Abstract
The reported rate of complications of reverse shoulder arthroplasty (RSA) seems to be higher than the complication rate of anatomical total shoulder arthroplasty.
The reported overall complication rate of primary RSA is approximately 15%; when RSA is used in the revision setting, the complication rate may approach 40%.
The most common complications of RSA include instability, infection, notching, loosening, nerve injury, acromial and scapular spine fractures, intra-operative fractures and component disengagement.
Careful attention to implant design and surgical technique, including implantation of components in the correct version and height, selection of the best glenosphere-humeral bearing match, avoidance of impingement, and adequate management of the soft tissues will hopefully translate in a decreasing number of complications in the future.
Cite this article: Barco R, Savvidou OD, Sperling JW, Sanchez-Sotelo J, Cofield RH. Complications in reverse shoulder arthroplasty. EFORT Open Rev 2016;1:72-80. DOI: 10.1302/2058-5241.1.160003.
Keywords: Complications, reverse shoulder arthroplasty, instability, fracture, loosening, notching
Reverse shoulder arthroplasty (RSA) was initially designed to address rotator cuff tear arthropathy in elderly patients.1,2 Over time, indications of RSA were expanded to other conditions with various degrees of cuff deficiency, such as irreparable rotator cuff tears without osteoarthritis,3 inflammatory arthritis,4 fracture sequelae,5 tumour resection,6 failed hemiarthroplasty after fracture,7 failed hemiarthroplasty with cuff deficiency,8 failure after total shoulder arthroplasty9 and deep infection.10 Other indications now include the treatment of complex fractures of the proximal humerus in the elderly,11 as well as osteoarthritis with posterior subluxation and a biconcave glenoid.12 Since RSA is commonly used to salvage complex conditions, not surprisingly the reported complication rate is relatively high.13
Most published studies on RSA have reported on either a so-called Grammont-style RSA (medialised centre of rotation) or a glenoid-based lateralised RSA. Lessons learned using both styles of prosthesis have led to the introduction more recently of new designs with a steeper joint line, multiple options for glenosphere offset and eccentricity, and humeral-based lateralisation, but the available literature on these designs is still scarce. The introduction of lateralised glenospheres, lateralised humeral components, and various humero-diaphyseal joint line angulations translates into different biomechanics compared to the first generation of RSA; the rate and type of complications may change in the future to some degree.
The purpose of this article is to provide a review of the complication rates reported after implantation of an RSA, taking into account the timing of the complications, the underlying diagnoses, and the various designs used.
Definition and incidence
The definition of a complication varies between authors.14-16 Zumstein et al. defined ‘complication’ as any intra-operative or post-operative event that was likely to have a negative influence on the final outcome (infection, dislocation, nerve problems, aseptic loosening of any component, disassociation of the components or glenoid screw problems).14 They used the term ‘problem’ to refer to those events perceived as adverse, but unlikely to affect the final outcome (notching, hematoma, heterotopic ossification, algodystrophy, intra-operative fracture, cement extravasation or glenoid lucent lines). Some of these decisions are arbitrary; for example, notching could be considered a complication by those who believe it leads to worse clinical outcomes, and as a problem by those who believe it is inconsequential. Other authors have used different criteria for the definition of an intra-operative or post-operative complication.
The reported rate of complications has varied substantially amongst authors, and it seems to be influenced substantially by the underlying indication and the mix of primary and revision procedures included in each study. Other factors that influence complication rates include component design and surgeon experience.13,17,18 Wall et al reported a 13% complication rate for primary RSA and a 37% complication rate for revision RSA.11 Wierks et al reported 33 complications in 15 patients; the most frequent complications were neuropathies, intra-operative fracture and dislocation, with the primary cause for revision surgery being dislocation.15 Other authors have reported even higher complication rates, in some studies as high as 68% for primary RSA.13 Walch et al reported an incidence of 19% in primary RSA and 24% in revision RSA with a rate of revision surgery of 7.5%.17 The reported complication and revision rates in the meta-analysis by Zumstein et al were 24% and 10%, respectively.14
The impact of the learning curve on complication rates is unclear.19,20 Groh et al reported an overall complication rate of 7%, and failed to show an effect of their learning curve.20 Kempton et al have established an early complication-based learning curve for RSA of approximately 40 cases, whereas other authors have reported that the complication rate decreases after the first 17 cases.19,21
Understanding the intricacies of a specific implant and its application for the different encountered surgical scenarios may take a number of cases. As surgeons expand their use of RSA to more complex indications, complication rates may vary significantly. Walch et al17 reviewed their experience with a Grammont-style RSA and analysed their complication rate at two time points. They showed a decreased rate of complications from 19% to 10%, mainly due to a decrease in the rate of infection and instability. The authors argued that the most probable cause for the decrease in their complication rates was a change in indication, with fewer revision cases being performed using a RSA. However, this may not be the case for surgeons with less experience and lower volume practices.22
Intra-operative fractures
Intra-operative fractures (Fig. 1) can happen on the glenoid or humeral side. Wierks et al reported six glenoid fractures and two humeral fractures in a series of 20 patients.15 Valenti et al reported three glenoid fractures in 39 patients, and Boileau reported one glenoid fracture in a series of 45 patients.23 Recommendations to decrease the rate of glenoid fractures include starting power reaming prior to placing the reamer on the face of the glenoid, and avoidance of over-reaming. The absence of glenoid arthritis (i.e., RSA after a proximal humerus fracture) translates into minimal subchondral sclerosis; special care must be taken when reaming the glenoid. Substantial glenoid fractures may make it impossible to achieve component fixation and require intra-operative conversion to a hemiarthroplasty.
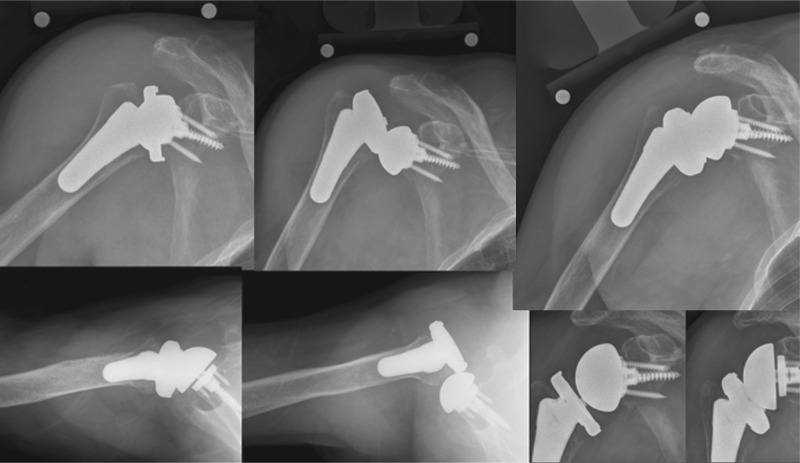
Fig. 1.
This figure shows the case of a patient with an intra-operative fracture. An anteroposterior radiograph of the right shoulder of a female patient operated for revision arthroplasty of a cemented hemiarthroplasty for post-traumatic sequelae of a proximal humerus fracture is shown (left column). Revision arthroplasty is at greater risk of suffering intra-operative fractures when compared to primary arthroplasty. When the fracture is proximal to the tip of the stem, most may be treated successfully by circumferential cerclage. In this case a long stem was used in addition to bypass a cortical window needed for cement extraction of the previous implant (middle and right columns).
Fractures on the humeral side may happen during exposure in patients with either severe osteopenia or marked fibrosis, as seen in revision cases.24 Although most early RSA were initially designed for cemented fixation of the humeral component, cementless fixation has become very common. Excessive uncontrolled reaming for cementless fixation should be avoided, as it may produce a stress riser area at the end of the reaming area, and may increase the risk of periprosthetic fracture.25
Instability
Dislocation after reverse arthroplasty represents a major source of concern. Despite the semiconstrained nature of RSA, dislocations do happen, and sometimes it is extremely difficult to identify the causes and mechanisms. Some authors have proposed that dislocation occurs in abduction and extension. RSA as a concept relies on the effective lever arm of the deltoid to compensate for the absent rotator cuff; this is partly achieved by lengthening the deltoid. Failure to achieve this tension may place the implants at risk of instability. Medial centre of rotation RSA changes the line of pull of the deltoid, which may have a dislocating effect.18 However, dislocations are reported with both prosthesis styles.26 Factors that can influence the degree of stability of RSA are the soft tissue balance, glenosphere size, the inclination of the humeral articular joint line, the version of the humeral component and the position of the metaglene (Fig. 2).27,28 Impingement of either bone or soft-tissue structures may also contribute to dislocation.
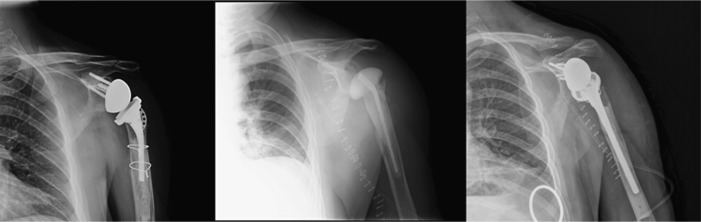
Fig. 2.
This figure demonstrates a patient with a right uncemented RSA with a short stem (left column) suffering a post-operative episode of anterior shoulder dislocation (middle column) after an initial satisfactory outcome. The patient underwent revision surgery and stability was achieved using an increased glenosphere size (right column). Further options may include lateralisation/distalisation of the glenosphere, the use of more constrained liners and correcting the height and version of the humeral stem, if anomalous.
Using a medial centre of rotation prosthesis and a deltopectoral approach, Edwards et al reported the incidence of instability without subscapularis repair to be double compared to when subscapularis repair was obtained.29 This information may not apply when a lateralised centre of rotation is used or when the RSA is implanted through a superior approach. Of note, repair of the subscapularis was associated with a greater improvement in range of motion in internal rotation when compared to patients without repair in a study by Wall et al.13 Trappey et al further analysed 284 arthroplasties and found 11 cases of instability in 212 primary cases (5.2%) and six cases in 72 revision arthroplasty cases (8.3%), and found a higher risk of dislocation when the subscapularis was irreparable and in fracture sequelae.30 Fracture sequelae, tumour surgery and instability arthropathy have shown the greatest incidence of instability.5,6,30 The primary diagnosis may affect the status of the subscapularis, the rate of impingement, and may increase the difficulty of assessing the correct height, version and adequate soft tissue tension, all of which can affect the stability of the arthroplasty.
To date, pre-operative templating with comparison of both arms remains the only objective evaluation to assess for the correct length of the arm at the time of arthroplasty. Intra-operative assessment of stability and impingement are advisable in all cases.31 When encountered, modern modular designs allow for a number of alternatives to improve stability, including sequentially increasing the height of the polyethylene, the use of a constrained polyethylene, the use of lateralised glenospheres or glenoid implants that extend distally.
When instability happens, it is usually in the first six months, and of those, half occur in the first three months.26 Conservative management can be successful in almost half of patients, and shoulders that remain stable after closed reduction have a similar outcome in terms of pain and motion. On the contrary, recurrent instability may lead to revision surgery. This may in turn increase the risk of infection (Fig. 3).26,30
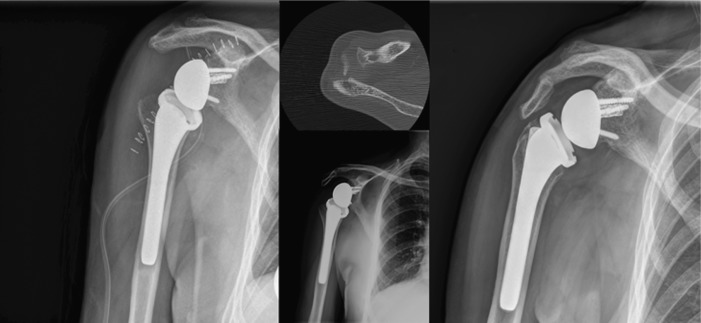
Fig. 3.
This figure shows an anteroposterior radiograph of a patient that had suffered a revision arthroplasty for failure of a hemiarthroplasty for fracture. A cement-in-cement fixation was used along with preventive cerclage due to the high risk of intra-operative fracture (left column). After 1.5 years the patient was diagnosed with a periprosthetic joint infection and underwent a two-stage revision arthroplasty. During the first surgery all the components were removed and an antibiotic-cemented spacer was used along with specific intravenous antibiotics targeting the intra-operative cultures (Staph. epidermidis)(middle column). After normalisation of PCR and ESR counts and a successful clinical course, the patient was revised to another cemented reverse shoulder arthroplasty. Intra-operative unexpected cultures grew (P. acnes) and antibiotic suppression was initiated (lateral column).
Infection
The reported rate of infection for RSA is higher than for anatomic shoulder arthroplasty. The reasons are not always clear. Factors that may explain the higher infection rate include increased implant surface, a larger dead space, patient factors and the complexity of some of the indications.16 The reported incidence in the literature varies from 1% to 15%. In a meta-analysis, Zumstein et al reported a mean infection rate of 3.8% in a systematic review including primary and revision RSA, with a higher rate in revision surgery.14 For non-reverse arthroplasty, lower rates of infection have been reported. In a single institution study, the rates were 0.7% (18/2512) for primary and 3.15% (7/22) for revision anatomic arthroplasty. Comparable rates have been reported in an integrated healthcare system (7.5% – 24/3014 in primary; 2.4% – 21/868 in revision anatomical arthroplasty).32,33
In a study involving 3906 patients, Richards et al reported a six times greater risk of infection when performing RSA, when compared to an unconstrained TSA.34 They found younger age and male gender to be risk factors for an infection, and this is consistent with other studies.30,34,35 A history of prior trauma or failed hemiarthroplasty has been shown in some studies to be a risk factor.30,34 Interestingly, smoking, rheumatoid arthritis or obesity did not increase the risk of infection in a single surgeon series when accounting for confounding variables.35 The incidence of positive cultures by Propionibacterium acnes is increased in shoulder surgery, but a true understanding of its significance in patients with minor symptoms is lacking.36
Mechanical failure
Mechanical failure may occur at the humeral or glenoid side. Due to the forces occurring at the glenoid, most early reports were wary of the outcome of these implants. Guery et al have shown a 91% implant survival rate at ten years, although it should be emphasised that most of the patients included in this study were elderly with low functional demands, and the primary diagnosis was rotator cuff tear arthropathy (Fig. 4).37
Fig. 4.
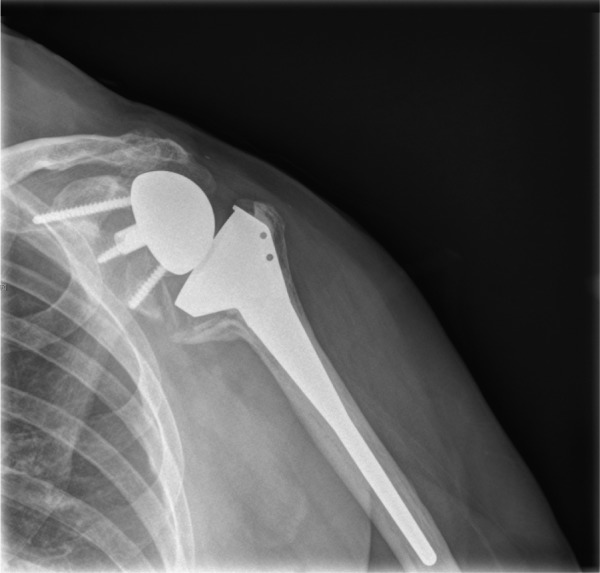
AP radiograph of a right shoulder in a patient with a RSA for rotator cuff arthropathy two years after implantation showing grade III scapular notching. The exact degree of scapular notching may be underdiagnosed if true AP views are not obtained. High-degree notching may be at risk for implant loosening and the patient must be closely monitored for evolving notching and advised of the possibility of component revision and bone grafting. Distalisation of the glenoid component with an eccentric component, along with the use of a humeral component with a more vertical joint line with or without humeral lateralisation may be advantageous in these situations.
Melis et al reported on the radiological findings of a multicentre study evaluating 122 RSA with eight years’ minimum follow-up.38 Cemented stems showed signs of radiolucency without implant migration in 20% of cases. In this study, eight of 34 uncemented humeral stems failed for aseptic loosening at eight years of follow-up. There were no glenoid failures. Uncemented stems showed proximal bone resorption and signs of stress shielding in 8% of cases, with stem diameter being related to the degree of bone resorption. The long-term effects of these changes are unknown, but they are probably the effect of the specific biomechanics and constraints of RSA. Other authors using a lateralised glenosphere have not reported humeral stem problems at short- and medium-term follow-up.39,40 Wiater et al have shown similar clinical and radiological outcomes in a cohort study comparing 37 patients with cemented RTSA, with 64 patients with cementless RTSA. None of the patients had humeral loosening or radiological signs of loosening at two years’ follow-up. Comparative long-term data were unavailable.
Glenoid loosening has been reported with both medialised and lateralised RSA to be 2.6% and 4.6 %, respectively, with an increased risk of revision surgery in lateralised designs.41 Significant mechanical stress at the bone-implant interface may influence bony ingrowth and may impact long-term stability.42 The addition of an hydroxyapatite coating and 5 mm peripheral screws reduced the rate of baseplate failure of a specific design of RSA, emphasising the importance of initial mechanical stability.43
Ek et al reported the results of patients undergoing RSA for massive irreparable cuff tears with a mean age of 60 years, at a mean follow-up of 93 months.3 Three of 46 implants (6.5%) required removal due to glenoid loosening, with an impact on outcome scores. The long-term implant survival at ten years was 91%, with 16% radiologic signs of glenoid loosening at ten years for older patients with rotator cuff arthropathy.37 It remains to be seen whether the long-term results of medialised RSA are replicated with more lateralised designs.
The rate of notching using an RSA with a medialised centre of rotation has been 47.3% in RSA with a medialised centre of rotation, with some studies reporting rates of up to 97%.2,23,44,46 The reported rate of notching of 4.6% with the use of lateralised RSA is significantly lower compared to medialised designs.27 Another radiological finding sometimes seen in the same location as notching is traction spurs in the inferior glenoid, which some authors have attributed to triceps traction enthesopathy due to insufficient release when using an antero-superior approach and heterotopic ossification, which is usually found in association with notching. Heterotopic ossification may be found distal to the glenoid and can limit range of motion.38
While some authors have suggested an increased risk of loosening with notching,44,47-49 others have not found such a relationship.13,14,38,41,50 The clinical implications of notching are controversial, and some authors have reported no effect over the clinical outcome,13,38,50 while others have reported that high grades of notching may be associated with a worse outcome.50,51 The use of an antero-superior approach, a high position of the metaglene on the glenoid, and superior tilting have all been associated with an increased rate of notching due to mechanical impingement with the arm in adduction.50
Eccentric glenospheres with an inferior offset and glenoid components with increased lateral offset (bony or metal) can reduce the rate of notching. Mizuno et al analysed the influence of an eccentric glenosphere in 47 consecutive cases compared with an historical group operated by the same surgeon.52 The rate of notching was not different, but the severity was reduced by the use of an eccentric glenosphere. Other authors have reported negligible rate of notching with the use of inferior offset component.53 Bony or metallic lateralisation of the glenosphere has shown decreased rates of notching.40,54
Glenosphere dissociation has been reported with a number of designs. Cusack et al reported on 13 patients with glenosphere dissociation using a lateralised centre of rotation RSA.55 The authors found that increasing the glenoid size led to an increased risk of component dissociation due to a higher surface for impinging with potentially improper taper engagement. Middernacht et al reported partial and complete disengagement using two different Grammont-type RSA implants.56 Revision was performed in two of the cases with complete disengagement. Different systems may have different modes of disassembly, and it remains to be seen whether this complication can be completely eliminated.
Neurological injuries
Subclinical neurological injuries with post-operative EMG changes are common after RSA, while the incidence of clinically evident neurological injury is much less frequent. They may also be under-reported due to the fact that spontaneous recovery happens in many cases.57 The most common nerve dysfunction after RSA involves the axillary nerve, although post-operative radial, ulnar, and musculocutaneous nerve palsies have been reported as well.58 Partial recovery of the axillary nerve may affect the clinical outcome, as it can affect deltoid strength.46 The suprascapular nerve and artery may be at risk at the spinoglenoid notch when drilling the posterior screw. Avoiding this complication is important, especially in cases where there is presence of a functional infraspinatus muscle.60
Excessive arm lengthening greater than 2 cm has been shown as a potential risk.31 Anatomical studies show that lateralisation is less harmful for the nerve than distalisation.59 Alentorn-Geli et al showed in their meta-analysis a 2.9% rate of neurological injury in medialised RSA versus 0.5% in lateralised COR. All surgeries included in this study were performed through a deltopectoral approach, which could potentially better isolate the effect of implant design on the rate of neurological deficit, albeit most complex and revision cases are performed through this approach.41 As in other shoulder surgeries, extreme positions of the arm may stretch the neurological structures; avoidance of unnecessary prolonged surgery and nerve ‘time-out’ recovery periods may prove beneficial to decrease the rate of nerve injuries.57
Acromion and scapular fractures
Excessive tensioning of the deltoid may place a weakened acromion at risk of fracture after the implantation of an RSA (Fig. 5). Mottier et al were the first to study the influence of acromial injuries on the outcome of RSA.61 In their study, the presence of pre-operative acromial injury (acromial stress fracture or os acromiale) did not influence the outcome, with comparable Constant Scores. However, two cases with post-operative scapular spine fractures were reported to have a poor outcome, with pain and poor motion. Walch et al studied the influence of an injury to the acromion in 457 consecutive RSA.62 Pre-operative acromial injuries did not affect the clinical outcome, but post-operative spine fractures were detrimental in regard to function. Pre-operative acromion tilt worsened after surgery, but without an impact on the Constant Score. Patients with post-operative scapular fractures were managed conservatively in three cases, and with ORIF in one case. The mean Constant Score and forward active elevation of this group was 35 and 81º, compared with 57 and 124º in the control group, with three being dissatisfied with the result. Post-operative fractures occurred without trauma in three of four cases and all appeared in the first post-operative year.
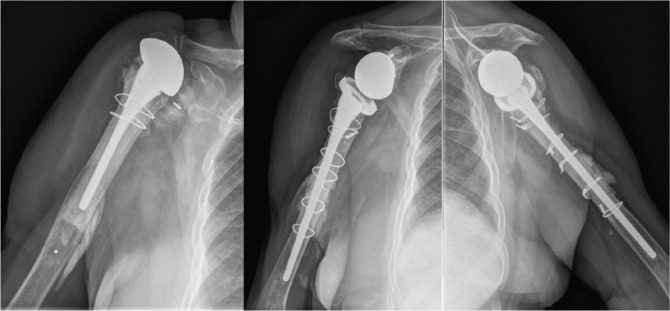
Fig. 5.
This figure shows the case of a patient that four months after uneventful RSA for rotator cuff arthropathy (left column) suffered a fall with acute onset of pain on the top of his right shoulder. Advanced imaging techniques are helpful in the diagnosis of acromial and scapular spine fractures (middle column). Subsequent evaluation of these patients shows a displaced acromion without functional impairment and a satisfied patient (right column). This may not be the case in patients with fractures of the scapular spine and it is yet not clear which patients may benefit from internal fixation.
The location of the acromial fracture may impact the outcome. Wahlquist et al reported on five cases with fractures of the base of the acromion with mean active forward elevation of only 43º and pain; after union occurred, pain improved and the mean arc of motion also improved to 83º of active elevation, so these fracture locations may not be benign.63
Crosby et al suggested a classification and treatment strategy on the basis of a retrospective review of 400 patients treated with RTSA over 4.5 years. They identified three discrete patterns: avulsion fractures of the anterior acromion (Type I); fractures of the acromion posterior to the acromioclavicular joint (Type II); and fractures of the scapular spine (Type III). They found eight type I, ten type II, and four type III fractures. Type I fractures were seen post-operatively while type II and III were seen at a mean of 10 months post-operatively. Non-operative management was used in all type I fractures and in low-demand patients in type II injuries, while surgical management was preferred in all type III and seven of the ten type II fractures. No functional data were reported, but all surgically treated fractures in types II and III united, and the authors recommend avoiding the superior screw in the metaglene because of concerns it could act as a stress riser.64 Otto et al also found that 14 of 16 scapular spine fractures arose from a screw location, but only found osteoporosis to be a risk factor. In common with other authors, they highlighted the difficulty in diagnosing these injuries upon presentation when they are undisplaced, and recommend advanced imaging for their prompt diagnosis.65
Summary
Complications after reverse shoulder arthroplasty (see Table 1) continue to be higher in primary and revision shoulder surgery when compared to total shoulder arthroplasty. Despite continued experience and better knowledge of the basic concepts of RSA, complications still occur, even in the most experienced hands. The rate of complications is influenced by many factors. As the concept and design of the reverse shoulder is evolving, the rate and type of complication may change over time.
Table 1.
Complications in reverse shoulder arthroplasty. A summarised review of the total rate of complications, reoperation rate, revision rate and specific complications of RSA
| Author | N | Total Complic-ation Rate (%) | Primary RSA complic-ation rate (%) | Revision RSA complic-ation Rate (%) | Reoperation Rate (%) | Revison Rate (%) | Specific Complications | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instability (%) | Infection (%) | Notching (%) | Mechanical failure (glenoid/humeral) (%) | Nerve Injury (%) | Acromial/Spine Fracture (%) | Intraoperative Fracture (%) | Component disassembly (%) | |||||||
| Alentorn-Geli et al. (2015) | 1188 | - | - | - | 4.8 | 5.6 | 4.4 | 2.8 | 43.8 | 1.8/1 | 2.9 | 1.2 | - | - |
| 219 | - | - | - | 7.8 | 10.5 | 3.2 | 3.7 | 4.6 | 4.6/0.9 | 0.5 | 2.3 | - | - | |
| 1721 | - | - | - | 3.7 | 3.8 | 2.8 | 3.4 | 50.8 | 3.4/2.1 | 0.6 | 0.6 | - | - | |
| Zummstein et al. (2011) | 782 | 25 | - | 33.3 | 3.5 | 10 | 6.9 | 5.6 | 51.8 | 6.9 | 1.7 | 2.2 | 4.3 | 4.4 |
| Wierks et al. (2009) | 20 | 165 | 10 | - | 10 | 5 | 5 | 55 | 0 | 0 | 5 | 0 | ||
| Cuff et al. (2008) | 94 | 9.5 | - | - | 3.1 | 3.1 | 4.2 | 1.1 | 0 | 1.1 | 0 | 1.1 | - | - |
| Wall et al.(2007) | 199 | 15 | 13 | 37 | - | - | 7.5 | 4.0 | 50.7 | <2.5 | <2.5 | <2.5 | <2.5 | <2.5 |
| Guery et al. (2006) | 60 | 15 | - | - | 6 | 6 | 3 | 4.5 | - | 6 | - | - | - | - |
| Boileau et al. (2006) | 45 | 24 | 25 | 45 | 9 | 13 | 6 | 6 | 68 | 4 | 2 | 4 | 4 | - |
| Werner et al. (2005) | 58 | 47 | - | - | 33 | - | 8.5 | 3.4 | - | 5.1/1.07 | - | - | - | - |
Footnotes
Conflict of interest: J.W. Sperling has received royalties from Zimmer-Biomet; J. Sanchez-Sotelo has received royalties from Stryker and Zimmer-Biomet, and has carried out consultancy for Stryker; R.H. Cofield has received royalties from Smith and Nephew.
Funding
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article. In addition, benefits have been or will be directed to a research fund, foundation, educational institution, or other non-profit organisation with which one or more of the authors are associated.
References
- 1. Baulot E, Sirveaux F, Boileau P. Grammont’s idea: the story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res 2011;469:2425-2431. PMID:21210311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65-68. PMID:8421661. [DOI] [PubMed] [Google Scholar]
- 3. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013;22:1199-1208. PMID:23385083. [DOI] [PubMed] [Google Scholar]
- 4. Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg 2010;19:1076-1084. PMID:20363159. [DOI] [PubMed] [Google Scholar]
- 5. Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae). J Bone Joint Surg [Am] 2014;96:2070-2076. PMID:25520341. [DOI] [PubMed] [Google Scholar]
- 6. De Wilde LF, Plasschaert FS, Audenaert EA, Verdonk RC. Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orthop Relat Res 2005;(430):156-162. PMID:15662318. [DOI] [PubMed] [Google Scholar]
- 7. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg [Am] 2007;89:292-300. PMID:17272443. [DOI] [PubMed] [Google Scholar]
- 8. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg [Br] 2007;89:189-195. PMID:17322433. [DOI] [PubMed] [Google Scholar]
- 9. Abdel MP, Hattrup SJ, Sperling JW, Cofield RH, Kreofsky CR, Sanchez-Sotelo J. Revision of an unstable hemiarthroplasty or anatomical total shoulder replacement using a reverse design prosthesis. Bone Joint J 2013;95-b(5):668-72. [DOI] [PubMed] [Google Scholar]
- 10. Cuff DJ, Virani NA, Levy J, et al. The treatment of deep shoulder infection and glenohumeral instability with debridement, reverse shoulder arthroplasty and postoperative antibiotics. J Bone Joint Surg [Br] 2008;90:336-342. PMID:18310757. [DOI] [PubMed] [Google Scholar]
- 11. Wall B, Walch G. Reverse shoulder arthroplasty for the treatment of proximal humeral fractures. Hand Clin 2007;23:425-430, v-vi. v-vi. PMID:18054669. [DOI] [PubMed] [Google Scholar]
- 12. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg [Am] 2013;95:1297-1304. PMID:23864178. [DOI] [PubMed] [Google Scholar]
- 13. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg [Am] 2007;89:1476-1485. PMID:17606786. [DOI] [PubMed] [Google Scholar]
- 14. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-157. PMID:21134666. [DOI] [PubMed] [Google Scholar]
- 15. Wierks C, Skolasky RL, Ji JH, McFarland EG. Reverse total shoulder replacement: intraoperative and early postoperative complications. Clin Orthop Relat Res 2009;467:225-234. PMID:18685908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2011;19:439-449. PMID:21724923. [PubMed] [Google Scholar]
- 17. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg 2012;21:1470-1477. PMID:22365818. [DOI] [PubMed] [Google Scholar]
- 18. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14(suppl S):147S-161S. PMID:15726075. [DOI] [PubMed] [Google Scholar]
- 19. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res 2011;469:2496-2504. PMID:21328021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:388-394. PMID:24021159. [DOI] [PubMed] [Google Scholar]
- 21. Riedel BB, Mildren ME, Jobe CM, Wongworawat MD, Phipatanakul WP. Evaluation of the learning curve for reverse shoulder arthroplasty. Orthopedics 2010;33. PMID:20415301. [DOI] [PubMed] [Google Scholar]
- 22. Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg [Am] 2003;85-a(12):2318-24. [DOI] [PubMed] [Google Scholar]
- 23. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (5 years) In: Walch G., Boileau P., Mole D, eds. Shoulder prosthesis: two to ten year follow-up. Montpellier: Sauramps, 2001:253–259. [Google Scholar]
- 24. Wagner ER, Houdek MT, Elhassan BT, Sanchez-Sotelo J, Cofield RH, Sperling JW. What are risk factors for intraoperative humerus fractures during revision reverse shoulder arthroplasty and do they influence outcomes? Clin Orthop Relat Res 2015;473:3228-3234. PMID:26162412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee M, Chebli C, Mounce D, Bertelsen A, Richardson M, Matsen F., III Intramedullary reaming for press-fit fixation of a humeral component removes cortical bone asymmetrically. J Shoulder Elbow Surg 2008;17:150-155. PMID:18029200. [DOI] [PubMed] [Google Scholar]
- 26. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:621-627. PMID:25441563. [DOI] [PubMed] [Google Scholar]
- 27. Gutiérrez S, Comiskey CA, IV, Luo ZP, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg [Am] 2008;90:2606-2615. PMID:19047705. [DOI] [PubMed] [Google Scholar]
- 28. Roche C, Flurin PH, Wright T, Crosby LA, Mauldin M, Zuckerman JD. An evaluation of the relationships between reverse shoulder design parameters and range of motion, impingement, and stability. J Shoulder Elbow Surg 2009;18:734-741. PMID:19250845. [DOI] [PubMed] [Google Scholar]
- 29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:892-896. PMID:19282204. [DOI] [PubMed] [Google Scholar]
- 30. Trappey GJ, IV, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res 2011;469:2505-2511. PMID:21104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lädermann A, Edwards TB, Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. Int Orthop 2014;38:991-1000. PMID:24271331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dillon MT, Ake CF, Burke MF, et al. The Kaiser Permanente shoulder arthroplasty registry: results from 6,336 primary shoulder arthroplasties. Acta Orthop 2015;86:286-292. PMID:25727949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res 2001;(382):206-216. PMID:11153989. [DOI] [PubMed] [Google Scholar]
- 34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res 2014;472:2809-2815. PMID:24906812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris BJ, O’Connor DP, Torres D, Elkousy HA, Gartsman GM, Edwards TB. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:161-166. PMID:25168350. [DOI] [PubMed] [Google Scholar]
- 36. Foruria AM, Fox TJ, Sperling JW, Cofield RH. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:620-627. PMID:22981448. [DOI] [PubMed] [Google Scholar]
- 37. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg [Am] 2006;88:1742-1747. PMID:16882896. [DOI] [PubMed] [Google Scholar]
- 38. Melis B, DeFranco M, Lädermann A, et al. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg [Br] 2011;93:1240-1246. PMID:21911536. [DOI] [PubMed] [Google Scholar]
- 39. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg [Am] 2005;87:1697-1705. PMID:16085607. [DOI] [PubMed] [Google Scholar]
- 40. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg [Am] 2008;90:1244-1251. PMID:18519317. [DOI] [PubMed] [Google Scholar]
- 41. Alentorn-Geli E, Guirro P, Santana F, Torrens C. Treatment of fracture sequelae of the proximal humerus: comparison of hemiarthroplasty and reverse total shoulder arthroplasty. Arch Orthop Trauma Surg 2014;134:1545-1550. PMID:25138037. [DOI] [PubMed] [Google Scholar]
- 42. Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in “reverse” total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg 2005;14(suppl S):162S-167S. PMID:15726076. [DOI] [PubMed] [Google Scholar]
- 43. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg [Am] 2010;92:2544-2556. PMID:21048173. [DOI] [PubMed] [Google Scholar]
- 44. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg [Am] 2005;87:1476-1486. PMID:15995114. [DOI] [PubMed] [Google Scholar]
- 45. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg [Br] 2004;86:388-395. PMID:15125127. [DOI] [PubMed] [Google Scholar]
- 46. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006;15:527-540. PMID:16979046. [DOI] [PubMed] [Google Scholar]
- 47. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129-133. PMID:11866145. [DOI] [PubMed] [Google Scholar]
- 48. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg 2004;70:219-225. PMID:15287400. [PubMed] [Google Scholar]
- 49. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg [Br] 2004;86:1187-1191. PMID:15568535. [DOI] [PubMed] [Google Scholar]
- 50. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2008;17:925-935. PMID:18558499. [DOI] [PubMed] [Google Scholar]
- 51. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg [Am] 2007;89:588-600. PMID:17332108. [DOI] [PubMed] [Google Scholar]
- 52. Mizuno N, Denard PJ, Raiss P, Walch G. The clinical and radiographical results of reverse total shoulder arthroplasty with eccentric glenosphere. Int Orthop 2012;36:1647-1653. PMID:22534957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg 2012;96(suppl 1):S27-S34. PMID:22528848. [DOI] [PubMed] [Google Scholar]
- 54. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011;469:2558-2567. PMID:21286887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cusick MC, Hussey MM, Steen BM, et al. Glenosphere dissociation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:1061-1068. PMID:25655458. [DOI] [PubMed] [Google Scholar]
- 56. Middernacht B, De Wilde L, Molé D, Favard L, Debeer P. Glenosphere disengagement: a potentially serious default in reverse shoulder surgery. Clin Orthop Relat Res 2008;466:892-898. PMID:18288559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nagda SH, Rogers KJ, Sestokas AK, et al. Neer Award 2005: peripheral nerve function during shoulder arthroplasty using intraoperative nerve monitoring. J Shoulder Elbow Surg 2007;16(suppl):S2-S8. PMID:17493556. [DOI] [PubMed] [Google Scholar]
- 58. Lädermann A, Lübbeke A, Mélis B, et al. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg [Am] 2011;93:1288-1293. PMID:21792494. [DOI] [PubMed] [Google Scholar]
- 59. Marion B, Leclère FM, Casoli V, et al. Potential axillary nerve stretching during RSA implantation: an anatomical study. Anat Sci Int 2014;89:232-237. PMID:24497198. [DOI] [PubMed] [Google Scholar]
- 60. Hart ND, Clark JC, Wade Krause FR, Kissenberth MJ, Bragg WE, Hawkins RJ. Glenoid screw position in the Encore Reverse Shoulder Prosthesis: an anatomic dissection study of screw relationship to surrounding structures. J Shoulder Elbow Surg 2013;22:814-820. PMID:23158042. [DOI] [PubMed] [Google Scholar]
- 61. Mottier F, Wall B, Nove-Josserand L, Galoisy Guibal L, Walch G. [Reverse prosthesis and os acromiale or acromion stress fracture]. Rev Chir Orthop Repar Appar Mot 2007;93:133-141. [In French] [DOI] [PubMed] [Google Scholar]
- 62. Walch G, Mottier F, Wall B, Boileau P, Molé D, Favard L. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg 2009;18:495-502. PMID:19250846. [DOI] [PubMed] [Google Scholar]
- 63. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg 2011;20:1178-1183. PMID:21493106. [DOI] [PubMed] [Google Scholar]
- 64. Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011;469:2544-2549. PMID:21448773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Otto RJ, Virani NA, Levy JC, Nigro PT, Cuff DJ, Frankle MA. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg 2013;22:1514-1521. PMID:23659805. [DOI] [PubMed] [Google Scholar]