Abstract
Acetabular bone loss is a relevant concern for surgeons dealing with a failed total hip arthroplasty.
Since the femoral head is no longer available, allografts represent the first choice for most reconstructive solutions, either as a structural buttress or impacted bone chips.
Even though fresh-frozen bone is firmly recommended for structural grafts, freeze-dried and/or irradiated bone may be used alternatively for impaction grafting. Indeed, there are some papers on freeze-dried or irradiated bone impaction grafting, but their number is limited, as is the number of cases.
Xenografts do not represent a viable option based on the poor available evidence but bioactive bioceramics such as hydroxyapatite and biphasic calcium phosphates are suitable bone graft extenders or even substitutes for acetabular impaction grafting.
Bone-marrow-derived mesenchymal stem cells and demineralised bone matrix seem to act as reliable bone graft enhancers, i.e. adjuvant therapies able to improve the biological performance of standard bone grafts or substitutes. Among these therapies, platelet-rich plasma and bone morphogenetic proteins need to be investigated further before any recommendations can be made.
Cite this article: EFORT Open Rev 2016;1:431-439. DOI:10.1302/2058-5241.160025
Keywords: revision, total hip arthroplasty, acetabulum, bone graft, impaction grafting, bone graft substitute, bone graft extenders, bone graft enhancers, bioceramics, mesenchymal stem cells
Introduction
Bone loss is a major concern of revision total hip arthroplasty (THA). While on the femoral side the problem is commonly solved by passing the defect through long stems seeking distal fixation in the healthy diaphysis, on the acetabular side any bone defect might severely affect the chance of achieving an adequate component stability, and an acceptable reconstruction of the hip anatomy and of the centre of rotation. Acetabular bone deficiencies are almost always managed with bone stock-restoring techniques (namely bone grafting), although in the last decade the availability of modular porous metal augments1,2 has introduced a viable alternative, especially attractive for older patients unlikely to undergo further revisions.
From a biological point of view, the femur and acetabulum are quite different in the revision scenario. The former is composed mostly of sclerotic cortical bone in which the osteogenic/osteo-inductive properties are limited, and is significantly devascularised with surgical exposure, whichever approach is chosen. The latter is biologically richer in cells and growth factors, since the pelvis is mostly made up of cancellous bone with bone marrow content and blood vessels and the exposure rarely affects both sides of the innominate bone. These considerations, together with morphology of the acetabular bone defects (more often cavitary and suitable for containing bone grafts rather than femoral defects), have contributed to the fact that bone deficiencies are restored on the acetabular side as commonly as they are bypassed on the femoral side, although in the hands of experienced surgeons cemented femoral impaction bone grafting restores effectively the bone stock and achieves a reliable fixation.3-5
Autologous bone represents the ‘gold standard’ source whenever a skeletal deficiency needs to be grafted. It fulfils all three ideal requisites of a bone graft, being osteoconductive, osteoinductive and osteogenic.6 Osteoconductivity is the passive ability of a scaffold to be progressively substituted by viable bone; osteoinductivity is the capacity to stimulate the osteoblastic differentiation of local adult stem cells (i.e. mesenchymal stem cells, MSCs) through specific growth factors, such as bone morphogenetic proteins (BMPs); and osteogenesis is the ability to form new bone from the living osteoblasts and MSCs present within the graft material. Moreover, the autograft is non-immunogenic and cannot transmit infectious agents unless contaminated during surgery. Although it still represents the first choice in several procedures such as fracture nonunion surgery,7 spinal fusion8 and primary THA in severely dysplastic hips,9 it is rarely employed in hip revision arthroplasty due to the relatively large amount of bone needed, along with the possibility that harvesting from the iliac crest might weaken the only reliable support for difficult acetabular reconstructions. Further drawbacks of iliac crest autografts are the increase in blood loss, the lengthening of the surgical procedure and the donor site morbidity, especially in older patients who are the usual candidates for revision surgery. For all the above reasons, allografts represent the standard choice in acetabular reconstruction. However, concerns about limited availability and disease transmission have induced consideration of different scaffolds as bone graft extenders (or expanders), which are mixed with the bone graft to augment its volume, or bone graft substitutes, that may be used alone in place of the bone graft. Recently, new medical products have been introduced that may be considered bone graft enhancers. They represent adjuvant therapies aimed at improving the biological performance of the bone grafts by adding cells or growth factors.
Allografts
Allogenic bone graft is essentially a biocompatible scaffold through which revascularisation, resorption and finally new bone apposition occur. In other words, it is osteoconductive, but not osteogenic nor osteoinductive. Its strength depends on the composition (cancellous, cortico-cancellous or cortical), on the bone bank processing (fresh-frozen or freeze-dried, irradiated or not) and on surgical handling (structural or morcellisd).
Structural allograft
Structural allografts are fixed to the peri-acetabular bone by press-fit or screws and act as support for the implantation of the new prosthetic socket. They not only restore the bone stock, but also provide a suitable buttress to lower the centre of rotation. The limited surface-to-volume ratio affects the extent of creeping substitution, with portions of ‘internal repair’ as little as 20% in massive allografts five years after oncological limb reconstruction.10 This percentage is expectedly higher in cortico-cancellous structural allografts used in acetabular revision arthroplasty, yet nevertheless is far below 100%. The persistence of a necrotic core explains the development of microfractures and the decrease of bone mineral density, which reportedly results in halving the mechanical strength ten years after implantation.11
Femoral heads or femoral head sections easily match the acetabular defect, especially if this is prepared with a hemispherical reamer approximately of the same diameter of the available head. The surgeon needs only to remove the chondral layer to obtain an adequate bone-to-bone contact. On the other hand, a distal femoral epiphysis needs to be carefully re-shaped to match the defect according to the technique proposed by Sporer et al to deal with severe dome deficiencies (Paprosky type IIIa).12 In the authors’ hands, this complex technique led to 26% of failures at ten years. Subsequently its use has been limited to very young patients who require effective bone stock restoration. In selected cases, a total acetabulum may be grafted within the defect, although this technique is generally reserved for oncological reconstructions.13
Minor column allografts14 are used to restore the hip centre of rotation in isolated anterosuperior or postero-superior defects of the acetabulum (i.e. Paprosky type II). They are usually obtained from femoral head sections and are screwed to the ilium to allow the press-fit implantation of a hemispherical prosthetic cup, often supplemented with transacetabular screws. Conversely, major column allografts15 are employed in massive peri-acetabular deficiencies (i.e. type III) and often require dedicated revision devices (cages, rings, etc.).
The more the cup is supported by the bone graft, the higher the risk of failure.16 The prognosis of bulky allografts is strongly influenced by the amount of load transmitted or bypassed by the hardware. Garbuz et al15 reported only a 55% survival rate at seven years after major column allograft (considering survival as a reconstruction that guarantees an improvement of the modified Harris Hip Score (mHHS) of 20 points at least, a stable cup and a united graft). However, they noticed that bone grafts protected by cages or rings had better results than the unprotected ones. From a series of 71 cases, with 56 hips available at a mean follow-up of 11.7 years, all of which received a Burch-Schneider anti-protrusio cage along with a major column allograft, Regis et al17 reported an 87.5% overall survival, confirming the hypothesis that load protection strongly improves the durability of massive allografts. In 2010, Lee et al18 reviewed a series of 85 minor column allografts associated with cemented and cementless hemispherical cups at five to 25 years of follow-up and reported a 55% 20-year Kaplan–Meier survivorship (with end-point re-revision for any cause). In this series, the shelf allograft was employed to treat uncontained defects in the range of 30% to 50% of the acetabulum.
Nowadays the use of modular porous metal augments has become widespread, and mid-term results are extremely encouraging.1,2 A recent report by Whitehouse et al19 documented a 92% survivorship of trabecular metal augments at ten years. However, highly porous metals do not allow bone substitution, but only bone ingrowth and may represent a safer option than bulky allografts only in older patients, who do not require bone stock restoration since future re-revisions are unlikely. For younger patients, structural bone grafts still represent a viable option for acetabular reconstruction when precise indication and surgical technique are followed and an appropriate bone graft is available.
Morcellised allograft
When cancellous and corticocancellous allografts are ground, the result is a morcellised allograft (commonly referred to as ‘bone chips’, with a size in the range of 1 mm to 10 mm) that may be packed to fill cavitary defects. The smaller the bone chips, the faster the graft union but also the more unstable the construct. The impaction increases the mechanical strength of the grafted acetabulum, often allowing the implantation of hemispherical cups with sufficient primary stability. The original technique of impaction bone grafting (IBG) can require a titanium mesh to convert a segmental defect into a cavitary one, it relies on a vigorous compaction and is followed by cement fixation of an all-poly socket (Fig. 1).20 On the femoral side nowadays using larger chips is recommended (at least 5 mm proximally and 2 mm to 3 mm distally), as is washing fat and soft tissue off the bone chips.21,22 More recently, uncemented IBG has gained popularity (Fig.1).23 An appropriate compaction force is necessary also in the case of cementless cups. The high surface-to-volume ratio enhances the creeping substitution, obtaining wider incorporation and reduced risk of delayed collapse when compared with bulky allografts, although the retention of nonunited bone chips surrounded by fibrous tissue is documented.24 Such bone-fibrous tissue composites seem not to compromise the stability of cemented components,25 while it might reasonably affect the secondary fixation of cementless acetabular cups.
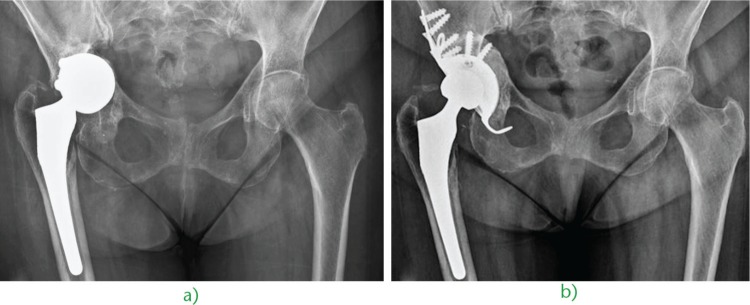
Fig. 1.
a) Superior and medial migration of a cementless cup in a 71-year-old woman. The bone loss is addressed with cemented impaction fresh-frozen bone grafting and an all-polyethylene cup. b) The post-operative radiograph shows an intra-operative trochanteric fracture fixed with two Kirschner wires. c) The five-year radiograph confirms the healing of both the grafted socket and the femoral fracture.
Schreurs et al26 reported a 20-year survivorship of cemented IBGs as high as 87% with end-point re-revision for aseptic loosening, and 75% with end-point re-revision for any reason. However, other authors reported inferior results, both in major bone loss and without correlation with the type of defect.27,28 The surgeon’s experience makes the difference in this demanding technique. Iwase reported favourable results only in cases of moderate bone loss with a maximum depth of 20 mm and without multiple segmental defects.29 Palm et al30 reported a nine-year survivorship of cementless IBGs as high as 94% with end-point re-revision for aseptic loosening, and 90.5% with end-point re-revision for any reason. This series received a hemispherical porous-coated hydroxyapatite-coated cup, but uncemented IBGs may be extended to tri-flange devices, cup-cage constructs and stemmed cups (Figs 2 and 3). Reliable primary stability in the host bone is required and porous coated implants have improved the results. In the past, 50% of contact was considered the cut-off for better results,31,32 while more recent reports with a titanium porous surface demonstrate that less than 50% can be acceptable for long-term stability if there is a good rim fit and host-bone contact is achieved with at least at part of the dome and of the posterior column.33 For massive bone defects, the use of bone impaction grafting and a cemented cup is particularly indicated in the absence of pelvic discontinuity and in the presence of an adequate support on the medial wall with a metallic mesh, and on the lateral rim of the acetabulum, with eventual trabecular augments.
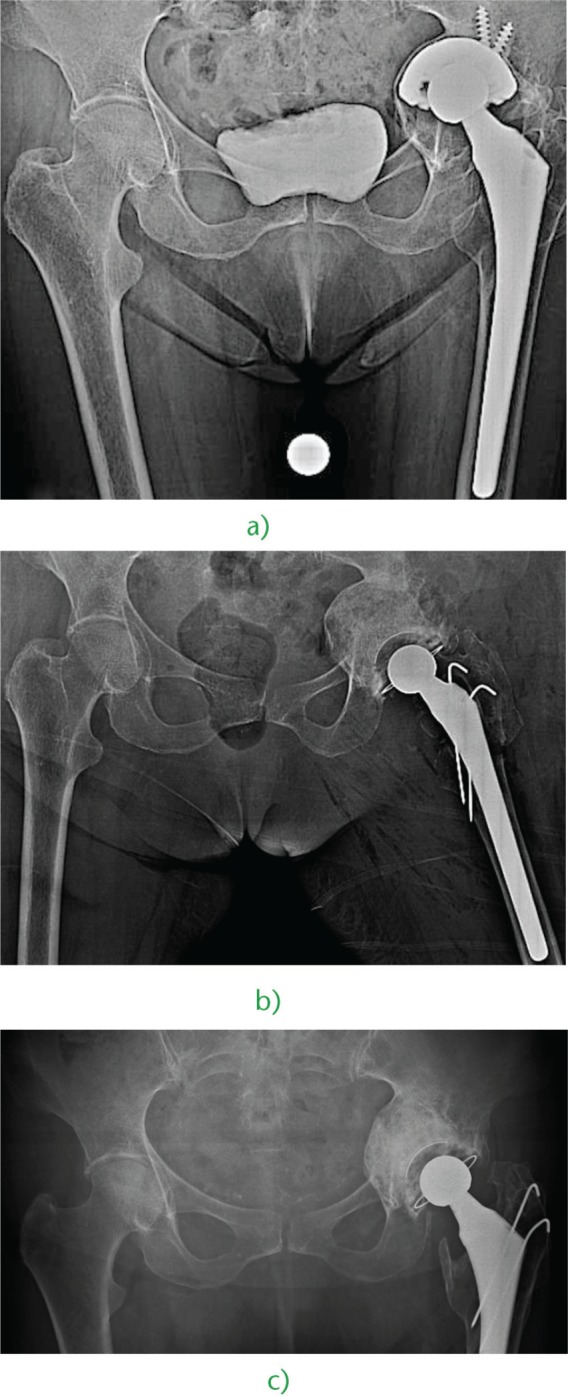
Fig. 2.
a) Medial wall bone loss caused by aseptic loosening and superomedial migration of the prosthetic cup in an 81-year-old woman affected by rheumatoid arthritis. b) Three years after revision with a cementless ‘bridging cup’ and IBG with fresh-frozen allograft, we observe perfect reconstruction of the bone stock, restoration of the hip geometry and healing of the medial wall discontinuity.
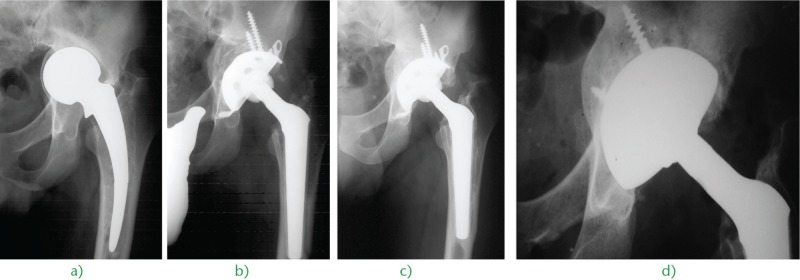
Fig. 3.
Monoblock unipolar hemiarthroplasty implanted in a 39-year-old man, revised for a) deep acetabular wear and stem breakage with b) cementless impaction bone grafting, revision acetabular implant and conic stem. c) Eight years later, we can appreciate the union of the graft and the perfect reconstruction of the medial wall. d) When a second revision was needed, the bone stock of the socket was so reconstituted that the surgeon could implant a simple hemispherical cup with screws.
Antibiotic supplementation of the allografts34 may enlarge the field of application of IBGs to the second stage of septic revisions when severe bone loss has to be addressed.
Allograft processing: fresh-frozen versus freeze-dried
Fresh-frozen allogenic bone is the standard source both for structural and for morcellised grafting. However, the growing need for this material for several surgical applications and difficult storage makes the freeze-dried bone an attractive alternative. Freeze-drying, or lyophilisation, consists of freezing the bone, placing it under a vacuum and obtaining the direct conversion of ice to vapour. The result is a bone graft that can be stored for a long time at room temperature, but is significantly weaker than fresh or fresh-frozen bone and then is scarcely suitable for structural grafting.35
However, its use for IBGs still represents an option, as demonstrated by the favourable experiences of Thien et al,36 who reported an 86% survival rate at a mean seven-year follow-up following acetabular cemented impaction grafting, and of Galia et al,37 who reported a graft survival rate of 90%, on average, eight years after cemented impaction grafting of femoral and/or acetabular components, with radiographic outcomes slightly better on the acetabular side.
Allograft processing: irradiated versus non-irradiated
Irradiation is an effective method of sterilising the musculoskeletal tissues whenever the process of harvesting and/or storage is suspected to be contaminated. The bone is exposed to a Cobalt-60 source of gamma radiation, obtaining a bactericidal activity for doses of about 20 kGy to 30 kGy and an anti-viral activity for doses of 60 kGy or higher.38 Unfortunately, high doses also produce a relevant decrease in mechanical strength.39,40 This is the reason why the actual standard gamma dose is about 25 kGy in most tissue banks, and recent research is lowering the dose threshold even further, as long as the required SAL of 10-6 (sterility assurance level), namely the chance of having a viable micro-organism on the bone graft after sterilisation, is obtained.41 The prevention of transmission of viruses and prions relies usually on donor screening and on laboratory testing, although some tissue banks have implemented chemical treatments to inactivate these agents.
Irradiated bone grafts may be used for acetabular reconstructions, but users should be aware that their mechanical properties might be impaired. This detrimental side effect is dose-dependent and is greater for freeze-dried bone than for the deep-frozen bone, since the release of free radicals is strictly related to the temperature.42
Mehendale et al43 reported a survival rate of 90% at 45 months after acetabular IBG and mostly cemented re-implantation, with end-point re-revision for any reason. Despite 70% of good to excellent clinical results, the frequency of incorporation and remodelling was as low as 20/50 and 3/50, respectively. Emms et al44 reported a survival rate of 83.3% at ten years after cemented acetabular IBG with end-point re-revision for any reason. Interestingly, in this report, 64 of 67 surviving reconstructions showed a united graft.
Xenografts
Xenografts, or bone grafts from non-human sources, are rarely employed due to concerns about immunogenicity and transmission of prions. Surgibone (Unilab Inc., Hillside, New Jersey) is a product obtained from calf bone through protein denaturation with 20% hydrogen peroxide and ether extraction of lipids, terminally sterilised with ethylene oxide. What remains is a scaffold made of hydroxyapatite and proteins without any viable cells. It was used as an autograft extender in hip revision surgery (mostly on the acetabular side and uncemented), but one case out of four failed due to graft resorption, early loosening, infection or foreign body reaction.45 On the other hand, Rosito et al reported similarly satisfactory clinico-radiographic results after acetabular revision arthroplasty with re-inforcement cage and IBG, employing alternatively human freeze-dried bone graft and bovine freeze-dried bone graft, both terminally sterilised by autoclave.46 At the present time, there is insufficient evidence for or against the use of xenograft in acetabular reconstruction, and further clinical trials are needed.
Bioceramics
Bioceramics are biocompatible ceramics, i.e. non-metallic inorganic materials that may be implanted in the human body with a very low risk of adverse reactions. The bioceramics used to treat bone deficiencies are defined as bio-active, as they attach to the bone with chemical bonding and act as a scaffold for bone regeneration; other bioceramics that do not show these features are defined as bio-inert and are used in orthopaedics to manufacture arthroplasty hard bearings.47
The most common bio-active bioceramics are bio-active glass, calcium carbonate, calcium sulphate and calcium phosphates. This last family of products is further classified as hydroxyapatite (HA), calcium-deficient apatite (CDA), β-tri-calcium phosphate (βTCP) and biphasic calcium phosphates (BCP) with a variable HA:βTCP ratio. Some of these ceramics have a natural origin, such as coralline calcium carbonate, coralline hydroxyapatite and bovine bone apatite, but most of them are synthetic. On the market, some bioceramics are available as composites: BCP + collagen; HA + calcium sulphate. For acetabular reconstruction powders, granules, pellets and cements are used, while blocks and wedges are dedicated to different applications such as osteotomies and spinal fusions.
Although bio-active bioceramics are traditionally considered only osteoconductive, recent experiments demonstrated the possibility of inducing ectopic bone formation in muscular tissue when particles of microporous BCP are implanted.48 The mechanism of osteo-induction is still under investigation, but might depend on the special geometry of the pores able to trap the circulating growth factors.
Bioceramics represent useful graft extenders for acetabular bone stock restoration, since their use mixed with allograft bone is well documented with satisfactory results. McNamara et al49 reported the clinical survivorship of all of the 50 acetabular reconstructions performed with cemented IBG (13 complex primary THAs and 37 revision THAs), on average five years after surgery, using a 1:1 mixture of irradiated deep-frozen allograft and Apapore 60 (ApaTech Ltd, Elstree, UK), pure HA with 60% porosity. Whitehouse et al50 reviewed a series of 43 hips which had received mostly uncemented acetabular revision with IBG and a 1:1 mixture of allograft and BoneSave (Stryker, Newbury, UK)—a BCP made of 80% βTCP and 20% HA with 50% porosity. The Kaplan–Meier curve showed 94% survivorship at seven years with end-point acetabular re-revision.
The same group of investigators estimated a 98% survivorship at seven years after cementless IBG with BoneSave employed alone as a graft substitute from another series of 43 revised acetabula followed up for four years on average, although in this cohort only cavitary defects were included.51
Adjuvant therapies
Adjuvant therapies or bone graft enhancers are products that may improve the graft incorporation by adding cells or growth factors. A combination of scaffold (osteoconductive), cells (osteogenic) and humoral factors (osteo-inductive) is a promising strategy of tissue engineering for the reconstruction of large bone defects. So far, demineralised bone matrix (DBM), platelet-rich plasma (PRP), MSCs and BMPs have been tested in addition to grafts and substitutes.
Demineralised bone matrix
DBM is essentially the proteinaceous component of bone, obtained from human bone through hydrochloric acid exposure (to extract the mineral component) and subsequent freeze-drying.52 For this reason, it is also referred to as demineralised freeze-dried bone allograft (DFDBA). It is available as strips, putty and paste. Besides collagen and other non-collagenic proteins, cell debris and mineral remnants, DBM includes and releases several growth factors (BMPs, TGF-β, IGF-1, etc.) that justify its osteo-inductive features in addition to the osteoconductive ones. Lacking the mineral component, DBM has no inherent structural rigidity and is not suitable for load-bearing applications. That is why its usage in revision THA is limited to mixtures with bone allografts, where it acts as both extender and as enhancer. Etienne et al53 reported the incorporation of IBG with bone chips and DBM (AlloMatrix Bone Putty; Wright Medical Technology, Inc., Arlington, Tennessee) in 18 out of 20 revised acetabula at a mean 27-month follow-up. They implanted hemispherical cups in all cases. Hamadouche et al54 used a disc of flexible DBM, the Grafton A Flex (Osteotech Inc., Eatontown, New Jersey), to line the acetabulum prior to placing a Kerboull cage and further structural allografts to complete the reconstruction. They report a survival rate of about 92% at 13 years, but most methods and results are absent from this surgical technique paper.
Platelet-rich plasma
PRP is an autologous blood-derived product that should release in situ several growth factors potentially involved in osteogenesis (PDGF, TGF-β, VEGF, IGF-1, IGF-2, FGF, some BMPs, etc.) when platelets are activated with calcium chloride and thrombin. Unfortunately, it has not been sufficiently tested in hip revision applications to suggest its routine use, with only a small pilot study published on retro-acetabular osteolytic lesions behind well fixed cups.55
Bone morphogenetic proteins
BMPs are autocrine and paracrine cytokines belonging to the TGF-β superfamily, released by MSC, osteoprogenitor cells, chondrocytes, osteoblasts, endothelial cells and platelets. Many of the over 20 BMPs identified so far have an osteo-inductive activity, promoting MSC differentiation into osteoblasts.56 Some of them can be produced as recombinant human proteins (rh).
Two BMPs are commercially available, rhBMP-2 and rhBMP-7 (also known as Osteogenic Protein 1 or OP-1). Unfortunately, to the best of our knowledge rhBMP-2 was reported as being applied to revision THA only in a single case of implant-saving treatment of a retro-acetabular osteolytic lesion, in conjunction with morcellised autograft and MSCs,57 while rhBMP-7 was reported as being applied in a series of revision THAs with unfavourable outcomes.58 This study compared standard IBG (control group) with IBG enhanced with rhBMP-7 (study group) and documented higher stem micromotions in the study group (measured with radiostereometric assay), along with more re-revisions, both on the acetabular and femoral sides.
In light of the available literature, BMPs do not have a proven role in periprosthetic bone loss reconstruction.
Mesenchymal stem cells
Bone marrow is the most studied and validated source of MSCs for bone regeneration, although different sources like adipose tissue are under evaluation. MSCs are the ideal osteogenic enhancer of bone grafts and their substitutes since they are multipotent cells able to differentiate into osteoblasts in an adequate humoral environment. Despite in vitro and in vivo animal experiments showing promising results,59,60 there is a lack of clinical data.
MSC-loaded allografts were used in at least two comparative clinical studies with favourable results. Hernigou et al compared 30 acetabular revisions performed with standard structural frozen-irradiated allograft and Kerboull cages with 30 similar procedures performed with MSC-‘supercharged’ allografts.61 All cases showed severe acetabular bone loss (Paprosky type IIIA and IIIB). Autologous MSCs were obtained from the iliac crest by multiple aspiration and concentrated; they were then infiltrated into the allogenic femoral heads prior to graft preparation. The authors reported a significantly lower resorption in the study group and a superior survivorship at a minimum 12-year follow-up (nine failures in the control group versus no failures in the study group). Ochs et al62 compared a standard fresh-frozen non-irradiated bone graft (41 hips) with MSC-loaded freeze-dried irradiated and chemically treated bone graft (38 hips). In this study, the marrow aspirate was not concentrated and was directly injected into the allograft prior to milling. Both groups underwent IBG and received a Burch-Schneider re-inforcement ring. At the latest follow-up, no component appeared to be loose and all the grafts united. The authors concluded that the safer but weaker and biologically poorer freeze-dried irradiated chemically treated allograft may become equivalent to a fresh-frozen allograft after MSC infiltration.
Conclusions
Bone stock restoration is a primary goal in acetabular revision. Since autologous bone is commonly unavailable, allografts represent the first choice for skeletal reconstruction. Structural allografts act as useful supports to reposition the centre of rotation and may effectively address segmental deficiencies of the acetabular rim, but should be protected by re-inforcement rings and cages to avoid late collapse and implant migration; in older patients, they can be favourably substituted by modular porous metal augments. Morcellised allografts can be impacted providing the reconstruction with adequate stability, but segmental deficiencies need first to be converted into cavitary ones prior to implementing this technique. Although impaction grafting was originally conceived for use with cemented sockets, nowadays it is commonly performed in conjunction with cementless devices. While structural allografts should preferably be fresh-frozen and non-irradiated, morcellised allografts may be freeze-dried and/or irradiated as second choice.
For the time being, xenografts cannot be recommended for acetabular reconstruction as the limited available reports provide conflicting conclusions about their safety and effectiveness. Conversely, bioceramics, especially HA and BCP, have proved to be suitable extenders and potential substitutes when impaction grafting techniques are employed.
Finally, bone graft enhancers represent an emerging field of interest for bone regeneration. However, only DBM and bone marrow-derived MSCs have demonstrated some promising contributions to bone graft performance, while PRP and rhBMPs are either insufficiently studied or potentially detrimental, and their application other than in clinical trials should be discouraged.
Footnotes
Conflict of Interest: None
Funding Statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Sporer SM, Paprosky WG. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J Arthroplasty 2006;21;(6Suppl 2):83-86. [DOI] [PubMed] [Google Scholar]
- 2. Van Kleunen JP, Lee GC, Lementowski PW, Nelson CL, Garino JP. Acetabular revisions using trabecular metal cups and augments. J Arthroplasty 2009;24;(6Suppl):64-68. [DOI] [PubMed] [Google Scholar]
- 3. Gie GA, Linder L, Ling RS, et al. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg [Br] 1993;75-B:14-21. [DOI] [PubMed] [Google Scholar]
- 4. Halliday BR, English HW, Timperley AJ, Gie GA, Ling RS. Femoral impaction grafting with cement in revision total hip replacement. Evolution of the technique and results. J Bone Joint Surg [Br] 2003;85-B:809-817. [PubMed] [Google Scholar]
- 5. Ornstein E, Linder L, Ranstam J, et al. Femoral impaction bone grafting with the Exeter stem - the Swedish experience: survivorship analysis of 1305 revisions performed between 1989 and 2002. J Bone Joint Surg [Br] 2009;91-B:441-446. [DOI] [PubMed] [Google Scholar]
- 6. Burwell RG. The function of bone marrow in the incorporation of a bone graft. Clin Orthop Relat Res 1985;200:125-141. [PubMed] [Google Scholar]
- 7. Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 2007;38(Suppl 1):S75-S80. [DOI] [PubMed] [Google Scholar]
- 8. Kannan A, Dodwad SN, Hsu WK. Biologics in spine arthrodesis. J Spinal Disord Tech 2015;28:163-170. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi S, Saito N, Nawata M, et al. Total hip arthroplasty with bulk femoral head autograft for acetabular reconstruction in developmental dysplasia of the hip. J Bone Joint Surg [Am] 2003;85-A:615-621. [DOI] [PubMed] [Google Scholar]
- 10. Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg [Am] 2001;83-A:971-986. [PubMed] [Google Scholar]
- 11. Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res 2005;435:36-42. [DOI] [PubMed] [Google Scholar]
- 12. Sporer SM, O’Rourke M, Chong P, Paprosky WG. The use of structural distal femoral allografts for acetabular reconstruction. Surgical technique. J Bone Joint Surg [Am] 2006;88-A(Suppl 1 Pt 1):92-99. [DOI] [PubMed] [Google Scholar]
- 13. Bradford MS, Paprosky WG. Total acetabular transplant allograft reconstruction of the severely deficient acetabulum. Semin Arthroplasty 1995;6:86-95. [PubMed] [Google Scholar]
- 14. Woodgate IG, Saleh KJ, Jaroszynski G, et al. Minor column structural acetabular allografts in revision hip arthroplasty. Clin Orthop Relat Res 2000;371:75-85. [DOI] [PubMed] [Google Scholar]
- 15. Garbuz D, Morsi E, Gross AE. Revision of the acetabular component of a total hip arthroplasty with a massive structural allograft. Study with a minimum five-year follow-up. J Bone Joint Surg [Am] 1996;78-A:693-697. [DOI] [PubMed] [Google Scholar]
- 16. Paprosky WG, Martin EL. Structural acetabular allograft in revision total hip arthroplasty. Am J Orthop (Belle Mead NJ) 2002;31:481-484. [PubMed] [Google Scholar]
- 17. Regis D, Magnan B, Sandri A, Bartolozzi P. Long-term results of anti-protrusion cage and massive allografts for the management of periprosthetic acetabular bone loss. J Arthroplasty 2008;23:826-832. [DOI] [PubMed] [Google Scholar]
- 18. Lee PT, Raz G, Safir OA, Backstein DJ, Gross AE. Long-term results for minor column allografts in revision hip arthroplasty. Clin Orthop Relat Res 2010;468:3295-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitehouse MR, Masri BA, Duncan CP, Garbuz DS. Continued good results with modular trabecular metal augments for acetabular defects in hip arthroplasty at 7 to 11 years. Clin Orthop Relat Res 2015;473:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slooff TJ, Huiskes R, van Horn J, Lemmens AJ. Bone grafting in total hip replacement for acetabular protrusion. Acta Orthop Scand 1984;55:593-596. [DOI] [PubMed] [Google Scholar]
- 21. Howie DW, Callary SA, McGee MA, Russell NC, Solomon LB. Reduced femoral component subsidence with improved impaction grafting at revision hip arthroplasty. Clin Orthop Relat Res 2010;468:3314-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McNamara IR. Impaction bone grafting in revision hip surgery: past, present and future. Cell Tissue Bank 2010;11:57-73. [DOI] [PubMed] [Google Scholar]
- 23. Ibrahim MS, Raja S, Haddad FS. Acetabular impaction bone grafting in total hip replacement. Bone Joint J 2013;95-B(Suppl A):98-102. [DOI] [PubMed] [Google Scholar]
- 24. van der Donk S, Buma P, Slooff TJ, Gardeniers JW, Schreurs BW. Incorporation of morselized bone grafts: a study of 24 acetabular biopsy specimens. Clin Orthop Relat Res 2002;396:131-141. [DOI] [PubMed] [Google Scholar]
- 25. Linder L. Cancellous impaction grafting in the human femur: histological and radiographic observations in 6 autopsy femurs and 8 biopsies. Acta Orthop Scand 2000;71:543-552. [DOI] [PubMed] [Google Scholar]
- 26. Schreurs BW, Bolder SB, Gardeniers JW, et al. Acetabular revision with impacted morsellised cancellous bone grafting and a cemented cup. A 15- to 20-year follow-up. J Bone Joint Surg [Br] 2004;86-B:492-497. [PubMed] [Google Scholar]
- 27. van Haaren EH, Heyligers IC, Alexander FG, Wuisman PI. High rate of failure of impaction grafting in large acetabular defects. J Bone Joint Surg [Br] 2007;89-B:296-300. [DOI] [PubMed] [Google Scholar]
- 28. Kostensalo I, Seppänen M, Virolainen P, et al. Acetabular reconstruction with impaction bone grafting and cemented polyethylene socket in total hip revision arthroplasty. Scand J Surg 2015;104:267-272. [DOI] [PubMed] [Google Scholar]
- 29. Iwase T, Ito T, Morita D. Massive bone defect compromises postoperative cup survivorship of acetabular revision hip arthroplasty with impaction bone grafting. J Arthroplasty 2014;29:2424-2429. [DOI] [PubMed] [Google Scholar]
- 30. Palm L, Jacobsson SA, Kvist J, et al. Acetabular revision with extensive allograft impaction and uncemented hydroxyapatite-coated implants. Results after 9 (7-11) years follow-up. J Arthroplasty 2007;22:1083-1091. [DOI] [PubMed] [Google Scholar]
- 31. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Trabecular metal cups for acetabular defects with 50% or less host bone contact. Clin Orthop Relat Res 2009;467:2318-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sternheim A, Backstein D, Kuzyk PR, et al. Porous metal revision shells for management of contained acetabular bone defects at a mean follow-up of six years: a comparison between up to 50% bleeding host bone contact and more than 50% contact. J Bone Joint Surg [Br] 2012;94-B:158-162. [DOI] [PubMed] [Google Scholar]
- 33. Patel S, Sukeik M, Haddad FS. Initial implant stability predicts migration but not failure in cementless acetabular revision with bone grafting. J Arthroplasty 2013;28:832-837. [DOI] [PubMed] [Google Scholar]
- 34. Buttaro MA. Bone grafting and two-stage revision total hip arthroplasty. Hip Int 2012;22(Suppl 8):S69-S74. [DOI] [PubMed] [Google Scholar]
- 35. Nather A, Thambyah A, Goh JC. Biomechanical strength of deep-frozen versus lyophilized large cortical allografts. Clin Biomech (Bristol, Avon) 2004;19:526-533. [DOI] [PubMed] [Google Scholar]
- 36. Thien TM, Welten ML, Verdonschot N, et al. Acetabular revision with impacted freeze-dried cancellous bone chips and a cemented cup: a report of 7 cases at 5 to 9 years’ follow-up. J Arthroplasty 2001;16:666-670. [DOI] [PubMed] [Google Scholar]
- 37. Galia CR, Macedo CA, Rosito R, et al. Femoral and acetabular revision using impacted nondemineralized freeze-dried bone allografts. J Orthop Sci 2009;14:259-265. [DOI] [PubMed] [Google Scholar]
- 38. Hernigou P, Gras G, Marinello G, Dormont D. Inactivation of HIV by application of heat and radiation: implication in bone banking with irradiated allograft bone. Acta Orthop Scand 2000;71:508-512. [DOI] [PubMed] [Google Scholar]
- 39. Anderson MJ, Keyak JH, Skinner HB. Compressive mechanical properties of human cancellous bone after gamma irradiation. J Bone Joint Surg [Am] 1992;74-A:747-752. [PubMed] [Google Scholar]
- 40. Currey JD, Foreman J, Laketić I, et al. Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res 1997;15:111-117. [DOI] [PubMed] [Google Scholar]
- 41. Nguyen H, Morgan DA, Forwood MR. Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank 2007;8:81-91. [DOI] [PubMed] [Google Scholar]
- 42. Hamer AJ, Stockley I, Elson RA. Changes in allograft bone irradiated at different temperatures. J Bone Joint Surg [Br] 1999;81-B:342-344. [DOI] [PubMed] [Google Scholar]
- 43. Mehendale S, Learmonth ID, Smith EJ, et al. Use of irradiated bone graft for impaction grafting in acetabular revision surgery: a review of fifty consecutive cases. Hip Int 2009;19:114-119. [DOI] [PubMed] [Google Scholar]
- 44. Emms NW, Buckley SC, Stockley I, Hamer AJ, Kerry RM. Mid- to long-term results of irradiated allograft in acetabular reconstruction: a follow-up report. J Bone Joint Surg [Br] 2009;91-A:1419-1423. [DOI] [PubMed] [Google Scholar]
- 45. Charalambides C, Beer M, Cobb AG. Poor results after augmenting autograft with xenograft (Surgibone) in hip revision surgery: a report of 27 cases. Acta Orthop 2005;76:544-549. [DOI] [PubMed] [Google Scholar]
- 46. Rosito R, Galia CR, Macedo CA, et al. Acetabular reconstruction with human and bovine freeze-dried bone grafts and a reinforcement device. Clinics (Sao Paulo) 2008;63:509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. LeGeros RZ, Daculsi G, LeGeros JP. Bioactive bioceramics. In: Pietrzak WS, ed. Musculoskeletal tissue regeneration: biological materials and methods. New York: Springer Science & Business Media Humana Press, 2008:153-181. [Google Scholar]
- 48. Le Nihouannen D, Daculsi G, Saffarzadeh A, et al. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone 2005;36:1086-1093. [DOI] [PubMed] [Google Scholar]
- 49. McNamara I, Deshpande S, Porteous M. Impaction grafting of the acetabulum with a mixture of frozen, ground irradiated bone graft and porous synthetic bone substitute (Apapore 60). J Bone Joint Surg [Br] 2010;92-B:617-623. [DOI] [PubMed] [Google Scholar]
- 50. Whitehouse MR, Dacombe PJ, Webb JC, Blom AW. Impaction grafting of the acetabulum with ceramic bone graft substitute mixed with femoral head allograft: high survivorship in 43 patients with a median follow-up of 7 years: a follow-up report. Acta Orthop 2013;84:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whitehouse MR, Dacombe PJ, Webb JC, Blom AW. Impaction grafting of the acetabulum with ceramic bone graft substitute: high survivorship in 43 patients with a mean follow-up period of 4 years. Acta Orthop 2013;84:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 2012;64:1063-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Etienne G, Ragland PS, Mont MA. Use of cancellous bone chips and demineralized bone matrix in the treatment of acetabular osteolysis: preliminary 2-year follow-up. Orthopedics 2004;27(suppl):S123-S126. [DOI] [PubMed] [Google Scholar]
- 54. Hamadouche M, Karoubi M, Dumaine V, Courpied JP. The use of fibre-based demineralised bone matrix in major acetabular reconstruction: surgical technique and preliminary results. Int Orthop 2011;35:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pierannunzii L, Fischer F, d’Imporzano M. Retroacetabular osteolytic lesions behind well-fixed prosthetic cups: pilot study of bearings-retaining surgery. J Orthop Traumatol 2008;9:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oryan A, Alidadi S, Moshiri A, Bigham-Sadegh A. Bone morphogenetic proteins: a powerful osteoinductive compound with non-negligible side effects and limitations. Biofactors 2014;40:459-481. [DOI] [PubMed] [Google Scholar]
- 57. Jäger M, Emami R, Thorey F, Krauspe R. Saving implants BMP-2 application in revision total hip surgery. Int J Biomed Sci 2006;2:187-195. [PMC free article] [PubMed] [Google Scholar]
- 58. Kärrholm J, Hourigan P, Timperley J, Razaznejad R. Mixing bone graft with OP-1 does not improve cup or stem fixation in revision surgery of the hip: 5-year follow-up of 10 acetabular and 11 femoral study cases and 40 control cases. Acta Orthop 2006;77:39-48. [DOI] [PubMed] [Google Scholar]
- 59. Lopa S, Mercuri D, Colombini A, et al. Orthopedic bioactive implants: hydrogel enrichment of macroporous titanium for the delivery of mesenchymal stem cells and strontium. J Biomed Mater Res A 2013;101:3396-3403. [DOI] [PubMed] [Google Scholar]
- 60. Lovati AB, Lopa S, Talò G, et al. In vivo evaluation of bone deposition in macroporous titanium implants loaded with mesenchymal stem cells and strontium-enriched hydrogel. J Biomed Mater Res B Appl Biomater 2015;103:448-456. [DOI] [PubMed] [Google Scholar]
- 61. Hernigou P, Pariat J, Queinnec S, et al. Supercharging irradiated allografts with mesenchymal stem cells improves acetabular bone grafting in revision arthroplasty. Int Orthop 2014;38:1913-1921. [DOI] [PubMed] [Google Scholar]
- 62. Ochs BG, Schmid U, Rieth J, et al. Acetabular bone reconstruction in revision arthroplasty: a comparison of freeze-dried, irradiated and chemically-treated allograft vitalised with autologous marrow versus frozen non-irradiated allograft. J Bone Joint Surg [Br] 2008;90-B:1164-1171. [DOI] [PubMed] [Google Scholar]