Abstract
Objectives:
We aimed to set up a robust multi-centre clinical fMRI and neuropsychological platform to investigate the neuropharmacology of brain processes relevant to addiction – reward, impulsivity and emotional reactivity. Here we provide an overview of the fMRI battery, carried out across three centres, characterizing neuronal response to the tasks, along with exploring inter-centre differences in healthy participants.
Experimental design:
Three fMRI tasks were used: monetary incentive delay to probe reward sensitivity, go/no-go to probe impulsivity and an evocative images task to probe emotional reactivity. A coordinate-based activation likelihood estimation (ALE) meta-analysis was carried out for the reward and impulsivity tasks to help establish region of interest (ROI) placement. A group of healthy participants was recruited from across three centres (total n=43) to investigate inter-centre differences.
Principle observations:
The pattern of response observed for each of the three tasks was consistent with previous studies using similar paradigms. At the whole brain level, significant differences were not observed between centres for any task.
Conclusions:
In developing this platform we successfully integrated neuroimaging data from three centres, adapted validated tasks and applied whole brain and ROI approaches to explore and demonstrate their consistency across centres.
Keywords: Brain, human, magnetic resonance imaging, substance-related disorders
Introduction
Addiction is a major global health problem, with illicit drug and alcohol use disorders contributing to approximately 20% of the burden from mental health disorders (Whiteford et al., 2013). Of concern is the lack of effective interventions for these disorders, whilst the prevalence of alcohol, opioid and cocaine addiction is increasing (Lingford-Hughes et al., 2012; Whiteford et al., 2013). The growing knowledge about the brain mechanisms underpinning addiction offers an important opportunity to develop new treatments. Studying the neurobiology of addiction can be challenging due to its common relapsing–remitting clinical course. To address this, a collaboration between Imperial College London, the University of Cambridge and the University of Manchester (ICCAM; http://www.bbmh.manchester.ac.uk/ICCAM/) was formed under a Medical Research Council (MRC) addiction initiative to maximize the existing magnetic resonance imaging (MRI) and clinical infrastructure and expertise available in the UK. Establishment of a platform is necessary to provide suffi-cient throughput to rapidly evaluate potential pharmacological treatments in addiction to allow us the best chance of meeting this area of significant unmet need. Here, the term ‘platform’ refers to the concept of applying a framework of experimentation (i.e. the functional MRI (fMRI) tasks and associated measures) and analysis that can be applied under different conditions and on different groups to accelerate efforts to identify effective treatments for challenging diseases (Berry et al., 2015).
The rationale for the cognitive processes and neuropharmacology as well as the clinical population studied in the ICCAM platform have been described in detail elsewhere (Paterson et al., 2015). Briefly, the aim was to develop a neuroimaging platform to assess candidate brain pathways underpinning addiction and relapse using appropriate fMRI tasks, and assessing their modulation by different pharmacological challenges (antagonists of Dopamine Receptor D3 (DRD3), µ-opioid receptors and Neurokinin 1 (NK1) receptors) in alcohol, heroin and cocaine addiction. Here we describe the establishment of fMRI tasks in the three centres in healthy volunteers and investigate their properties. Results from the ICCAM platform with regard to brain responses to the tasks in addicts and modulation to pharmacological challenges will be reported elsewhere.
In addiction, common themes implicated in relapse involve difficulties with reward or motivation and impulse control, as well as stress-related emotional reactivity. There is a considerable body of evidence from neuroimaging studies that a dysregulated reward/motivation system in addiction as well as deficits in inhibitory control, poor decision making (Loree et al., 2014; Noel et al., 2013) and stress (Koob and Kreek, 2007; Sinha and Li, 2007) contribute to relapse. We therefore selected established fMRI tasks designed to elucidate the neural responses associated with these processes – reward/motivation, impulse control and emotional reactivity.
For reward, we chose the widely used monetary incentive delay task since it provides a measure of reward sensitivity with robust increases in striatal activity evident in healthy volunteers (Knutson et al., 2001). Striatal activity has been shown to be reduced in alcohol dependence (Wrase et al., 2007b), and in stimulant use related to treatment status (Bustamante et al., 2014; Jia et al., 2011; Schouw et al., 2013). Furthermore, ventral striatal activation in response to the task is sensitive to pharmacological modulation by amphetamines (Knutson et al., 2004), olanzapine (Schlagenhauf et al., 2008) and catecholamine depletion (Hasler et al., 2009).
For impulsivity, we chose the go/no-go task since it provides a measure of inhibitory control mediated by prefrontal–striatal circuits (Garavan et al., 2002, 2003). Neural responses during go/no-go have been shown to be altered in cocaine users (Connolly et al., 2012; Kaufman et al., 2003) and opiate addiction (Forman et al., 2004), and to be modulated by certain dopaminergic gene variants in heavy drinkers (Filbey et al., 2012).
To explore stress we exploited the associated emotional dysregulation, since amygdalar response is robustly observed and altered in a range of neuroimaging studies of addicts (Asensio et al., 2010; Gilman and Hommer, 2008; Li and Sinha, 2008). Therefore, in common with others, we used an evocative images task to assess emotional reactivity to contrasting aversive images with neutral images from the International Affective Picture System (IAPS) library. Photographs containing scenes of animate and inanimate objects or scenes were displayed in a block design, with each block containing either neutral or distressing images of an injurious or threatening nature. In addition, due to studying addiction to different substances and therefore potentially variable cue-reactivity, images had no explicit alcohol/drug content. Due to time constraints within the imaging session, positive images were not included. Similar tasks have been shown to elicit amygdala responses and have been employed to demonstrate enhanced responses in alcohol dependence (Gilman and Hommer, 2008) that were decreased by an NK1 receptor antagonist (George et al., 2008). Whilst salient cues are strong triggers for relapse, we did not include such a task due to concerns about determining and optimizing salience for each participant, ensuring salience was equal across different substances, and habituation over five sessions. We also had to consider the time constraints of our imaging sessions which were developed to be tolerable for participants such that they would perform adequately.
In order to define a priori where responses were expected in the brain for each task, we carried out a coordinate-based meta-analysis of neuroimaging data using activation likelihood estimation (ALE) (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2002, 2012). Such an approach overcomes potential bias of choosing regions of interest (ROIs) based on an investigator’s knowledge of where a task has been found to modulate activity in their previous work. For instance, several studies have used these methods to establish locations of consistent response to reward (Bartra et al., 2013; Keuken et al., 2014), impulsivity (Criaud and Boulinguez, 2013; Simmonds et al., 2008) and emotional reactivity (Fusar-Poli et al., 2009). However, there is much variation in the specifics of fMRI tasks, even amongst those considered as ‘standard’, with meta-analyses often using relatively broad inclusion criteria. These have the advantage of increased statistical power at the expense of reduced specificity. Here we seek to establish not only the general neural correlates of the paradigms under investigation, but also those elicited by the specific versions of each task as they were implemented.
Whilst the advantages of multi-centre study designs are numerous and well-rehearsed (Paterson et al., 2015), the involvement of multiple acquisition centres introduces new factors that require appropriate consideration during subsequent analysis. In particular, the overall variance is inflated by a between-centre factor, and there is potential for bias should a sub-set of centres have significantly greater statistical power than the others.
In this paper, therefore, we detail both the specific versions of the tasks used in the platform fMRI study, along with their modelling, sufficiently to enable replication. Following this, and taking each task in turn, we establish their characteristics before investigating inter-centre differences.
Methods
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from West London and Gene Therapy Advisory Committee (GTAC) National Research Ethics Service (NRES) committee and relevant Research Governance and Participant Identification Centre approvals obtained. Data were collected at three UK centres: Imanova Limited, London; The Wolfson Brain Imaging Centre, University of Cambridge; and Salford Royal NHS Foundation Trust, Manchester.
Participants
Out of the 155 participants who had a full baseline imaging session in the main ICCAM study, 68 were healthy controls with no history of drug or alcohol dependence (19, 33 and 16 from London, Cambridge and Manchester, respectively) – only this group is examined further here. These were recruited from healthy volunteer databases, via multimedia advertising including fliers, posters, social media, local newspapers, websites, homepages and via word of mouth.
From this group of 68 a subgroup of 43 (n=15, 15 and 13 from London, Cambridge and Manchester, respectively) were chosen so that each centre had a similar distribution of gender and age. This group of 43 healthy individuals was used for both the task characterization and inter-centre variability investigations. Although not reported in this work, the majority of these participants took part in further imaging sessions in the main ICCAM study, beyond these baseline sessions.
fMRI task protocols
E-Prime 2.0 RC (version 2.0.8.90) was used to run all tasks. Tasks were adapted such that two runs of each (along with resting state and preliminaries) could be achieved within a one hour period.
Monetary incentive delay task
The monetary incentive delay task, designed to probe reward sensitivity, was modified from Knutson et al. (2001). Participants could win or lose money depending upon how quickly they reacted to a target stimulus. The task contained win, lose and neutral trials. For the win trials, participants could win £0.50 if they responded quickly enough; for the lose trials, participants lost £0.50 if they did not respond quickly enough; and for the neutral trials participants neither won nor lost money. During each run, 216 volumes were collected, for a run length of 7 min 12 s.
This task used an event related design, though with long mini-blocks (i.e. several TRs (repetition time) in length), carried out in two runs (for a combined length of 14 min 24 s). Each run contained 18 win trials, 18 neutral trials, and six lose trials. In total, the task contained 36 win trials, 36 neutral trials and 12 lose trials. The task was set to obtain approximately 66% accuracy for the win trials. Furthermore, the task was designed to give an approximate winnings total of £10 (a perfect, though unlikely, result would result in winnings of £18).
Participants were informed as to what trial they were about to perform via ‘cues’ that appeared on the screen for one second. Following the cues, there was an anticipation period (i.e. a blank black screen) before the target stimulus was presented. The duration of the anticipation period was randomly selected as 2, 3 or 4 s (with equal numbers of each period for each trial type). The anticipation period was immediately followed by the presentation of the target stimulus. The duration of the target stimulus differed depending upon the accuracy of participants.
The starting duration for the win and neutral trials was 280 milliseconds (ms) for both runs (i.e. the time allowed for a participant to press the button after the stimulus was displayed). For the individualized algorithm, if a participant responded in time for the target stimulus, the target duration dropped by 10 ms (until the floor duration of 150 ms was reached). If a participant missed a trial, the target duration increased by 10 ms (until the ceiling duration of 300 ms was reached). The duration of each of the target symbols for each trial (win, neutral, lose) was contingent upon the participant’s accuracy for the same trial type only – that is, win trial accuracy only affected stimulus duration of subsequent win trials, and not neutral or loss trials. Participants were informed if they were successful immediately (always 0.5 s after target presentation) after each trial, together with a display of their total winnings, which was shown for 2 s. For each trial type the interval between the end of this information/winnings display, and the onset of the next cue were 2.4, 3.4, or 4.4 s, with equal numbers of each period across trial types.
The starting duration for the loss trials was 240 ms for both runs. A reduced loss starting duration was chosen as we required participants to lose in order to increase the incentive salience of reward trials. A fixation cross was displayed for 12 s at the beginning of each run.
Go/no-go task
The go/no-go task, designed to probe impulsivity, was modified from Garavan et al. (2002). Participants were presented with an alternating series of letter Xs and letter Ys and asked to ‘respond as quickly as possible’ to the appearance of each letter presented (‘go’ trial), except when the alternating sequence was broken by the appearance of a letter the same as that presented previously (‘no-go’ trial). During each run, 131 volumes were collected, for a run length of 4 min 22 s.
This task used an event related design and was carried out in two runs. Each run contained 250 trials. 220 of these were ‘go’ trials where participants had to respond, and 30 of these trials were ‘no-go’ trials where the participant had to withhold a response (i.e. when the letter was the same as the previous letter). On average there was one ‘no-go’ trial every 8 s (range: 4–14 s).
Each letter was presented on the screen for 900 ms and was followed by a 100 ms inter-stimulus interval consisting of a blank screen. A fixation cross was displayed for 12 s at the beginning of each run.
Evocative images task
The evocative images task was designed to probe emotional reactivity. Participants were presented with aversive IAPS images containing scenes of injury or threat and neutral IAPS images containing scenes of animate and inanimate objects. Participants had to press their response pad to each image to ensure they were awake and attending to the images. During each run, 196 volumes were collected, for a run length of 6 min 32 s.
The task used a block design and was carried out in two runs. Each run contained four blocks of aversive images and four blocks of neutral images. Each block contained six images and each block was separated by a rest period to prevent carry-over effects. Images in each block were presented in a pseudorandomized order. The second run of the task contained the same images as the first run, but presented in a different order. Due to possible habituation effects, different images were presented at each session.
Each block was 32.4 s (six images of 5 s duration followed by a 400 ms inter-stimulus interval). Each rest period lasted 15 s. A fixation cross was displayed for 12 s at the beginning of each run.
Activation likelihood estimation meta-analyses
To identify appropriate regions of interest (ROIs) for specific analyses, activation likelihood estimation (ALE) meta-analyses (Eickhoff et al., 2009) of the literature were carried out using the BrainMap Project’s GingerALE version (2.3.1) for both the monetary incentive delay and go/no-go tasks.
The following general study selection criteria were applied: (1) participants’ mean age greater than 25 years (to preclude those studies focusing on young adults or children); (2) used only one form of response (i.e. a single button for input); (3) reported activation foci in either Talairach or Montreal Neurological Institute (MNI) space; (4) published in English; (5) appeared in a peer reviewed publication; (6) used human participants; (7) used greater than six participants; (8) published between January 2002 and April 2013. Only healthy control data was used if a study included other groups.
Monetary incentive delay studies were identified by searching the PubMed database using the terms: (‘monetary’ OR ‘money’ OR ‘anticipation’) AND (‘fMRI’ OR ‘neuroimaging’); and by searching the BrainMap database (Fox et al., 2005) using the filters ‘fMRI’ and ‘reward’. In order to identify previous studies with comparable versions of the monetary incentive delay task examined here, these further criteria were used: (1) participants actually paid their winnings; (2) loss trials present; (2) more than ten gain trials; (4) reward anticipation modelled against neutral anticipation.
Go/no-go studies were identified by searching the PubMed database using the terms: (‘go/no-go’ OR ‘response inhibition’) AND (‘fMRI’ or ‘neuroimaging’); and by searching the BrainMap database using the filters ‘fMRI’ and ‘go/no-go’. In order to identify previous studies with comparable versions of the go/no-go task examined here, these further criteria were used: (1) no-go trials make up fewer than 40% of all trials; (2) not include an oddball stimuli (i.e. not include go trials with a different letter/shape/image); (3) use only letters (not images); (4) use only one no-go cue; (5) correct no-go modelled against either correct go or an implicit baseline.
For studies that reanalysed previously used data, only the original studies were used. All coordinates were transformed into MNI space as necessary. ALE was performed for each task with a False discovery rate (FDR) of p<0.05 (corrected) and a minimum cluster volume of 600 mm3 (0.6 ml).
For each task, an ROI was made up of two 5 mm radius spheres placed bilaterally such that they overlapped with the weighted centre coordinates of the strongest bilateral ALE clusters, while robustly covering grey matter.
Defining regions of interest: evocative images task
Although emotional imaging tasks have been used in many previous studies, the considerably variability in the design (especially in the specific images used within) precludes a meta-analysis using the criteria used for the other tasks here. We therefore selected the bilateral amygdala as a key region of interest, based on the previous literature with a range of emotional tasks (Phan et al., 2002). Thus, the ROI for the evocative images task was made up of two 5 mm radius spheres centred at the MNI coordinates (±22 mm, −4 mm, −12 mm) so as to be robustly in the grey matter of the amygdala as defined functionally by the clusters reported in a previously published ALE meta-analysis of amygdala responsivity (Costafreda et al., 2008).
MRI data acquisition
All centres operated MRI machines with a main magnetic field of 3 tesla (T). Centres in London and Cambridge operated nominally identical 3T Siemens Tim Trio systems running the syngo MR B17 software with a Siemens 32 channel receive-only phased-array head coil. The Manchester centre operated a 3T Philips Achieva running version 2.6.3.5 software and an eight-element SENSE head coil.
At each visit the imaging session consisted of: localizer scans to set up the positioning of those that would follow; main magnetic field mapping; one run of resting state (360 s); two runs of the monetary incentive delay task (432 s each); two runs of the go/no-go task (262 s each); and two runs of the evocative images task (392 s each).
The tasks were presented to participants in the same order in which they have been covered in this work, namely the two runs of the monetary incentive delay task, followed by the two runs of the go/no-go task, followed by the two runs of the evocative images task. This was so that performance of the monetary incentive delay task would not be adversely affected by a changed emotional state following the presentation of aversive images during the evocative images task.
For each cohort, at the first visit only, a block of structural imaging was performed at the end of the session involving: a high resolution structural scan for anatomical registration and radiological reporting; a proton density scan to provide a second contrast for radiological reporting; and a diffusion tensor imaging sequence for analysis of white matter. The resting state and diffusion tensor data will not be described further here, but will be described elsewhere. Structural images were used in spatial registration, but analysis of structural differences is not described here.
Total in-scanner time was approximately 80 minutes at the first visit, and 60 minutes at all subsequent visits. At every visit, all tasks were practiced outside of the scanner immediately prior to the start of the imaging session.
Structural acquisition
At London and Cambridge (Siemens), high-resolution T1-weighted volumes were acquired using a magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR=2300 ms, TE=2.98 ms, TI=900 ms, flip angle =9°, field of view =256 mm, image matrix =240×256) with a resolution of 1 mm isotropic. For the volume, 160 abutting straight sagittal slices were collected in an interleaved right to left manner, resulting in whole head coverage. Parallel imaging using Generalized Autocalibrating Partially Parallel Acquisition (GRAPPA) with an acceleration factor of 2 was performed.
At Manchester (Philips), high-resolution T1-weighted volumes were also acquired using an MPRAGE sequence (TR=6.8 ms, TE=3.1 ms, TI=900 ms, flip angle =9°, field of view =270 mm, image matrix =256×256) with an in-plane resolution of 1.055×1.055 mm and a slice thickness of 1.200 mm. For the volume, 126 abutting straight sagittal slices were collected in an interleaved right to left manner, resulting in whole head coverage. Parallel imaging using Sensitivity Encoding (SENSE) with an S reduction of 1.8 was performed.
These T1-weighted volumes followed ADNI protocols (Jack et al., 2008) to minimize inter-centre differences.
Functional acquisition
At London and Cambridge (Siemens), functional imaging was performed using a multi-echo gradient echo echoplanar imaging (EPI) sequence (TR=2000 ms, TE=13 ms and 31 ms, flip angle =80°, field of view =225 mm, image matrix =64×64) with an in-plane resolution of 3.516×3.516 mm and a slice thickness of 3.000 mm. The phase encoding direction was anterior to posterior. Echo spacing was 0.52 ms. Only the second echo (TE=31 ms) was used in this work.
For each volume, 36 abutting oblique axial slices were collected in an ascending manner at an angle of around 30° to the anterior (AC) and posterior commissure (PC) line. This results in slightly less than whole brain coverage, with the most superior 9 mm not being imaged in most participants.
To achieve the desired resolution and repetition time, parallel imaging using GRAPPA with an acceleration factor of 2 was performed. The first three volumes of each functional run were automatically discarded to allow for T1 saturation effects and are not included in any number of volumes reported here.
At Manchester (Philips) identical parameters were used for EPI acquisition, but with 34 slices being collected and with acceleration achieved using SENSE.
Data processing
Structural and functional processing was carried out using Analysis of Functional NeuroImages (AFNI) (version AFNI_2011_12_21_1014), FreeSurfer (version freesurfer-x86_64-unknown-linux-gnu-stable5-20130513), Advanced Normalization Tools (ANTs) (version ANTs-1.9.v4-Linux), and FMRIB Software Library’s (FSL) (version 5.0.6) FMRI Expert Analysis Tool (FEAT) (version 6.00). All were run on CentOS 6.5 (version centos-release-6-5.el6.centos.11.2.x86_64).
T1 images were first corrected for intensity non-uniformity (AFNI’s 3dUniformize) before having extracerebral tissues removed (as part of FreeSurfer’s recon-all pipeline). The whole brain images were then non-linearly registered to the MNI ICBM152 non-linear 6th generation symmetric average brain stereotaxic registration model in a 2 mm isotropic voxel space (ANTs’ antsRegistration).
EPIs were corrected for slice timing effects (AFNI’s 3dTshift) before each volume was registered (AFNI’s 3dvolreg) to the volume most similar, in the least squares sense, to all others (in-house code). For each task a summary of movement was recorded as the speed of motion over the runs (i.e. the sum of framewise displacements (FD) over the time taken for the runs, measured in mm/s).
The residual extracerebral tissues were then removed using FSL’s Brain Extraction Tool (BET). Linear registration to the T1 image was achieved through a Boundary Based Registration (BBR) approach (FSL’s epi_reg) before combining transformations to bring the EPIs into the same standard stereotaxic space as the transformed T1 (ANTs’ antsApplyTransforms). Finally, these were smoothed with a three-dimensional Gaussian kernel of full width at half maximum of 6.0 mm (i.e. standard deviation =2.5 mm) (AFNI’s 3dBlurInMask).
fMRI task modelling
Task processing and modelling was carried out using E-Prime (version 2.0.8.90), Microsoft Office Excel 2007 (version 12.0.4518.1014), in-house Python (version 2.7.6) scripts, and FSL.
Data from task responses were processed into usable formats (E-Prime’s E-DataAid) before behavioural data and timings were extracted (Excel) and processed further (Python scripts) into three-column-format text files for each event type for compatibility with FEAT.
FMRIB’s Improved Linear Modelling (FILM) prewhitening was performed on all voxel time courses. Estimates of six motion parameters (translations in the three orthogonal directions along with pitch, roll and yaw) calculated during preprocessing (AFNI’s 3dvolreg) were included in each model as confounding explanatory variables.
In all models convolution with a haemodynamic response function (HRF) was performed, this being FSL’s commonly used gamma function with standard deviation 3 s and mean lag 6 s. No temporal derivatives were used in any model. All models had the same temporal filtering applied to them as was done to the image data.
Monetary incentive delay task
Nine explanatory variables were used for modelling the task itself. These were the three different general conditions – reward, neutral or loss – with each of these having three potential phases – anticipation, successful outcome or unsuccessful outcome. ‘Anticipation’ was modelled as a block beginning at the cue (an arrow or line) onset and ending at the trial (a star) onset (these blocks lasting between approximately 3 s and 5 s – that is, the combined time of the cue and blank screen before the star). ‘Outcome’ was modelled as an immediately abutting block beginning at the trial (a star) onset and ending two seconds later. A high-pass filter cut-off of periods above 50 s was applied to both the data and the model. The contrast further explored in this work is that of ‘reward anticipation’ compared with ‘neutral anticipation’, with ‘reward anticipation’ being expected to show greater BOLD response (Knutson et al., 2001).
Go/no-go task
Two explanatory variables were used for modelling the task itself, one for ‘successful no-go’ and the other for ‘unsuccessful no-go’. These were modelled against an implicit baseline of ‘go’. Both ‘successful no-go’ and ‘unsuccessful no-go’ were modelled as events lasting 0.1 s. A high-pass filter cut-off of periods above 120 s was applied to both the data and the model. The contrast further explored in this work is that of ‘successful no-go’ compared with the implicit baseline of ‘go’, with ‘successful no-go’ being expected to show greater BOLD response (Garavan et al., 2003).
Evocative images task
Two explanatory variables were used for modelling the task itself, one for ‘aversive’ images and the other for ‘neutral’ images. Both ‘aversive’ and ‘neutral’ were modelled as blocks lasting 32.4 s. A high-pass filter cut-off of periods above 100 s was applied to both the data and the model. The contrast further explored in this work is that of ‘aversive’ compared with ‘neutral’ images, with ‘aversive’ images being expected to show greater BOLD response (Asensio et al., 2010).
Higher level analysis
FEAT was used to run all the models discussed above within a general linear model framework. As each task was run twice in each imaging session the mean of the results for both runs (at the individual level) was used in all higher level analyses.
This voxelwise analysis was extended to a group level in a mixed-effects analysis using FSL’s FLAME 1 (one-sample t-test) controlling for centre, age and sex. In calculating the whole brain group maps as part of the task characterization investigation, data from the baseline (i.e. neither a drug nor placebo) session of the 43 inter-centre participants were used (a between-centre factor was included in the model). The Z statistic images shown in this work for the evocative and go/no-go tasks were thresholded using clusters determined by Z>3.1 (i.e. an initial uncorrected cluster forming threshold of p<0.001) and a (corrected) cluster significance threshold of p<0.05. These initial cluster thresholds are higher than those commonly seen, and follow the advice given by Woo et al. (2014) relating to minimum valid thresholds. The equivalent images for the monetary incentive delay task were thresholded using clusters determined by Z>4.5 and a (corrected) cluster significance threshold of p<0.05. This initial cluster threshold was raised compared with the other tasks due to the relatively stronger response expected in comparison to the other tasks, so that clusters would still be able to form and be interpretable. This group analysis was performed on the whole brain, insofar as including all those voxels which all participants had in common (areas outside this common coverage are shown masked in figures).
For the tasks which have temporal characteristics similar to block designs (monetary incentive delay and evocation) the contrasts’ mean percentage signal changes within their ROIs were calculated (FSL’s Featquery), while for the fast event-related design (go/no-go) arbitrary units based on the parameter estimates were used, as percentage signal change is not usefully interpretable in this case.
Inter-centre differences
Non-image statistical analysis was carried out using IBM SPSS Statistics (version 22.0). When appropriate, values are given as mean±standard deviation.
Between centre differences were tested for using one-way analysis of variance (ANOVA). When significant differences were found between centres Tukey’s honestly significant difference (HSD) was used as the post-hoc test. Heterogeneity of variance was examined using Levene’s test, and if found to be significant (p<0.05) Welch’s F was used. Post-hoc testing for data not meeting the homogeneity of variance assumption was carried out using the Games Howell method. Normality of data from each centre was tested using the Shapiro–Wilk method, and, if found to be significantly (p<0.05) skewed, a non-parametric Kruskal–Wallis test performed in place of an ANOVA. Post-hoc tests for data examined using a non-parametric approach were carried out using the Mann–Whitney U test. All reported p values are those before any correction for either the number of tasks, or the number of tests carried out on the behavioural and summary imaging measures of those tasks, but they have been corrected for the number of post-hoc tests carried out for a particular measure.
FEAT was used to perform a voxelwise ANOVA, examining between-centre differences to produce F statistic images of the whole brain for each task.
Results
Participants
A summary of the groupings and participant information is given in Table 1. No differences were found between centres for age, sex, or handedness, consistent with the matching process.
Table 1.
Participant information.
| London |
Cambridge |
Manchester |
ANOVA/χ2 |
Combined |
|
|---|---|---|---|---|---|
| (n=15) | (n=15) | (n=13) | (n=15, 15, 13) | (n=43) | |
| Age (years) | 40.5±8.5 (21–53) | 37.9±9.3 (22–52) | 41.0±9.3 (25–56) | F2,40=0.50, p=0.61 | 39.7±8.9 (21–56) |
| # female | 3 | 3 | 3 | χ2(2, N=43)=0.05, p=0.97 | 9 |
| # left handed /ambidextrous | 4/1 | 4/1 | 0/2 | χ2(4, N=43)=4.61, p=0.33 | 8/4 |
Each of the three tasks – monetary incentive delay, go/no-go and evocative images – will be fully covered in turn, with each broken down into its meta-analysis/ROI definition, task characterization, and inter-centre differences.
Monetary incentive delay – ALE meta-analysis
For the monetary incentive delay task, we identified an initial total of 487 studies from searches on PubMed, and 170 from the BrainMap database, with 156 of the latter being duplicates of the former. This left a total of 501 studies. After abstract screening (501 studies) and full-text review (90 studies), 17 studies remained, representing 292 healthy participants with a total of 170 activation foci, shown in Table 2. Four clusters were found after carrying out the ALE analysis, the two largest of these being focused on the anterior region of the left and right putamen and overlapping with portions of caudate, nucleus accumbens and globus pallidus (all bilaterally). All clusters found through ALE analysis are listed in Supplementary Table 1 and are shown in Figure 1. The ROI for this task was made up of bilateral 5 mm radius spheres centred at the co-ordinates ((L–R, P–A, I–S) in MNI space) (±14 mm, 12 mm, −4 mm); that is, striatum (dorsal putamen/caudate).
Table 2.
Studies included in the monetary incentive delay ALE meta-analysis.
| Year | Author | Participants | Foci | Design | Scanner strength (T) | Whole brain analysis |
|---|---|---|---|---|---|---|
| 2003 | Knutson et al. | 12 | 10 | Knutson | 1.5 | No |
| 2004 | Knutson et al. | 8 | 8 | Knutson | 3 | Yes |
| 2006 | Juckel et al. | 10 | 9 | Knutson | 1.5 | No |
| 2007a | Wrase et al. | 14 | 18 | Knutson | 1.5 | No |
| 2007b | Wrase et al. | 16 | 2 | Knutson | 1.5 | No |
| 2008 | Knutson et al. | 12 | 8 | Knutson | 1.5 | Yes |
| 2008 | Schlagenhauf et al. | 10 | 12 | Knutson | 1.5 | No |
| 2008 | Schmack et al. | 44 | 2 | Knutson | 1.5 | No |
| 2008 | Strohle et al. | 10 | 7 | Knutson | 1.5 | No |
| 2009 | Beck et al. | 19 | 6 | Knutson | 1.5 | No |
| 2010 | Bjork et al. | 24 | 10 | Bjork | 3 | No |
| 2011 | de Greck et al. | 20 | 12 | Knutson | 1.5 | Yes |
| 2012 | Balodis et al. | 14 | 7 | Knutson | 3 | No |
| 2013 | Cho et al. | 30 | 18 | Knutson | 3 | Yes |
| 2012 | Enzi et al. | 19 | 15 | Knutson | 1.5 | Yes |
| 2013 | Edel et al. | 12 | 4 | Knutson | 1.5 | No |
| 2013 | Saji et al. | 18 | 22 | Knutson | 1.5 | Yes |
| Total | 292 | 170 |
Figure 1.
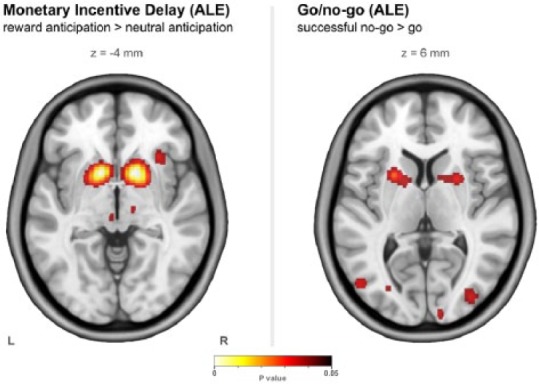
Clusters found through the activation likelihood estimation (ALE) meta-analyses. ALE was performed for each task with a false discovery rate (FDR) of p<0.05 (corrected) and a minimum cluster volume of 0.6 ml.
Monetary incentive delay – task characterization
Accuracy was not found to be different between the three types of trial (reward, neutral, and loss) (F2,126=1.33, p=0.27). Response time did differ (F2,126=4.31, p=0.015), with post-hoc analysis showing that response time of neutral trials was slower than loss trials (p=0.003) with no other differences apparent. Supplementary Table 6 lists behavioural results.
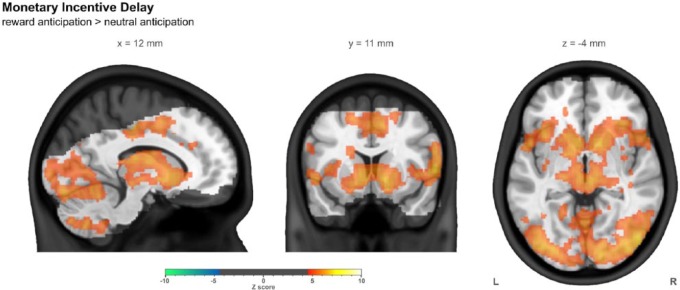
The strongest observed response to reward anticipation (in terms of Z statistics) was in the primary visual cortex, with other strong responses in the caudate and anterior insula bilaterally. A spatially widespread response was observed in other visual areas and a large group of regions incorporating the striatum, thalamus and insula, along with the supplementary motor area. No regions were seen to have a stronger response to neutral anticipation. Whole brain summary images of the reward anticipation > neutral anticipation contrast are shown in Figure 2, while more detailed images are shown in Supplementary Figure 1. Supplementary Table 3 lists the locations of clusters larger than 2 ml.
Figure 2.
The contrast of reward anticipation with neutral anticipation in the monetary incentive delay task in the combined group (n=43), controlling for centre, age and sex. Images were thresholded using clusters determined by Z>4.5 and a (corrected) cluster significance threshold of p<0.05. The slices shown were chosen such that all three intersect with the left side of the ROI used later in this work. The greyed out portion shows areas outside common coverage.
For this contrast, in the striatal ROI, the mean response (n=43) was 0.53%±0.05% with a mean Z statistic of 5.84±0.31. Supplementary Table 6 lists ROI results.
Monetary incentive delay – inter-centre differences
In the monetary incentive delay task the accuracy of loss trials was different between the centres (Kruskal–Wallis, p=0.006), with Manchester having lower accuracy than Cambridge (Mann–Whitney, p=0.007). The response time of successful loss trials was different between the centres (Kruskal–Wallis, p=0.004), with Manchester being slower than Cambridge (Mann–Whitney, p=0.012). Three of the other measures for the monetary incentive delay task – amount won, reward accuracy and neutral accuracy – had skewed distributions (Shapiro–Wilk test) and so a non-parametric (Kruskal–Wallis) test was performed (p=0.01, 0.04 and 0.03, respectively). These do not survive at the α=0.05 level after a Bonferroni correction for the number of tests performed on the behavioural measures of this task (approximately seven independent tests), but are reported here for completeness. Appropriately corrected Mann–Whitney U post-hoc tests reveal that Manchester participants won less than those in London (p=0.009), and had lower accuracy at reward trials than those in London (p=0.021).
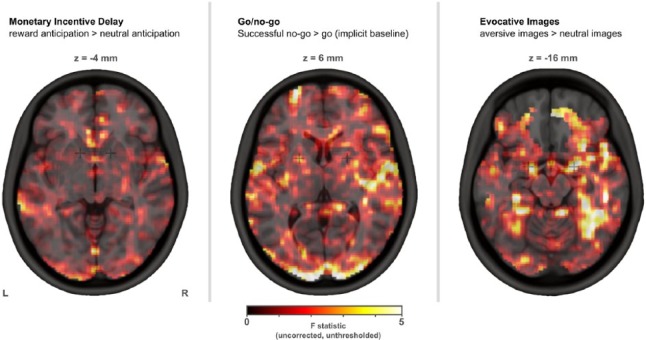
No imaging differences were found between centres at the whole brain (voxelwise) level. Unthresholded F maps are shown below in Figure 3.
Figure 3.
Unthresholded F maps exploring inter-centre differences. No significant imaging differences were found between centres at this whole brain (voxelwise) level.
No differences were found between centres with regard to the ROI results.
Go/no-go – ALE meta-analysis
For the go/no-go task, we identified an initial total of 353 studies from searches on PubMed, and 94 from the BrainMap database, with 80 of the latter being duplicates of the former. This left a total of 367 studies. After abstract screening (367 studies) and full-text review (189 studies), 12 studies remained, representing 243 healthy participants with a total of 180 activation foci, shown in Table 3. 12 clusters were found after carrying out the ALE analysis, distributed around the brain, but with a concentration around the striatum. All clusters found through ALE analysis are listed in Supplementary Table 2 and are shown in Figure 1. The ROI for this task was made up of bilateral 5 mm radius spheres centred at the co-ordinates (±22 mm, 8 mm, 6 mm); that is, striatum (dorsal putamen).
Table 3.
Studies included in the Go/no-go ALE meta-analysis.
| Year | Author | Participants | Foci | Design | Scanner strength (T) | Whole brain analysis |
|---|---|---|---|---|---|---|
| 2002 | Garavan et al. | 14 | 16 | X/Y Alternating | 1.5 | Yes |
| 2003 | Garavan et al. | 16 | 7 | X/Y Alternating | 1.5 | Yes |
| 2004 | Hester et al. | 15 | 21 | X/Y Alternating | 1.5 | Yes |
| 2004 | Kelly et al. | 15 | 23 | X/Y Alternating | 1.5 | Yes |
| 2005 | Maltby et al. | 11 | 5 | X is Go, K is No-go | 1.5 | Yes |
| 2007 | Epstein et al. | 9 | 15 | Multiple Go Cues, X is No-go | 1.5 | Yes |
| 2009 | Welander-Vatn et al. | 28 | 12 | Multiple Go Cues, V is No-go | 1.5 | Yes |
| 2012 | Bannbers et al. | 14 | 2 | X/Y Alternating | 3 | Yes |
| 2012 | Sebastian et al. | 24 | 19 | Multiple Go Cues, X is No-go | 3 | Yes |
| 2013a | Sebastian et al. | 49 | 26 | Multiple Go Cues, X is No-go | 3 | Yes |
| 2013b | Sebastian et al. | 24 | 25 | Multiple Go Cues, X is No-go | 3 | Yes |
| 2013 | van der Salm et al. | 24 | 9 | X/Y Alternating | 3 | Yes |
| Total | 243 | 180 |
Go/no-go – task characterization
Response time was found to be different between successful ‘go’ and unsuccessful ‘no-go’, with faster button presses for unsuccessful ‘no-go’ (t=6.69, p<0.0001, df=42). Supplementary Table 7 lists behavioural results.
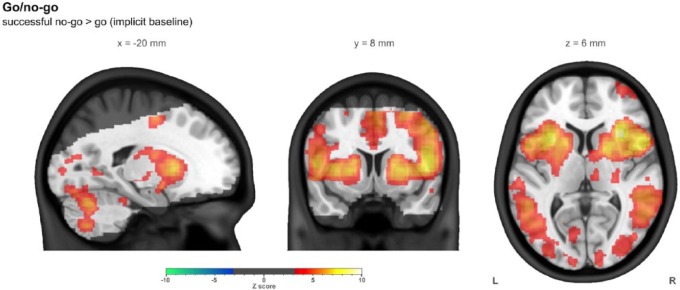
The strongest observed response to successful ‘no-go’ (in terms of Z statistics) was in the anterior insula bilaterally, with other strong responses in right inferior frontal gyrus, putamen and thalamus. A spatially widespread response was observed across the brain, including right dorsolateral prefrontal cortex and bilateral supplementary motor area. Only ventromedial prefrontal cortex was observed to have greater response to ‘go’ (implicit baseline). Whole brain summary images of the successful no-go>go (implicit baseline) contrast are shown in Figure 4, while more detailed images are shown in Supplementary Figure 2. Supplementary Table 4 lists the locations of clusters larger than 2 ml.
Figure 4.
The contrast of successful no-go with go (implicit baseline) in the go/no-go task in the combined group (n=43), controlling for centre, age and sex. Images were thresholded using clusters determined by Z>3.1 and a (corrected) cluster significance threshold of p<0.05. The slices shown were chosen such that all three intersect with the left side of the ROI used later in this work. The greyed out portion shows areas outside common coverage.
For this contrast, in the striatal ROI, the mean response (n=43) was 0.36 arbitrary units ±0.06, with a mean Z statistic of 6.27±0.47. Supplementary Table 7 lists ROI results.
Go/no-no – inter-centre differences
No behavioural differences were found between centres for the go/no-go task.
No imaging differences were found between centres at the whole brain (voxelwise) level. Unthresholded F maps are shown below in Figure 3.
No differences were found between centres with regard to the ROI results.
Evocative images – task characterization
Although the range of response times was large (280ש1373 ms for neutral images and 267–1651 for aversive images) there was a very strong correlation between the two times (r=0.94, p<0.00001, df=58). The difference in response time (21 ms) was not significant between the aversive and neutral images (t=1.86, p=0.068, df=42). Supplementary Table 8 lists behavioural results.
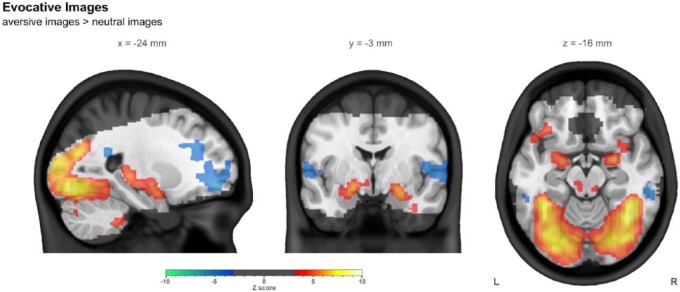
The strongest response observed to aversive images (in terms of Z statistics) was in visual cortex, with the strongest response outside of this region being in the amygdala bilaterally. Strong response was also observed in thalamus and medial hippocampus. Greater response to neutral images was observed in prefrontal and auditory cortices. Whole brain summary images of the aversive images > neutral images contrast are shown in Figure 5, while more detailed images are shown in Supplementary Figure 3. Supplementary Table 5 lists the locations of clusters larger than 2 ml.
Figure 5.
The contrast of aversive images with neutral images in the evocative images task in the combined group (n=43), controlling for centre, age, and sex. Images were thresholded using clusters determined by Z>3.1 and a (corrected) cluster significance threshold of p<0.05. The slices shown were chosen such that all three intersect with the left side of the ROI used later in this work. The greyed out portion shows areas outside common coverage.
For this contrast, in the amygdala ROI, the mean response (n=43) was 0.32%±0.09%, with a mean Z statistic of 3.90±1.06. Supplementary Table 8 lists ROI results.
Rate of motion (i.e. mm/s) was not found to differ significantly for different tasks (F2,126=2.61, p=0.08).
Evocative images – inter-centre differences
No behavioural differences were found between centres for the evocative images task.
No imaging differences were found between centres at the whole brain (voxelwise) level. Unthresholded F maps are shown in Figure 3.
In the evocative images task, mean response (percentage signal change) within the amygdala ROI was found to differ between the centres (F2,40=5.06, p=0.01), along with, as one would expect given the signal change, the mean Z statistic in this region (F2,40=5.98, p=0.005). This was due to the Manchester participants having a lower response for this aversive images > neutral images contrast.
Discussion
We report here the establishment of an fMRI platform, ICCAM, to study mechanisms of relevance to relapse in addiction. Across three tasks investigating reward sensitivity, inhibitory control and emotional reactivity, we have examined their characteristics, and inter-centre differences for behavioural, whole brain and ROI measures. This study raised a number of issues, which we now discuss in turn.
Importantly our three tasks resulted in the expected pattern of brain responses consistent with existing evidence. Thus the monetary incentive delay task resulted in responses in regions such as the visual cortex, striatum, prefrontal and insula cortices consistent with previous studies (Knutson et al., 2001). The influence of variations in the task on the patterns of brain responses have been described elsewhere (Hommer et al., 2011; Limbrick-Oldfield et al., 2013). Though many people use the monetary incentive delay task, most adapt it to some extent so that it is no longer a standardized task. For instance, the ICCAM version of the monetary incentive delay task prioritized imaging ‘anticipation of reward’ since this primary contrast has been found altered in addiction and is of relevance to relapse. Therefore we were less interested in brain responses to loss or outcomes.
The pattern of brain response elicited by our monetary incentive delay task was consistent with that derived from the meta-analysis. Many fMRI studies of the monetary incentive delay task used spatially constrained approaches –that is, analyses performed within ROIs of varying size, focused on striatal regions. Out of the 17 studies used here, 11 were not ‘whole brain’ analyses. Indeed, in the original fMRI monetary incentive delay study (Knutson et al., 2001), a limited acquisition of coronal slices was used, limiting coverage to a block including the striatum, and our own coverage is itself limited, as can be seen throughout the figures (such as Figure 2). By comparison, of the 12 go/no-go studies included in the meta-analysis, none used such a spatially constrained approach.
The responses to the go/no-go task in inferior frontal gyrus, striatum, insula and thalamus were consistent with previous studies (Luijten et al., 2014; Steele et al., 2013). Our meta-analysis of similar go/no-go tasks resulted in a striatal ROI, though this was more dorsal than the one derived from the meta-analysis of the monetary incentive delay task. This association between ventral striatum associated with reward processing and dorsal striatum with habit or compulsive behaviours, and the importance of fronto-corticostriatal loops in inhibitory control, have been well documented (Everitt and Robbins, 2005; Koob and Volkow, 2010).
Although there is often a focus on the inferior frontal gyrus (IFG) when discussing go/no-go tasks, this did not emerge in our ALE meta-analysis (which closely follow the results presented in the task characterization here). IFG response was observed in our task, though was weaker than insular or striatal responses. This might be explained by the ‘simple’ design task used here (and thus the strict criteria in our meta-analysis), while the majority of those in the literature used more complex designs (Criaud and Boulinguez, 2013). In the extensive ALE meta-analysis performed by Criaud and Boulinguez (2013) examining several facets of fMRI go/no-go tasks, it is also suggested that typical no-go activity is mostly driven by attention, not inhibition, though this is still a current topic of debate (Aron et al., 2014).
Both the go/no-go and monetary incentive delay task resulted in robust responses in the insula, particularly anterior insula. This brain region has been shown to be involved in self-regulation and reward seeking, as well as in emotional awareness, through integrating sensory information into cognitive, affective and physiological processes, along with being part of a task general network (Gu et al., 2013; Menon and Uddin, 2010; Nelson et al., 2010). With regard to addiction, the insula appears also to be involved with critical functions such as craving, and the landmark description that damage to its structure substantially increased the likelihood of smoking cessation (Garavan, 2010; Naqvi and Bechara, 2009).
The evocative images task produced a robust response in the dorsal amygdala, along with inferior portions of the globus pallidus, with the highest response near the amygdala overlapping with the predetermined amygdala ROI. Such a pattern is consistent with previous studies using an evocative task or one that requires emotional processing (Costafreda et al., 2008; Sergerie et al., 2008). We were particularly interested in demonstrating a robust response in the amygdala, since dysregulation in this region is implicated in relapse vulnerability in addiction, in particular those involving stress (Koob et al., 2014).
Even though this comparison between centres did not utilize a travelling participants design, such as those of Friedman et al. (2008), Gee et al. (2015) and Suckling et al. (2012), it demonstrates that different groups of participants at different centres produce markedly similar patterns of response to the tasks in our ICCAM platform. Recent explorations of both functional and structural neuroimages acquired from multiple centres have unequivocally demonstrated high levels of within- and between-centre reliability, as well as small between-centre variances relative to the total variance (Gee et al., 2015; Suckling et al., 2012).
However, the lack of significant whole brain differences in the participants examined here does not necessarily imply that with larger groupings and different patient populations there would not be differences observed.
Although much effort was made to run the study in as similar a manner possible at each centre, there were inevitably slight differences between the experimental set-ups which may have driven differences. In the monetary incentive delay task a lower accuracy led to slightly less money being won by the Manchester group, though brain response wasn’t observed to be different. In the evocative images task a centre difference was found in the amygdala ROI (Supplementary Table 8) and was driven by the Manchester group (at the whole brain level, no significant differences were observed). One factor may have been the means by which images were projected, which was almost identical in London and Cambridge but differed in Manchester, where images were projected in a different manner, creating a less bright and so possibly less salient image, creating a smaller difference in activity between the aversive and neutral images.
Although in this analysis we have explored differences between centres, in the patient study itself participants were recruited so that there would be a roughly equal proportion of cases to controls at each centre. Centre was used as a covariate in the characterization of the tasks. Thus, although small differences were observed in the monetary incentive delay task behavioural results, and the evocative images task neuroimaging results, these may be regarded as effects of centre (which is used as a covariate in all analyses).
Conclusion
We have demonstrated here the establishment of an fMRI platform involving three different tasks, repeated at multiple sessions and at three different centres. The establishment of this platform was critical to provide a framework to explore three key processes in the neurobiology of relapse vulnerability in addiction: reward, inhibitory control and emotional regulation. This allows for an evidence base to inform future development in treatment to be provided within reasonable time periods. Future papers will present the results of these tasks in our healthy and patient groups, and under pharmacological modulation.
Supplementary Material
Acknowledgments
We wish to thank all the participants who took part in this study.
We wish to thank research assistants Claire Whitelock, Heather Agyepong, Rania Christoforou and Natalie Cuzen for their help with data collection, and MR technician Jonathan Howard for his assistance with MR acquisition and task set-up.
We wish to thank our recruitment partners: Imperial College Healthcare NHS Trust, Central and North West London NHS trust, Camden and Islington NHS trust, Cambridge University Hospitals NHS Foundation Trust, Norfolk and Suffolk NHS Foundation Trust, Cambridge and Peterborough NHS Foundation Trust, South Staffordshire and Shropshire NHS Foundation Trust, Manchester Mental Health NHS and Social Care Trust, Greater Manchester West NHS Foundation Trust, Pennine Care NHS Foundation Trust, Salford Royal NHS Foundation Trust, Addaction, Foundation 66 and CRI (Crime Reduction Initiative).
The research was carried out at the NIHR/Wellcome Trust Imperial Clinical Research Facility, the NIHR/Wellcome Trust Cambridge Research Facility and Clinical Trials Unit at Salford Royal NHS Foundation Trust, and is supported by the North West London, Eastern and Greater Manchester NIHR Clinical Research Networks. The views expressed are those of the author(s) and not necessarily those of the Medical Research Council, the NHS, the NIHR or the Department of Health.
Footnotes
ICCAM platform collaborators: David Nutt, Anne Lingford-Hughes, Louise Paterson, John McGonigle, Remy Flechais, Csaba Orban, Bill Deakin, Rebecca Elliott, Anna Murphy, Eleanor Taylor, Trevor Robbins, Karen Ersche, John Suckling, Dana Smith, Laurence Reed, Filippo Passetti, Luca Faravelli, David Erritzoe, Inge Mick, Nicola Kalk, Adam Waldman, Liam Nestor, Shankar Kuchibatla, Venkataramana Boyapati, Antonio Metastasio, Yetunde Faluyi, Emilio Fernandez-Egea, Sanja Abbott, Barbara Sahakian, Valerie Voon, Ilan Rabiner
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Liam J Nestor was a Senior Research Scientist employed by GlaxoSmithKline during this work.
Trevor W Robbins has research grants with Eli Lilly and Company and Lundbeck, has received royalties from Cambridge Cognition, has received editorial honoraria from Springer Verlag, Elsevier, Society for Neuroscience; has performed educational lectures for Merck Sharp & Dohme, and performs consultancy work for Cambridge Cognition, Eli Lilly and Company, Lundbeck, Teva Pharmaceutical Industries and Shire Pharmaceuticals.
JF William Deakin currently advises or carries out research funded by Autifony Therapeutics, Sunovion Pharmaceuticals, Lundbeck, AstraZeneca and Servier. All payment is to the The University of Manchester.
David J Nutt is an advisor to British National Formulary, Medical Research Council, General Medical Council, and Department of Health (UK), is President of the European Brain Council, past President of the British Neuroscience Association and European College of Neuropsychopharmacology, chair of the Independent Scientific Committee on Drugs (UK), is a member of the International Centre for Science in Drug Policy, advisor to Swedish government on drug, alcohol and tobacco research, editor of the Journal of Psychopharmacology, sits on advisory Boards at Lundbeck, Merck Sharp & Dohme, Nalpharm, Orexigen Therapeutics, Shire Pharmaceuticals, has received speaking honoraria (in addition to above) from Bristol-Myers Squibb/Otsuka, GlaxoSmithKline, Eli Lilly and Company, Janssen, Servier, is a member of the Lundbeck International Neuroscience Foundation, has received grants or clinical trial payments from P1vital, Medical Research Council, National Health Service, Lundbeck, has share options with P1vital, has been expert witness in a number of legal cases relating to psychotropic drugs and has edited/written 27 books – some purchased by pharmaceutical companies.
Anne R Lingford-Hughes has received honoraria from Lundbeck and research support from GlaxoSmithKline for a PhD studentship.
All other authors declared no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article presents independent research funded by the Medical Research Council as part of their addiction initiative (grant number G1000018).
GlaxoSmithKline kindly funded the functional and structural MRI scans that took place at the London centre (Imanova Limited/Imperial College London) and provided the GSK598809 and vofopitant medication in the main study, of which the data presented here makes up only a subset.
References
- Aron AR, Robbins TW, Poldrack RA. (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18: 177–185. [DOI] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, et al. (2010) Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol 15: 504–516. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, et al. (2009) Ventral Striatal Activation During Reward Anticipation Correlates with Impulsivity in Alcoholics. Biol Psychiatry 66: 734–742. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. (2013) The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76: 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, et al. (2012) Diminished Frontostriatal Activity During Processing of Monetary Rewards and Losses in Pathological Gambling. Biol Psychiatry 71: 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SM, Connor JT, Lewis RJ. (2015) The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 313: 1619–1620. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, et al. (2010) Adolescents, Adults and Rewards: Comparing Motivational Neurocircuitry Recruitment Using fMRI. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante JC, Barros-Loscertales A, Costumero V, et al. (2014) Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict Biol 19: 885–894. [DOI] [PubMed] [Google Scholar]
- Bannbers E, Gingnell M, Engman J, et al. (2012) The effect of premenstrual dysphoric disorder and menstrual cycle phase on brain activity during response inhibition. Journal of Affective Disorders 142: 347–350. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, et al. (2012) The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend 121: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, et al. (2008) Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58: 57–70. [DOI] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, et al. (2013) Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage 66: 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P. (2013) Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev 37: 11–23. [DOI] [PubMed] [Google Scholar]
- de Greck M, Scheidt L, Bolter AF, et al. (2011) Multimodal psychodynamic psychotherapy induces normalization of reward related activity in somatoform disorder. World Journal of Biological Psychiatry 12: 296–308. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, et al. (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, et al. (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30: 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi B, Edel MA, Lissek S, et al. (2012) Altered ventral striatal activation during reward and punishment processing in premanifest Huntington’s disease: A functional magnetic resonance study. Experimental Neurology 235: 256–264. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, et al. (2007) ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. Journal of Child Psychology and Psychiatry 48: 899–913. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Edel MA, Enzi B, Witthaus H, et al. (2013) Differential reward processing in subtypes of adult attention deficit hyperactivity disorder. Journal of Psychiatric Research 47: 350–356. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Morgan M, et al. (2012) Dopaminergic genes modulate response inhibition in alcohol abusing adults. Addict Biol 17: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, et al. (2004) Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiat 55: 531–537. [DOI] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, et al. (2005) BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Map 25: 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Stern H, Brown GG, et al. (2008) Test-retest and between-site reliability in a multicenter fMRI study. Hum Brain Map 29: 958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. (2009) Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiat Neurosci 34: 418–432. [PMC free article] [PubMed] [Google Scholar]
- Garavan H. (2010) Insula and drug cravings. Brain Struct Funct 214: 593–601. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, et al. (2003) A midline dissociation between error-processing and response-conflict monitoring. Neuroimage 20: 1132–1139. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, et al. (2002) Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage 17: 1820–1829. [DOI] [PubMed] [Google Scholar]
- Gee DG, McEwen SC, Forsyth JK, et al. (2015) Reliability of an fMRI paradigm for emotional processing in a multisite longitudinal study. Hum Brain Map 36: 2558–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, et al. (2008) Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319: 1536–1539. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. (2008) Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol 13: 423–434. [DOI] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, et al. (2013) Anterior insular cortex and emotional awareness. J Comp Neurol 521: 3371–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Luckenbaugh DA, Snow J, et al. (2009) Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biol Psychiatry 66: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. (2004) Individual differences in error processing: A review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex 14: 986–994. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Bjork JM, Gilman JM. (2011) Imaging brain response to reward in addictive disorders. Ann NY Acad Sci 1216: 50–61. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, et al. (2008) The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imag 27: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, et al. (2011) An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiat 70: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, et al. (2006) Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 187: 222–228. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, et al. (2003) Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci 23: 7839–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, et al. (2004) Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience 19: 3105–3112. [DOI] [PubMed] [Google Scholar]
- Keuken MC, Muller-Axt C, Langner R, et al. (2014) Brain networks of perceptual decision-making: an fMRI ALE meta-analysis. Front Hum Neurosci 8: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, et al. (2001) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, et al. (2003) A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18: 263–272. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bjork JM, Fong GW, et al. (2004) Amphetamine modulates human incentive processing. Neuron 43: 261–269. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. (2008) Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B-Biological Sciences 363: 3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, et al. (2014) Addiction as a stress surfeit disorder. Neuropharmacology 76: 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND.(2010) Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R. (2008) Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev 32: 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbrick-Oldfield EH, van Holst RJ, Clark L. (2013) Fronto-striatal dysregulation in drug addiction and pathological gambling: consistent inconsistencies? Neuroimage Clin 2: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Welch S, Peters L, et al. (2012) BAP updated guidelines: evidence-based guidelines for the pharmacological management of substance abuse, harmful use, addiction and comorbidity: recommendations from BAP. J Psychopharmacol 26: 899–952. [DOI] [PubMed] [Google Scholar]
- Loree AM, Lundahl LH, Ledgerwood DM. (2014) Impulsivity as a predictor of treatment outcome in substance use disorders: review and synthesis. Drug Alcohol Rev 34: 119–134. [DOI] [PubMed] [Google Scholar]
- Luijten M, Machielsen MW, Veltman DJ, et al. (2014) Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci 39: 149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, et al. (2005) Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage 24: 495–503. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. (2009) The hidden island of addiction: the insula. Trends Neurosci 32: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, et al. (2010) Role of the anterior insula in task-level control and focal attention. Brain Struct Funct 214: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A. (2013) A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol 23: 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson LM, Flechais RS, Murphy A, et al. (2015) The Imperial College Cambridge Manchester (ICCAM) platform study: an experimental medicine platform for evaluating new drugs for relapse prevention in addiction. Part A: study description. J Psychopharmacol 29: 943–960. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, et al. (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Saji K, Ikeda Y, Kim W, et al. (2013) Acute NK1 receptor antagonist administration affects reward incentive anticipation processing in healthy volunteers. International Journal of Neuropsychopharmacology 16: 1461–1471. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, et al. (2008) Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 196: 673–684. [DOI] [PubMed] [Google Scholar]
- Schouw ML, De Ruiter MB, Kaag AM, et al. (2013) Dopaminergic dysfunction in abstinent dexamphetamine users: results from a pharmacological fMRI study using a reward anticipation task and a methylphenidate challenge. Drug Alcohol Depend 130: 52–60. [DOI] [PubMed] [Google Scholar]
- Schmack K, Schlagenhauf F, Sterzer P, et al. (2008) Catechol-O-methyltransferase Val(158)met genotype influences neural processing of reward anticipation. Neuroimage 42: 1631–1638. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. (2008) The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32: 811–830. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Gerdes B, Feige B, et al. (2012) Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Research-Neuroimaging 202: 132–141. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Baldermann C, Feige B, et al. (2013a) Differential effects of age on subcomponents of response inhibition. Neurobiology of Aging 34: 2183–2193. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Pohl MF, Kloppel S, et al. (2013b) Disentangling common and specific neural subprocesses of response inhibition. Neuroimage 64: 601–615. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. (2008) Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. (2007) Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev 26: 25–31. [DOI] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Wrase J, et al. (2008) Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 39: 966–972. [DOI] [PubMed] [Google Scholar]
- Steele VR, Aharoni E, Munro GE, et al. (2013) A large scale (N=102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav Brain Res 256: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling J, Barnes A, Job D, et al. (2012) The Neuro/PsyGRID calibration experiment: identifying sources of variance and bias in multicenter MRI studies. Hum Brain Map 33: 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, et al. (2002) Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, et al. (2012) Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Map 33: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Salm SMA, van der Meer JN, Nederveen AJ, et al. (2013) Functional MRI study of response inhibition in myoclonus dystonia. Experimental Neurology 247: 623–629. [DOI] [PubMed] [Google Scholar]
- Welander-Vatn AS, Jensen J, Lycke C, et al. (2009) No altered dorsal anterior cingulate activation in bipolar II disorder patients during a Go/No-go task: an fMRI study. Bipolar Disorders 11: 270–279. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, et al. (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. (2014) Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schiagenhauf F, et al. (2007a) Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage 36: 1253–1262. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, et al. (2007b) Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35: 787–794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.