Abstract
Objective
Evidence regarding the age at which autism spectrum disorder (ASD) is identified is essential for improving early detection, yet many extant studies have not applied time-to-event analyses, which accounts for statistical biases that arise from sampling in cross-sectional surveys by adjusting for child age at time of parental report. Our objective was to estimate age distributions for first identification of ASD in national parent surveys using time-to-event analyses.
Method
We conducted time-to-event analyses of responses to identical questions in the 2011–2012 National Survey of Children’s Health (n=95,677) and the 2009–2010 National Survey of Children with Special Health Care Needs (n=371,617).
Results
Parents in both surveys reported that a minority of ASD cases were identified before age 3 years, and that 1/3 to 1/2 of cases were identified after 6 years. In both surveys, a majority of parents described their child’s ASD severity as mild, and these parents reported the oldest age-at-identification (mean = 5.6 and 8.6 years). In contrast, parents who described their child’s ASD as severe reported earlier age-at-identification (mean = 3.7 and 4.5 years). Time-to-event analyses yielded older estimates of age at identification than analyses based on raw distributions.
Conclusion
In two national surveys, a majority of parents of children with ASD reported identification after 3 years, when eligibility for Early Intervention services expires, and many reported identification of ASD after school age. Later identification of children with milder symptoms highlights the need for early screening that is sensitive to all forms of ASD, regardless of severity.
Keywords: autism spectrum disorder, epidemiology, mass screening, survival analysis, age of diagnosis
INTRODUCTION
In the United States, there is a strong focus on reducing the age at which children with autism spectrum disorder (ASD) are first identified, diagnosed, and treated. The Centers for Disease Control and Prevention promote a campaign to “Know the signs; act early,”1 and state and local governments are increasingly passing legislation to promote early detection.2 Despite the emphasis on early identification, a large body of evidence demonstrates that many children remain undiagnosed until school age.3–6 Moreover, there is significant variation in the age at which ASD is first identified and diagnosed, with systematic delays in diagnosis for children from minority race/ethnicity groups, with low socioeconomic status, and/or with milder severity of ASD symptoms.3–6
Many studies on age at ASD identification suffer from methodological limitations. For example, a majority of published studies report age at ASD identification as a mean or median value.6 Unfortunately, measures of central tendency omit important information about the full distribution of ages at which children are diagnosed with ASD. For example, the mean or median age at identification may not correspond with the ages at which identification is most likely, and dramatically different distributions may have equivalent measures of central tendency.7 Ideally, published studies would report estimates of the full distribution of ages at which children in the general population are identified with ASD.
Moreover, published studies often use sampling methods when estimating age at identification that can introduce bias. Fortunately, more appropriate statistical analyses, including time-to-event analyses, are available that can minimize sampling biases. As a simple example, consider a disease that is diagnosed, on average, at three years of age. Now consider estimating this mean age with two different samples of children, one that includes only 2-year-olds and one that includes only 4-year-olds. Clearly, the average age of diagnosis among the 2-year-olds will be less than 2 years, while the average among 4-year-olds will be higher. This difference in estimates reflects bias that is purely an artifact of the sampling strategies. Ideally, analyses would account for the fact that the 2-year-old children have had less time to develop and be diagnosed with the disease than the 4-year-old children, and that children in both groups may yet be diagnosed in the future.
Challenges such as these are common to all types of time to event data, which in this case refers to the time between birth and a diagnosis, but may also refer to other situations such as the time between treatment and death (i.e., survival data). Fortunately, explicit methods to analyze time-to-event data are widely available and have been used previously to analyze age at onset and diagnosis for various forms of psychopathology.8–10
If researchers only assessed age at identification among samples of older children for whom there was no chance of future diagnosis, special statistical methods would not be needed. However, bias can result when samples include children who are assessed when they are only part-way through the period during which they are at risk of receiving a diagnosis (i.e., window of risk). Data collected from such participants are useful for estimating risk up until the age at which they are sampled, but after this age they contribute no new information to the analysis. Referring back to the example above, the sample of 2-year-olds can tell us a lot about the risk of diagnosis in the first 2 years of life, but it can tell us nothing about the risk of diagnosis at age 3 years. In the language of time to event analyses, these children are censored from the sample at age 2 years because they contribute no data after this point. In mixed samples that include children of many different ages, time-to-event analyses (e.g., survival analyses) can account for the fact that children without diagnoses are censored at different ages.
In ASD research, many cross-sectional surveys sample parents of children across a wide age range (e.g., 3 to 17 years) and participants who have not been identified with ASD at the time of the survey may still be within the window of risk for receiving a diagnosis. Because the age range in which ASD is identified substantially overlaps the age range sampled by most national surveys,3,4,11–14 statistical analyses need to adjust for censoring.7,15 Thus, if parents answer “No” when asked whether they have ever been told that their child has autism, their response may be best interpreted and analyzed as “Not yet,” with the probability of eventually obtaining a diagnosis depending on the age of the child. Importantly, time-to-event analyses minimize biases in estimating age at identification. Using appropriate analyses for time-to-event data in ASD research can therefore improve estimates and inform public policy.
For example, consideration of age-at-identification allows for greater clarity in interpreting prevalence data. Two prominent and ongoing studies provide a case in point. From 2007 to 2015, the CDC reported increasing rates of ASD, most recently documented as 1 in 68 children.16 Based on a review of medical and educational records through the Autism and Developmental Disabilities Monitoring Network (ADDM; CDC, 2015),16,17 the validity of this prevalence estimate rests on the assumption that most cases of ASD have been identified by 8 years of age. In contrast, a recent publication based on the National Health Interview Study (NHIS) suggested ASD prevalence of 1 in 45.14 While this estimate was based on parent report only, it also differed from the CDC ADDM study in that its estimates were derived from children who ranged in age from 3 to 17 years. Given that the ages at which children are diagnosed with ASD may overlap across these prominent samples, accounting for age-at-diagnosis and where children are within the window of risk may offer perspective on the influence of sampling methodology on estimates of ASD prevalence.
Finally, in addition to valuing less biased estimates, policy makers are interested not only in the mean or median age at diagnosis, but also in the proportion of children who are identified at different ages, such as before eligibility for Part C Early Intervention (EI) services passes or after entry to formal schooling.18 Recent studies suggest that until children reach stages of development when deficits in social reciprocity are more likely to be evident, some ASD symptoms are unlikely to be observed by trained examiners or documented in medical records by community providers.19–21 Thus, early identification of ASD is dependent on both the emergence and the detection of symptoms among young children. Direct estimates of the proportion of children who are identified early enough to receive EI services has clear relevance to policy.
While time-to-event analyses have been used in cross-sectional surveys to analyze age at diagnosis for ASD and age-of-onset of other psychiatric disorders,8–10 we know of no example of their use for analyzing age-at-identification in the National Survey of Children’s Health (NSCH)12 or the National Survey of Children with Special Health Care Needs (NS-CSHCN) from the US.13 While the NHIS does not include data on age at ASD identification, the NSCH and the NS-CSHCN do include such data. In this paper, we used time-to-event analyses to characterize the full distribution of ages at which parents report identification of ASD in these two national surveys. Such analyses account for the cross-sectional sampling strategies employed in these surveys and thereby address the potential for bias. Consistent with past reports, we hypothesized that unadjusted analyses would result in downward bias of estimates of median age at identification,15,22 and that application of time-to event analyses would yield more accurate estimates across the full distribution of ages at which children are identified with ASD.
METHOD
Study Design and Participants
Full descriptions for the NSCH and the NS-CSHCN are available elsewhere.23–25 Briefly, the 2011–2012 NSCH is a population-based survey of children’s health and experiences with the health care system in which 95,677 parents or guardians were interviewed by cellphone or landline telephone in English or Spanish. The survey focused on one child selected randomly from each household. The 2009–2010 NS-CSHCN is very similar to the NSCH with the following exceptions. Screens for children with special health care needs (CSHCN)26 were conducted in 371,617 households. No further data were collected from households without CSHCN. For 40,242 households with CSHCN, one child with a special health care need was selected at random as the focus of the interview. Thus, separate datasets estimate the proportion of CSHCN in the general population and the prevalence of various health variables among CSHCN.
Measures
Primary questions and response options were identical in both surveys. Whether parents were told their child had ASD was coded based on the question, “Has a doctor or other health care provider ever told you that [your child] had...autism, Asperger's disorder, pervasive developmental disorder, or other autism spectrum disorder?” Current ASD was coded conditional on an affirmative response to this question and the question, “Does [your child] currently have autism or an autism spectrum disorder?” The age at which parents were first told their child had ASD was defined by responses to the question, “How old was [your child] when you were first told by a doctor or other health care provider that [he/she] had autism or autism spectrum disorder?” Primary analyses of age at ASD identification relied on the same question, but limited cases to those participants who also reported current ASD. ASD severity was defined by responses to the question, “Would you describe [his/her] autism or ASD as mild, moderate, or severe?” Severity therefore reflects the perception of the parent at the time of the survey.
Analyses
Following recommendations specific to each survey,23,24 all analyses incorporate appropriate survey weights and account for stratification. A half-year correction was applied to children’s ages under the assumption that reported ages were best represented by their midpoint (e.g., the average age of 2-year-olds is 2.5 years). Sample ages were otherwise treated as exact ages. All primary analyses were based on the hazard function for being identified with ASD at each age, estimated using the Nelson-Aalen equation:
Where d = number identified at time i, and n = number eligible for identification at time i. Whereas kernel smoothing is typically used to account for variability in estimates in smaller samples,27 the large sample sizes afforded by the NSCH and NS-SHCN allow for direct estimation of the hazard distribution. Given evidence suggesting that age-at-identification varies based on ASD severity and because parents’ reports of ASD severity fell into mutually exclusive categories, mild, moderate, and severe ASD were modeled as competing risks.6 Therefore, cumulative incidence was estimated as the product of the hazard rate and survival through the previous time period.28,29 Median age-at-identification was defined as the age by which at least 50% cases had been identified. Mean age-at-identification was defined as the sum of the products of age and hazard divided by cumulative hazard through age 17.
We conducted two sets of analyses:
Overall hazard and cumulative incidence distributions were calculated for cases in which parents reported current ASD and a special health care need. This approach yielded estimates that are directly comparable between the NSCH and the NS-CSHCN.
To demonstrate the bias caused by neglecting censoring in time-to-event data, the hazard distribution for “age first told” was calculated using descriptive statistics and then using appropriate time-to-event analyses that account for censoring. Because data on “age first told” were not available for all children in the NS-CSHCN, these analyses were limited to the NSCH.
In the NSCH, hazards and their confidence intervals were calculated by estimating the proportion of children identified at each age among those who were eligible for identification at that age. Results were verified by comparison to a life-table constructed based on prevalence estimates at each age. To obtain population-level estimates that are commensurate with those derived from the NSCH, additional methods were required for the NS-CSHCN. Instead of directly surveying a random sample from the general population, the NS-CSHCN uses a 2-step method in which households are first screened for CSHCN and then full interviews (including question regarding ASD) are conducted among those who screen positive in a second stage. Sampling weights are provided for each stage individually, but not for analyses of the combination. Thus, most analyses of the NS-CSHCN generalize not to the full population, but only to children with special health care needs. To develop estimates for the general population, NS-CSHCN documentation states that analysts should multiply “the weighted proportional estimate based on the interview weights and the weighted number of CSHCN in the population of interest based on the screener weights.”23 We thus multiplied each proportion estimated among CSHCN by the proportion of the general population at each age who reported a special health care need. To estimate confidence intervals, 2,500 bootstrap samples were drawn for each relevant estimate for each stage of the survey.30 Individual bootstrap estimates were multiplied to create a distribution of estimates for each sample age. Data across sample ages were then weighted and summed to estimate hazards. Within both the first and second stage samples, results of this approach were verified against results estimated by calculating proportions directly using methods identical to those described above for the NSCH.
Despite using the same screener for CSHCN, the NSCH and the NS-CSHCN have been found to yield different prevalence estimates for CSHCN. These differences have been attributed to differences in the survey’s respective sampling methods based on data suggesting that CSHCN with milder severity are less likely to be identified in the NS-CSHCN than in the NSCH. Although both surveys use identical questions, the NS-CSHCN uses the CSHCN screen to determine eligibility, perhaps discouraging some participants from endorsing questions.31,32 Because our estimate of ASD is linked to CSHCN status, variation between the two surveys may reflect not only differences in ASD, but also differences in sensitivity in detecting CSHCN. To account for this variation, the prevalence of CSHCN in the NS-CSHCN was adjusted at each sample age to be equivalent to the prevalence of CSHCN found in the NSCH. The Tufts Medical Center Human Subjects Review Board and University of Massachusetts Boston Institutional Review Board approved analysis of these data as exempt.
RESULTS
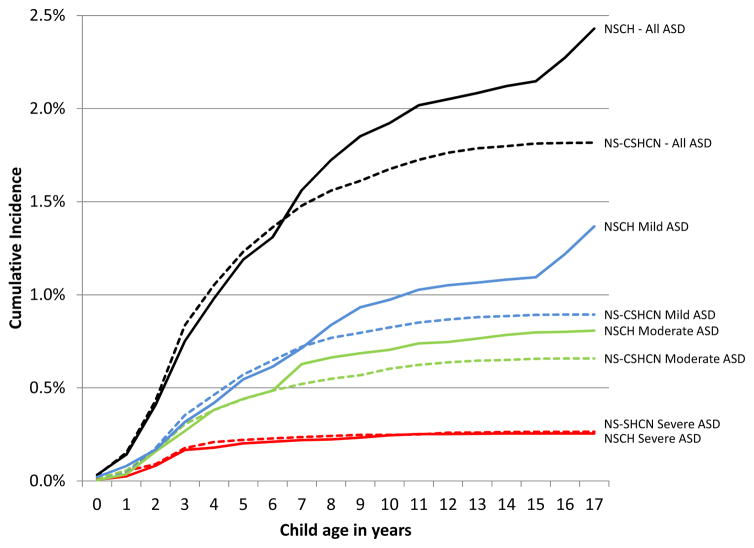
Figure 1 displays the hazard distributions for all parents who reported that their child currently has ASD and a special health care need in both the NSCH and the NS-CSHCN. Estimates of central tendency vary markedly between the NSCH and the NS-CSHCN. In the NSCH, median age at identification is 6 years and mean age is 7.4 years, while in the NS-CSHCN, median age at identification is 4 years and mean age is 5.4 years. Inspection of cumulative incidence distributions in Figure 2 reveals that the higher means in the NSCH are the result of slightly lower hazards at younger ages combined with much higher hazards at older ages. Moreover, inspection of cumulative incidence curves by severity in Figure 2 reveals close correspondence between the two surveys with regard to ASD described by parents as severe, with greater discrepancies for moderate and mild ASD, especially at older ages. Thus, variations in age at identification between the two surveys appear to be driven more by discrepancies in ASD described as mild or moderate than ASD described as severe. As is typical of hazard distributions,24 confidence intervals become larger at older ages as sample sizes become smaller and thus should be interpreted with caution.
Figure 1.
Age of first identification among all parents who were told by a professional that their children had autism and reported current autism and reported a special health care need. Note: ASD = autism spectrum disorder.
Figure 2.
Cumulative incidence of autism identification in two national surveys. Note: Solid lines represent the National Survey of Children’s Health (NSCH); dotted lines represent the National Survey of Children with Special Health Care Needs (NS-CSHCN). Blue lines depict autism spectrum disorder (ASD) described as mild; green lines depict ASD described as moderate; red lines depict ASD described as severe; black lines depict all ASD.
Table 1 offers further comparisons between the two surveys’ hazard distributions. Overall, 17-year cumulative incidence varies markedly between the two surveys (2.4% vs 1.8%), but both estimates are high, reflecting 1 in 41 and 1 in 55 children, respectively. A majority of parents in both surveys described their child’s ASD as mild. Consistent with the cumulative incidence distributions depicted in Figure 2, the two surveys for ASD yield close correspondence for children described by parents as severe: cumulative incidence by 17 years and median age at identification are almost identical (i.e., both round to 0.26%), while mean age differs by only 0.8 years (mean age = 3.7 and 4.5 years). In contrast, estimates differ markedly for children with ASD described by parents as mild: cumulative incidence by 17 years differs by approximately 50%, while mean and median ages at identification each differ by 3 years. Estimates for moderate ASD fall in between these two extremes. Notably, results from both surveys suggest that only a minority of children with ASD are identified before age three (17% and 23%), although the estimates of the proportion of child identified before age three are both higher and more consistent for ASD described as severe (32% and 34%). Despite marked variance between the surveys, both agree that a substantial proportion of children with ASD are identified after age 6 years (51% and 33%) and even age 8 years (29% and 15%).
Table 1.
Comparison of Hazard and Cumulative Incidence Distributions Derived From Two National Surveys
|
|
||||
|---|---|---|---|---|
| Any ASD | ASD described as “mild” | ASD described as “moderate” | ASD described as “severe” | |
|
|
||||
| Frequency | ||||
|
|
||||
| NSCH | 1,615 | 889 | 537 | 189 |
|
|
||||
| NS-CSHCN | 3,037 | 1,587 | 1,092 | 358 |
|
|
||||
| Cumulative incidence by 17 y | ||||
|
|
||||
| NSCH | 2.47% | 1.39% | 0.82% | 0.26% |
|
|
||||
| NS-CSHCN, adjusted | 1.82% | 0.91% | 0.66% | 0.26% |
|
|
||||
| RR | 1.4 | 1.5 | 1.2 | 1.0 |
|
|
||||
| Median age of 1st identification, y | ||||
|
|
||||
| NSCH | 6 | 7 | 5 | 3 |
|
|
||||
| NS-CSHCN, adjusted | 4 | 4 | 4 | 3 |
|
|
||||
| Difference | 2 | 3 | 1 | 0 |
|
|
||||
| Mean age of 1st identification, y | ||||
|
|
||||
| NSCH | 7.4 | 8.6 | 6.3 | 4.5 |
|
|
||||
| NS-CSHCN, adjusted | 5.4 | 5.6 | 5.5 | 3.7 |
|
|
||||
| Difference | 2 | 3 | 0.8 | 0.8 |
|
|
||||
| Proportion identified < 3 y | ||||
|
|
||||
| NSCH | 17% | 12% | 19% | 32% |
|
|
||||
| NS-CSHCN, adjusted | 23% | 20% | 24% | 34% |
|
|
||||
| Proportion identified 3–5 y | ||||
|
|
||||
| NSCH | 32% | 27% | 35% | 47% |
|
|
||||
| NS-CSHCN, adjusted | 44% | 44% | 42% | 48% |
|
|
||||
| Proportion identified 6–8 y | ||||
|
|
||||
| NSCH | 22% | 21% | 28% | 8% |
|
|
||||
| NS-CSHCN, adjusted | 18% | 22% | 17% | 9% |
|
|
||||
| Proportion identified >8 y | ||||
|
|
||||
| NSCH | 29% | 39% | 18% | 13% |
|
|
||||
| NS-CSHCN, adjusted | 15% | 14% | 17% | 10% |
|
|
||||
Note. ASD = Autism Spectrum Disorder; NSCH = National Survey of Children’s Health; NS-CSHCN = National Survey of Children with Special Health Care Needs; RR = risk ratio.
As a point of comparison, we also calculated the hazard distribution for all parents in the NSCH who reported that they had been told that their child had ASD, irrespective of whether they later reported current ASD or a special health care need. The cumulative incidence of being told a child has ASD by 17 years of age is 3.2%. The median age at which parents were told is 6 years and the mean is 7.4 years. Compared to analyses based on the question regarding current ASD, cumulative incidence was markedly higher, but mean and median age of diagnosis were similar. Notably, analyses based on a raw distribution of ages that did not account for censoring yielded a median age of 4 years and a mean of 5.4 years, thus demonstrating the effect of time-to-event analyses in reducing bias.
DISCUSSION
While hazard and cumulative incidence distributions for identification of ASD differ across the NSCH and the NS-CSHCN, both are consistent in several ways that have important implications for policy. First, parents in both surveys report that only a minority of children with ASD are identified before age 3 years, while they are still eligible for Part C Early Intervention services—a result that was consistent for ASD described as mild, moderate, or severe. Moreover, results indicate that 1/3 to 1/2 of children are identified after 6 years, an age when children are typically enrolled in school. If early identification of children with ASD is a policy goal, these results suggest a need for further improvements.
Consistent with previous literature,15,22 time-to-event analyses were useful in addressing the potential for bias resulting from censoring. When analyses were conducted on data from prevalent cases without accounting for censoring, the mean and median age at which parents were first told their child had ASD were underestimated by two years, suggesting that early detection efforts are more successful than is likely true. As expected, analyses revealed that bias resulted from underestimation of the hazard rate at older ages.
Cumulative incidence distributions also have clear implications for interpreting studies of ASD prevalence. In the NSCH, the cumulative incidence for being identified with ASD by 17 years was 2.4%—notably higher than a previous estimate of prevalence of 2.0%.33 Technically, both estimates are correct and each may be useful for different purposes. The prevalence estimate correctly reflects the fact that 2% of parents report having a child with ASD. Our estimate of cumulative incidence accounts for the fact that some children whose parents who do not report ASD may still be identified in the future; thus, 2.4% offers a better estimate of the risk of being identified with ASD throughout childhood than does the previously reported prevalence rate.
Examination of cumulative incidence distributions makes clear that for ASD, both prevalence and cumulative incidence depend on age. This observation is consistent with emerging evidence that until children reach stages of development when deficits in social reciprocity are more likely to be evident, some ASD symptoms are unlikely to be observable by trained examiners or documented in medical records by community providers.19–21 Time lags resulting from the variable effectiveness of systems to detect emergent cases of ASD widen the age window in which ASD is detected still further. In this light, it is notable that although the CDC’s ADDM carefully titled their most recent report “Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years,” results are widely disseminated as evidence of ASD prevalence in the US without reference to age. Evidence from both the NSCH and NS-CSHCN suggesting that a substantial proportion of children with ASD are identified after 8 years (15% and 29%) may have implications for the interpretation of the ADDM estimate. On the one hand, the ADDM’s careful evaluation of medical and (when available) educational records often results in identification of cases that had not previously been diagnosed.34 On the other hand, if symptoms of children with ASD who are not identified by age 8 are less likely to be documented in medical or educational records, then ADDM estimates may under-represent true prevalence.
Finally, results highlight the importance of severity in the epidemiology of ASD. Consistent with previous reports,6 milder severity was reported by a majority of parents of children with ASD and was associated with later ages at identification. Moreover, whereas the cumulative incidence of ASD described as severe was consistent across the NSCH and the NS-CSHCN, estimates for ASD described as mild or moderate were markedly less so. If detection of milder variants of ASD is sensitive to methodological differences in national surveys, the same may be true of screening instruments or surveillance systems designed to improve early detection. These results suggest that severity should be considered in future studies that seek to improve identification of ASD among young children.
We note several limitations to our study. Because the surveys we analyzed rely on parent report, ASD diagnoses cannot be confirmed. Therefore, rates should be interpreted as counting both true and false positive cases and omitting false negative cases. Consideration of additional factors that may influence age at identification, such as race/ethnicity and family socioeconomic status, fell beyond the scope of the present study. Similarly, our estimates of hazard distributions did not consider either cohort or period effects, both of which are likely to influence the detection of ASD.35–37 Further analyses of factors such as these will increase the utility of evidence from the NSCH and NS-CSHCN for policy makers.
In conclusion, the current findings have significant implications for both researchers and policymakers. After considering the distributions of ages at which children are identified with ASD reported in this paper, policy makers should consider approaches to improve early detection that address the full range of severity reported by parents of children with ASD. For example, children with milder symptoms, who are typically identified at older ages, may benefit from new screening instruments that are sensitive to milder symptoms at young ages, as well as specialized intervention approaches that are appropriate for their needs. They may also benefit from later screening than is typically recommended—as long as screening is effective in detecting children before they would otherwise be identified, the age of identification will be reduced.
Researchers should use appropriate statistical methods when estimating age of identification, and they should also use caution when comparing results across studies that include samples of children with varying age distributions and that employed different sampling and statistical methods. In addition, findings underscore the importance of severity for understanding the epidemiology and treatment of ASD. Given the late age-of-identification among children whose ASD was described by parents as mild, more research is needed to better understand the utility of current detection and intervention strategies for this population, and whether new approaches may be needed.
Clinical Guidance.
Parents report that children with ASD are identified at a wide range of ages
A minority of children with ASD are identified before three years of age, and many are identified after age 6 years
Parents who describe their child’s ASD as mild report much later age of identification than parents who describe their child’s ASD as severe
Different strategies for screening, diagnosis, and treatment may be warranted for children with milder forms of ASD
Acknowledgments
This research was supported in part by a National Institute of Mental Health (NIMH) grant to the University of Massachusetts Boston and Tufts University (#R01MH104400) and an Autism Speaks Dennis Weatherstone Predoctoral Fellowship to Melissa P. Maye, MA at the University of Massachusetts Boston (#9117).
Dr. Sheldrick served as the statistical expert for this research.
The authors would like to acknowledge Victoria E. Sanchez, BA, of the University of Massachusetts Boston, for her contribution in preparing this manuscript for publication.
Footnotes
Ms. Maye reports no biomedical financial interests or potential conflicts of interest.
Disclosure: Dr. Sheldrick has received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the WT Grant Foundation, and the JPB Foundation. Dr. Carter has received funding from the Health Resources and Services Administration and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. R. Christopher Sheldrick, Tufts Medical Center, Boston.
Ms. Melissa P. Maye, University of Massachusetts, Boston.
Dr. Alice S. Carter, University of Massachusetts, Boston.
References
- 1.Centers for Disease Control and Prevention. [Accessed June 30, 2016];Act Early Initiative website. 2015 Feb 23; http://www.cdc.gov/ncbddd/actearly/about-initiative.html.
- 2.National Conference of State Legislatures. [Accessed June 30, 2016];Autism website. 2016 Feb; http://www.ncsl.org/research/health/autism-policy-issues-overview.aspx.
- 3.Mandell DS, Wiggins LD, Carpenter LA, et al. Racial/Ethnic Disparities in the Identification of Children With Autism Spectrum Disorders. Am J Public Health. 2011;99:493–498. doi: 10.2105/AJPH.2007.131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandell DS, Morales KH, Xie M, Lawer LJ, Stahmer AC, Marcus SC. Age of diagnosis among Medicaid-enrolled children with autism, 2001–2004. Psychiatr Serv. 2010;61:822–9. doi: 10.1176/appi.ps.61.8.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Facts About ASD. [Accessed January 25, 2017];ASD Homepage. http://www.cdc.gov/ncbddd/autism/facts.html. Published 2016.
- 6.Daniels AM, Mandell DS. Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism. 2013;18:583–597. doi: 10.1177/1362361313480277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 8.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 9.Hansen SN, Schendel DE, Parner ET, et al. Explaining the Increase in the Prevalence of Autism Spectrum Disorders. JAMA Pediatr. 2015;169:56. doi: 10.1001/jamapediatrics.2014.1893. [DOI] [PubMed] [Google Scholar]
- 10.Parner ET, Schendel DE, Thorsen P. Autism prevalence trends over time in Denmark: changes in prevalence and age at diagnosis. Arch Pediatr Adolesc Med. 2008;162:1150–6. doi: 10.1001/archpedi.162.12.1150. [DOI] [PubMed] [Google Scholar]
- 11.Mandell DS, Listerud J, Levy SE, Pinto-Martin JA. Race differences in the age at diagnosis among medicaid-eligible children with autism. J Am Acad Child Adolesc Psychiatry. 2002;41:1447–1453. doi: 10.1097/00004583-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Child and Adolescent Health Measurement Initiative. Fast Facts: 2011/12 National Survey of Children’s Health. Data Resource Center; [Accessed July 7, 2016]. www.childhealthdata.org. Published 2013. [Google Scholar]
- 13.Child and Adolescent Health Measurement Initiative. National Survey of Children with Special Health Care Needs (2009/10 NS-CSHCN): Fast facts about the survey. Data Resource Center; [Accessed July 7, 2016]. www.childhealthdata.org. Published 2010. [Google Scholar]
- 14.National Center for Health Statistics. Survey Description, National Health Interview Survey, 2014. Hyattsville, Maryland: 2015. [Accessed July 7, 2016]. cdc.gov/nchs/nhis.htm. [Google Scholar]
- 15.Faraone SV, Tsuang MT, Tsuang MT. Methods in psychiatric genetics. In: Faraone SV, Tsuang MT, Tsuang MT, editors. Textbook in psychiatric epidemiology. Hoboken, NJ: John Wiley and Sons; 2002. pp. 81–134. [Google Scholar]
- 16.Christensen DL, Baio J, Braun KVN, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. [Accessed June 30, 2016];Autism and Developmental Disabilities Moniroting (ADDM) website. 2016 Mar 13; http://www.cdc.gov/ncbddd/autism/addm.html.
- 18.Rosenberg SA, Robinson CC, Shaw EF, et al. Part C early intervention for infants and toddlers: percentage eligible versus served. Pediatrics. 2013;131:38–46. doi: 10.1542/peds.2012-1662. [DOI] [PubMed] [Google Scholar]
- 19.Ozonoff S, Young GS, Belding A, et al. The broader autism phenotype in infancy: When does it emerge? J Am Acad Child Adolesc Psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szatmari P, Chawarska K, Dawson G, et al. Prospective Longitudinal Studies of Infant Siblings of Children With Autism: Lessons Learned and Future Directions. J Am Acad Child Adolesc Psychiatry. 2016;55:179–187. doi: 10.1016/j.jaac.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maenner MJ, Schieve LA, Rice CE, et al. Frequency and pattern of documented diagnostic features and the age of autism identification. J Am Acad Child Adolesc Psychiatry. 2013;52:401–413. e8. doi: 10.1016/j.jaac.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen WJ, Faraone SV, Orav EJ, Tsuang MT. Estimating age at onset distributions: the bias from prevalent cases and its impact on risk estimation. Genet Epidemiol. 1993;10:43–59. doi: 10.1002/gepi.1370100106. [DOI] [PubMed] [Google Scholar]
- 23.Bramlett MD, Blumberg SJ, Ormson AE, et al. Design and operation of the National Survey of Children with Special Health Care Needs, 2009–2010. [Accessed June 30, 2016];Vital Health Stat. 2014 1(57):1–271. http://www.ncbi.nlm.nih.gov/pubmed/25383698. [PubMed] [Google Scholar]
- 24.Blumberg SJ, Foster EB, Frasier AM, et al. [Accessed July 7, 2016];Design and Operation of the National Survey of Children’s Health, 2007. 2012 http://www.ncbi.nlm.nih.gov/pubmed/22834229. [PubMed]
- 25.Centers for Disease Control and Prevention. National Survey of Children’s Health. National Center for Health Statistics; http://www.cdc.gov/nchs/slaits/nsch.htm. Published 2013. [Google Scholar]
- 26.Bethell CD, Read D, Stein REK, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002;2:38–48. doi: 10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S, May S. Regression Modeling of Time-to-Event Data. 2. Hoboken, NJ: John Wiley and Sons; 2008. Applied Survival Analysis. [Google Scholar]
- 28.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter J, Bithell J. Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Bethell CD, Read D, Blumberg SJ, Newacheck PW. What is the prevalence of children with special health care needs? Toward an understanding of variations in findings and methods across three national surveys. Matern Child Health J. 2008;12:1–14. doi: 10.1007/s10995-007-0220-5. [DOI] [PubMed] [Google Scholar]
- 32.Bethell CD, Blumberg SJ, Stein REK, Strickland B, Robertson J, Newacheck PW. Taking stock of the CSHCN screener: A review of common questions and current reflections. Acad Pediatr. 2015;15:165–176. doi: 10.1016/j.acap.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in prevalence of parent-reported autism spectrum disorder in school-aged US children: 2007 to 2011–2012. Natl Health Stat Report. 2013;65:1–11. [PubMed] [Google Scholar]
- 34.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 35.Fombonne E. The changing epidemiology of autism. Journal of Applied Research in Intellectual Disabilities. 2005;18:281–294. [Google Scholar]
- 36.Keyes KM, Susser E, Cheslack-Postava K, Fountain C, Liu K, Bearman PS. Cohort effects explain the increase in autism diagnosis among children born from 1992 to 2003 in California. Int J Epidemiol. 2012;41:495–503. doi: 10.1093/ije/dyr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idring S, Lundberg M, Sturm H, et al. Changes in Prevalence of Autism Spectrum Disorders in 2001–2011: Findings from the Stockholm Youth Cohort. J Autism Dev Disord. 2015;45:1766–73. doi: 10.1007/s10803-014-2336-y. [DOI] [PubMed] [Google Scholar]