Abstract
Lumbar spinal stenosis has become one of the most disabling pathologies in the elderly population.
Some additional conditions such as foraminal stenosis or degenerative spondylosis with a history of back pain and leg pain must be considered before treatment.
A completely appropriate protocol and unified management of spinal stenosis have not yet been well defined.
The objective of this literature review is to provide evidence-based recommendations reflected in the highest-quality clinical literature available to address key clinical questions surrounding the management of degenerative lumbar spinal stenosis.
Cite this article: Covaro A, Vilà-Canet G, García de Frutos A, Ubierna MT, Ciccolo F, Caceres E. Management of degenerative lumbar spinal stenosis: an evidence-based review article. EFORT Open Rev 2016;1:267-274. DOI: 10.1302/2058-5241.1.000030.
Keywords: spinal stenosis, degenerative spondylolisthesis, management
Methods
A comprehensive search was performed to identify previous studies of spinal degenerative stenosis with and without spondylolisthesis in PubMed, Cochrane and EMBASE databases. The selection included studies published in the last ten years and was conducted following PRISMA recommendations for systematic reviews.1
Disease and clinical diagnosis
Degenerative processes of the lumbar spine causing stenosis are one of the major causes of pain and dysfunction in the elderly, having a much stronger negative impact on health-related quality of life (HR-QoL), in comparison with other comorbid conditions such as osteoarthritis of the knee and hip, cardiovascular disease, cerebrovascular disease or respiratory disease.2
Congenital or acquired lumbar spinal stenosis (LSS) was well described by Verbiest and Epstein.3-7 More commonly, lumbar stenosis is the result of degenerative changes. This degenerative process is thought to be initiated by disc dehydration and bulging, and collapse of the disc space which leads to the narrowing of the space, resulting in an increased transfer of stress to the facet joints. This accelerates facet joint cartilage degeneration and osteophyte formation.8,9
The combination of degenerative changes in the disc and facet joints can lead to central canal or lateral recess stenosis, which may also result in vertebral displacement leading to degenerative spondylolisthesis (Table 1).3-7 These stenotic changes can cause neural compression that presents clinically as variable degrees of back and leg pain, numbness and weakness, as well as gait deterioration.10
Table 1.
Sub-types of spinal canal stenosis, which can be alone or combined
| Stenosis types (by anatomical site) | Causes | Root affected |
|---|---|---|
| Central | Segmental slip in spondylolisthesis, flavum bulging or facet joint hypertrophy, congenital | Descending root |
| Sub-articular | ||
| Foraminal | Bone spurs from facet joints, bulging or herniated discs, ligamentous flavum hypertrophy | Emerging root |
| Extraforaminal (far lateral) |
Patients with central lumbar spondylitic stenosis most commonly present with neurogenic claudication and report discomfort whist standing or mantaining extension posture, as well as diminished walking capacity. However, their ability to walk distances can be increased by ambulating with the spine in a flexed forward posture such as that used when pushing a shopping trolley.10,11
It is well known that radiographic changes do not always correlate with symptoms, explaining why diagnosis is typically based on clinical history and physical examination and is confirmed using imaging studies.12 Radiographic LSS (lumbar spinal stenosis) commonly occurs in the elderly; however, the exact prevalence of symptomatic LSS has not been well defined.13,14 Typically, radiographic evaluation starts with plain standing anterior–posterior (AP) and lateral radiographs, which may demonstrate narrowing of the disc space, end-plate sclerosis, osteophytes, facet hypertrophy and also the presence of degenerative spondylolisthesis, which is most common at L4-L5.15 Lateral flexion-extension radiographs may be helpful in determining whether spondylolisthesis is mobile and also demonstrate the slip that is not visible on the plain standing lateral view. CT scans can also be performed to assess the extent of facet joint arthritis and foraminal compromise by osteophytes. The degree of spinal stenosis is best evaluated on MRI because it can demonstrate disc degeneration or herniation, hypertrophy of the ligamentum flavum and facet capsule, and narrowing of the central canal and lateral recess. The absence of normal sedimentation on the lumbar nerve roots is a positive sign of LSS and is shown to have high intra-observer reliability and acceptable inter-observer reliability (Fig. 1).16
Fig. 1.
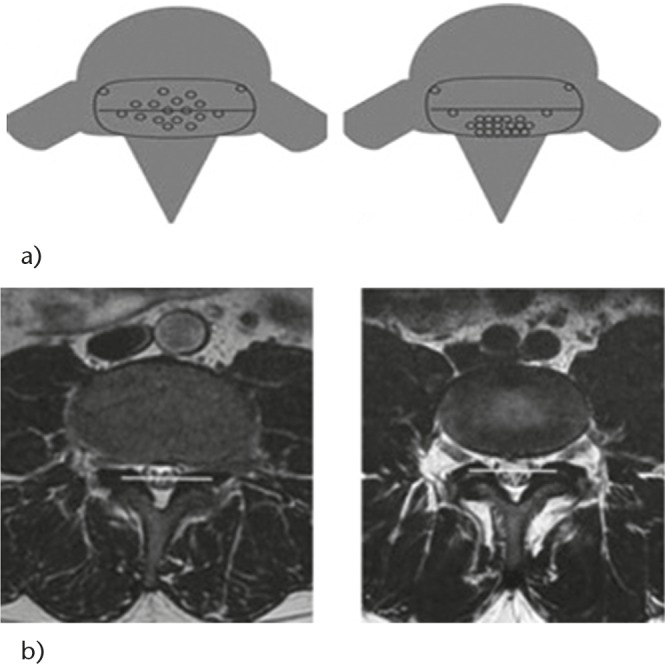
Comparative axial T2 MRI, showing a) positive sedimentation sign; b) negative sedimentation sign.
Larger ligamentum flavum cross-sectional areas and ligamentum flavum thicknesses at the most stenotic intervertebral level are asociated with higher disability (Fig. 2).17
Fig. 2.
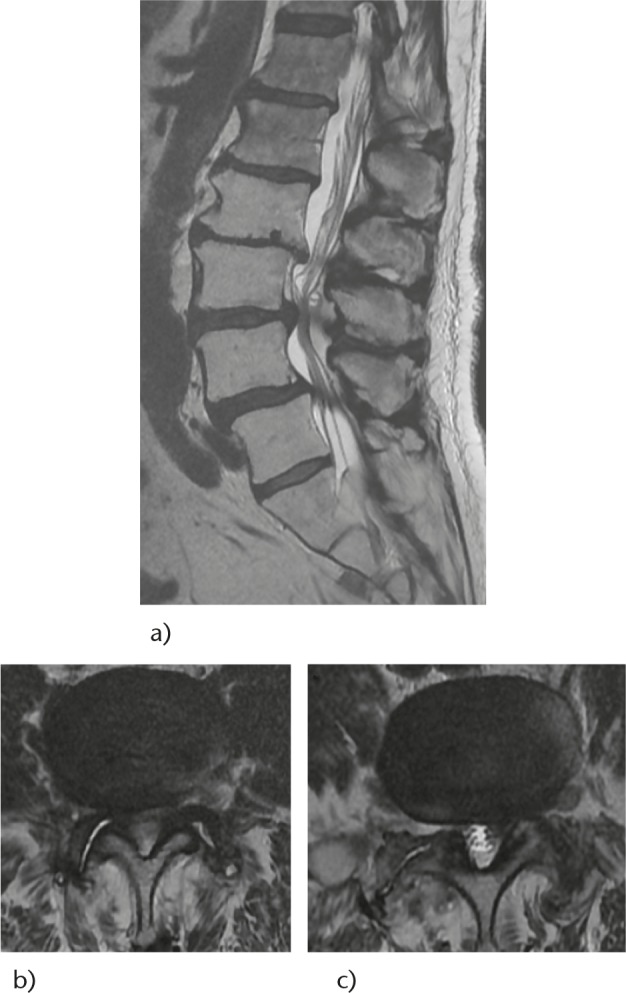
A 79-year-old male with severe radiculopathy of the right thigh and left leg in the standing position: a) sagittal MRI showing spondylolis with central L3-L4-L5 stenosis, b) axial L3-L4: central and right lateral recess stenosis, c) axial L4-L5: left lateral recess stenosis.
When MRI cannot be performed, CT myelography can provide reproducible measurements of intra-canal dimensions and flavum thickness, but this is an invasive procedure with several potential complications.18 Upright, standing or positional MRI (uMRI) is a type of vertical, open MRI developed in recent years. The proposed advantages of uMRI are based on the ability to scan the spine (or joints) in different positions (including the position where clinical symptoms are more pronounced) and assess the effects of weight-bearing, position and dynamic movement. There is insufficient scientific evidence to make any conclusions about the true effectiveness of this type of MRI and whether it can replace other tests, or whether it results in equivalent or better diagnostic outcomes.19
Management
Conservative treatment
The objectives of non-surgical treatment are to alleviate pain and improve function. The initial treatment of LSS is non-surgical. The most effective non-surgical treatment is a comprehensive combination of anti-inflammatory drugs, physical therapy and conditioning, and epidural injections.
Non-steroidal anti-inflammatory drugs (NSAIDs) and narcotic analgesics may temporarily alleviate pain, but their role is limited due to potential adverse effects, especially in the elderly population.20,21
Physical therapy was shown to improve physical function score on the Short Form-36 Health Survey at two years.22 In a subcategory of the SPORT study, results showed that physical therapy used in the first six weeks of enrolment was associated with a reduced likelihood of crossover to surgery after one year (21% versus 33%, p = 0.045), and greater reductions on the Short Form-36 physical functioning scale after one year (mean difference 6.5, 95% CI 0.6 to 12.4).23
Although epidural injections of local anaesthetic have been shown to improve pain and function in LSS, these benefits seem to be short-lived. The available evidence does not strongly support the addition of steroids to local anaesthetic agents.24 However, epidural injections may be considered as an effective procedure for a select group of patients who have chronic function-limiting lower back and lower extremity pain secondary to LSS.25
There are still controversies due to the lack of moderate- to high-grade evidence for non-operative treatment for short-term outcomes and results favouring decompression at long term-outcomes.26
Surgical treatment
Patient who fail non-operative treatment should be considered for surgery.27 The surgical procedure depends upon the location and character of the stenosis. Decision-making in order to obtain a good surgical result is based on a careful clinical assessment of motor weakness or radicular symptoms along with specific nerve root distribution affection corresponding with the imaging location of central or lateral recess and foramen compression (Table 2).
Table 2.
Decompression types and techniques (alone or combined)
| Laminotomy / foraminotomy | Partial removal of the laminae or the articular process into the lateral recess. |
| Laminectomy | Complete removal of the laminae. Can be unilateral or central, including spinous process. |
| Discectomy | Removal of part of the disc that is compressing the root. |
A prospective study by Amundsen et al,28 a randomised study by Malmivaara et al29 and the Spine Patient Outcomes Research Trial (SPORT)30 demonstrated that patients treated surgically had a significantly better outcome than those treated with non-surgical care at two-year follow-up. It has been reported that patients with predominant leg pain rather than back pain had better surgical outcomes.31 The most significant randomised controlled trials comparing conservative treatment versus surgery are presented in Table 3.
Table 3.
Surgical versus conservative randomised controlled trials (RCTs)
| Author | Journal | Patients (n) | With/without spondylolisthesis | Measurement tool | Follow-up (years) | Results | Risk bias |
| Atlas (Maine Lumbar Study Group)32 | Spine, 2005 | 148 | Not specified | Bothersomeness scale for leg/back pain and weakness. SF-36, Modified Roland scale. | 8-10 | Better leg pain relief and back-related functional status in surgically-treated. | Non-random height rate loss follow-up. Various levels of decompression. |
| Amundsen28 | Spine, 2000 | 100 | Not specified | Intensity of pain (light/moderate/severe). Patients reported results after surgery (worse/unchanged/fair/excellent). Daily activity. Neurological status. Walking distance. |
4-10 | Most favourable surgically-treated results. | Only 31 randomised lost follow-up. |
| Malmivaara29 | Spine, 2007 | 94 | Both | ODI, VAS leg/back.Walking ability. |
2 | Better improvement for surgical group for leg/back pain and disability. | Crossover. |
| Weinstein et al (SPORT)30 | N Engl J Med, 2008 | 365 | Without spondylolisthesis | ODI, SF-36. | 4 | Better surgical results. As treated analysis. | Crossover. |
| Kovacs et al33 | Spine, 2011 | Review 5 RCTs | Both | ODI, SF-36.VAS leg/back.Walking ability. | 4 | Surgery more effective than conservative. treatment in patients with neurogenic claudication. | Heterogeneous population and interventions. |
Although evidence from current studies suggested that surgical intervention is effective, the same studies showed that patients treated non-operatively also improve initially. Patients selected for surgical treatment were more likely to be younger, had worse back pain, physical function and disability, and worse central stenosis index and lateral recess stenosis.34
Spinal stenosis with degenerative spondylolisthesis
Studies on the use of decompression alone to manage degenerative spondylolisthesis were published by Epstein35 and Kristof,36 with good to excellent outcomes in older patients without dynamic instability on lateral radiographs (82.0% and 73.5%, respectively). When decompression alone is performed, preservation of the facet joints leads to better outcomes and less risk of slip progression.37 Two randomised controlled trials compared decompression alone with decompression and posterolateral fusion, finding that patients in whom fusion was added had greater functional improvement.38,39 Martin et al studied the surgical outcomes in degenerative spondylolisthesis and found that spinal decompression and fusion led to better clinical outcomes than decompression alone (RR 1.40; 95% CI 1.04 to 1.89) and that fusion was improved with instrumentation (RR 1.37; 95% CI 1.07 to 1.75), although instrumentation did not correlate with better clinical outcomes (RR 1.19; 95% CI 0.92 to 1.54).40 Recently, a meta-analysis published by Ye et al concluded that the inclusion of instrumentation to fusion surgery for lumbar spondylolisthesis provided no benefit in patient-reported outcomes, with a higher functional disability and no difference in pain change and satisfaction at two-year follow-up.41 In recent years, transforaminal lumbar interbody fusion (TLIF) has gained popularity due to increased mechanical strength compared with posterolateral fusion alone, segmental lordosis and disc high restoration and indirect foraminal decompression, but this technique does not seem to improve functional outcomes compared with instrumented posterolateral fusion alone, with increased operation time and blood loss for the TLIF group,42 and a tendency to be more costly in terms of bed days and production loss at two years.43 In patients with back pain due to dynamic instability, instrumented fusion can be an option (Fig. 3).
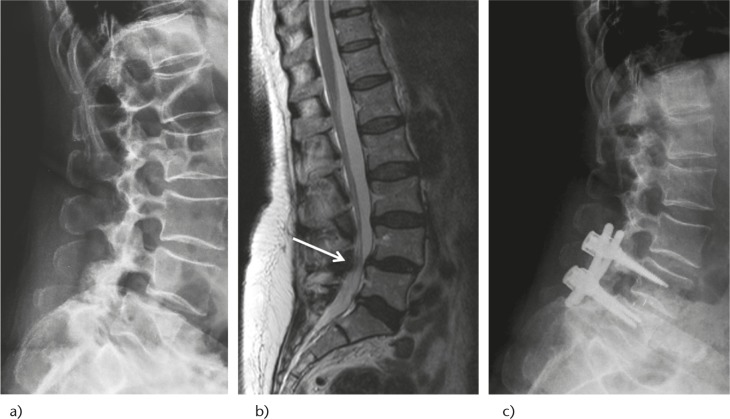
Fig. 3.
A 58-year-old female with neurogenic claudication and right leg radiculopathy in the standing position. a) Lateral radiographs show L4-L5 degenerative spondylolisthesis Grade I; b) sagittal MRI with central canal stenosis (white arrow); c) decompression and instrumented 360° fusion with TLIF technique with a PEEK cage on the right side.
Spinal stenosis without degenerative spondylolisthesis
Sigmundsson et al studied a cohort of 9051 patients in the Swedish Spine Register of patients with lumbar stenosis without spondylolisthesis, finding that patients with a predominant back pain pattern were associated with inferior outcomes, and the addition of spinal fusion could only provide a small benefit in patients with predominant back pain rather than leg pain.44 Recently, the multicentre SPORT study reported outcomes at eight years for patients with stenosis and without spondylolisthesis, showing no differences between surgery and conservative treatment in ‘intent-to-treat’ analysis. However, 47% were lost to follow-up and a large number of patients (52%) who initially enrolled for conservative treatment underwent surgery. In the ‘as-treated’ analysis, those that underwent surgery showed significantly greater improvement in pain, function, satisfaction and self-rated progress during the eight years than patients treated non-operatively.45
Instrumented or non-instrumented fusion?
The use of posterior instrumentation with fusion has become the standard of care; several studies show higher fusion rates with the use of instrumentation. However, its effect on clinical outcomes remains unclear.46 The literature supports fusion surgery as a viable treatment option for reducing pain and improving function in patients with chronic lower back pain refractory to non-surgical care when a diagnosis of disc degeneration can be made;47 however, there is lack of evidence regarding whether tests can identify which sub-group of back pain patients can best benefit from spinal fusion.48 Kleinstueck et al found better results in terms of back pain reduction when fusion was added to decompression in patients with stenosis and degenerative spondylolisthesis, but patients who underwent fusion had worse back pain pre-operatively.49 Försth et al studied the data of 5390 patients of the National Swedish Spine Register for Spinal Surgery (Swespine) who underwent decompressive surgery alone, or decompression and fusion for spinal stenosis, finding no differences in patient satisfaction regardless of the presence of pre-operative spondylolisthesis. Also, they did not find any significant differences between instrumented and non-instrumented fusion and the rate of further surgery.50 Regardless of better fusion rates in instrumented patients, no better outcomes were found compared with non-fusion, even in patients with spondylolisthesis (Fig. 4).41
Fig. 4.
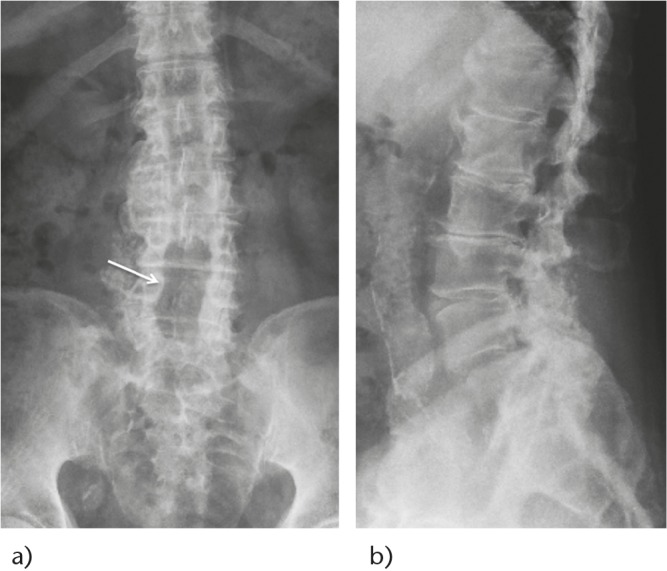
Post-operative radiographs showing central decompression alone (white arrow) without instrumentation.
Complications
Surgical treatment of LSS by decompression with or without fusion has a number of potential complications such as infection, dural tear, epidural haematoma and instability. An incidence of 2% of reported deep infection required debridement.41 Incidence of durotomy of patients undergoing laminectomy for lumbar degenerative spondylolisthesis reported in the SPORT trial was 5–10%, but this does not seem to affect pain and function in the long term.51 Reported incidence of epidural haematoma is in the range of 0%–1%, with requiring re-operation.45,52 Kornblum et al demonstrated the long-term outcomes in degenerative spondylolisthesis patients operated on with pseudarthrosis, who did not do as well as those treated with solid fusion. The authors concluded that solid fusion was beneficial for good long-term results and that consideration should be given to adding spinal instrumentation at the index procedure.53
Minimally invasive techniques
In an ageing population, and in patients with multiple co-morbidities who are at higher risk for complications, minimally-invasive (MIS) techniques may result in lower complication rates and lower hospital resource utilisation, as has been previously reported for open surgery.54,55 However, MIS spinal procedures carry an inherently difficult learning curve.56 New techniques of posterior decompression have been developed to preserve spinal integrity and to minimise tissue damage by limiting bony decompression and avoiding removal of the mid-line structures (i.e. spinous process, vertebral arch and interspinous and supraspinous ligaments). Parker et al, in an effectiveness and cost-utility analysis, reported that MIS-TLIF is a more cost-effective treatment than open-TLIF for patients with degenerative spondylolisthesis at two-year follow-up.57 Recently, in a Cochrane systematic review, Overdevest et al compared the effectiveness of different posterior decompression techniques with the ‘gold standard’ conventional laminectomy for lumbar stenosis.58 Proposed advantages of these techniques regarding the lower incidence of iatrogenic instability and post-operative back pain were reported, but definitive conclusions are limited by poor methodology, and poor reports of the outcome measures in the included studies. Future research is necessary to establish the incidence of iatrogenic instability using standardised definitions of radiological and clinical instability at comparable follow-up intervals.58 Long-term results with these techniques to asses the clinical benefits are currently lacking.
Interspinous devices
Flexion tends to relieve symptoms for some patients due to widening the spinal canal.59,60 Therefore, interspinous dynamic devices (ID) have been designed to limit spinal extension.61-63 Three high-quality reviews, according to Jacobs et al,64 compared interspinous process distraction devices with conservative treatment, finding better Zurich claudication questionnaire scores when ID were used, but long-term outcomes and cost-effectiveness need to be assessed.4,7,12 Although patients may obtain some benefits from interspinous spacers implanted through a MIS technique, ID use is associated with a higher incidence of re-operation and higher costs. Recently, a systematic review and meta-analysis compared ID placement versus laminectomy, found no differences in clinical outcomes, higher complication rates and significantly higher re-operation rates than laminectomy patients (12.6% versus 5.8%, p = 0.026), and incurred higher cumulative costs than laminectomy patients at 12-month follow-up.65 In the last ten years, the use of these implants has become very common but to date, no long-term follow-ups regarding clinical and radiological aspects are available. The higher re-operation rate, recurrence of symptoms and progression of degenerative changes is evident in the literature, therefore the indications, risks and benefits of using an ID should be carefully considered before surgery.
Conclusions
Surgical decompression for patients with predominant radicular pain has been shown to offer the most beneficial long-term outcomes. Additional instrumentation could be added in patients with a history of back pain and those with disc and facet joint degeneration with associated spondylolisthesis at the index level. More long-term studies are needed to assess the benefit of MIS techniques.
Footnotes
Conflict of Interest: None declared.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Otani K, Kikuchi S, Yabuki S, et al. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: an epidemiological cross-sectional study of 1862 community-dwelling individuals. Scientific World Journal 2013;2013:590652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg [Br] 1954;36-B:230-7. [DOI] [PubMed] [Google Scholar]
- 4. Verbiest H. Chapter 16. Neurogenic intermittent claudication in cases with absolute and relative stenosis of the lumbar vertebral canal (ASLC and RSLC), in cases with narrow lumbar intervertebral foramina, and in cases with both entities. Clin Neurosurg 1973;20:204-14. [DOI] [PubMed] [Google Scholar]
- 5. Epstein JA. Diagnosis and treatment of painful neurological disorders caused by spondylosis of lumbar spine. J Neurosurg 1960;17:991-1001. [Google Scholar]
- 6. Epstein JA, Epstein BS, Rosenthal AD, Carras R, Lavine LS. Sciatica caused by nerve root entrapment in the lateral recess: the superior facet syndrome. J Neurosurg 1972;36:584-9. [DOI] [PubMed] [Google Scholar]
- 7. Epstein JA, Epstein BS, Lavine LS, et al. Lumbar nerve root compression at the intervertebral foramina caused by arthritis of the posterior facets. J Neurosurg 1973;39:362-9. [DOI] [PubMed] [Google Scholar]
- 8. Verbiest H. Pathomorphologic aspects of developmental lumbar stenosis. Orthop Clin North Am 1975;6:177-96. [PubMed] [Google Scholar]
- 9. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307-14. [DOI] [PubMed] [Google Scholar]
- 10. Issack PS, Cunningham ME, Pumberger M, Hughes AP, Cammisa FP. Degenerative lumbar spinal stenosis: evaluation and management. J Am Acad Orthop Surg 2012;20:527-35. [DOI] [PubMed] [Google Scholar]
- 11. Djurasovic M, Glassman SD, Carreon LY, Dimar JR. Contemporary management of symptomatic lumbar spinal stenosis. Orthop Clin North Am 2010;41:183-91. [DOI] [PubMed] [Google Scholar]
- 12. Amundsen T, Weber H, Lilleås F, et al. Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976) 1995;20:1178-86. [DOI] [PubMed] [Google Scholar]
- 13. Jönsson B, Strömqvist B. Symptoms and signs in degeneration of the lumbar spine. A prospective, consecutive study of 300 operated patients. J Bone Joint Surg [Br] 1993;75:381-5. [DOI] [PubMed] [Google Scholar]
- 14. Miyakoshi N, Hongo M, Kasukawa Y, Ishikawa Y, Shimada Y. Prevalence, spinal alignment, and mobility of lumbar spinal stenosis with or without chronic low back pain: a community-dwelling study. Pain Res Treat 2011;2011:340629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denard PJ, Holton KF, Miller J, et al. Lumbar spondylolisthesis among elderly men: prevalence, correlates, and progression. Spine (Phila Pa 1976) 2010;35:1072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomkins-Lane CC, Quint DJ, Gabriel S, Melloh M, Haig AJ. Nerve root sedimentation sign for the diagnosis of lumbar spinal stenosis: reliability, sensitivity, and specificity. Spine (Phila Pa 1976) 2013;38:E1554-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim YU, Kong Y-G, Lee J, et al. Clinical symptoms of lumbar spinal stenosis associated with morphological parameters on magnetic resonance images. Eur Spine J 2015;24:2236-43 [DOI] [PubMed] [Google Scholar]
- 18. Ogura H, Miyamoto K, Fukuta S, Naganawa T, Shimizu K. Comparison of magnetic resonance imaging and computed tomography-myelography for quantitative evaluation of lumbar intracanalar cross-section. Yonsei Med J 2011;52:137-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karadimas EJ, Sodium M, Smith FW, et al. Positional MRI changes in supine versus sitting postures in patients with degenerative lumbar spine. J Spinal Disord Tech 2006;19:495-500. [DOI] [PubMed] [Google Scholar]
- 20. Kanno H, Endo T, Ozawa H, et al. Axial loading during magnetic resonance imaging in patients with lumbar spinal canal stenosis: does it reproduce the positional change of the dural sac detected by upright myelography? Spine (Phila Pa 1976) 2012;37:E985-92. [DOI] [PubMed] [Google Scholar]
- 21. De Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med 2013;126(Suppl 1):S3-11. [DOI] [PubMed] [Google Scholar]
- 22. Delitto A, Piva SR, Moore CG, et al. Surgery versus nonsurgical treatment of lumbar spinal stenosis: a randomized trial. Ann Intern Med 2015;162:465-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fritz JM, Lurie JD, Zhao W, et al. Associations between physical therapy and long-term outcomes for individuals with lumbar spinal stenosis in the SPORT study. Spine J 2014;14:1611-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manchikanti L, Cash KA, McManus CD, et al. The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: a randomized, equivalence controlled trial. Pain Physician 2009;12:E341-54. [PubMed] [Google Scholar]
- 25. Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician 2012;15:371-84. [PubMed] [Google Scholar]
- 26. Ammendolia C, Stuber KJ, Rok E, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev 2013;8:CD010712. [DOI] [PubMed] [Google Scholar]
- 27. Parker SL, Godil SS, Mendenhall SK, et al. Two-year comprehensive medical management of degenerative lumbar spine disease (lumbar spondylolisthesis, stenosis, or disc herniation): a value analysis of cost, pain, disability, and quality of life: clinical article. J Neurosurg Spine 2014;21:143-9. [DOI] [PubMed] [Google Scholar]
- 28. Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine (Phila Pa 1976) 2000;25:1424-35; discussion 1435-6. [DOI] [PubMed] [Google Scholar]
- 29. Malmivaara A, Slätis P, Heliövaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976) 2007;32:1-8. [DOI] [PubMed] [Google Scholar]
- 30. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008;358:794-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearson A, Blood E, Lurie J, et al. Predominant leg pain is associated with better surgical outcomes in degenerative spondylolisthesis and spinal stenosis. Spine (Phila Pa 1976) 2011;36:219-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atlas SJ, Keller RB, Yen AW, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine Lumbar Spine Study. Spine (Phila Pa 1976) 2005;30:936-943. [DOI] [PubMed] [Google Scholar]
- 33. Kovaks FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis. Spine (Phila Pa 1976) 2011;36:E1335-E1351. [DOI] [PubMed] [Google Scholar]
- 34. Kurd MF, Lurie JD, Zhao W, et al. Predictors of treatment choice in lumbar spinal stenosis: a spine patient outcomes research trial study. Spine (Phila Pa 1976) 2012;37:1702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Epstein NE. Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord 1998;11:116-22; discussion 123. [DOI] [PubMed] [Google Scholar]
- 36. Kristof RA, Aliashkevich AF, Schuster M, et al. Degenerative lumbar spondylolisthesis-induced radicular compression: nonfusion-related decompression in selected patients without hypermobility on flexion-extension radiographs. J Neurosurg 2002;97(3 Suppl):281-6. [DOI] [PubMed] [Google Scholar]
- 37. Lombardi JS, Wiltse LL, Reynolds J, Widell EH, Spencer C. Treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976) 1985;10:821-7. [DOI] [PubMed] [Google Scholar]
- 38. Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg [Am] 1991;73:802-8. [PubMed] [Google Scholar]
- 39. Ghogawala Z, Benzel EC, Amin-Hanjani S, et al. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J Neurosurg Spine 2004;1:267-72. [DOI] [PubMed] [Google Scholar]
- 40. Martin CR, Gruszczynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine (Phila Pa 1976) 2007;32:1791-8. [DOI] [PubMed] [Google Scholar]
- 41. Ye Y, Chen D, Xu H. The comparison of instrumented and non-instrumented fusion in the treatment of lumbar spondylolisthesis: a meta-analysis. Eur Spine J 2014;23:1918-26. [DOI] [PubMed] [Google Scholar]
- 42. Høy K, Bünger C, Niederman B, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: a randomized clinical trial with 2-year follow-up. Eur Spine J 2013;22:2022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christensen A, Høy K, Bünger C, et al. Transforaminal lumbar interbody fusion vs. posterolateral instrumented fusion: cost-utility evaluation along side an RCT with a 2-year follow-up. Eur Spine J 2014;23:1137-43. [DOI] [PubMed] [Google Scholar]
- 44. Sigmundsson FG, Jönsson B, Strömqvist B. Preoperative pain pattern predicts surgical outcome more than type of surgery in patients with central spinal stenosis without concomitant spondylolisthesis. Spine (Phila Pa 1976) 2014;39:E199-210. [DOI] [PubMed] [Google Scholar]
- 45. Lurie JD, Tosteson TD, Tosteson A, et al. Long-term outcomes of lumbar spinal stenosis: eight-year results of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976) 2015;40:63-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Resnick DK, Watters WC, Mummaneni PV, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: lumbar fusion for stenosis without spondylolisthesis. J Neurosurg Spine 2014;21:62-6. [DOI] [PubMed] [Google Scholar]
- 47. Phillips FM, Slosar PJ, Youssef JA, Andersson G, Papatheofanis F. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine (Phila Pa 1976) 2013;38:E409-22. [DOI] [PubMed] [Google Scholar]
- 48. Willems PC, Staal JB, Walenkamp GHIM, de Bie RA. Spinal fusion for chronic low back pain: systematic review on the accuracy of tests for patient selection. Spine J 2013;13:99-109. [DOI] [PubMed] [Google Scholar]
- 49. Kleinstueck FS, Fekete TF, Mannion AF, et al. To fuse or not to fuse in lumbar degenerative spondylolisthesis: do baseline symptoms help provide the answer? Eur Spine J 2012;21:268-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Försth P, Michaëlsson K, Sandén B. Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis?: A two-year follow-up study involving 5390 patients. Bone Joint J 2013;95-B:960-5. [DOI] [PubMed] [Google Scholar]
- 51. Desai A, Ball PA, Bekelis K, et al. Surgery for lumbar degenerative spondylolisthesis in Spine Patient Outcomes Research Trial: does incidental durotomy affect outcome? Spine (Phila Pa 1976) 2012;37:406-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kao F-C, Tsai T-T, Chen L-H, et al. Symptomatic epidural hematoma after lumbar decompression surgery. Eur Spine J 2015;24:348-57. [DOI] [PubMed] [Google Scholar]
- 53. Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976) 2004;29:726-33; discussion 733-4. [DOI] [PubMed] [Google Scholar]
- 54. Kalanithi PS, Patil CG, Boakye M. National complication rates and disposition after posterior lumbar fusion for acquired spondylolisthesis. Spine (Phila Pa 1976) 2009;34:1963-9. [DOI] [PubMed] [Google Scholar]
- 55. O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine 2009;11:471-6. [DOI] [PubMed] [Google Scholar]
- 56. Ahn J, Iqbal A, Manning BT, et al. Minimally invasive lumbar decompression - the surgical learning curve. Spine J 2015. DOI: 10.1016/j.spinee2015.07.455. [DOI] [PubMed] [Google Scholar]
- 57. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg 2014;82:230-8. [DOI] [PubMed] [Google Scholar]
- 58. Overdevest GM, Jacobs W, Vleggeert-Lankamp C, et al. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database Syst Rev 2015;3:CD010036. [DOI] [PubMed] [Google Scholar]
- 59. Kanbara S, Yukawa Y, Ito K, Machino M, Kato F. Dynamic changes in the dural sac of patients with lumbar canal stenosis evaluated by multidetector-row computed tomography after myelography. Eur Spine J 2014;23:74-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miao J, Wang S, Park WM, et al. Segmental spinal canal volume in patients with degenerative spondylolisthesis. Spine J 2013;13:706-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine (Phila Pa 1976) 2007;32:1345-8. [DOI] [PubMed] [Google Scholar]
- 62. Sobottke R, Schlüter-Brust K, Kaulhausen T, et al. Interspinous implants (X Stop, Wallis, Diam) for the treatment of LSS: is there a correlation between radiological parameters and clinical outcome? Eur Spine J 2009;18:1494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park SC, Yoon SH, Hong YP, et al. Minimum 2-year follow-up result of degenerative spinal stenosis treated with interspinous U (Coflex). J Korean Neurosurg Soc 2009;46:292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jacobs WCH, Rubinstein SM, Willems PC, et al. The evidence on surgical interventions for low back disorders, an overview of systematic reviews. Eur Spine J 2013;22:1936-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu A-M, Zhou Y, Li Q-L, et al. Interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PLoS One 2014;9:e97142. [DOI] [PMC free article] [PubMed] [Google Scholar]