Abstract
Benign abnormalities of bone are common, especially in children.
Malignant bone sarcomas are aggressive and have a poor outcome, particularly if treatment is delayed or initiated in a non-specialist centre.
Conversely, specialist tumour centres are overwhelmed with referrals for benign disease, a predictable outcome of an increasingly litigious medical environment. This review aims to arm the general orthopaedic surgeon or general practitioner with information to better discern a benign bone lesion from a malignant one, and explain the process of investigation and onward referral for those in whom malignant disease is suspected.
Cite this article: Plant J, Cannon S. Diagnostic work up and recognition of primary bone tumours: a review. EFORT Open Rev 2016;1:247-253. DOI: 10.1302/2058-5241.1.000035.
Keywords: bone tumour, sarcoma, diagnosis, recognition, sarcoma referral
Introduction
Primary bone tumours are rare, with an incidence in the United Kingdom of around six cases per million of the population. The general orthopaedic surgeon may only encounter one or two in their life’s practice. It is therefore necessary to maintain a high index of suspicion for tumours in order to not miss the diagnosis when it arises.
Sarcomas are malignant tumours of mesodermal origin. They are frequently aggressive, and more prevalent in a younger patient group compared to carcinoma. The age of the patient is a very useful step in formulating a differential diagnosis for bone tumours in general (Table 1); note that there can always be an exception to the rule.
Table 1.
The more likely differential diagnoses by age
| 0-5 years | Osteomyelitis Osteofibrous dysplasia Metastatic neuroblastoma Metastatic rhabdomyosarcoma Leukaemia |
| 5-20 years | Langerhans cell histiocytosis (Eosinophilic granuloma) Osteomyelitis Fibrous dysplasia Osteosarcoma Ewing’s tumour Leukaemia |
| > 40 years | Metastatic bone disease Myeloma Lymphoma Paget’s disease Bone infarct Chondrosarcoma Pleomorphic undifferentiated sarcoma (Malignant fibrous histiocytoma) |
As soon as tumour is suspected, early referral to a specialist centre is critical. The cornerstone of cancer care is the multidisciplinary meeting involving surgeons, oncologists, radiologists, pathologists, specialist nurses and support workers. Local and systemic staging should be completed prior to biopsy, as general anaesthetic and local soft tissue insult can confound interpretation of the subsequent imaging.
Diagnosis requires correlation of clinical setting and radiological findings. Biopsy is used to grade, clarify and confirm a provisional diagnosis. Correct diagnosis becomes essential to guide management.
History and clinical examination
Like other orthopaedic sub-specialities, oncological practice requires specific questions to elicit a complete and relevant history. Current symptoms of malaise, fevers or unremitting pain, especially at night, should ring alarm bells for neoplasia.
The pain of primary bone tumours and metastatic bone disease is similar. Initially, a deep-seated dull pain can be intermittent and related to activity and loading. It can be mistakenly attributed to an innocuous trauma or sporting injury. Unlike these transient musculoskeletal disorders, the pain of cancer progresses to become constant without relief, even using simple analgesia. Conversely, the pain of a primary bone tumour can be masked by inappropriate prescribing of strong analgesia without first establishing a diagnosis. If a mass is present, the duration of its existence and rapidity of growth must be elucidated.
Past history of malignancy is crucial information, as is a history of specific predisposing genetic conditions (Maffuccis syndrome, Li- Fraumeni, neurofibromatosis) or other diseases such as Paget’s disease of the bone. Metastatic disease and myeloma are common, and are far more likely to be the cause of a bone lesion seen in an elderly skeleton.
Gout can mimic tumour, and this should be considered and queried in the adult history. Infection is also a great mimic. This should always be in the differential diagnosis, and is far more common than sarcoma, especially when considering a lesion in a child.
Examination is, as ever, tailored to the history. The area of tenderness, swelling or deformity is inspected and palpated. Skin changes are noted and masses are initially characterised by assessing size and depth in relation to fascia. Accurate measurements should be recorded. A useful rule of thumb is that masses greater than 5 cm in maximum dimension and depth to fascia should be considered malignant until proven otherwise. A golf ball is a useful comparator. Mass consistency, position, and mobility in relation to surrounding tissues are evaluated.
Local lymph nodes should be palpated for adenopathy and appropriate examination of other systems (thyroid, breast, abdomen, prostate) should be performed if metastatic disease is suspected.
Imaging
For efficiency of service, imaging studies can be completed prior to clinical examination by a tumour specialist, assuming the imaging does not delay referral.
Orthogonal radiographs of the local area should be completed for all lesions. A chest radiograph should be requested if malignant disease is suspected.
Ultrasound is a useful initial investigation of superficial and soft tissue tumours as it is harmless, inexpensive, non-invasive and readily available. Increased heterogeneity of a lesion is concerning for a malignant process. Ultrasound is a dynamic imaging modality and is ideal for diagnosing and assessing vascular lesions, as an experienced sonographer can gauge compressibility and flow. However it is of little use in the investigation of bone tumours.
Interpretation of radiographs
Almost all general practitioners and practicing orthopaedic surgeons have access to radiographic studies and may be required to interpret them. There is a danger of becoming deskilled by relying on a radiologist’s report; it is therefore important for any practicing doctor to be able to discern a concerning lesion from a benign one.
William Enneking first proposed asking certain pertinent questions when suspicion of a bone tumour occurs.1 These can be ordered as:
Where is the lesion? In which bone and within which anatomical region of that bone?
What is it doing to the bone?
How is the bone responding?
What is in the lesion (any clues to the histology)?
An appreciation of these questions helps to understand the differing appearances of lesions on radiographs.
Where is the lesion?
Not only which bone, but the location of the anomaly within that bone. The site of the lesion is sometimes pathognomonic. Giant cell tumour (a usually benign but locally aggressive disease) classically crosses the physis into the epiphysis and extends through the subchondral bone to the underside of the articular cartilage (Fig. 1).
Fig. 1.
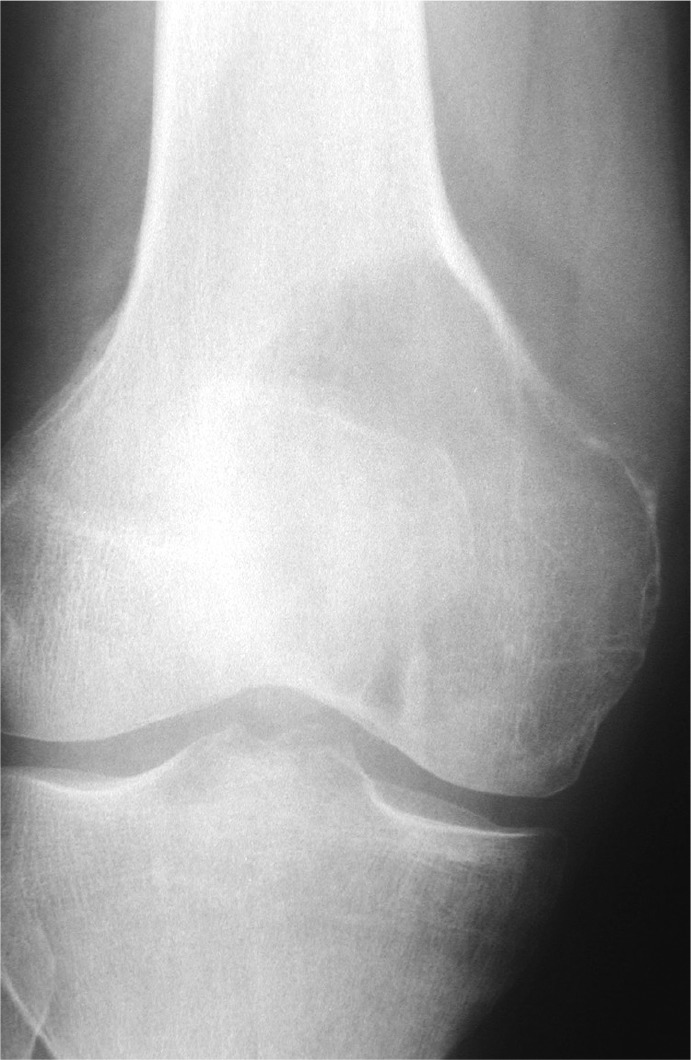
Classic subchondral location of a giant cell tumour.
The majority of simple (unicameral) bone cysts are in the metaphysis of the proximal humerus, moving to the proximal diaphysis with skeletal growth.
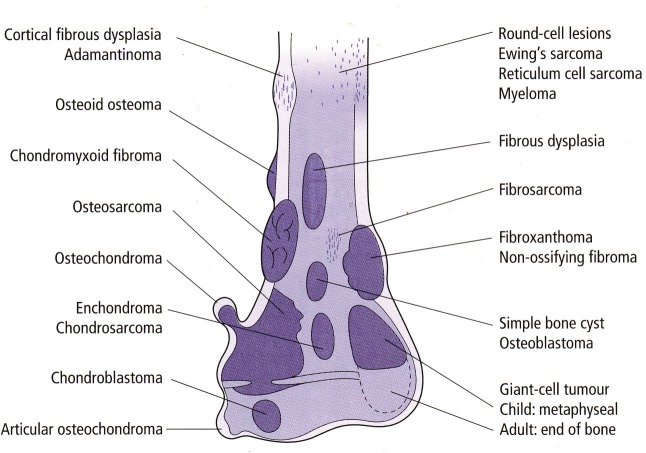
Figure 2 describes the common anatomical location of bone tumours.2
Fig. 2.
Common anatomical location of bone tumours.2
What is the lesion doing to the bone?
Some tumours are osteolytic; others are osteoblastic. Some, notably prostate or breast metastases, can be mixed. Osteolysis caused by tumour is, at a cellular level, performed by osteoclasts. The tumour cells themselves cannot resorb bone but can up-regulate the osteoclasts via the RANK ligand pathway. Note that for a lytic lesion to be seen on a radiograph, at least 50% of the bone matrix (compared to the surrounding bone) must have been resorbed.2
There are three patterns of bone destruction that give a clue to the aggressive nature of an osteolytic lesion:
Geographic bone destruction
Geographic bone destruction implies a lesion with a sharply-defined border. This can also be described as a narrow zone of transition between pathologic and normal bone. A geographic appearance implies a benign or less aggressive tumour. The bone has had time to respond, and does so by reacting to and containing the tumour. Examples of geographic lesions include non-ossifying fibroma (Fig. 3), chondromyxoid fibroma and Langerhans cell histiocytosis (eosinophilic granuloma).
Fig. 3.
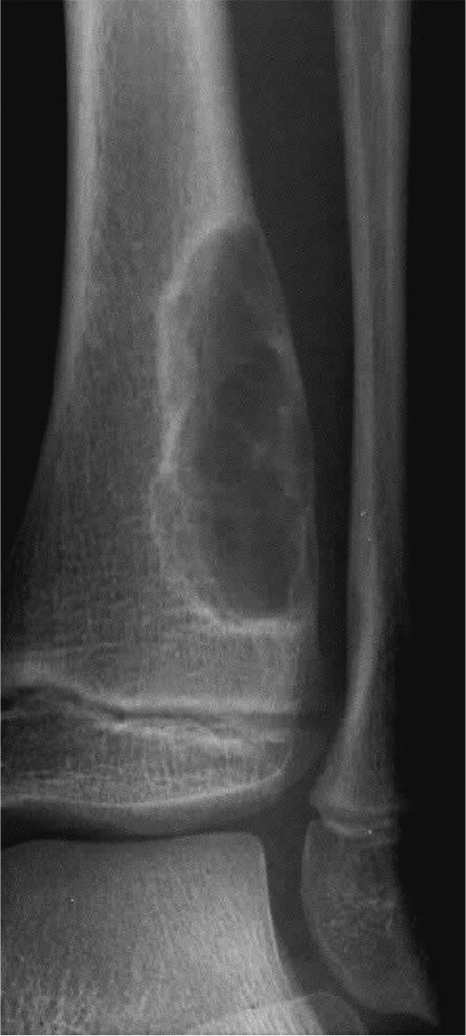
Note the well-demarcated rim of a non-ossifying fibroma.
Medullary lesions can result in cortical resorption of bone from within. This can be seen as endosteal scalloping (Fig. 4).
Fig. 4.
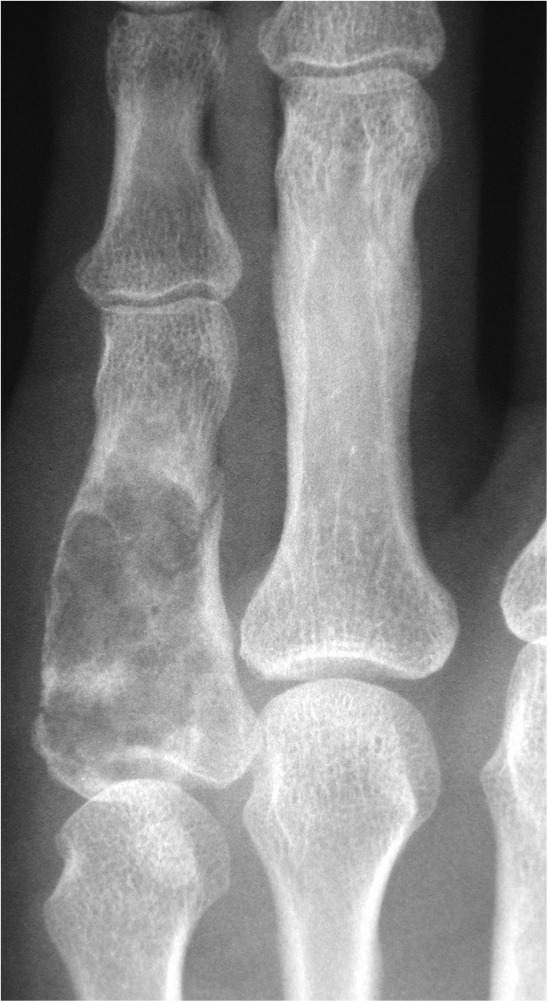
Endosteal scalloping and bone expansion from a phalangeal enchondroma.
Moth-eaten appearance
This refers to areas of bone destruction with ragged edges. This is indicative of a malignant process rapidly expanding into the bone. Metastatic disease, myeloma, lymphoma and Ewing’s sarcoma commonly have a moth-eaten appearance. Telangectactic osteosarcoma is a rare form of osteosarcoma and has a lytic appearance on plain films (Fig. 5).
Fig. 5.
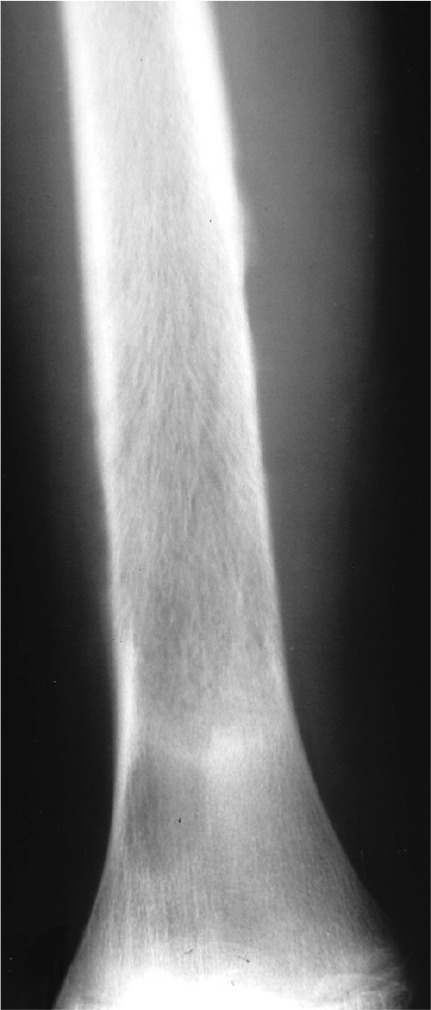
Telangectatic osteosarcoma is lytic with a broad zone of transition.
A permeative appearance
Often confused with moth-eaten, a permeative appearance implies that the tumour is moving through the bone without destroying all the trabeculae. There is, therefore, an ill-defined wide zone of transition between pathologic and normal bone. This classically is characteristic of round cell tumours. Examples of permeative lesions include lymphoma, leukaemia, Ewing’s sarcoma, myeloma, osteomyelitis and neuroblastoma.
What is the bone doing?
This question is closely related to the above. The response of bone depends on the tumour histology and grade, including the rapidity of its growth.
The universal response of bone to disease or injury is to make more bone. A benign lesion may not normally elicit a response from the bone or periosteum. Some lesions, such as osteoid osteoma, may cause a solid thickening of the bone around them.
Tumours which are rapidly increasing in size give the most impressive and characteristic Roentgenogram changes. The tumour is growing too fast for the periosteum to produce bone to contain them. As layers of partially-formed and partly calcified bone are laid down, the tumour expands through them, resulting in a radiological appearance known as onion-skinning (Fig. 6). At the corners where the periosteum is being lifted off the bone, the infill with calcified osteoid results in Codman triangles on the radiograph.
Fig. 6.
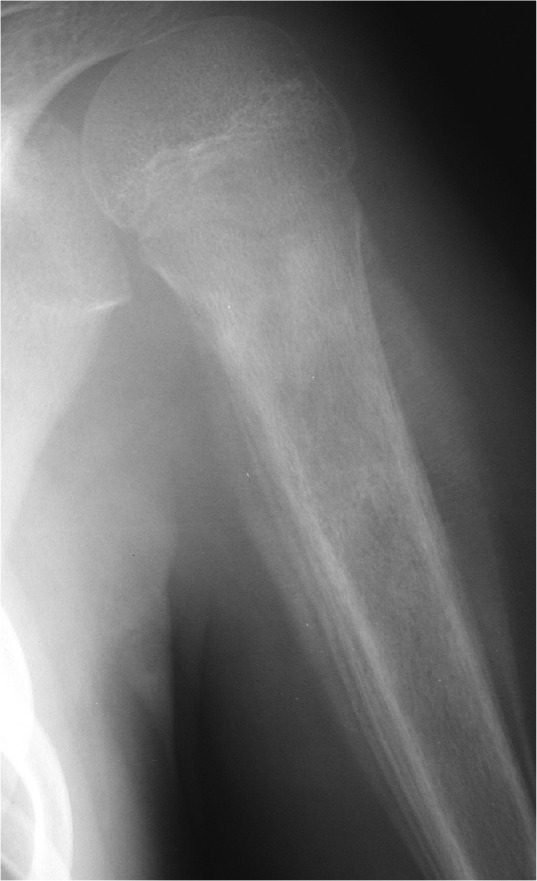
The ‘onion skin’ appearance, in this case from a Ewing’s sarcoma of the proximal humerus.
In aggressive tumours, the attempted vertical calcification of osteoid as the tumour rapidly expands can also result in a sunburst, or hair-on-end, appearance on plain films. Figure 7 demonstrates a classic osteosarcoma with both Codman triangles and the sunburst appearance.
Fig. 7.
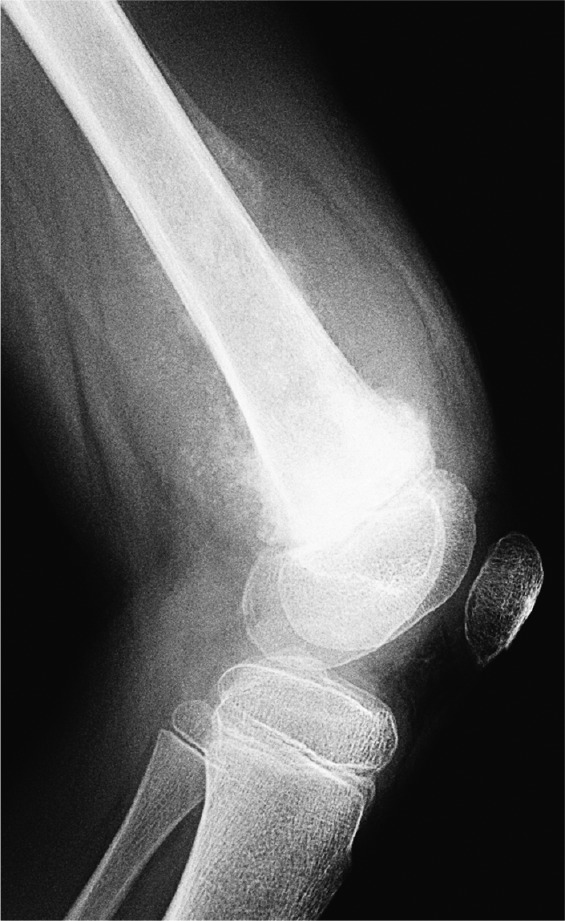
Classic osteosarcoma of the femur in a child.
What is in the lesion?
An assessment of the matrix of a lesion can give a clue to the histological diagnosis. An osseous matrix appears fluffy and white on a radiograph. A chondroid matrix has a classical appearance of ‘rings and arcs’ on plain film (Fig. 8).
Fig. 8.
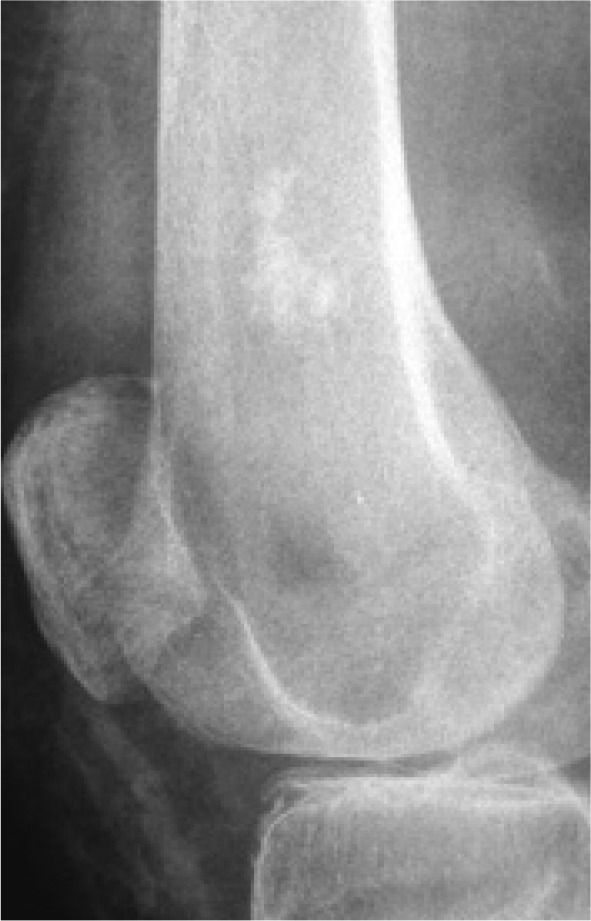
A common location and appearance of a chondroid series tumour.
Investigations
In suspected neoplasia, certain blood tests must be performed. Full blood count, inflammatory markers and a bone profile should always be sent for analysis. In older patients, serum electrophoresis with or without urine analysis for Bence-Jones protein, is necessary to exclude or confirm a myeloproliferative disorder. Prostate-specific antigen should be quantified if metastatic prostate disease is suspected. Urea, electrolytes and liver function assays are useful to record baseline renal and hepatic function prior to commencing cytotoxic therapy. Uric acid levels can help exclude gout, and parathyroid function tests are performed if a brown tumour (of hyperparathyroidism) is suspected.
Staging
Whilst plain radiographs offer the best imaging to characterise a lesion, magnetic resonance imaging (MRI) is the gold standard modality for local assessment and staging. MRI scanners are becoming increasingly accessible, but this investigation is usually best performed by a specialist institution with experienced radiographers, radiologists and superior MRI equipment. Designated tumour MRI scanning protocols exist which may not be applied in a peripheral centre.
The entire bone that contains the lesion should be imaged using MRI. This is very sensitive for detecting skip lesions in the bone, and also for assessing the soft tissue component of the tumour. MRI can sometimes be useful in characterising lesions, and is crucial for deciding the direction of biopsy and assessing the involvement of neurovascular structures by an adjacent tumour. The MRI scan is also used to assess any tumour infiltration of nearby joints.
MRI works by detecting energy released by protons as they realign to the magnetic field, in which the body part of interest lies, following a pulse of radiofrequency energy. Different tissue types can be enhanced by differential weighting of the MRI ‘time to echo’ (time taken to detect the signal echo) and ‘time to repeat’ (of the radiofrequency pulse). Only two dimensional images are obtained, necessitating repeated scans to view the anatomy in other planes.
Tumours have a similar signal intensity to muscle on T1 weighted images (short time to echo (TE) < 60 ms, short time to repeat (TR) < 1000 ms). The fat of marrow is bright on T1 and gives excellent contrast to the hypointense tumour.3
Administration of intravenous contrast can also be used with T1-weighted scans to better delineate enhancement of a tumour, especially when combined with a fat-supressed sequence.
The most sensitive MRI sequence is the short tau inversion recovery (STIR), a method of fat suppression with T1 imaging. Proton density (PD) fat saturated images have largely replaced T2-weighted MRI sequences in tumour protocols. In both these studies, tumour appears hyperintense, especially if there is a cartilaginous or myxoid component. The surrounding oedema is also hyperintense.
More recently a role has been developing for dynamic contrast-enhanced MRI scanning. This refinement is useful in sarcoma follow-up, in that it is more easily able to distinguish tumour recurrence from post-operative scarring.4 Dynamic MRI has also been shown to better assess the response of a bone tumour to chemotherapy than static MRI.5,6
In certain circumstances, computerised tomographic (CT) scans of the tumour can be very useful. A fine slice CT has better resolution that an MRI scan, and can be very useful for particular conditions. The nidus of an osteoid osteoma is better seen on CT than with any other form of imaging. CT is useful for assessing bony destruction when it comes to planning reconstructive surgery (Fig. 9), and is commonly used to guide biopsy.
Fig. 9.
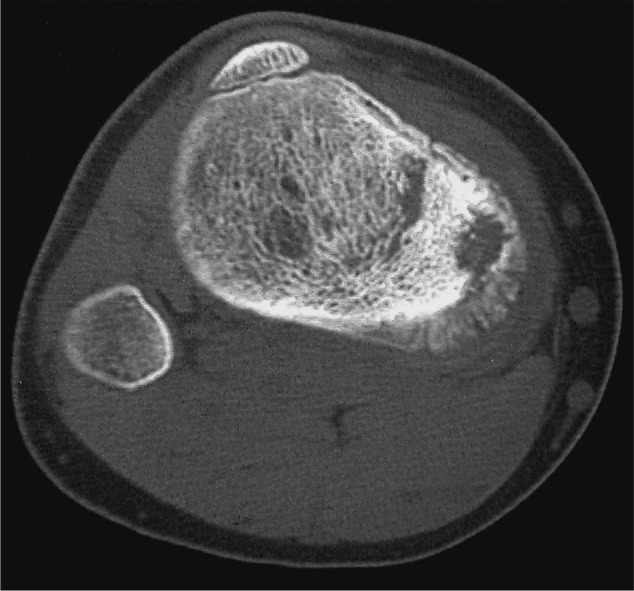
A CT scan of osteosarcoma, showing bony formation and destruction more clearly than MRI.
A lesion which appears to be aggressive on radiographs and MRI should initiate systemic staging. This always consists of a chest CT scan, with an abdominal and pelvic CT scan if metastatic disease is suspected.
Whole body imaging has long been the domain of the Technetium-99m bone scan. This scanning modality initially displays blood flow to the tissues, then some hours later osteoblast activity, as the radiolabelled methylene-diphosphonate is utilised. Though a bone scan is a highly sensitive investigation, it has low specificity. A superscan refers to a bone scan with universal high uptake that can then be erroneously calibrated to appear normal. The clue is the relative hypoperfusion of the kidneys, which should appear dark as they excrete the tracer.
As scintigraphy relies on osteoblast activity to signal metabolic activity, lesions with minimal osteoblastic response can be missed. Multiple myeloma deposits will often not be visible on a bone scan. Scintigraphy is useful to distinguish an active lesion from an inactive one, increasing suspicion of sinister intent if active. Early disease or very aggressive disease can however be ‘cold’ on scintigraphy if there is little osteoblastic activity.7
Tumours are obligate utilisers of glucose. For this reason there is a growing trend towards using positron emission tomography (PET) with a fluorine-18 labelled fluoro-deoxy-glucose (FDG) for systemic staging. The positron (a positively-charged electron) is emitted from the radiolabeled glucose analogue, reacting with a nearby electron to result in two high-energy (annihilation radiation energy) photons moving in opposite directions. Three-dimensional imaging is acquired by detecting photons moving at 180° to each other, an hour after the introduction of the labelled glucose analogue.
The standard uptake value (SUV) is used to quantify the 18F-FDG avidity. Benign disease such as infection, rheumatoid arthritis, fibrous dysplasia and Paget’s disease of bone can be glucose avid. An SUVmax above 3.7 has been proposed to distinguish a malign bone process from a benign one.8 Despite very low-grade tumours potentially giving false-negative results, PET scan has a 97% specificity for ruling out malignant disease.9
CT or MRI imaging is used to ’co-register’ with the PET scan, allowing the glucose avid hotspots to be correlated to anatomic location. The contrast-enhanced CT scan is performed first, with the patient lying still until the PET scan is complete shortly afterwards. The images can be evaluated separately or fused together (Fig. 10).
Fig. 10.
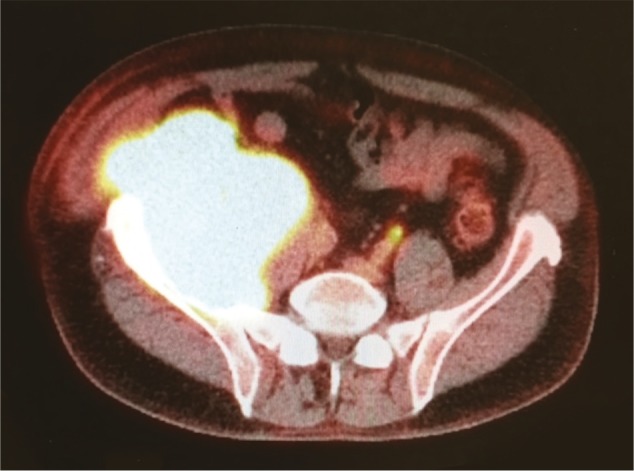
Metabolic and anatomic information is amalgamated with a PET-CT scan.
Biopsy
Biopsy must always be performed under the direct instruction of a surgeon at a specialist bone tumour unit. It is well-known that biopsies performed in a non-specialist centre can lead to diagnostic errors, and can cause a change in the treatment plan, increased local recurrence and may also result in unnecessary amputation.10
Histological analysis is required to grade the lesion. If there is any doubt regarding the diagnosis, a biopsy is indicated. Biopsy techniques may be percutaneous, incisional or excisional. Excisional biopsy is reserved for lesions with very low malignant potential on their imaging. Lesions that are potentially malignant but too small to biopsy percutaneously are excised with a wide local margin of normal tissue, so if malignant disease is confirmed histologically, an oncologic operation has already been safely performed.
Percutaneous biopsy is the usual technique employed to obtain diagnostic tissue. A Tru-cut biopsy or Jamshidi needle (Beckton Dickinson, Franklin Lakes, NJ) is passed into the lesion to obtain a core of diagnostic tissue.11 The central part of the lesion is often necrotic, so it is important that the relevant imaging is discussed by the treating surgeon, the interventional radiologist and the expectant histopathologist to plan the site, direction and target of the biopsy needle. If malignant disease is confirmed, the biopsy tract requires excision at the time of surgery.
Percutaneous biopsy can be carried out accurately using ultrasound, CT or MRI guidance. In a specialist centre, a 98% diagnostic accuracy can be recorded in peripheral malignancies.
Incisional biopsy is used rarely; and usually when the radiologists cannot safely access a lesion or if the lesion is too small to accept the ‘throw’ of a biopsy needle. Open biopsy increases the risks of local contamination and clinical morbidity, with a worse prognosis in 8% of cases. Definitive surgery can be made more difficult following an open biopsy, and there is an increased risk of amputation. There are strict oncologic principles regarding biopsy surgery, of which the surgeon must be cognisant.12
Conclusion
General orthopaedic surgeons and general practitioners do not need an encyclopaedic knowledge of bone tumour diagnoses. It is, however, appropriate that they are able to recognise a suspicious lesion and refer appropriately to a specialist centre.
It is imperative that a clear explanation of the process during the diagnosis and staging of a lesion is given. The patient will be anxious, and needs the reassurance that an organised and logical process to investigate and treat them is in place.
Footnotes
Authors’ Note: The authors would like to thank Dr A. Saifuddin (Royal National Orthopaedic Hospital, Stanmore) for many of the radiology figures.
Conflict of Interest: None declared.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Enneking WF. Clinical musculoskeletal pathology. Gainesville, Florida: Storer Printing Co, 1970. [Google Scholar]
- 2. Ramachandran M. Basic orthopaedic sciences: the Stanmore guide. London: Hodder Arnold, 2007. [Google Scholar]
- 3. de Kerviler E, Cuenod CA, Clément O, et al. Qu’est-ce qui est blanc en T1? [What is bright on T1 MRI scans?]. J Radiol 1998;79:117-26. [In French] [PubMed] [Google Scholar]
- 4. Del Grande F, Subhawong T, Weber K, et al. Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology 2014;271:499-511. [DOI] [PubMed] [Google Scholar]
- 5. Amit P, Patro DK, Basu D, Elangovan S, Parathasarathy V. Role of dynamic MRI and clinical assessment in predicting histologic response to neoadjuvant chemotherapy in bone sarcomas. Am J Clin Oncol 2014;37:384-90. [DOI] [PubMed] [Google Scholar]
- 6. Amit P, Malhotra A, Kumar R, et al. Evaluation of static and dynamic MRI for assessing response of bone sarcomas to preoperative chemotherapy: correlation with histological necrosis. Indian J Radiol Imaging 2015;25:269-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blake GM, Park-Holohan SJ, Cook GJ, Fogelman I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin Nucl Med 2001;31:28-49. [DOI] [PubMed] [Google Scholar]
- 8. Shin D-S, Shon O-J, Han D-S, et al. The clinical efficacy of (18)F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008;22:603-9. [DOI] [PubMed] [Google Scholar]
- 9. Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, et al. The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med 2002;43:510-8. [PubMed] [Google Scholar]
- 10. Mankin HJ, Mankin CJ, Simon MA, Members of the Musculoskeletal Tumor Society. The hazards of the biopsy, revisited. J Bone Joint Surg [Am] 1996;78:656-63. [DOI] [PubMed] [Google Scholar]
- 11. Stoker DJ, Cobb JP, Pringle JAS. Needle biopsy of musculoskeletal lesions. A review of 208 procedures. J Bone Joint Surg [Br] 1991;73:498-500. [DOI] [PubMed] [Google Scholar]
- 12. Briggs T, Miles J, Aston W. Operative orthopaedics: the Stanmore guide. London: Hodder Arnold, 2010. [Google Scholar]