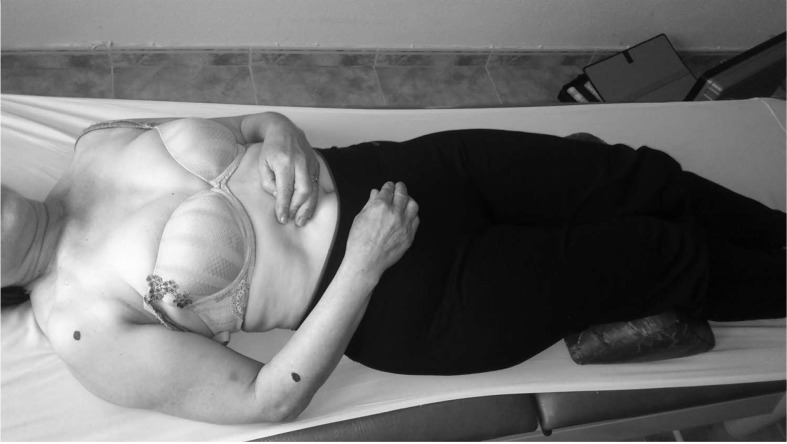
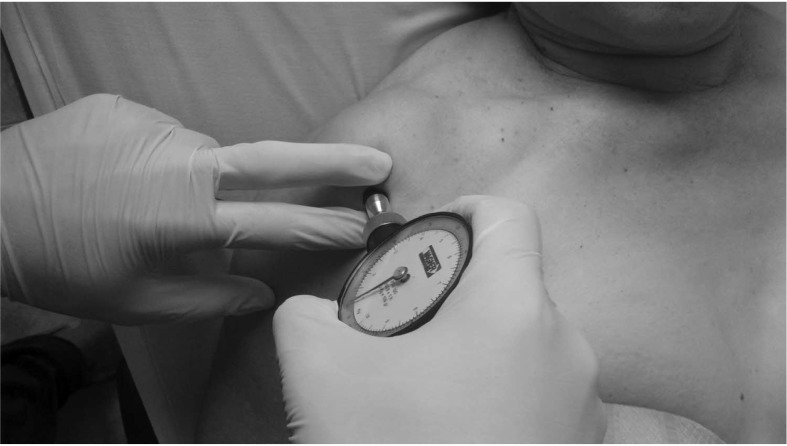
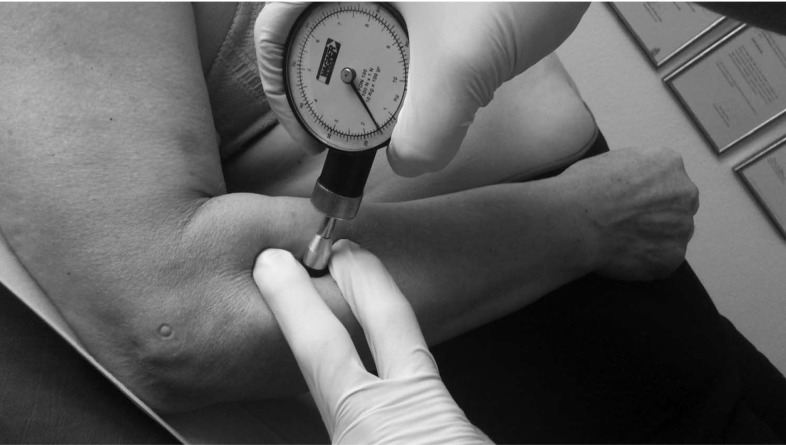
The PPT, measured with a WAGNER FDK/FDN series Force Dial analogue Fisher algometer (Wagner Instruments, Greenwich, CT), varies between 0 and 10 kg/cm2 and has recently been shown to be superior in reliability, reproducibility, and sensitivity concerning a controlled automated system of deformation.34 The algometrical reliability, validity, and reproducibility in different age and sex groups were reported in some studies.35–37 Neziri et al38 observed that sex, age, and/or the interaction of age with sex are the only variables that consistently affected pain measures. In particular, women are more sensitive to pain than men, and the sex influence decreases as age increases. Fisher35 proposed a critical PPT level of 2 kg/cm2 lower with regard to the normal control point in MPS; this level is considered clinically relevant. After indicating the most hyperalgesic latent MTrPs of anterior deltoid and extensor carpi radialis brevis muscles, the examiner determined PPT for these points. Both the blind examiner and the participant were trained to recognize the PPT on the contralateral side. This procedure was recommended by Fisher.35 The position used is shown in Figure 1. The PPT measuring procedures of latent MTrPs on the anterior deltoid and extensor carpi radialis brevis muscles are shown in Figures 2 and 3, respectively. Three repeated measurements were performed at the same place within an interval of 30 to 60 seconds, and the average value was used for data analysis. A filled-in circle was drawn in permanent marker to indicate latent MTrPs for the anterior deltoid and extensor carpi radialis brevis muscles homolateral to the painful shoulder. To calculate the algometric measurement of A2, both points were periodically reviewed during the week after the intervention, and participants were asked to cover the points with tape.
Figure 1.
Position to determine the localization and PPT of latent MTrPs in the anterior deltoid and extensor carpi radialis brevis. MTrPs, myofascial trigger points; PPT, pressure pain threshold.
Figure 2.
Procedure to determine the PPT of latent MTrP in anterior deltoid. MTrPs, myofascial trigger points; PPT, pressure pain threshold.
Figure 3.
Procedure to determine the PPT of latent MTrP in the extensor carpi radialis brevis. MTrPs, myofascial trigger points; PPT, pressure pain threshold.
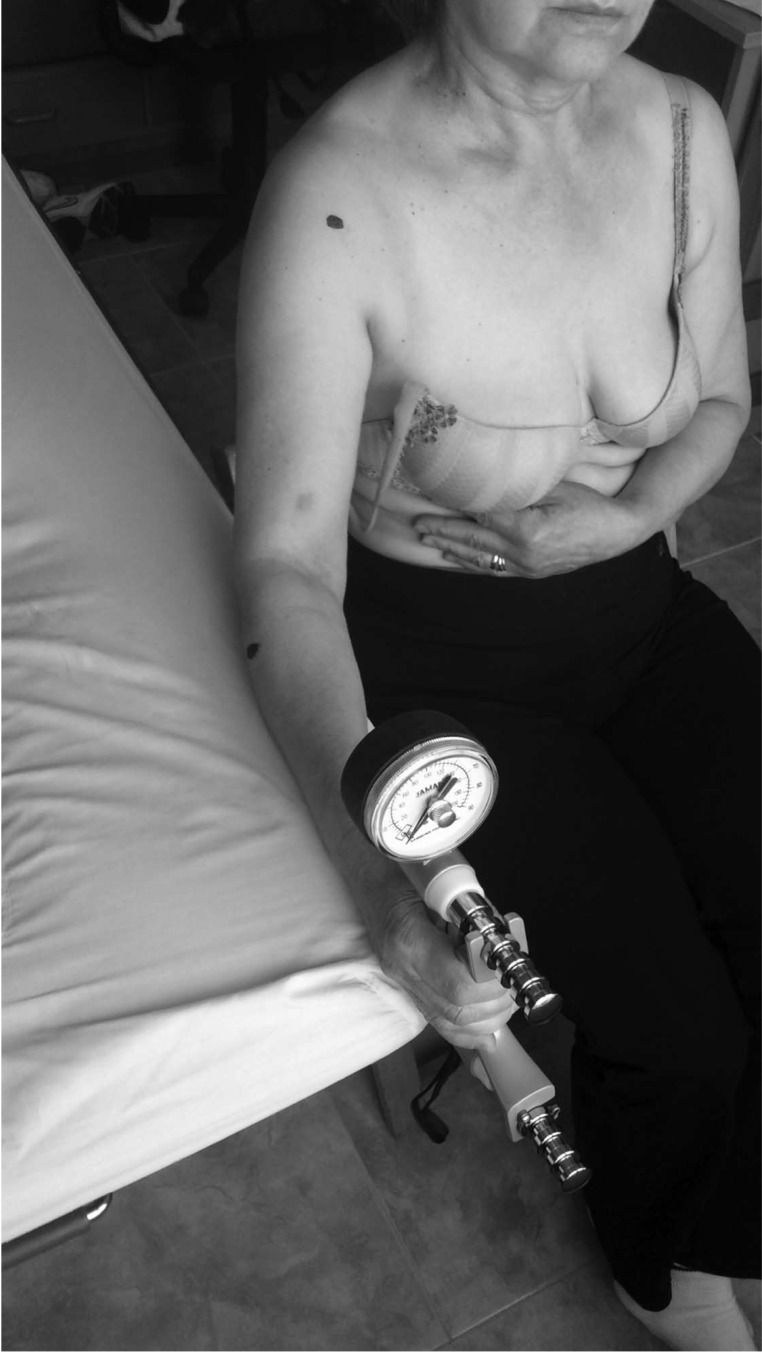
Grip strength, defined as the maximum isometric muscular force in kilogram was measured with a JAMAR hydraulic manual analogical dynamometer (Sammons Preston Rolyan, Bolingbrook, IL) with an interval of 0 to 90 kg to evaluate the weakness of latent forearm MTrPs. The validity, reproducibility, and reliability of the maximum grip strength in different elderly groups were demonstrated by Abizanda et al,39 and the least relevant clinical difference is 42.17%.40 The procedure for measuring the grip strength involved previously training the participant on the contralateral side; the participants were required to use their maximum grip strength during an interval of 5 to 10 seconds (Figure 4). On the basis of the fatigability of older adults, a single measurement was performed.39
Figure 4.
Position and procedure to determine the maximum grip strength.
Procedures and Intervention
This study was carried out by 2 experienced physical therapists (>4 years clinical experience) in MPS. Physical therapist 1 (CCL), who was blinded to patients' group allocations, carried out the assessments at baseline (A0), immediately after the intervention (A1), and 1 week after the intervention (A2) to collect sociodemographic and primary outcomes measurements.
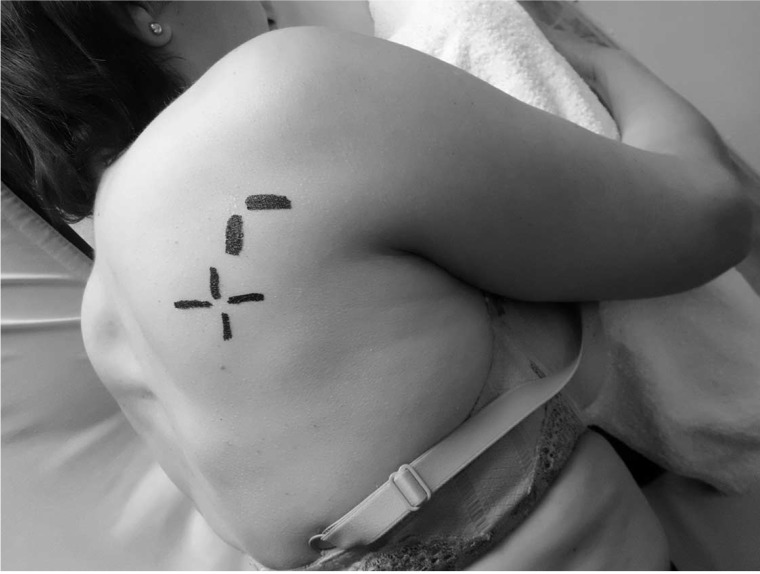
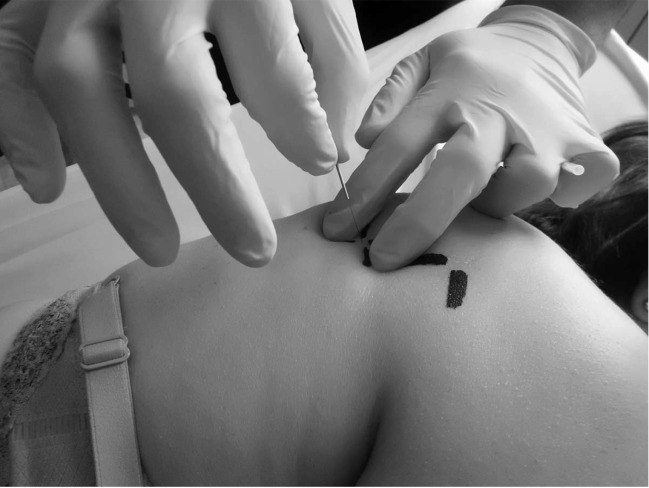
Physical therapist 2 (EHH) performed the physical therapy interventions in each group. The CG received a single session physical therapy intervention that comprised DDN of the active MTrP that was most hyperalgesic to palpation of the infraspinatus muscle homolateral to the painful shoulder. If there was more than 1 active MTrP, the most hyperalgesic MTrP was considered the one that elicited the highest pain intensity in the NRS under the same pressure. The EG received the same treatment described for CG combined with DDN of the most hyperalgesic latent MTrP, both being located in the infraspinatus muscle homolateral to the painful shoulder. To identify the infraspinatus MTrPs that were homolateral to the painful shoulder, a grid with 4 perpendicular lines drawn using a permanent marker to determine the active MTrP and a grid of 2 perpendicular lines to determine the most mechanosensitive latent MTrPs (Figure 5). A headless 0.32 × 40 mm needle (AGU-PUNT) was inserted perpendicularly to the scapula toward the MTrP located between the fingers of the subdominant hand, and the guide tube was removed. By means of metacarpophalangeal flexion/extension of the first and second fingers of the dominant hand (Figure 6), the area was probed in different directions until a minimum of 1 LTR, a local pain response, and usually the referred pain pattern of the MTrP were obtained.15 The penetration depth varied according to subject,22 and Hong's fast-in and fast-out technique was used.10,20–24 The observation or sensitivity to 1 LTR is considered an indispensable confirmatory criterion when performing DDN in both active and latent MTrPs. Furthermore, in those cases where it is possible and based on clinical use, DDN technique was performed until reaching LTR exhaustion or the patient's tolerance limit.15,20 Each complete DDN procedure on each localized MTrP had a duration of 1 to 2 minutes, being similar to a previous study.22 After extracting the needle from the dominant hand, hemostasis was applied with the fingers for 1 minute.15
Figure 5.
Position and procedure to determine the active (a grid with 4 perpendicular lines) and latent (a grid with 2 perpendicular lines) MTrPs in the infraspinatus. MTrPs, myofascial trigger points.
Figure 6.
Intervention by deep dry needling in the active and latent infraspinatus MTrPs. MTrPs, myofascial trigger points.
Statistical Procedure
SPSS version 18.0 for Windows (IBM, Chicago: SPSS Inc) was used for statistical analysis. At the beginning, Shapiro-Wilks test was used to assess normal distribution of the used variables. At first, such statistics were computed taking into account all the subjects together, and afterwards, considering the treatment of each group independently.
Descriptive statistics were calculated to describe the data outcomes in the 3 measurements carried out (A0, A1, and A2). The mean and the standard deviation (SD) were used in the case of the variables that were adjusted to normal. The median and interquartile range were used in the case of variables that were not normally distributed. These results are shown in Table A1 in Appendix 1. Moreover, the mean and SD of PPT by sex in the control and EGs were added, according to Neziri et al.38
Afterward, the homogeneity of both the sociodemographic variables and baseline data (A0) was studied within both groups that received treatment. For the “sex” outcome, homogeneity was assessed using the Fisher exact test. For the age and A0 outcomes (pain intensity, PPT, and grip strength), the Student t test for independent samples was used to check the homogeneity between both groups because the outcomes were adjusted to the normal distribution.
Repeated-measures analysis of variance (ANOVA) with 2 factors, 2 (group) × 3 (time), were carried out. The values for the primary outcomes in the 3 measurements carried out (A0, A1, and A2) by independently analyzing each of the treatment groups were obtained as follows: depending on the normality of the variables, repeated-measures ANOVA, completed with Simple and Helmert contrasts, was used for parametric data. For nonnormally distributed variables, the repeated-measures Friedman test, complemented with the Dunn multiple comparison test (by means of the GraphPad InStat 3.06 statistic package), was used.
Finally, the values of the different variables (A1-A0, A2-A0) and the efficacies of the 2 treatment groups were compared; the Student t test for independent samples was employed because all variables conformed to the normal curve. This significance test was complemented by adding the effect size using the formula d = 2t/√gl.
The analysis was performed “per intent-to-treat.” The statistical tests were performed considering a confidence interval of 95% (P value <0.05).
RESULTS
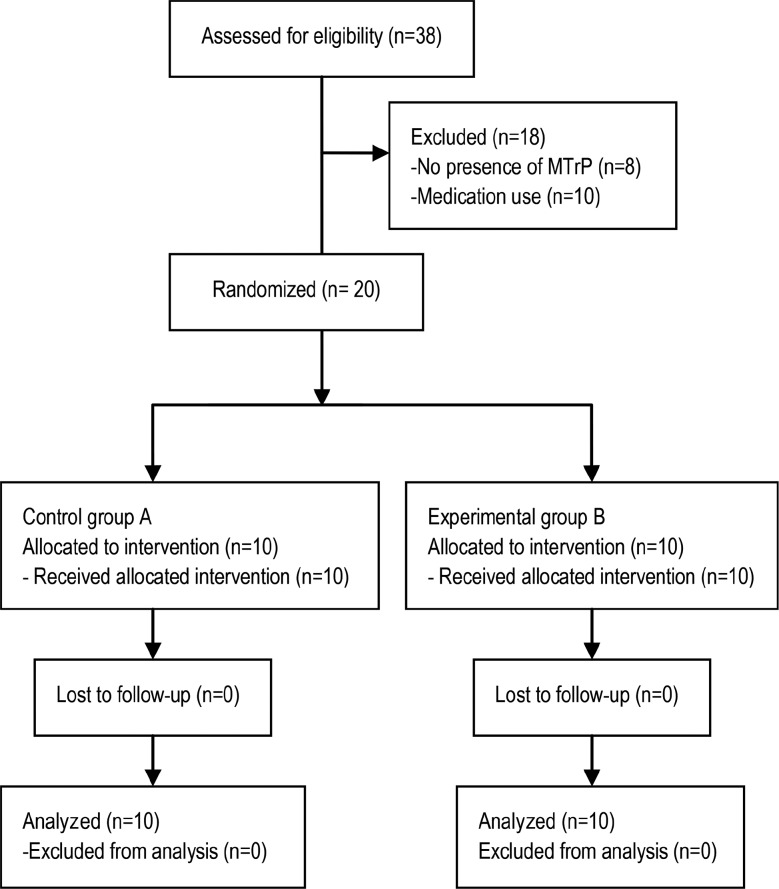
Of the 38 participants who were assessed for eligibility, 18 were excluded (n = 8 did not present MTrPs, and n = 10 were taking medication). Of the 20 participants, 10 patients were randomized to the EG, and 10 were randomized to the CG. All of the participants in both groups received the allocated intervention and completed the 1-week analysis (Figure 7).
Figure 7.
CONSORT 2010 flow diagram.
Baseline Description
The baseline characteristics of the 2 groups are summarized in Table 1. There were no significant differences (P < .05) for the sociodemographic and primary outcomes. The EG was younger than the CG and had a higher mean baseline pain intensity, anterior deltoid PPT, extensor carpi radialis brevis PPT, and grip strength. The 2 groups did not differ by sex, and there were more women (70%) than men (30%) overall.
Table 1. Baseline Characteristics of the Sample According to the Intervention Groups.
| Baseline Characteristics | Experimental Group | Control Group | P |
|---|---|---|---|
| Mean (SD) or Percentage | |||
| Age, y | 77.45 (7.6) | 81.77 (8.7) | .252a |
| Sex, % | 1.000b | ||
| Women | 70 | 70 | |
| Men | 30 | 30 | |
| Pain intensity | 4.40 (1.0) | 3.60 (1.7) | .202a |
| Anterior deltoid PPT | 3.01 (0.6) | 2.68 (0.6) | .248a |
| Women | 2.85 (0.7) | 2.37 (0.5) | |
| Men | 3.39 (0.1) | 3.40 (0.2) | |
| Extensor carpi radialis brevis PPT | 2.49 (0.8) | 2.35 (0.6) | .654a |
| Women | 2.14 (0.5) | 2.09 (0.5) | |
| Men | 3.30 (0.7) | 2.95 (0.4) | |
| Grip strength, kg | 19.55 (8.6) | 16.20 (10.0) | .431a |
Abbreviations: PPT, pressure pain threshold (kg/cm2); SD, standard deviation.
aThe Student t test.
b The Fisher exact test.
Efficacy of the Intervention in Each Group
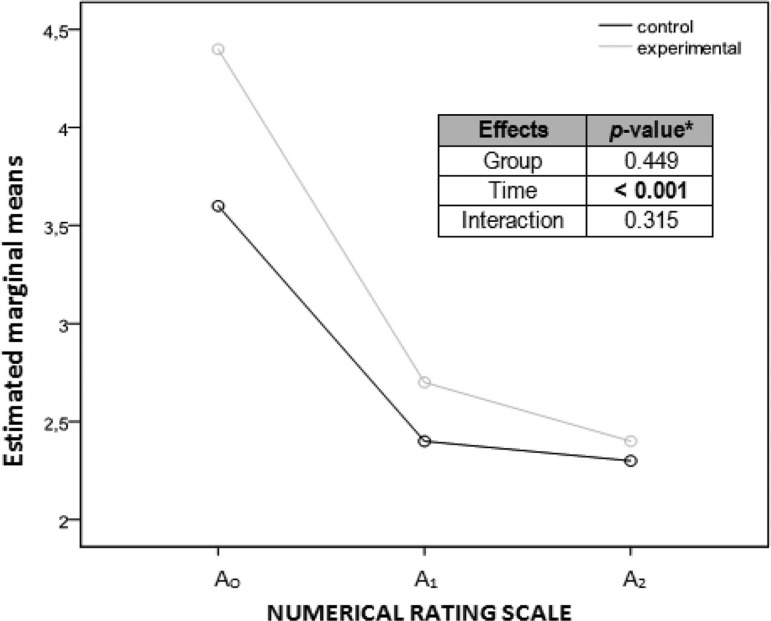
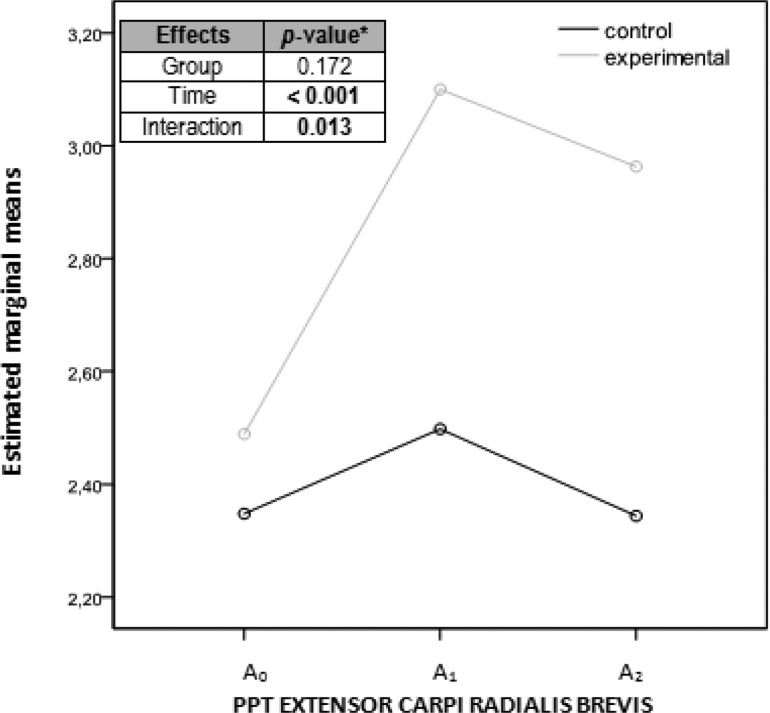
Considering each group separately, the differences among the 3 assessments (A0, A1, A2) for each primary outcome are detailed in Table 2. Marginal mean graphs (Figures 8–11) were added to illustrate the difference found in the assessments for each outcome, as well as for each group separately. The P values of the 3 comparisons by repeated-measures ANOVA with 2 factors, 2 (group) × 3 (time), were included in these figures; there were significant differences (P = .013) for the extensor carpi radialis brevis PPT interaction (Figure 10).
Table 2. Efficacy of Intervention in Each Group.
| Primary Outcomes | Mean (SD) | ANOVA of Repeated Measurements, P | Simple and Helmert Contrasts, P | ||||
|---|---|---|---|---|---|---|---|
| A0 | A1 | A2 | A1-A0 | A2-A0 | A2–A1 | ||
| Experimental group | |||||||
| Pain intensity | 4.5 (1)a | 2.5 (1)a | 2 (1)a | .0014b | .022 | .008 | .518 |
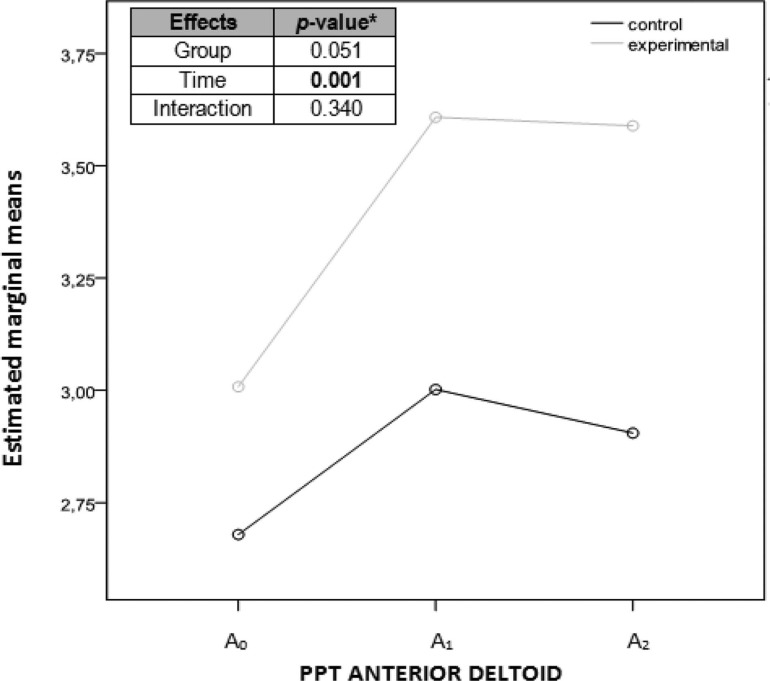
| Anterior deltoid PPT | 3.28 (0.8)a | 3.58 (0.8)a | 3.6 (1.1)a | .0655b | .082 | .054 | .799 |
| Extensor carpi radialis brevis PPT | 2.49 (0.7) | 3.10 (1.0) | 2.96 (0.8) | .005 | .009 | .001 | .347 |
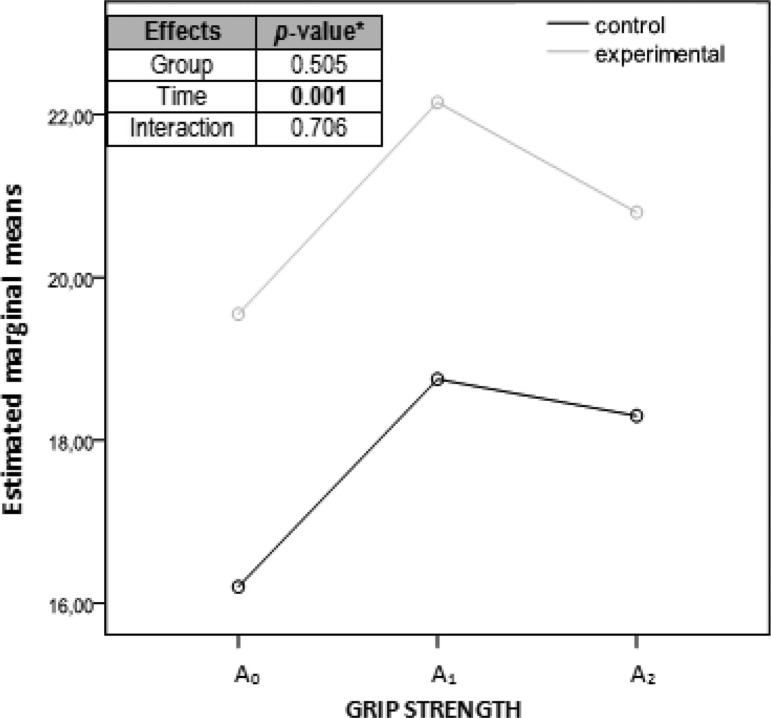
| Grip strength (kg) | 19.5 (8.6) | 22.1 (10.1) | 20.8 (9.1) | .049 | .045 | .130 | .147 |
| Control group | |||||||
| Pain intensity | 3 (2)a | 2 (3)a | 2 (2)a | .0008b | .020 | .020 | .564 |
| Anterior Deltoid PPT | 2.68 (0.6) | 3.00 (0.8) | 2.90 (0.8) | .071 | .050 | .148 | .244 |
| Extensor carpi radialis brevis PPT | 2.35 (0.6) | 2.49 (0.6) | 2.34 (0.7) | .212 | .144 | .968 | .137 |
| Grip strength (kg) | 16.2 (10.0) | 18.7 (11.3) | 18.3 (12.1) | .010 | .010 | .029 | .566 |
P < .05
Abbreviations: A0, baseline assessment; A1, assessment immediately after the intervention; A2, assessment a week after the intervention; ANOVA, analysis of variance; PPT, pressure pain threshold (kg/cm2); SD, standard deviation.
aMedian and the interquartile range.
bThe Friedman test completed with the Dunn multiple comparisons test.
Figure 8.
Graph showing marginal means of the numerical rating scale measurements considering each group separately. Abscissa axis: horizontal with the 3 evaluation moments; ordinate axis: score. aANOVA (analysis of variance) of repeated measurements with 2 factors, 2 (group) × 3 (time). A0, before the intervention; A1, immediately after the intervention; A2, a week after the intervention; NRS, Numerical Rating Scale.
Figure 11.
Graph showing marginal medians of the grip strength measurements considering each group separately. Abscissa axis: horizontal with the 3 evaluation moments; ordinate axis: kg. aANOVA (analysis of variance) of repeated measurements with 2 factors, 2 (group) × 3 (time). A0, before the intervention; A1, immediately after the intervention; A2, a week after the intervention.
Figure 10.
Graph showing marginal means of the pressure pain threshold measurements of the extensor carpi radialis brevis considering each group separately. Abscissa axis: horizontal with the 3 evaluation moments; ordinate axis: kg/cm2. aANOVA (analysis of variance) of repeated measurements with 2 factors, 2 (group) × 3 (time). A0, before the intervention; A1, immediately after the intervention; A2, a week after the intervention; PPT, pressure pain threshold.
Figure 9.
Graph showing marginal means of the pressure pain threshold measurements of the anterior deltoid considering each group separately. Abscissa axis: horizontal with the 3 evaluation moments; ordinate axis: kg/cm2. aANOVA (analysis of variance) of repeated measurements with 2 factors, 2 (group) × 3 (time). A0, before the intervention; A1, immediately after the intervention; A2, a week after the intervention; PPT, pressure pain threshold.
There was significant difference for the pain intensity between the EG and CG in A1 (immediately after intervention) and A2 (1 week after intervention), respectively. For extensor carpi radialis brevis PPT, significant differences were found in the EG in A1 and A2. For grip strength, there were significant differences for the experimental and control groups in A1 and for the CG in A2.
Efficacy of the Intervention Compared Between Groups
Regarding the extensor carpi radialis brevis PPT, the results in Table 3 show the existence of significant differences favoring the EG for the differences obtained in A1 and A2. For the rest of the primary outcomes, no significant differences were found between the groups. Furthermore, the effect size values were small for strength, moderate for pain intensity and anterior deltoid PPT, and large for extensor carpi radialis brevis PPT in A1 and A2, respectively.
Table 3. Efficacy of Interventions Comparing Groups.
| Difference Variables | Experimental Group | Control Group | Pa | Effect Sizeb |
|---|---|---|---|---|
| Mean (SD) | ||||
| Pain intensity | ||||
| A1-A0 | 1.70 (1.4) | 1.20 (0.8) | .171 | 0.46 |
| A2-A0 | 2 (0.94) | 1.30 (10.0) | .057 | 0.78 |
| Anterior deltoid PPT | ||||
| A1-A0 | 0.60 (0.7) | 0.32 (0.5) | .157 | 0.49 |
| A2-A0 | 0.58 (0.7) | 0.23 (0.5) | .089 | 0.66 |
| Extensor carpi radialis brevis PPT | ||||
| A1-A0 | 0.61 (0.6) | 0.15 (0.3) | .019 | 1.06 |
| A2-A0 | 0.47 (0.3) | −0.00 (0.3) | .002 | 1.58 |
| Grip strength | ||||
| A1-A0 | 2.60 (3.5) | 2.55 (2.5) | .485 | 0.02 |
| A2-A0 | 1.25 (2.4) | 2.10 (2.6) | .225 | 0.36 |
Abbreviations: A0, baseline assessment; A1, assessment immediately after the intervention; A2, assessment a week after the intervention; PPT, pressure pain threshold (kg/cm2); SD, standard deviation.
aThe Student t test.
bThe d Cohen (2t/√gl).
DISCUSSION
A single DDN intervention of the most hyperalgesic infraspinatus muscle latent MTrP may reduce the mechanosensitivity of the extensor carpi radialis brevis muscle immediately after the intervention and in the short term in people older than 65 years with nonspecific shoulder pain.
Concerning shoulder pain, EG participants experienced a greater reduction in the pain intensity than subjects in the CG, although no significant differences were found between the groups. The effect size was moderate, which may be due to the small sample size. Therefore, it would be beneficial to perform a similar study with a larger sample. In a previous study, Hsieh et al23 carried out a similar intervention with the same DDN technique on 1 active MTrP in subjects with bilateral shoulder pain, using each subject's nontreated shoulder as a control for the treatment. The immediate results were significant compared with the control side and with regard to the pain intensity after the intervention. Williamson and Hoggart31 determined that a minimum difference in the NRS of approximately 30% to 33% suggested a better response to treatment. In this study, both interventions achieved this level.
Regarding mechanosensitivity, there were no significant differences for the anterior deltoid muscle upon comparing both groups, and the effect size value was moderate. Furthermore, the PPT decreases with age,37 and the critical PPT level was less than that of 2 kg/cm2 proposed by Fisher35; therefore, no clinically relevant findings for the PPT were observed. According to Neziri et al,38 sex, age and/or the interaction of age with sex may consistently affect pain measures. Nevertheless, for the extensor carpi radialis brevis, significant differences were observed, and effect size value was large. Hsieh et al23 used the extensor carpi radialis longus muscle to measure mechanosensitivity in the forearm, whereas, in this study, the extensor carpi radialis brevis was measured. In the literature, this muscle has been used in peripheral sensitivity models of C5-6 by injecting algogenic substances into latent MTrPs of the infraspinatus. Fernández-Carnero et al41 stated that the infraspinatus muscle is innervated by the C4 to C6 segments and that the extensor carpi radialis brevis afferences converge in the same intrasegmental area of the spinal cord. According to Jayakumar et al,42 the origin of the innervation to the extensor carpi radialis brevis varies according to previous studies; it originates from the radial nerve trunk (0%-20%), the superficial (24%-56%), or the deep (32%-58%) branch of the radial nerve or the angle of bifurcation of the radial nerve (0%-22%), which is between its superficial and deep branches. However, several studies in the literature affirm this wide variability in the percentage of innervations. Bevelaqua et al43 elucidated the relationship with C6-C7 roots using needle electromyography. The axillary nerve is one of the terminal branches of the posterior cord of the brachial plexus and usually contains fibers from C5 to C6 ventral rami. It innervates the teres minor and deltoid muscles, skin over the shoulder, and the glenohumeral joint.44 In this study, the infraspinatus, anterior deltoid, and extensor carpi radialis brevis were addressed for 2 reasons—first, because of the C6 innervation relationship,22,23,41–44 and second because the anterior deltoid and extensor carpi radialis brevis muscles are inside of the referred pain pattern area of infraspinatus MTrPs.15,22–24 In this study, infraspinatus PPT was not assessed to place a greater importance on assessing the same level of innervation and the referred pain distribution.
For grip strength, the EG treatment did not produce better values than those in the CG, and the effect size was small. This may be due to small sample size, and the treatment of a proximal muscle in the upper limb may have influenced this result. In 2011, Celik and Yeldan27 indicated that although differences in strength on the dominant and the nondominant sides are not important, muscular strength is significantly lower on both sides in subjects with latent MTrPs in the shoulder girdle compared with healthy subjects. In contrast, Dhara et al40 found a variation of 42.17% in the manual grip strength of people with different orthopedic alterations in the upper limbs compared with healthy subjects. The results of this study did not reach this relevant minimum difference. However, Bohannon45 found that low grip strength was consistently associated with a greater likelihood of premature mortality, the development of disability, and an increased risk of complications or a prolonged length of stay after hospitalization or surgery in older adults.
Considering the internal validity, as some daily activities can influence shoulder pain in older adults, and as the daily activities are different whether the patient is living in a care center or at home, the sample included patients with both lifestyles. To control for bias in measurements or diagnosis, the tools were calibrated and validated according to a special protocol for measuring the outcomes. Because of surveillance, bias due to the Hawthorne effect, wherein the normal conduct of the patient may be modified if/when feeling observed, had the potential to occur. To control for this, an explanation protocol for the test was designed with instructions that examiners had to provide to patients under the same conditions.
With regard to the external validity, this study was limited to older adults, had a single blind examiner, and did not have a placebo CG. Importantly, DDN presents difficulties in blinding patients due to referred pain and postneedling pain. However, Mayoral et al21 demonstrated that DDN is superior to placebo in older adults by achieving the double blinding of patients by treating under anesthesia. However, the reliability of the detection of trigger points remains questioned.29,30 Because needle electromyography was not used in this study, there may be a lack of objective findings to detect MTrPs because no specific tools for the gold standard were used. Needle electromyography determines the noise of the motor end plate, which represents the spontaneous electrical activity of acetylcholine (Ach), but requires suitable training and equipment, which are not generally available.15,17 Simons et al15 recommend this finding as confirmatory criteria.
The aim of this study was to evaluate the treatment of nonspecific shoulder pain; this coincides with daily clinical practice due to the frequent diagnostic difficulties in determining a specific structure responsible for the shoulder pain.8 However, the majority of studies relate MPS with subacromial impingement and rotator cuff tendonopathies.10–14 Neither the nature nor the temporality of pain was limited in this research to independently test the efficacy of the proposed intervention of acute or chronic pain. Nevertheless, authors such as Bron et al12–14 have focused on nontraumatic chronic shoulder pain. Combinations with different methods or therapeutic agents are recommended, but this study emphasizes the evaluation of DDN in isolation.20
This study considered the observation or sensitivity of an LTR on both latent and active MTrPs as an indispensable confirmatory criterion when performing DDN because it was performed by reproducing its clinical use until the LTR was exhausted or the patient's tolerance limit was reached.15,20 Notably, 1 participant of the EG did not obtain an LTR during DDN treatment of an infraspinatus latent MTrP. Nevertheless, 95% of participants obtained an LTR in all of the other MTrPs; this coincided with previous studies on older adults. Ga et al46 performed a study on the upper trapezius muscle on people with an average age of 78 years and demonstrated a greater presence (97.5%) of LTRs in older adults than in subjects with an average age of 40 years. This may be an important consideration for performing DDN in older adults.
Considering the mechanisms and implications of MPS in an elderly population as well as in the general population,22,23 this study did not assess the influence of other central mechanisms of pain modulation because it was limited to the evaluation of MTrPs of the C6 segmentary level. Mense47 describes allodynia, hyperalgesia and referred pain of the MTrPs as changes due to an increase in the synaptic efficacy of central connections. The inactivation of latent MTrPs using DDN may prevent the development of active MTrPs and reduce their nociceptive input, normalize synaptic efficacy, and reduce central and peripheral sensitization.26,48 This study supports the influence of latent MTrPs on nonspecific shoulder pain disorders in elderly people.
Finally, this study considers the addition of DDN of latent MTrPs in the physical therapy treatment of active MTrPs.
CONCLUSIONS
A single physical therapy intervention with deep dry needling on 1 latent MTrP in conjunction with 1 active MTrPs in the infraspinatus muscle may increase the PPT of the extensor carpi radialis brevis muscle area immediately and 1 week after the intervention in older adults with nonspecific shoulder pain. This study may contribute to the consideration of latent MTrPs DDN to reduce the mechanosensitivity of the distal musculature of the upper limb.
Appendix 1. Descriptive Analysis
Table A1. Descriptive Outcome Data in the 3 Measurements Carried Out (A0, A1, A2).
| Primary Outcomes | Group | Mean | SD |
|---|---|---|---|
| A0 pain intensity | Control | 3.0a | 2.0a |
| Experimental | 4.40 | 0.97 | |
| A1 pain intensity | Control | 2.0a | 3.0a |
| Experimental | 2.5a | 1.0a | |
| A2 pain intensity | Control | 2.30 | 1.34 |
| Experimental | 2.0a | 1.0a | |
| A1-A0 pain intensity | Control | 1.20 | 0.79 |
| Experimental | 1.70 | 1.42 | |
| A2-A0 pain intensity | Control | 1.30 | 0.95 |
| Experimental | 2.0a | 1.25a | |
| A0 PPT anterior deltoid | Control | 2.68 | 0.63 |
| Experimental | 3.28a | 0.84a | |
| A1 PPT anterior deltoid | Control | 3.00 | 0.75 |
| Experimental | 3.61 | 0.64 | |
| A2 PPT anterior deltoid | Control | 2.90 | 0.75 |
| Experimental | 3.59 | 0.57 | |
| A1-A0 PPT anterior deltoid | Control | 0.31a | 0.42a |
| Experimental | 0.60 | 0.71 | |
| A2-A0 PPT anterior deltoid | Control | 0.23 | 0.45 |
| Experimental | 0.58 | 0.66 | |
| A0 PPT extensor carpi radialis brevis | Control | 2.35 | 0.63 |
| Experimental | 2.49 | 0.75 | |
| A1 PPT extensor carpi radialis brevis | Control | 2.49 | 0.59 |
| Experimental | 3.10 | 1.03 | |
| A2 PPT extensor carpi radialis brevis | Control | 2.34 | 0.62 |
| Experimental | 2.96 | 0.78 | |
| A1-A0 PPT extensor carpi radialis brevis | Control | 0.15 | 0.29 |
| Experimental | 0.61 | 0.58 | |
| A2-A0 PPT extensor carpi radialis brevis | Control | −0.004 | 0.31 |
| Experimental | 0.47 | 0.33 | |
| A0 grip strength | Control | 16.20 | 9.99 |
| Experimental | 19.55 | 8.55 | |
| A1 grip strength | Control | 18.75 | 11.31 |
| Experimental | 22.15 | 10.09 | |
| A2 grip strength | Control | 18.30 | 12.10 |
| Experimental | 20.80 | 9.11 | |
| A1-A0 grip strength | Control | 2.55 | 2.48 |
| Experimental | 2.60 | 3.53 | |
| A2-A0 grip strength | Control | 2.10 | 2.56 |
| Experimental | 1.25 | 2.37 |
Abbreviations: A0, baseline assessment; A1, assessment immediately after the intervention; A2, assessment a week after the intervention; PPT, pressure pain threshold; SD, standard deviation.
aMedian and interquartile range were used.
Footnotes
The authors declare no conflict of interest.
Richard W Bohannon was the Decision Editor.
REFERENCES
- 1.Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb G, Ranberg KA, Jentoft AC, O'Neill D, Topinkova E, Michel JP. Geriatric care in Europe—the EUGMS Survey part I: Belgium, Czech Republic, Denmark, Germany, Ireland, Spain, Switzerland, United Kingdom. Eur Geriatric Med. 2011;2(5):290–295. [Google Scholar]
- 3.IMSERSO. Encuesta Mayores 2010. http://www.imserso.es/InterPresent2/groups/imserso/documents/binario/presentacionencuestamayores_20.pdf. Published 2010. Accessed April 3, 2013.
- 4.Mitchell C, Adebajo A, Hay E, Carr A. Shoulder pain: diagnosis and management in primary care. Clinical review. BMJ. 2005;331(7525):1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33(2):73–81. [DOI] [PubMed] [Google Scholar]
- 6.Tekavec E, Jöud A, Rittner R, et al. Population-based consultation patterns in patients with shoulder pain diagnoses. BMC Musculoskelet Disord. 2012;13:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miranda H, Punnett L, Juntura EV, Heliövaara M, Knekt P. Physical work and chronic shoulder disorder. Results of a prospective population-based study. Ann Rheum Dis. 2008;67(2):218–223. [DOI] [PubMed] [Google Scholar]
- 8.Alqunaee M, Galvin R, Fahey T. Diagnostic accuracy of clinical tests for subacromial impingement syndrome: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2012;93(2):229–236. [DOI] [PubMed] [Google Scholar]
- 9.Faber E, Kuiper JI, Burdorf A, Miedema HS, Verhaar JAN. Treatment of impingement syndrome: a systematic review of the effects on functional limitations and return to work. J Occup Rehabil. 2006;16(1):6–25. [DOI] [PubMed] [Google Scholar]
- 10.Palomares SP, Blázquez BO, Burró AMA, et al. Contributions of myofascial pain in diagnosis and treatment of shoulder pain. A randomized control trial. BMC Musculoskelet Disord. 2009;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas KR, Polus BI, Rich P. Latent myofascial trigger points: their effects on muscle activation and movement efficiency. J Bodyw Mov Ther. 2004;8(3):160–166. [Google Scholar]
- 12.Bron C, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. High prevalence of shoulder girdle muscles with myofascial trigger points in patients with shoulder pain. BMC Musculoskelet Disord. 2011:12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bron C, Franssen J, Wensing M, Oostendorp RA. Interrater reliability of palpation of myofascial trigger points in three shoulder muscles. J Man Manip Ther. 2007;14(4):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bron C, de Gast A, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. Treatment of myofascial trigger points in patients with chronic shoulder pain: a randomized, controlled trial. BMC Med. 2011;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simons DG, Travell JG, Simons LS. Travell & Simons: Dolor y Disfunción Miofascial: El Manual de los Puntos Gatillo: Volumen 1: Mitad Superior del Cuerpo. 2nd ed. Madrid, Spain: Panamericana; 2002. [Google Scholar]
- 16.Bron C, Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep. 2012;16(5):439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Partanen JV, Ojala TA, Arokoski JP. Myofascial syndrome and pain: a neurophysiological approach. Pathophysiology. 2010;17(1):19–28. [DOI] [PubMed] [Google Scholar]
- 18.Shah JP, Danoff JV, Desai MJ, et al. Biochemical associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. [DOI] [PubMed] [Google Scholar]
- 19.Desai MJ, Saini V, Saini S. Myofascial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayoral O. Dry needling treatments for myofascial trigger points. J Musculoskelet Pain. 2010;18(4):411–416. [Google Scholar]
- 21.Mayoral O, Salvat I, Martín MT, et al. Efficacy of myofascial trigger point dry needling in the prevention of pain after total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. Evid Based Complement Alternat Med. 2013;2013:694941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srbely J, Dickey J, Lee D, Lowerison M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med. 2010;42(5):463–468. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh Y-L, Kao M-J, Kuan T-S, Chen S-M, Chen J-T, Hong C-Z. Dry needling to a key myofascial trigger point may reduce the irritability of satellite myofascial trigger points. Am J Phys Med Rehabil. 2007;86(5):397–403. [DOI] [PubMed] [Google Scholar]
- 24.Ge HY, Fernández-de-Las-Peñas C, Madeleine P, Arendt-Nielsen L. Topographical mapping and mechanical pain sensitivity of myofascial trigger points in the infraspinatus muscle. Eur J Pain. 2008;12(7):859–865. [DOI] [PubMed] [Google Scholar]
- 25.Kuan T-S, Hong C-Z, Chen S-M, et al. Myofascial pain syndrome: correlation between the irritability of trigger points and the prevalence of local twitch responses during trigger point injection. J Musculoskelet Pain. 2012;20(4):250–256. [Google Scholar]
- 26.Ge HY, Arendt-Nielsen L. Latent myofascial trigger points. Curr Pain Headache Rep. 2011;15(5):368–392. [DOI] [PubMed] [Google Scholar]
- 27.Celik D, Yeldan I. The relationship between latent trigger point and muscle strength in healthy subjects: a double-blind study. J Back Musculoskelet Rehabil. 2011;24(4):251–256. [DOI] [PubMed] [Google Scholar]
- 28.International Association for the Study of Pain IASP. Global year against musculoskeletal pain: shoulder pain. http://www.iasppain.org/Content/NavigationMenu/GlobalYearAgainstPain/20092010MusculoskeletalPain/FactSheets/default.htm. Published October 2009. Accessed April 3, 2013.
- 29.Tough EA, White AR, Richards S, Campbell J. Variability of criteria used to diagnose myofascial trigger point pain syndrome—evidence from a review of the literature. Clin J Pain. 2007;23(3):278–286. [DOI] [PubMed] [Google Scholar]
- 30.Lucas N, Macaskill P, Irwig L, Moran R, Bogduk N. Reliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literature. Clin J Pain. 2009;25(1):80–89. [DOI] [PubMed] [Google Scholar]
- 31.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. [DOI] [PubMed] [Google Scholar]
- 32.Taylor LJ, Harris J, Epps C, Herr K. Psychometric evaluation of selected pain intensity scales for use in cognitively impaired and cognitively intact older adults. Rehabil Nurs. 2005;30(2):55–61. [DOI] [PubMed] [Google Scholar]
- 33.Kahl C, Cleland J. Visual analogue scale, numeric pain rating scale and the McGill pain questionnaire: an overview of psychometric properties. Phys Ther Rev. 2005;10(2):123–128. [Google Scholar]
- 34.Koo TK, Guo J, Brown CM. Test-retest reliability, repeatability, and sensitivity of an automated deformation-controlled indentation on pressure pain threshold measurement. J Man Manip Ther. 2013;36(2):84–90. [DOI] [PubMed] [Google Scholar]
- 35.Fisher A. Algometry in diagnosis of musculoskeletal pain and evaluation of treatment outcome: an update. J Musculoskelet Pain. 1998;6(1):5–32. [Google Scholar]
- 36.Park G, Kim CD, Park SB, Kim MJ, Jang SH. Reliability and usefulness of the pressure pain threshold measurement in patients with myofascial pain. Ann Rehabil Med. 2011;35(3):412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–418. [DOI] [PubMed] [Google Scholar]
- 38.Neziri AY, Scaramozzino P, Andersenc OK, Dickensond AH, Arendt-Nielsenc L, Curatoloa M. Reference values of mechanical and thermal pain tests in a pain-free population. Eur J Pain. 2010;15(4):376–383. [DOI] [PubMed] [Google Scholar]
- 39.Abizanda P, Navarro JL, García-Tomás MI, López-Jiménez E, Martínez-Sánchez E, Paterna G. Validity and usefulness of hand-held dynamometry for measuring muscle strength in community-dwelling older persons. Arch Gerontol Geriatr. 2012;54(1):21–27. [DOI] [PubMed] [Google Scholar]
- 40.Dhara PC, De S, Pal A, Sengupta P, Roy S. Assessment of hand grip strength of orthopedically challenged persons affected with upper extremity. J Life Sci. 2009;1(2):121–127. [Google Scholar]
- 41.Fernández-Carnero J, Ge HY, Kimura Y, Fernández-de-Las-Peñas C, Arendt-Nielsen L. Increased spontaneous electrical activity at a latent myofascial trigger point after nociceptive stimulation of another latent trigger point. Clin J Pain. 2010;26(2):138–143. [DOI] [PubMed] [Google Scholar]
- 42.Jayakumar VR, Roshni B, Shubha R. The nerve supply to extensor carpi radialis brevis muscle—a study. Int J Cur Res Rev. 2013;5(17):116–123. [Google Scholar]
- 43.Bevelaqua AC, Hayter CL, Feinberg JH, Rodeo SA. Posterior interosseous neuropathy: electrodiagnostic evaluation. HSSJ. 2012;8(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tubbs S, Tyler-Kabara E, Aikens A, et al. Surgical anatomy of the axillary nerve within the quadrangular space. J Neurosurg. 2005;102(5):912–914. [DOI] [PubMed] [Google Scholar]
- 45.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. [DOI] [PubMed] [Google Scholar]
- 46.Ga H, Choi JH, Park CH, Yoon HJ. Dry needling of trigger points with and without paraspinal needling in myofascial pain syndromes in elderly patients. J Altern Complement Med. 2007;13(6):617–624. [DOI] [PubMed] [Google Scholar]
- 47.Mense S. How do muscle lesions such as latent and active trigger points influence central nociceptive neurons? J Musculoskelet Pain. 2010;18(4):348–353. [Google Scholar]
- 48.Dommerholt J. Dry needling—peripheral and central considerations. J Man Manip Ther. 2011;19(4):223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]