Abstract
The soluble fraction of brain samples from patients with Alzheimer’s disease contains highly biologically active amyloid-β seeds. In this study, we sought to assess the potency of soluble amyloid-β seeds derived from the brain and cerebrospinal fluid. Soluble Alzheimer’s disease brain extracts were serially diluted and then injected into the hippocampus of young, APP transgenic mice. Eight months later, seeded amyloid-β deposition was evident even when the hippocampus received subattomole amounts of brain-derived amyloid-β. In contrast, cerebrospinal fluid from patients with Alzheimer’s disease, which contained more than 10-fold higher levels of amyloid-β peptide than the most concentrated soluble brain extracts, did not induce detectable seeding activity in vivo. Similarly, cerebrospinal fluid from aged APP-transgenic donor mice failed to induce cerebral amyloid-β deposition. In comparison to the soluble brain fraction, cerebrospinal fluid largely lacked N-terminally truncated amyloid-β species and exhibited smaller amyloid-β-positive particles, features that may contribute to the lack of in vivo seeding by cerebrospinal fluid. Interestingly, the same cerebrospinal fluid showed at least some seeding activity in an in vitro assay. The present results indicate that the biological seeding activity of soluble amyloid-β species is orders of magnitude greater in brain extracts than in the cerebrospinal fluid.
Keywords: biomarker, dementia, amyloid, seeding
Introduction
Alzheimer’s disease is the most common form of senile dementia and its incidence increases with age. The defining neuropathological hallmarks are cerebral deposits of amyloid-β peptide and filamentous tau lesions (neurofibrillary tangles) (Hardy and Selkoe, 2002; Holtzman et al., 2011). The aggregation of amyloid-β peptide is thought to be an early event that drives Alzheimer’s disease pathogenesis and begins at least a decade before clinical symptoms are detected (Hardy and Selkoe, 2002; Holtzman et al., 2011; Bateman et al., 2012; Villemagne et al., 2013). To diagnose patients at a preclinical stage, disease biomarkers are crucial. Reduced levels of amyloid-β42 (the amyloid-β peptide that ends with amino acid 42) and increased total tau levels in CSF as well as increased retention of amyloid-β binding agents such as Pittsburgh compound B in brain using PET are used as diagnostic tools to identify preclinical Alzheimer’s disease patients (Brys et al., 2009; Blennow et al., 2012). It is thought that the changes in CSF amyloid-β and tau levels and Pittsburgh compound B retention become significant only when there is already robust amyloid deposition in the brain (Blennow et al., 2012; Johnson et al., 2012; Maia et al., 2013).
Amyloid formation in vitro starts with a slow nucleation phase that leads to formation of the initial nucleus of an amyloid fibril (‘amyloid-β seed’) (Harper and Lansbury, 1997; Eisenberg and Jucker, 2012). After this slow nucleation phase amyloid formation occurs fairly rapidly, i.e. soluble amyloid-β peptide is added to the amyloid-β seed by conformational conversion. With increasing size, the amyloid aggregate/fibril has a tendency to break and form new amyloid-β seeds. These in vitro observations of amyloid formation have recently been translated to in vivo models by demonstrating that amyloid-β aggregation can be induced (and accelerated) in young (pre-depositing) APP transgenic mice through the intracerebral injections of brain extract containing aggregated amyloid-β (i.e. amyloid-β seeds) (Kane et al., 2000; Meyer-Luehmann et al., 2006). Subsequently, it could be demonstrated that the most potent amyloid-β seeds in brain extracts from APP23 transgenic mice are small and soluble amyloid-β containing assemblies (Langer et al., 2011). These findings raise the possibility that soluble amyloid-β assemblies in the human brain also are potent amyloid-β seeds, and that such seeds may occur in bodily fluids and could serve as an early diagnostic biomarker for cerebral β-amyloidogenesis. To test this hypothesis we first assessed the in vivo seeding capacity of soluble human Alzheimer’s disease brain-derived amyloid-β seeds in young APP23 transgenic mice. We then examined the seeding efficacy of human CSF from subjects with and without Alzheimer’s disease from 50 to 80 years of age.
Materials and methods
Mice
For all experiments, 2.5 to 4-month-old APP23 transgenic mice were used (Sturchler-Pierrat et al., 1997). Male and female mice were used and have been randomly assigned to the groups. Consistent with earlier observations we did not find an obvious gender difference in the induction of β-amyloidosis, and thus males and females were combined for the analyses. All mice have been backcrossed with C57BL/6J mice for >20 generations [C57BL/6J-tg(Thy1-APPK670N;M671L)23]. All mice were kept under specific pathogen-free conditions.
Human brain fractions
Human brain tissue (frontal cortex) was obtained from autopsy material of histopathologically diagnosed Alzheimer’s disease patients (mean age 78 years, CERAD C/Braak V-VI stadium; see Supplementary Table 1 for details). The tissue was immediately frozen (post-mortem delay 17–42 h) and stored at −80°C until use. Brain tissue was homogenized at 10% (w/v) in sterile PBS (Lonza) and the formic-acid soluble brain fraction was prepared as previously described for APP transgenic mice (Maia et al., 2013). The soluble brain fraction was obtained by a 100 000g centrifugation and using the supernatant as previously described for mice (Langer et al., 2011). The dilutions described in this manuscript refer to the supernatant fraction.
Human CSF samples
CSF was obtained from a total of 14 human cases, eight Alzheimer’s disease and six non-Alzheimer’s disease controls, ranging from 52 to 87 years of age (Supplementary Table 1). CSF was obtained by lumbar puncture and collected in polypropylene vials. Samples were then centrifuged (1600–4000g for 10–15 min at either room temperature or at 4°C), aliquoted, frozen, and stored at −80°C. To investigate the influence of a freeze-thaw cycle CSF was collected and stored at 4°C until use. Selected CSF samples from Alzheimer’s disease and control patients (900 μl starting material) were concentrated (15-fold) before the injection using a Speed Vac concentrator (Thermo Scientific).
Murine CSF samples
CSF samples from 24-month-old APP23 transgenic mice and age-matched non-transgenic wild-type mice were collected as previously described (Maia et al., 2013).
Electrochemiluminescence-linked immunoassay for amyloid-β
Amyloid-β peptide (amyloid-βx-38, amyloid-βx-40, amyloid-βx-42) concentrations in CSF (1:14 dilution), supernatant fraction, and formic acid fraction were determined with an electrochemiluminescence-linked immunoassay using the MSD® 96-well Human (6E10) amyloid-β peptide Triplex Assay (Meso Scale Discovery) as previously described (Langer et al., 2011; Maia et al., 2013). For total amyloid-β peptide levels amyloid-βx-40 and amyloid-βx-42 peptides were added (amyloid-βx-38 peptide of the supernatant fraction mostly fell below the limit of detection and thus was not further considered).
Sample preparation and mass spectrometric analysis
Immunoprecipitation of CSF and the brain supernatant fraction was performed using a KingFisher™ magnetic particle processor (Thermo Scientific) as described earlier (Portelius et al., 2007). Briefly, 4 μg each of antibodies 6E10 and 4G8 (raised against amyloid-β peptide epitopes 4–9 and 18–22, respectively; Signet Laboratories, Inc) were separately added to 25 μl Dynabeads M-280 (Dynal) coated with sheep anti-mouse antibodies. The immunoprecipitations were conducted on 940 μl CSF and 3–4 ml supernatant, to which Tween-20 (Bio-Rad) had been added (final concentration 0.025%). The beads/sample solution was transferred to the KingFisher™ magnetic particle processor (polypropylene tubes, Thermo Scientific) for washing and elution in a five-step procedure. The supernatant was dried in a vacuum centrifuge and redissolved in 5 μl 0.1% formic acid in 20% acetonitrile. Analysis was done by MALDI TOF/TOF (Autoflex, Bruker Daltonics) in reflector mode. FlexAnalysis (version 3.3, Bruker Daltonics) was used for automated integration of the peaks for each spectrum. All solvents were of HPLC quality.
Stereotactic surgery
APP23 transgenic mice were anaesthetized with a mixture of ketamine/xylazine (ketamine 100 mg/kg, xylazine 10 mg/kg). Brain supernatant fraction or CSF (2.5 μl) were stereotactically placed with a Hamilton syringe into the hippocampus (antero-posterior − 2.5 mm, lateral ± 2.0 mm, dorso-ventral − 1.8 mm). Injection speed was 1.25 μl/min and the needle was kept at the injection site for an additional 2 min before withdrawal. The surgical area was cleaned with sterile PBS and the incision was sutured. The mice were kept under infrared light and monitored until they had recovered from anaesthesia.
Histology and immunohistochemistry
Mice were deeply anaesthetized with ketamine (250 mg/kg)/xylazine (25 mg/kg) and sacrificed by transcardial perfusion with 50 ml ice-cold PBS. Brains were immersion-fixed for 48 h in 4% paraformaldehyde in PBS, then cryoprotected in 30% sucrose in PBS for additional 48 h. Brains were cut into 25 μm-thick coronal sections using a freezing-sliding microtome (Leica SM 2000R). Sections were collected in cryoprotectant (35% ethylene glycol, 25% glycerol in PBS) and stored at −20°C until use. Immunohistochemistry was performed according to standard protocols with the Vectastain Elite ABC Kit (Vector Laboratories). The polyclonal rabbit CN3 antibody to amyloid-β was used as previously described (Eisele et al., 2010). Sections were counterstained with Congo red according to standard protocols.
Quantitative analysis of total amyloid load
Total amyloid-β load was quantified on a CN3/Congo red-immunostained set of every 12th systematically sampled, serial, coronal section throughout the entire hippocampus. A microscope equipped with a motorized x-y-z stage coupled to a video-microscopy system and the Stereo Investigator software (MicroBrightField, Inc) was used as and previously described (Bondolfi et al., 2002). The investigators who performed analysis were blind to the inoculation groups. The total amyloid-β load (%) was determined by calculating the areal fraction occupied by CN3- and Congo red-positive immunostaining in 2D sectors (× 20/0.45 objective).
In vitro seeding analysed by super-resolution fluorescence microscopy
Monomeric amyloid-β1-40 peptide (Bachem) was prepared by sequential treatment with trifluoroacetic acid and hexafluoroisopropanol (Teplow, 2006). The brain supernatant fraction or CSF (25 μl) was incubated at 37°C for 72 h together with 25 μM of amyloid-β1-40 peptide, 10% of which was labelled with Hilyte™ Fluor 647 (HF647; Anaspec, Cambridge Bioscience). Final total volume was 50 μl. After incubation, 25 μl were deposited onto a Lab-Tek II chambered cover-glass (NUNC, Thermo Fisher Scientific) and incubated for 30 min at 37°C in order for the aggregates/seeds to settle on the glass surface before immunofluorescence staining was performed. The solutions were blocked for 1 h in 5% normal donkey serum/PBS and then probed with anti-Aβ antibody CN3 (1:300) and secondary Alexa Fluor® 568 (AF568) labelled antibodies (1:200) (Life Technologies). After washing with 0.05% Tween/PBS, photo-switching buffer was added followed by super-resolution imaging using a Nikon Eclipse TE 300 inverted widefield microscope, and a ×100, 1.49 NA TIRF objective lens (Kaminski Schierle et al., 2011; Pinotsi et al., 2014). Imaging was in highly inclined illumination mode to image deeper into the amyloid-β seeds/aggregates. The imaged field covered 128 × 128 camera pixels corresponding to an area on the sample of ∼ 20 × 20 μm. Typically, 10 000 fluorescence frames with 10 ms exposure time were recorded. From each image stack a reconstructed dSTORM image was generated by using the open source rainSTORM software developed in-house and written in MATLAB (The MathWork Inc.). Analysis of the aggregate size was performed using ImageJ (NIH).
Study approval
The experimental procedures using mice were carried out in accordance with the veterinary office regulations of Baden-Württemberg (Germany) and Basel (Switzerland) and approved by the local Animal Care and Use Committees (N6/10; N7/12; BS2471). The study using human material was approved by the ethics committees of the Medical Faculty, University of Tübingen, Germany (94/2013BO2) and of the Medical Faculty, University of Duisburg-Essen, Germany (14-5860-BO and 14-5861-BO).
Results
Amyloid-β seeding activity of diluted soluble fractions from Alzheimer’s disease brains
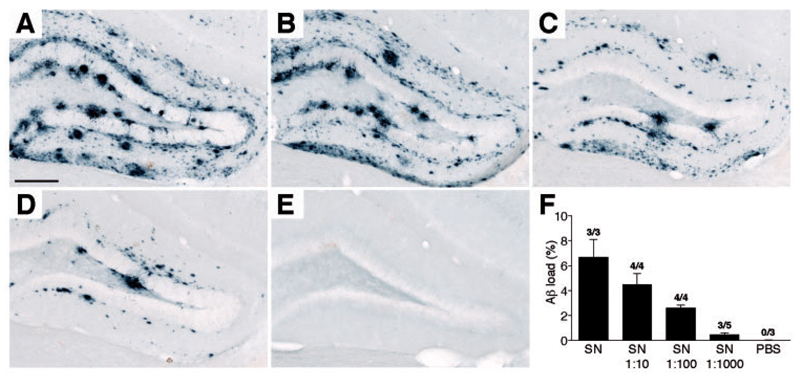
Amyloid-β peptide measurements of the PBS-soluble 100 000g supernatant fraction of fresh-frozen autopsied Alzheimer’s disease neocortical brain tissue revealed 512 ± 193 pg/ml amyloid-β peptide (i.e. total amyloid-βx-40 and amyloid-βx-42) (Table 1), which is < 0.2% of the total (formic acid-soluble) amyloid-βx-40 and amyloid-βx-42 in the same brain tissue (259 228 ± 65 097 pg/ml; n = 4, Supplementary Table 1). Mass spectrometric analysis of the supernatant fraction indicated that the major amyloid-β species were amyloid-β1-40 peptide, and a variety of species truncated at amino acid 4, in particular amyloid-β4-40 and amyloid-β4-42 peptides (Supplementary Fig. 1A). Overall this was very similar to previously published findings for the formic acid soluble brain fraction (Portelius et al., 2010). The supernatant fraction was further diluted in PBS (1:10, 1:100 and 1:1000) and 2.5 μl were injected into the hippocampus of young, pre-depositing APP23 transgenic mice. Eight months later the hippocampi were immunohistochemically analysed for amyloid-β deposition. Results revealed a dilution-dependent induction of amyloid-β deposition (Fig. 1). Strikingly, even the 1:1000 dilutions, containing ∼500 fg/ml amyloid-β peptide (i.e. ∼ 1 fg amyloid-β peptide/per injected hippocampus) still induced appreciable amyloid-β deposition throughout the hippocampus (Fig. 1). The induced amyloid-β deposition was largely diffuse in nature, consistent with previous studies using soluble amyloid-β seeds from APP transgenic mice (Langer et al., 2011).
Table 1. Testing of CSF for amyloid-β seeding capacity.
| Sex | Mean age | Amyloid-β42 | Total amyloid-β | Seeding activity |
|||
|---|---|---|---|---|---|---|---|
| Fraction | (m/f) | (years) | (pg/ml) | (pg/ml) | Incubation | Induction* | |
| Alzheimer’s disease brain SN | n = 3 | 2/1 | 78 | 73 ± 18 | 512 ± 193 | 6–8 months | 12/12 |
| Alzheimer’s disease CSF | n = 8 | 4/4 | 66 | 514 ± 199 | 8644 ± 1514 | 6–8 months | 0/27 |
| Control CSF | n = 6 | 3/3 | 63 | 717 ± 111 | 6834 ± 1835 | 6–8 months | 0/26 |
| CSF APP transgenic | n = 2 | 2/- | 2 | 2750 ± 323 | 31455 ± 2508 | 8 months | 0/3 |
| CSF wild-type | n = 2 | 2/- | 2 | n.d. | n.d. | 8 months | 0/1 |
The total number of animals with induced amyloid-β deposition/total number of animals tested is indicated (for more detailed information about subjects, clinical diagnosis and biochemical measurements see Supplementary Table 1).
SN = supernatant; m = male; f = female; n.d. = not detected.
Figure 1.
Induction of amyloid-β deposition by a PBS-soluble Alzheimer’s disease brain fraction. The soluble fractions of brain tissue from two different Alzheimer’s disease patients (SN = 100 000 g supernatant of 1:10 brain homogenate; 333 and 898 pg amyloid-β peptide/ml, respectively) were further diluted 1:10, 1:100 and 1:1000 in PBS and 2.5 μl was bilaterally injected into the hippocampus of young, pre-depositing 4-month-old APP23 transgenic mice. Animals were immunohistochemically analysed for amyloid-β deposition after an 8-month incubation period. Shown is a representative overview of the hippocampal area. No obvious differences in the induced amyloid-β deposition were noted between the supernatant of the two patients. (A) supernatant; (B) supernatant 1:10; (C) supernatant 1:100; and (D) supernatant 1:1000; (E) PBS. (F) Quantitative stereological analysis of the amyloid-β deposition (amyloid-β load in the hippocampus, n = 3 – 5 mice/group, mean ± SEM). The numbers above the bars indicate the number of animals with induced amyloid-β deposition/total number of animals injected. Scale bar = 200 μm.
Human CSF lacks amyloid-β seeding activity in vivo
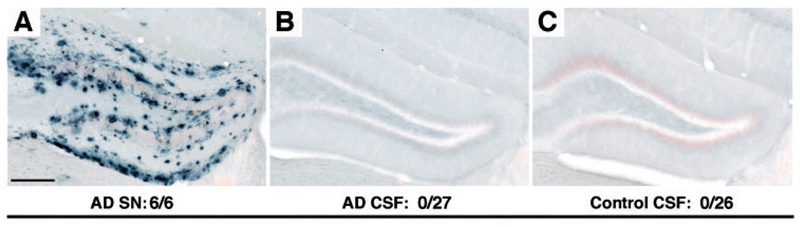
CSF samples from eight patients with Alzheimer’s disease and six age-matched non-Alzheimer’s disease control subjects were collected and revealed >10-fold higher amyloid-β peptide concentration compared to the supernatant fraction of the Alzheimer’s disease cortex (Table 1). Mass spectrometric analysis showed amyloid-β peptides starting at amino acid 1, the major species being amyloid-β1-17, amyloid-β1-38, and amyloid-β1-40 (Supplementary Fig. 1B), similar to previously published findings for other CSF samples (Portelius et al., 2007). To determine whether human CSF harbours amyloid-β-inducing activity, 2.5 μl of undiluted human CSF was injected into the hippocampus of young, pre-depositing APP23 transgenic mice, and compared with the amyloid-β-inducing activity of the supernatant fraction from Alzheimer’s disease brain (Fig. 2). Although the supernatant revealed the expected induction of amyloid-b deposition, neither the CSF samples from patients with Alzheimer’s disease nor from control patients induced any amyloid-β deposition after a 6–8 month incubation period (Fig. 2 and Table 1). Selected CSF samples (Alzheimer’s disease and control subjects) were also concentrated 15-fold using a Speed Vac concentrator but also the concentrated samples did not induce amyloid-β deposition 6 months later (n = 3; results not shown).
Figure 2.
Human CSF does not induce cerebral β-amyloidosis. The soluble fraction of an Alzheimer’s disease brain extract (supernatant, see Fig. 1) and undiluted human CSF were injected into young, pre-depositing 3- to 4-month-old APP23 transgenic mice (2.5 μl bilaterally). Brains were immunohistochemically analysed for amyloid-β deposition after a 6–8 month incubation period (Supplementary Table 1). (A) The supernatant (SN) fraction of an Alzheimer’s disease patient induced the expected amyloid-β deposition (see Fig. 1A). (B and C) Neither CSF from Alzheimer’s disease patients (shown is A023) nor control subjects (shown is A009) revealed any amyloid-β deposition. CSF from eight Alzheimer’s disease patients and six control subjects was tested in 2–10 mice per CSF sample. Two CSF samples were tested both fresh and frozen (for details see Supplementary Table 1). The total number of animals with induced amyloid-β deposition/total number of animals tested is indicated. Scale bar = 200 μm. AD = Alzheimer’s disease.
Freeze-thaw cycles may change the amyloid-β concentration in CSF and/or its seeding activity (Schoonenboom, 2004). To exclude this possibility, CSF from two of the patients with Alzheimer’s disease was either frozen or kept at 4°C until use (within 20 h). However, fresh CSF did not reveal any amyloid-β seeding activity either (Fig. 2 and Supplementary Table 1).
It has been reported that the CSF environment is inhibitory for amyloid-β fibril formation (Wisniewski et al., 1993) although such findings are controversial (Ikeda et al., 2010). Nevertheless, to test the possibility that the activity of amyloid-β seeds in CSF is masked by inhibitory factors, the supernatant fraction of the Alzheimer’s disease cortex was mixed (1:1) with Alzheimer’s disease CSF and injected into the hippocampus of young APP23 transgenic mice. Results revealed robust induction of amyloid-β deposition with the supernatant/CSF mixture (which was indistinguishable from a supernatant/PBS mixture) but not with the corresponding CSF alone (Supplementary Fig. 2).
Murine CSF also lacks amyloid-β seeding activity in vivo
To exclude species differences as a reason for the lack of amyloid-β seeding with human CSF, murine CSF from amyloid-β laden 2-year-old APP23 transgenic mice and age-matched wild-type control mice were collected and immediately injected into the hippocampus of pre-depositing APP23 transgenic mice. The CSF amyloid-β peptide concentration of the aged APP23 transgenic mice was 4.5 times higher than the amyloid-β peptide concentration in human CSF (Table 1) which mainly reflects the 5 - to 7-fold overexpression of APP in APP23 transgenic mice (Sturchler-Pierrat et al., 1997). Immunohistological analysis of the inoculated mice 8 months later revealed no amyloid-β deposition (Supplementary Fig. 3). To determine whether longer incubation periods would be necessary for the induction of amyloid-β deposition, young pre-depositing APP23 transgenic mice were unilaterally inoculated with murine CSF derived from five additional aged APP23 transgenic mice. Unilateral injections were necessary to compare the injected side with the non-injected side since in this mouse model, endogenous amyloid-β deposition in the hippocampus begins to appear at 8–10 months of age. At 14 and 21 months postinoculation there was no appreciable difference in amyloid-β deposition between the injected versus non-injected side, and no sign of amyloid-β seeding at or near the injection sites (n = 10 host mice, results not shown).
Amyloid-β particle size in CSF and soluble brain fraction
Stimulated by a recent study reporting in vitro amyloid-β seeding activity of Alzheimer’s disease CSF (Salvadores et al., 2014), we tested two Alzheimer’s disease CSF samples and two brain supernatant fractions for seeding activity. We employed super-resolution imaging to visualize amyloid-β-positive particles and their elongation by synthetic amyloid-β peptide (Supplementary Fig. 4). Results revealed that amyloid-β-positive particles (presumably amyloid-β aggregates) in Alzheimer’s disease CSF were smaller than those derived from the brain supernatant fraction. Interestingly, there was at least some elongation (seeding) of the CSF amyloid-β-positive particles in this in vitro assay, albeit less compared to the elongation in the brain supernatant fraction (Supplementary Fig. 4).
Discussion
Early diagnosis and treatment of Alzheimer’s disease remains challenging. This is of interest, as therapeutic interventions for Alzheimer’s disease are expected to be most effective when applied at an early stage before neurodegeneration has occurred. amyloid-β seeds are among the few candidates for both a therapeutic target and early biomarker as they are predicted to form very early in Alzheimer’s disease pathogenesis (Jucker and Walker, 2013). In the present study we demonstrate that the soluble Alzheimer’s disease brain fraction contains highly active amyloid-β seeds that induce β-amyloidosis. Indeed, intrahippocampal injections of the 1000-fold diluted supernatant fraction (i.e. 10 000-fold diluted soluble Alzheimer’s disease brain material) containing only ∼1 fg amyloid-β (corresponding to ∼130 000 monomeric amyloid-β molecules) induced appreciable amyloid-β deposition after an 8-month incubation period in at least 50% of the APP23 transgenic host mice. The induction of amyloid-β deposition in APP23 transgenic mice using more highly concentrated extract followed a hyperbolic function. Our data show that in vivo testing of the amyloid-β-inducing activity is a highly sensitive bioassay.
Small amyloid-β species ranging from dimers to larger oligomers, and protofibrils have been suggested to be the most pathogenic species (Mucke and Selkoe, 2012). Consistently, in Alzheimer’s disease post-mortem brain samples, soluble amyloid-β species were found to correlate with diagnosis and severity of Alzheimer’s disease dementia (Shankar et al., 2008; Noguchi et al., 2009; Tomic et al., 2009; Mc Donald et al., 2010; Esparza et al., 2013; Lesné et al., 2013). The soluble amyloid-β assemblies that manifest this exceptionally potent amyloid-β-inducing activity in vivo have so far not been identified, but likely consist also of amyloid-β peptide multimers. Therefore it is likely that the amount of the most potent amyloid-inducing subpopulation of amyloid-β required for seeding is considerably below the 1 fg of total amyloid-β peptide injected in the present studies.
In contrast to the soluble brain fraction, no amyloid-β-inducing activity of CSF was found in our in vivo assay, even though the amyloid-β peptide concentrations in CSF were at least 10-fold higher compared to the amyloid-β peptide concentrations in the soluble brain fraction. When soluble, brain-derived seeds were mixed with CSF, the seeding potency of the brain seeds was maintained, indicating that the CSF per se does not inhibit seeding, at least under our experimental conditions. Some previous studies reported a lack of amyloid-β oligomers in CSF using assays with a detection limit in the low pg/ml range (Esparza et al., 2013; Yang et al., 2013) whereas others reported amyloid-β oligomers in CSF at a concentration of 0.5–2.5 pg/ml (Hölttä et al., 2013). The present data show that our in vivo assay can detect amyloid-β seeding activity at 0.5 pg/ml amyloid-β peptide and likely below. If present at such concentrations in CSF, oligomeric amyloid-β species very likely are not seeding-competent in vivo. It is possible that N-terminally truncated amyloid-β species, such as those beginning at position 4, contribute directly or indirectly to the in vivo seeding activity of the soluble Alzheimer’s disease brain fraction because amyloid-β peptide N-terminal truncations were largely absent in the CSF.
The finding that the same CSF samples that lack in vivo seeding are capable of at least some seeding activity in vitro, though unexpected, was consistent with a recent study that also reported amyloid-β seeding activity by Alzheimer’s disease CSF in vitro (Salvadores et al., 2014). The reasons for the discrepant in vivo and in vitro seeding activity of CSF will require further study, but an important factor may relate to the much higher concentration of (monomeric) amyloid-β peptide available under in vitro conditions. Our results also revealed that the amyloid-β peptide aggregates in the CSF were smaller than those in the soluble brain fraction, and thus are presumably less stable which, in turn, may also explain their lack of seeding activity in vivo (Weber et al., 2006).
While the lack of detectable in vivo amyloid-β-inducing activity in human CSF can be seen as a negative result in terms of developing an early and/or predictive Alzheimer’s disease biomarker assay, it is reassuring that human CSF, which is routinely handled in many laboratories, does not seem to contain the self-propagating amyloid-β seeds that have been shown to be transmissible under laboratory conditions (Jucker and Walker, 2013).
Supplementary Material
Supplementary material is available at Brain online.
Acknowledgements
We would like to thank Lary Walker (Atlanta), Yvonne Eisele (San Diego), Jonas Neher and Frank Baumann (Tübingen) for comments to the manuscript; Hermann Esselmann (Essen), Anja Schneider (Göttingen), Jörg Odenthal, Michael Hruscha, Marius Lambert, Claudia Schäfer, and Ulrike Obermüller (Tübingen) for experimental help.
Funding
This work was supported by grants to M.J. from the Competence Network on Degenerative Dementias (BMBF-01GI0705), and the Alzheimer Forschung Initiative (AFI-11816); and to D.T.W. from the Swiss National Science Foundation (32323B-123812) and Velux-Foundation.
References
- Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H, Fagan AM. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006221. doi: 10.1101/cshperspect.a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Calhoun M, Ermini F, Kuhn HG, Wiederhold K-H, Walker L, et al. Amyloid-associated neuron loss and gliogenesis in the neocortex of amyloid precursor protein transgenic mice. J Neurosci. 2002;22:515–22. doi: 10.1523/JNEUROSCI.22-02-00515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30:682–90. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–82. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM, et al. Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013;73:104–19. doi: 10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–56. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harper JD, Lansbury PT. Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Trans Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä M, Hansson O, Andreasson U, Hertze J, Minthon L, Nägga K, et al. Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS One. 2013;8:e66381. doi: 10.1371/journal.pone.0066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Ono K, Elashoff D, Condron MM, Noguchi-Shinohara M, Yoshita M, et al. Cerebrospinal Fluid from Alzheimer’s disease patients promotes amyloid beta-protein oligomerization. J Alzheimers Dis. 2010;21:81–6. doi: 10.3233/JAD-2010-100075. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski Schierle GS, van de Linde S, Erdelyi M, Esbjörner EK, Klein T, Rees E, et al. In situ measurements of the formation and morphology of intracellular β-amyloid fibrils by super-resolution fluorescence imaging. J Am Chem Soc. 2011;133:12902–5. doi: 10.1021/ja201651w. [DOI] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, et al. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–11. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. Soluble A{beta} seeds are potent inducers of cerebral {beta}-Amyloid deposition. J Neurosci. 2011;31:14488–95. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, et al. Brain amyloid-β oligomers in ageing and Alzheimer’s disease. Brain. 2013;136:1383–98. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia LF, Kaeser SA, Reichwald J, Hruscha M, Martus P, Staufenbiel M, et al. Changes in Amyloid-β and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Trans Med. 2013;5:194re2. doi: 10.1126/scitranslmed.3006446. [DOI] [PubMed] [Google Scholar]
- Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, et al. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–41. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–84. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A, Matsumura S, Dezawa M, Tada M, Yanazawa M, Ito A, et al. Isolation and characterization of patient-derived, toxic, high mass amyloid beta-protein (Abeta) assembly from Alzheimer disease brains. J Biol Chem. 2009;284:32895–905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotsi D, Buell AK, Galvagnion C, Dobson CM, Kaminski Schierle GS, Kaminski CF. Direct observation of heterogeneous amyloid fibril growth kinetics via two-color super-resolution microscopy. Nano Lett. 2014;14:339–45. doi: 10.1021/nl4041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portelius E, Bogdanovic N, Gustavsson MK, Volkmann I, Brinkmalm G, Zetterberg H, et al. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2010;120:185–93. doi: 10.1007/s00401-010-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portelius E, Tran AJ, Andreasson U, Persson R, Brinkmalm G, Zetterberg H, et al. Characterization of amyloid beta peptides in cerebrospinal fluid by an automated immunoprecipitation procedure followed by mass spectrometry. J Proteome Res. 2007;6:4433–9. doi: 10.1021/pr0703627. [DOI] [PubMed] [Google Scholar]
- Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F, Soto C. Detection of misfolded Aβ oligomers for sensitive biochemical diagnosis of Alzheimer’s disease. Cell Rep. 2014;7:261–8. doi: 10.1016/j.celrep.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Schoonenboom NSM. Effects of Processing and Storage Conditions on Amyloid (1-42) and Tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin Chem. 2004;51:189–95. doi: 10.1373/clinchem.2004.039735. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–92. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplow DB. Preparation of amyloid beta-protein for structural and functional studies. Methods Enzymol. 2006;413:20–33. doi: 10.1016/S0076-6879(06)13002-5. [DOI] [PubMed] [Google Scholar]
- Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–8. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Weber P, Giese A, Piening N, Mitteregger G, Thomzig A, Beekes M, et al. Cell-free formation of misfolded prion protein with authentic prion infectivity. Proc Natl Acad Sci USA. 2006;103:15818–23. doi: 10.1073/pnas.0605608103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Castano E, Ghiso J, Frangione B. Cerebrospinal fluid inhibits Alzheimer beta-amyloid fibril formation in vitro. Ann Neurol. 1993;34:631–3. doi: 10.1002/ana.410340422. [DOI] [PubMed] [Google Scholar]
- Yang T, Hong S, O’Malley T, Sperling RA, Walsh DM, Selkoe DJ. New ELISAs with high specificity for soluble oligomers of amyloid {beta}-protein detect natural A{beta} oligomers in human brain but not CSF. Alzheimers Dement. 2013;9:99–112. doi: 10.1016/j.jalz.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]