Abstract
Rotator cuff lesions (RCL) have considerable variability in location, tear pattern, functional impairment, and repairability.
Historical classifications for differentiating these lesions have been based upon factors such as the size and shape of the tear, and the degree of atrophy and fatty infiltration. Additional recent descriptions include bipolar rotator cuff insufficiency, ‘Fosbury flop tears’, and musculotendinous lesions.
Recommended treatment is based on the location of the lesion, patient factors and associated pathology, and often includes personal experience and data from case series. Development of a more comprehensive classification which integrates historical and newer descriptions of RCLs may help to guide treatment further.
Cite this article: Lädermann A, Burkhart SS, Hoffmeyer P, et al. Classification of full thickness rotator cuff lesions: a review. EFORT Open Rev 2016;1:420-430. DOI: 10.1302/2058-5241.1.160005.
Keywords: rotator cuff lesion, repair, tear pattern, classification, massive rotator cuff tear, repairable and non-repairable, shoulder imaging
Introduction
A clear consensus on the classification of rotator cuff lesions (RCLs) does not yet exist. A valuable classification system would be reproducible, encourage communication among surgeons and would provide more precise information regarding treatments and outcomes. As new types of RCLs have recently been identified, some of the previous definitions and treatment options are no longer valid. Advances in imaging and the advent of arthroscopy in particular have provided opportunities to better understand RCLs, and led to additions to historical descriptors. RCLs can be treated with a wide variety of approaches including non-operative management, open or arthroscopic repair and shoulder arthroplasty. Given this variability in management options, precise and comprehensive classifications are not only mandatory for deciding which treatment is the best for patients but also for comparing published outcomes.
This paper provides a comprehensive review of current classification systems for RCLs, and describes our method for the comprehension and treatment of full-thickness lesions based on isolated or combined involvement of the bone, tendon, musculotendinous junction, or muscle.
Definition and classification
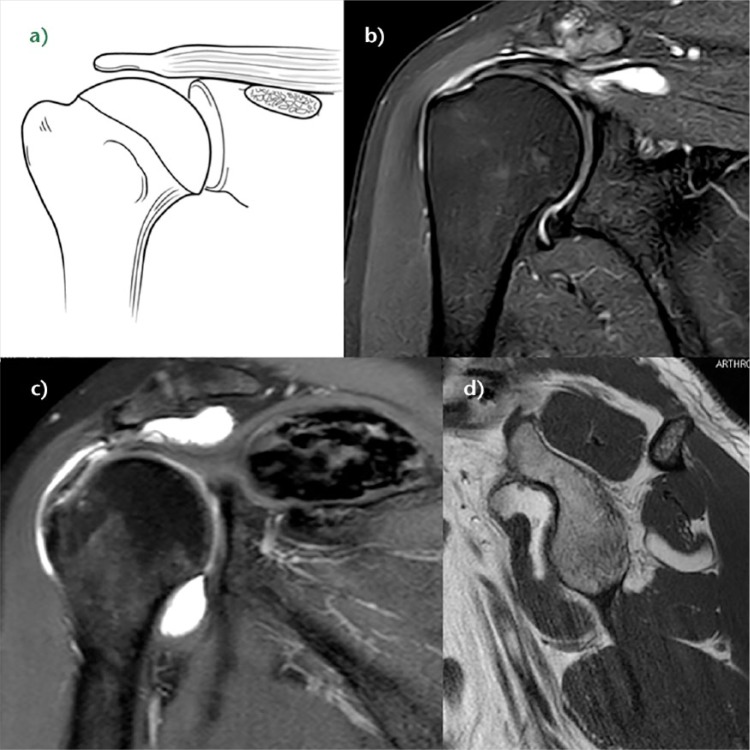
All full-thickness or equivalent RCLs treated with surgery by one of the authors (AL) between April 2012 and December 2015 were reviewed. A total of 368 RCLs were treated during this period. The mean age was 56 years at the time of surgery (22 to 83). There were 172 women and 196 men. The RCLs were categorised into three major groups based on involvement of the bone (type A), tendon (type B) or musculotendinous junction (type C). In addition, muscle insufficiency (type D) was noted when present (Fig. 1). The tear pattern distribution is summarised in Table 1.
Fig. 1.
Classification of the rotator cuff, representing a) the bone, b) the tendon, c) the musculotendinous junction and d) the muscle, respectively.
Table 1.
Prevalence of full-thickness rotator cuff lesions (RCLs) or equivalent, operated for an open or arthroscopic rotator cuff repair
| Type | RCL | Prevalence of full-thickness RCLs on a series of 368 (%)* |
|---|---|---|
| Bony rotator cuff A1 A2 A3 |
Acute bony involvement Tuberosity malunion/nonunion Tuberosity insufficiency |
27 (7.3%) Greater tuberosity 12 (3.2%) Lesser tuberosity 4 (1.1%) 5 (1.3%) 6 (1.6%) |
| Full-thickness tendon lesion B1 B2 B3 B4 |
Avulsion of tendinous attachments Midsubstance tear Fosbury flop tear Bony adhesions |
334 (90.7%) Posterosuperior cuff 287 (85.4%) Subscapularis 32 (8.7%) 7 (1.9%) 6 (1.6%) 2 (0.5%) |
| Musculotendinous junction lesion C C |
Infraspinatus Supraspinatus |
4 (1.1%) 3 (0.8%) 1 (0.2%) |
| Muscle insufficiency D1 D2 D3 |
Fatty infiltration > stage 2, muscle atrophy Neurological impairment Tumour, masses |
49 (13.3%) 34 (9.2%) 1 (0.2%) 3 (0.8%) |
Multiple diagnosis possible
Type A: bony involvement
While the majority of RCLs involve the tendinous insertion, bony involvement is an important consideration in a comprehensive classification. Bony involvement includes acute fractures, malunion/nonunion, and chronic bony insufficiency.
A1: acute bony involvement (fractures and avulsions)
Isolated greater tuberosity fractures are considered uncommon, representing less than 5% of all operatively treated proximal humeral fractures.1 Isolated lesser tuberosity fractures are generally considered rare. In our practice, type A lesions of the greater or lesser tuberosity represent approximately 3.2% and 1.1%, respectively, of surgically treated RCLs (see Table 1). Tuberosity fractures are included in the accepted classification for proximal humeral fractures by Neer,2 in itself a modification of Codman’s original description. As the greater and lesser tuberosity form the insertion site of the rotator cuff, even small tuberosity fractures or avulsions can represent substantial disruption of the rotator cuff and lead to functional impairment if displaced and left untreated. Historically, Neer proposed 10 mm of displacement as a threshold for operative intervention.2 However, more recent investigation has recommended that a threshold of 5 mm should be used.3 Displacement of greater than 5 mm can lead to bony impingement with loss of range of motion as well as loss of strength from compromise in the normal length-tension relationship of the rotator cuff.4 A traumatic mechanism is a typical cause of this injury, such as violent muscular contraction, impaction of the greater tuberosity beneath the acromion or shearing against the anterior glenoid rim during a glenohumeral dislocation. Thorough patient evaluation is required to make an appropriate treatment recommendation. Conservative therapy is limited to non- or minimally displaced fractures. The ongoing development of arthroscopic techniques has led to multiple reports of arthroscopically-assisted or total arthroscopic techniques in the treatment of these injuries.5
A2: tuberosity malunion/nonunion
Tuberosity malunion or nonunion can be a sequel of either conservative or surgical treatment of acute injuries (Fig. 2). As noted previously, displacement effectively shortens the muscle—tendon unit such that the rotator cuff cannot function properly (Fig. 2). Various open techniques have been described for the management of the malunion of proximal humeral fractures, including prosthetic reconstruction, open corrective osteotomy6 or arthroscopic capsular release followed by takedown of the rotator cuff from the malunited proximal humerus, tuberoplasty and rotator cuff advancement.4 Although the latter technique is technically demanding, it allows preservation of the native humeral head which is associated with a low complication rate, and avoids concerns about long-term prosthetic survival in young patients (Fig. 2).
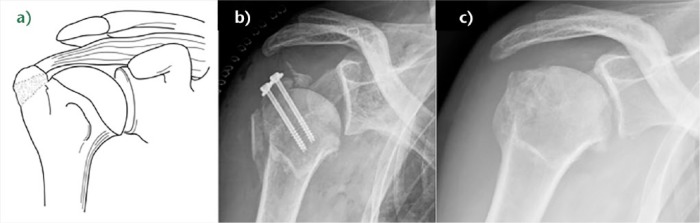
Fig. 2.
a) Schema of right greater tuberosity malunion resulting in dysfunction of the rotator cuff because the length-tension relationship is altered. b) Post-operative anteroposterior radiograph of a right shoulder showing proximal humeral screw osteosynthesis of an unreduced greater tuberosity (A2 RCL (rotator cuff lesion)) and an inferior subluxation due to operative nerve block. c) Post-operative anteroposterior radiograph of the same patient after revision surgery that included arthroscopic hardware removal, arthrolysis, rotator cuff detachment, tuberoplasty with a burr, and repair with restoration of the normal length-tension relationship of the rotator cuff. Lateral acromioplasty should have been added.
A3: tuberosity insufficiency
Tuberosity insufficiency can range from contained cystic bony defects within the tuberosity to the absence of the entire tuberosity. Cystic bony defects are often encountered during primary or revision rotator cuff repair. Such defects may be idiopathic, related to the patient’s rotator cuff disease, or secondary to osteolysis from breakdown of bioresorbable anchors. These osseous defects reduce biological healing capacity and may decrease repair fixation strength. Bone grafting techniques are needed to address these defects.7
We have also observed occasional complete resorption of the tuberosities (Fig. 3). In such a situation, a simple tendon rotator repair is usually unsuccessful, as a large bony defect significantly lowers the prognosis for primary repair.8 Therefore, reconstruction of this combined bony and tendon defect may require both tendinous and bony reconstruction. In older patients, such insufficiency is most reliably addressed with reverse shoulder arthroplasty (RSA). However, RSA is not ideal for young patients as multiple studies have demonstrated increased complications in this patient population.9,10 Recently, a fresh frozen bony-tendinous allograft of the calcaneum and Achilles tendon has been used effectively to address this difficult problem (Video 1).11
Fig. 3.
a) Schema of an A3B1 rotator cuff lesion (RCL) (combined bony and tendinous insufficiency). b) Example of a 46-year-old man who sustained a left fracture of the greater tuberosity treated by plating. Plate removal revealed massive humeral head bone loss. A tentative repair had been unsuccessful with persistent pain and pseudoparalysis. c) A fresh frozen allograft of calcaneum and Achilles tendon was used to compensate for this deficiency.
Type B: full thickness tendon lesion
B1: lateral tendinous disruption
Full thickness disruption of the lateral tendon stump is the most frequent type of RCL, comprising approximately 90.1% of all surgically treated lesions (Table 1). Tendinous lesions most commonly involve the posterosuperior cuff. Subscapularis tears are nevertheless found in 59% of arthroscopic rotator cuff repairs.12 However, such tears are only full-thickness in 8.7% of cases, and are rarely isolated.
Size of tendon lesion
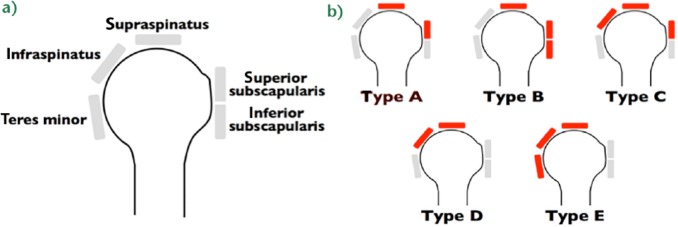
Classifications for tear size include measurement in centimetres13,14 or number of tendons involved.15-17 We believe that size classification should take into account three-dimensional information, as proposed by Davidson and Burkhart14 and Lädermann et al.17 This information can be derived from arthroscopy or magnetic resonance imaging and is used to offer guidance on treatment and prognosis. Once size is identified, it can be further classified according to Collin et al.18 In this classification, the rotator cuff is divided into five components: supraspinatus; superior subscapularis; inferior subscapularis; infraspinatus; and teres minor (Fig. 4a). Rotator cuff tear patterns can then be classified into five types: type A, supraspinatus and superior subscapularis tears; type B, supraspinatus and entire subscapularis tears; type C, supraspinatus, superior subscapularis, and infraspinatus tears; type D, supraspinatus and infraspinatus tears and type E, supraspinatus, infraspinatus, and teres minor tears (Fig. 4b).18 This classification not only subclassifies massive tears but has also been linked to function, particularly the maintenance of active elevation,18 giving more information than the traditional six sagittal segments of Patte’s classification.19
Fig. 4.
a) In the Collin et al classification, the rotator cuff is divided into five components: supraspinatus; superior subscapularis; inferior subscapularis; infraspinatus; and teres minor. b) Rotator cuff tears classified by the involved components: type A, supraspinatus and superior subscapularis tears; type B, supraspinatus and entire subscapularis tears; type C, supraspinatus, superior subscapularis, and infraspinatus tears; type D, supraspinatus and infraspinatus tears; and type E, supraspinatus, infraspinatus, and teres minor tears (reprinted with permission from Elsevier from Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195–1202).
Tendon retraction
Patte devised a method of classifying tendon coronal retraction19 that is often used for research purposes. The retraction is due to tendon and muscle shortening that are not synchronous after tendon tear.20 Substance loss in the later stages of musculotendinous retraction may be because of active shortening of the tendon substance, suggesting that over-reduction and lateral transposition of the tendon over the greater tuberosity may be unphysiological.
Tear pattern
Full-thickness posterosuperior tears come in a variety of patterns. The most common categories include crescent tears, L- and reverse L-shaped tears, and U-shaped tears accounting respectively for 40%, 30% and 15% of posterosuperior RCLs.14 Recognition of these tear patterns is most useful for anatomical restoration during repair. Crescent tears have good medial-to-lateral mobility and are amenable to a double-row repair. Longitudinal tears (L- and reverse L-shaped tears; U-shaped tears) have greater mobility in one plane and typically require margin convergence to achieve complete repair. Finally, massive contracted tears have also been described. These tears have limited medial-to-lateral and anterior-to-posterior mobility and typically require advanced mobilisation techniques (i.e. interval slides) to achieve repair.
Full-thickness subscapularis tears are classically described as types II to V according to Lafosse et al.21 A type II is a full thickness tear of the superior one-third of the tendon. A type III tear includes the superior two-thirds. Type II and III tears correspond to the upper subscapularis of Collin et al.18 Type IV tears involve the entire subscapularis and type V tears are type IV tears with > grade III fatty degeneration; these lesions are equivalent to the subscapularis minor described by Collin et al (Fig. 4).22 Subscapularis tendon tears can be successfully repaired arthroscopically23 and the improvement in functional outcome is durable in the long term.24
B2: medial tendinous disruption
Disruption of the tendon medial to an intact lateral tendon stump has been reported in primary chronic and acute cases25,26 or post-operatively as a failure medial to the medial row.27 Regarding the former, it is now understood that the infraspinatus insertion is quite broad and wraps around posterior to anterior to occupy much of the lateral greater tuberosity. Therefore, such descriptions of a lateral tendon stump remaining may in fact represent a torn supraspinatus with an intact infraspinatus.
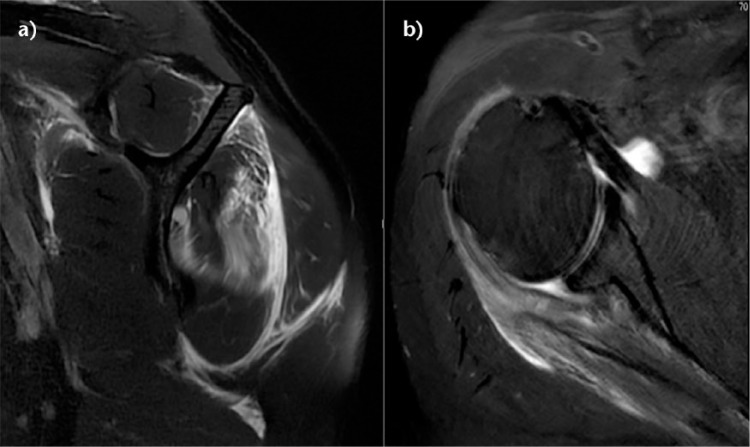
Full thickness defects medial to an intact footprint of the rotator cuff can be seen following a rotator cuff repair (Fig. 5). Trantalis et al described five patients with medial failure following a double-row rotator cuff repair.27 Such failure results from over-tensioning during repair and is very difficult to manage with revision repair. These lesions do not produce muscular oedema, except in traumatic cases with important and acute retraction of the muscle and the remnant of the tendon (Fig. 6). Its origin is then either retraction, which may appear after some hours, or neurological lesions, which are noted after some weeks.28
Fig. 5.
a) Schema of a B2 rotator cuff lesion (RCL) (full-thickness defects medial to an intact footprint of the rotator cuff). b) Coronal T2-weighted FATSAT MRI image after a right arthroscopic double-row rotator cuff repair showing an intact rotator cuff footprint but full-thickness defect in the rotator cuff medial to an intact rotator cuff footprint.
Fig. 6.
Sagittal and axial T2-weighted FATSAT MRI images demonstrating oedema of the infraspinatus. During surgery, a tendon stump confirming a B2 lesion was found and allowed a side-to-side repair.
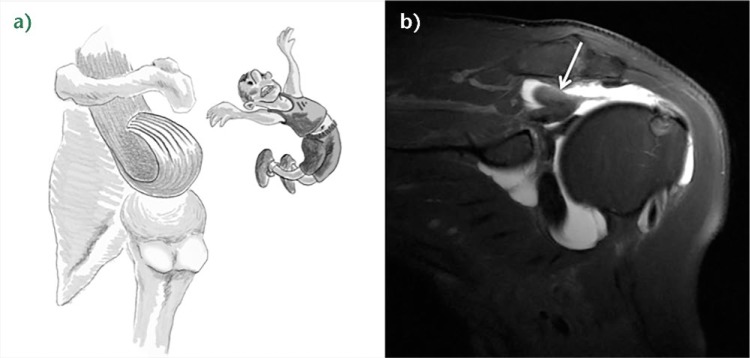
B3: tendon-to-tendon adhesion: ‘Fosbury flop tear’
The Fosbury flop tear is a newly–described lesion which occurs from a full-thickness tear that has flipped upon itself and adhered medially (Fig. 7). In a prospective study of 97 patients spanning one year, Lädermann, Denard and Kolo29 reported five patients with full- or partial-thickness RCLs (a 5% incidence rate). Radiologically, these lesions showed a thicker than normal tendon stump on the bursal side of the retracted supraspinatus tendon, in a superomedial orientation. Additionally, patients with this lesion were also found to have an accumulation of fluid in the superomedial part of the subacromial bursa as well as adhesions between the wall of the subacromial bursa and the tendon of the supraspinatus. All cases were successfully repaired arthroscopically (see Video 2).29 Since the original description, another group verified the same entity.30
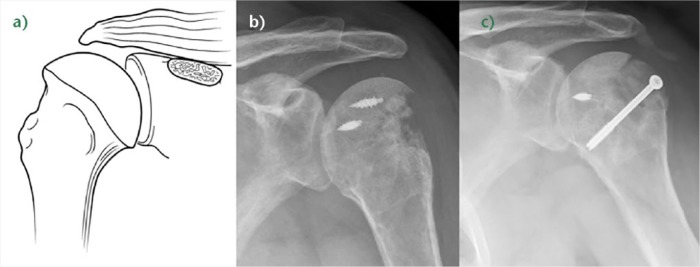
Fig. 7.
a) Illustration of a right B3 rotator cuff lesion (RCL) (‘Fosbury flop tear’). b) Coronal T2-weighted FATSAT MRI image of the right shoulder. Adherences between the bursal tendon side and the wall of the subacromial bursa, fluid in the subacromial bursa, and abnormal orientation of the fibres in the tendon stump (write arrow) are noted.
B4: tendon-to-acromion adhesion
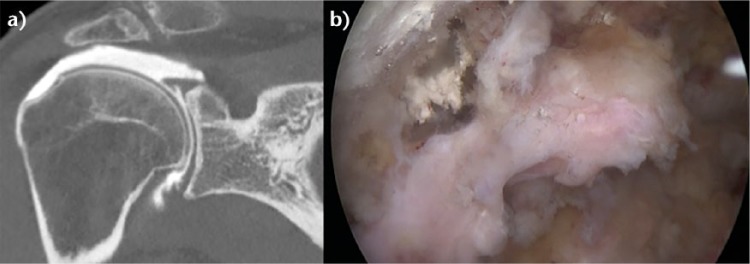
Disruption of the lateral tendon stump can be followed by adhesion under the acromion, the coracoid process or the coraco-acromial arch (Fig. 8).31 These adhesions are most pronounced in revision situations but may also be observed in primary cases, particularly in the setting of a massive contracted rotator cuff tear.
Fig. 8.
a) A coronal view of a right shoulder CT arthrogram shows a probable B2 rotator cuff lesion (RCL) with a Patte 3 retraction. b) The arthroscopic view through lateral portal revealed after partial debridment that the tendon was not retracted but actually had adhered under the acromion (B4 RCL).
Type C: musculotendinous junction lesion
Isolated ruptures of the musculotendinous junction are rare in the rotator cuff, but have a dramatic impact on functional outcome. Such lesions have been observed in all muscles of the rotator cuff, affecting the infraspinatus muscle in half of cases, supraspinatus in 31% of cases, subscapularis in 25% and the teres minor in 19% of cases (more than one muscle is occasionally involved).32 These lesions can be classified into three stages. Grade I injuries are a muscular strain that heals without adverse sequelae, grade II are partial ruptures without tendon retraction, and grade III classify complete ruptures at the musculotendinous junction.33 The acute phase of these injuries is associated with severe inflammation, leading to a highly characteristic bright signal on T2-weighted magnetic resonance imaging (MRI) (Fig. 9). Oedema of rotator cuff muscle with an intact tendon bone insertion is infrequent. It has been described in cases of denervation such as compression of the suprascapular nerve,34 in Parsonage-Turner syndrome35 and in other rare and non-specific conditions. Complete musculotendinous junction ruptures have only been described in the infraspinatus36 and the supraspinatus.37,38 Possible causes for musculotendinous junction infraspinatus lesion are calcific tendinitis or previous cortisone injection. On the other hand, rupture of the other muscles appears to be due to trauma or inlet impingement syndrome.32,38 There is little information about the clinical results of grade III musculotendinous junction lesions.
Fig. 9.
Axial and sagittal T2-weighted FATSAT MRI images demonstrating a type C rotator cuff lesion (RCL) with an intact tendon, a stage 3 rupture of the musculotendinous junction, and huge oedema of the muscle.
Type D: muscle insufficiency
D1: fatty infiltration and muscle atrophy
One of the most important prognostic factors for rotator cuff repair is non-functional muscle bellies.39 Muscle quality is most commonly classified according to Goutallier et al to determine the extent of injuries based upon the degree in which fat is present in the muscle. They proposed a five-stage classification system of fatty infiltration. Additionally, they demonstrated that multiple muscles develop fatty degeneration, even when not impacted directly by the original lesion.39 With the advent of MRI however, the classification was extrapolated to the most lateral parasagittal image on which the scapular spine was in contact with the scapular body (‘Y view’).40 The mean time to tendon rupture observed for stage II fatty infiltration is three years for the supraspinatus, and two and a half years for the infraspinatus and the subscapularis.41 The mean time observed to grade III and IV fatty infiltration is five, four and three years for the supraspinatus, the infraspinatus and the subscapularis, respectively.41
Zanetti, Gerber and Hodler42 described a radiographic tangent sign to quickly and reliably assess the presence or absence of supraspinatus atrophy on MRI scan. This sign is a reliable method for evaluating the presence or absence of muscle atrophy using the sagittal plane, and is moreover significantly related to the level of fatty infiltration within the supraspinatus muscle.43 It has been reported to be a predictor of whether a rotator cuff tear will be repairable.44 On the other hand, in a recent prospective analysis, Denard et al found that a complete repair could be achieved in over 90% of patients with this sign, which the authors attributed to repair of the subscapularis tendon and advanced mobilisation techniques.45
Thomazeau et al proposed calculating the occupation ratio of the supraspinatus muscle belly using MRI scan.46 This involved a comparison of the supraspinatus fossa volume with the total supraspinatus muscle belly volume and calculating the ratio. This ratio was found to be significantly decreased in patients with repairable rotator cuff tears.
In general, rotator cuff repair should be performed before the appearance of fatty infiltration (stage II) and atrophy (positive tangent sign), and as soon as possible in older patients when the tear involves multiple tendons.47
D2: neurological impairment
Isolated suprascapular nerve neuropathy is a condition associated with acute and chronic shoulder girdle traction injuries, compressive lesions such as paralabral cysts and compressive ligaments, as well as large or massive rotator cuff tears.48 In the latter situation, the proposed mechanism involves traction of the nerve caused by retraction of the supraspinatus against its fixed points on the suprascapular and spinoglenoid notches.49 However, clinical diagnosis is beset with uncertainties as the potential symptoms of suprascapular nerve neuropathy — namely, pain, weakness and atrophy — are inseparable from those of RCL-associated symptoms. Currently, there is no support for routine suprascapular nerve release as the prevalence of suprascapular nerve neuropathy in the setting of a massive rotator cuff tear was very low (2%) in a recent prospective study.48
D3: tumours
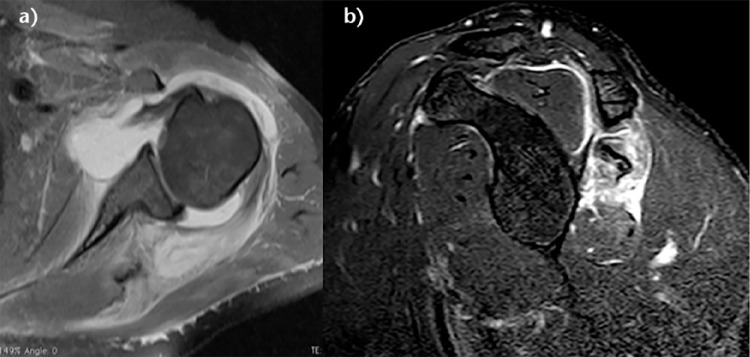
Many tumours such as an arthrosynovial cyst, intramuscular lipoma or calcified haematoma can develop at the expense of the muscular tissue and cause muscular insufficiency (Fig. 10).50 These impairments can be isolated, or may have other RCL associations. Management may require treatment of the associated mechanical stress (Video 3) in addition to rotator cuff repair.50
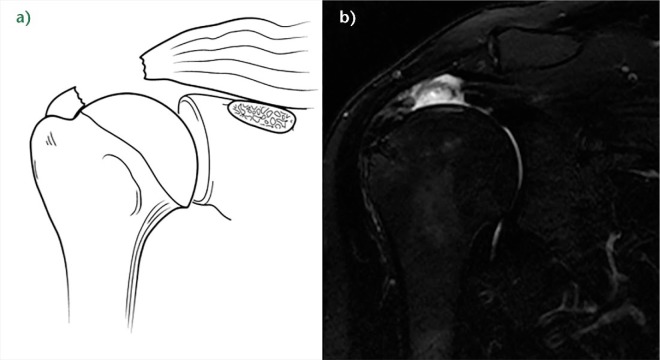
Fig. 10.
a) Schema of a D rotator cuff insufficiency. b) Coronal T2-weighted SPAIR MRI image of a right shoulder showing a B2D3 lesion with an intramuscular arthrosynovial cyst. c) Coronal T2-weighted PD and d) sagittal T1-weighted image (demonstrating D3 rotator cuff lesions with a calcified haematoma in the supraspinatus and an intramuscular lipoma of the subscapularis, respectively.
Discussion
This review summarises the current literature regarding full-thickness RCL classification. We have used a simple method (A-B-C-D), presenting different types of RCLs and allowing the classification of all types of lesions. Using historical methods, more than 10% of the RCLs observed in this series would not have been described.
One aim of this review was to describe RCL patterns. One point of interest is that bone involvement was found in around 7% of cases. This prevalence was unexpected and reiterates the importance of good pre-operative planning, as these lesions are less easily diagnosed and not treated in the same way as classical tendon lesions. One should anticipate osteosynthesis (open or arthroscopic) or reverse shoulder arthroplasty for fractures, whereas cancellous bone graft for cysts, or even bony and tendon grafts or reverse shoulder arthroplasty are required in the setting of tuberosity resorption.
Another point of interest in the present article is the importance of pre- and intra-operative comprehension of type B1 and B3 lesions, the lateral tendinous disruption and Fosbury flop tear. Successful repair of both types of lesions are dependent upon an understanding of tear patterns. With proper recognition, most RCLs in our hands are at least partially repairable. Nevertheless, even in the case of a partial repair, or only partial healing of the repair, the restoration of balanced force couples and the ‘suspension- bridge’ system of force transmission in the shoulder allowed successful results.51
The distinction between a B2 lesion (medial tendinous disruption) and a C lesion (musculotendinous junction lesion) remains extremely difficult. First, the stump of the tendon can be short and not always visible on MRI. Second, B2 lesions traditionally do not demonstrate typical muscular oedema.27 However, we have observed several cases of traumatic B2 lesions with substantial muscular oedema only some days after the trauma (Fig. 6) that we called oedema of retraction. Effectively, oedema of denervation does not appear until weeks later.28 Recently, Wieser et al analysed a sheep rotator cuff tear model and the development of muscular oedema after tenotomy. In their study design, the first MRI was performed immediately after surgery and after six and 16 weeks.52 Consequently, no comment about acute/retraction oedema could be made, the MRI having been performed too soon after the tenotomy. Research remains to be done in this domain to understand the precise cause of oedema and the timing of installation.
D lesions (muscle) remain challenging. First, we believe that there is confusion regarding the Goutallier et al39 classification, which classified muscle quality by the amount of fatty infiltration in the rotator cuff muscle as identified on CT in the axial plane, with a thorough analysis of the whole muscle belly. However, a sagittal MRI is currently used the most (Y view), which can overestimate fatty infiltration due to musculotendinous retraction frequently seen in rotator cuff tears. As a result, a normal muscle can be interpreted as completely fat-infiltrated if such MRI criteria are used, and conversely, supposed fat.infiltrated muscle can post-operatively appear normal. The muscle bellies should thus be assessed on both T1-weighted axial and sagittal views with cuts sufficiently medial to allow proper assessment, regardless of retraction. Secondly, there is no proof that repair of a tendon lesion, or tumour removal inducing mechanical stress, improves muscle status post-operatively. The best way to prevent irreversible fatty infiltration and atrophy remains rotator cuff repair. In the future, it is probable that other methods of prevention and treatment in certain groups of patients, such as administration of an anabolic steroid,53 may prevent the occurrence of these troublesome complications.
Conclusion
Rotator cuff lesions can involve one of four distinct anatomical segments: a) bone; b) tendon; c) musculotendinous junction; and d) muscle. A comprehensive classification system that incorporates the pertinent anatomical segment of the rotator cuff lesion provides a more complete description of the lesion than other classification systems.
Footnotes
Conflict of Interest: One or more of the authors has declared the following potential conflict of interest or source of funding:
S.S.B. is a consultant for and receives royalties from Arthrex, Inc. (Naples, Florida).
P.J.D. is a consultant for and receives research support from Arthrex, Inc.
Funding
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article. In addition, benefits have been or will be directed to a research fund, foundation, educational institution, or other nonprofit organisation with which one or more of the authors are associated.
Supplementary Material
The three videos cited in the text are available alongside the online version of this article at http://www.efortopenreviews.org/content/1/112/420.figures-only
References
- 1. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand 2001;72:365-371. [DOI] [PubMed] [Google Scholar]
- 2. Neer CS., II Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg [Am] 1970;52-A:1077-1089. [PubMed] [Google Scholar]
- 3. Bono CM, Renard R, Levine RG, Levy AS. Effect of displacement of fractures of the greater tuberosity on the mechanics of the shoulder. J Bone Joint Surg [Br] 2001;83-B:1056-1062. [DOI] [PubMed] [Google Scholar]
- 4. Lädermann A, Denard PJ, Burkhart SS. Arthroscopic management of proximal humerus malunion with tuberoplasty and rotator cuff retensioning. Arthroscopy 2012;28:1220-1229. [DOI] [PubMed] [Google Scholar]
- 5. Greiner S, Scheibel M. Bony avulsions of the rotator cuff: arthroscopic concepts. Der Orthopade 2011;40:21-4,26-30. [Article in German]. [DOI] [PubMed] [Google Scholar]
- 6. Bh B, Oberoi I, Tay A, Collin P. Osteotomy and re-fixation for treatment of malunited greater tuberosity of humerus. J Orthop Case Rep 2012;2:18-20. [PMC free article] [PubMed] [Google Scholar]
- 7. Burkhart SS, Klein JR. Arthroscopic repair of rotator cuff tears associated with large bone cysts of the proximal humerus: compaction bone grafting technique. Arthroscopy 2005;21:1149. [DOI] [PubMed] [Google Scholar]
- 8. Moore DR, Cain EL, Schwartz ML, Clancy WG., Jr Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med 2006;34:392-396. [DOI] [PubMed] [Google Scholar]
- 9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013;22:1199-1208. [DOI] [PubMed] [Google Scholar]
- 10. Sershon RA, Van Thiel GS, Lin EC, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg 2014;23:395-400. [DOI] [PubMed] [Google Scholar]
- 11. Lädermann A, Denard P, Abrassart S, Schwitzguébel A. Achilles tendon allograft for an irreparable massive rotator cuff tear with bony deficiency of the greater tuberosity: a case report. Knee Surg Sports Traumatol Arthrosc 2016. [Epub ahead of print] PMID: 26811033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barth JR, Burkhart SS, De Beer JF. The bear-hug test: a new and sensitive test for diagnosing a subscapularis tear. Arthroscopy 2006;22:1076-1084. [DOI] [PubMed] [Google Scholar]
- 13. Cofield RH. Rotator cuff disease of the shoulder. J Bone Joint Surg [Am] 1985;67-A:974-979. [PubMed] [Google Scholar]
- 14. Davidson J, Burkhart SS. The geometric classification of rotator cuff tears: a system linking tear pattern to treatment and prognosis. Arthroscopy 2010;26:417-424. [DOI] [PubMed] [Google Scholar]
- 15. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg [Am] 2000;82-A:505-515. [DOI] [PubMed] [Google Scholar]
- 16. Harryman DT, II, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg [Am] 1991;73-A:982-989. [PubMed] [Google Scholar]
- 17. Lädermann A, Denard PJ, Collin P. Massive rotator cuff tears: definition and treatment. Int Orthop 2015;39:2403-2414. [DOI] [PubMed] [Google Scholar]
- 18. Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195-1202. [DOI] [PubMed] [Google Scholar]
- 19. Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res 1990;254:81-86. [PubMed] [Google Scholar]
- 20. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med 2012;40:606-610. [DOI] [PubMed] [Google Scholar]
- 21. Lafosse L, Jost B, Reiland Y, et al. Structural integrity and clinical outcomes after arthroscopic repair of isolated subscapularis tears. J Bone Joint Surg [Am] 2007;89-A:1184-1193. [DOI] [PubMed] [Google Scholar]
- 22. Collin P, Lädermann A, Le Bourg M, Walch G. Subscapularis minor–an analogue of the Teres minor? Orthop Traumatol Surg Res 2013;99(Suppl):S255-S258. [DOI] [PubMed] [Google Scholar]
- 23. Denard PJ, Lädermann A, Burkhart SS. Arthroscopic management of subscapularis tears. Sports Med Arthrosc 2011;19:333-341. [DOI] [PubMed] [Google Scholar]
- 24. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy 2012;28:1587-1591. [DOI] [PubMed] [Google Scholar]
- 25. Loew M, Magosch P, Lichtenberg S, Habermeyer P, Porschke F. How to discriminate between acute traumatic and chronic degenerative rotator cuff lesions: an analysis of specific criteria on radiography and magnetic resonance imaging. J Shoulder Elbow Surg 2015;24:1685-1693. [DOI] [PubMed] [Google Scholar]
- 26. Teefey SA, Middleton WD, Bauer GS, Hildebolt CF, Yamaguchi K. Sonographic differences in the appearance of acute and chronic full-thickness rotator cuff tears. J Ultrasound Med 2000;19:377-378 quiz 383. [DOI] [PubMed] [Google Scholar]
- 27. Trantalis JN, Boorman RS, Pletsch K, Lo IK. Medial rotator cuff failure after arthroscopic double-row rotator cuff repair. Arthroscopy 2008;24:727-731. [DOI] [PubMed] [Google Scholar]
- 28. Fleckenstein JL, Watumull D, Conner KE, et al. Denervated human skeletal muscle: MR imaging evaluation. Radiology 1993;187:213-218. [DOI] [PubMed] [Google Scholar]
- 29. Lädermann A, Denard PJ, Kolo FC. A new tear pattern of the rotator cuff and its treatment: fosbury flop tears. Int J Shoulder Surg 2015;9:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamizo H. Arthroscopic repair for subacromial incarceration of a torn rotator cuff. AP-SMART 2015;2:90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romeo AA, Loutzenheiser T, Rhee YG, et al. The humeroscapular motion interface. Clin Orthop Relat Res 1998;350:120-127. [PubMed] [Google Scholar]
- 32. Taneja AK, Kattapuram SV, Chang CY, et al. MRI findings of rotator cuff myotendinous junction injury. AJR Am J Roentgenol 2014;203:406-411. [DOI] [PubMed] [Google Scholar]
- 33. Zarins B, Ciullo JV. ACute muscle and tendon injuries in athletes. Clin Sports Med 1983;2:167-182. [PubMed] [Google Scholar]
- 34. Ludig T, Walter F, Chapuis D, et al. MR imaging evaluation of suprascapular nerve entrapment. Eur Radiol 2001;11:2161-2169. [DOI] [PubMed] [Google Scholar]
- 35. Bredella MA, Tirman PF, Fritz RC, et al. Denervation syndromes of the shoulder girdle: MR imaging with electrophysiologic correlation. Skeletal Radiol 1999;28:567-572. [DOI] [PubMed] [Google Scholar]
- 36. Walch G, Nové-Josserand L, Liotard JP, Noël E. Musculotendinous infraspinatus ruptures: an overview. Orthop Traumatol Surg Res 2009;95:463-470. [DOI] [PubMed] [Google Scholar]
- 37. Hertel R, Lambert SM. Supraspinatus rupture at the musculotendinous junction. J Shoulder Elbow Surg 1998;7:432-435. [DOI] [PubMed] [Google Scholar]
- 38. Lädermann A, Christophe FK, Denard PJ, Walch G. Supraspinatus rupture at the musclotendinous junction: an uncommonly recognized phenomenon. J Shoulder Elbow Surg 2012;21:72-76. [DOI] [PubMed] [Google Scholar]
- 39. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994;304:78-83. [PubMed] [Google Scholar]
- 40. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 1999;8:599-605. [DOI] [PubMed] [Google Scholar]
- 41. Melis B, Nemoz C, Walch G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res 2009;95:319-324. [DOI] [PubMed] [Google Scholar]
- 42. Zanetti M, Gerber C, Hodler J. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Invest Radiol 1998;33:163-170. [DOI] [PubMed] [Google Scholar]
- 43. Williams MD, Lädermann A, Melis B, Barthelemy R, Walch G. Fatty infiltration of the supraspinatus: a reliability study. J Shoulder Elbow Surg 2009;18:581-587. [DOI] [PubMed] [Google Scholar]
- 44. Kissenberth MJ, Rulewicz GJ, Hamilton SC, Bruch HE, Hawkins RJ. A positive tangent sign predicts the repairability of rotator cuff tears. J Shoulder Elbow Surg 2014;23:1023-1027. [DOI] [PubMed] [Google Scholar]
- 45. Denard PJ, Lädermann A, Brady PC, et al. Pseudoparalysis from a massive rotator cuff tear is reliably reversed with an arthroscopic rotator cuff repair in patients without preoperative glenohumeral arthritis. Am J Sports Med 2015;43;10:2373-2378. [DOI] [PubMed] [Google Scholar]
- 46. Thomazeau H, Rolland Y, Lucas C, Duval JM, Langlais F. Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand 1996;67:264-268. [DOI] [PubMed] [Google Scholar]
- 47. Melis B, DeFranco MJ, Chuinard C, Walch G. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res 2010;468:1498-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Collin P, Treseder T, Lädermann A, et al. Neuropathy of the suprascapular nerve and massive rotator cuff tears: a prospective electromyographic study. J Shoulder Elbow Surg 2014;23:28-34. [DOI] [PubMed] [Google Scholar]
- 49. Albritton MJ, Graham RD, Richards RS, II, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg 2003;12:497-500. [DOI] [PubMed] [Google Scholar]
- 50. Lädermann A, Genevay M, Abrassart S, Schwitzguebel AJ. Supraspinatus intramuscular calcified hematoma or necrosis associated with tendon tear. Case Rep Orthop 2015:496313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Denard PJ, Koo SS, Murena L, Burkhart SS. Pseudoparalysis: the importance of rotator cable integrity. Orthopedics 2012;35:e1353-e1357. [DOI] [PubMed] [Google Scholar]
- 52. Wieser K, Meyer D, Flück M, et al. Muscle degeneration associated with rotator cuff tendon release and/or denervation in sheep. American Academy of Orthopaedic Surgeons 2016 Annual Meeting http://aaos2016.conferencespot.org/61513-aaos-1.2968416/t002-1.2975366/f002-1.2975367/a070-1.2975482/paper-878-1.2975497 (date last accessed 7 November 2016). [Google Scholar]
- 53. Gerber C, Meyer DC, Flück M, et al. Anabolic steroids reduce muscle degeneration associated with rotator cuff tendon release in sheep. Am J Sports Med 2015;43:2393-2400. [DOI] [PubMed] [Google Scholar]