Abstract
Chronic osteomyelitis represents a progressive inflammatory process caused by pathogens, resulting in bone destruction and sequestrum formation.
It may present with periods of quiescence of variable duration, whereas its occurrence, type, severity and prognosis is multifactorial.
The ‘gold standard’ for the diagnosis of chronic osteomyelitis is the presence of positive bone cultures and histopathologic examination of the bone.
Its management remains challenging to the treating physician, with a multidisciplinary approach involving radiologists, microbiologists with expertise in infectious diseases, orthopaedic surgeons and plastic surgeons.
Treatment should be tailored to each patient according the severity and duration of symptoms, as well as to the clinical and radiological response to treatment.
A combined antimicrobial and surgical treatment should be considered in all cases, including appropriate dead space management and subsequent reconstruction. Relapse can occur, even following an apparently successful treatment, which has a major impact on the quality of life of patients and is a substantial financial burden to any healthcare system.
Cite this article EFORT Open Rev 2016;1:128–135. DOI: 10.1302/2058-5241.1.000017.
Keywords: osteomyelitis, chronic, pathogenesis, diagnosis, imaging, antibiotics, surgical treatment, complications
Introduction
Osteomyelitis is an ancient disease, which has been present for the last 250 million years and was first described in humans by Hippocrates.1 It is a progressive inflammatory process caused by pathogens, resulting in bone destruction and sequestrum formation.2,3 The infection can be limited to the bone, or it can propagate to the bone marrow, the periosteum and the surrounding soft tissues.2,4 It represents a major financial burden for every health system and substantially affects the quality of life of the affected patients and their families.
Chronic osteomyelitis may present as a recurrent or intermittent disease. The symptoms and their duration may vary considerably, whereas periods of quiescence can also be of variable duration. The incidence of relapse following an apparently ‘successful’ treatment remains high, making its management challenging for the treating physician.5 Assumed ‘remission’ should only be claimed after at least 12 months of follow-up, while ‘cure’ of the disease cannot be safely declared.
Prompt diagnosis and aggressive management of chronic osteomyelitis are critical to the prognosis and final outcome. Treatment aims to achieve the resolution of the infection and restoration of function.6 Historically, lengthy antibiotic regimes in combination with extensive surgical debridement have been used for its management.6 Even though the antibiotic choice, delivery type and duration remains controversial,7 it is generally accepted that adequacy of debridement with wide excision remains the most important clinical predictor of a successful outcome.8
A good understanding of the aetiopathogenesis and pathophysiological features of chronic osteomyelitis, along with the understanding of the treatment principles and options, is necessary to guide the treating physician to a successful outcome.
Classification
Even though several classification systems have been suggested, there is no consensus on which one is the most appropriate to use. In general terms, osteomyelitis is characterised as acute or chronic, based on its histopathological findings, rather than the duration of the infection.9 Acute osteomyelitis typically presents two weeks after bone infection, characterised by inflammatory bone changes.9 By contrast, chronic osteomyelitis typically presents six or more weeks after bone infection and is characterised by the presence of bone destruction and formation of sequestra.9,10
The most widely-used classification system of chronic osteomyelitis in adults is the Cierny–Mader classification (Table 1).6 It incorporates prognostic factors and delineates treatment for each clinical stage according to the anatomical type and physiological class of the host.11
Table 1.
Cierny–Mader classification system6
| Anatomical type | |
|---|---|
| Type | Characteristics |
| I | Medullary osteomyelitis |
| II | Superficial osteomyelitis |
| III | Localised osteomyelitis |
| IV | Diffuse osteomyelitis |
| Physiological class | |
| Type | Characteristics |
| A | Good immune system and delivery |
| B | Compromised locally (BL) or systemically (BS) |
| C | Requires suppressive or no treatment; Minimal disability; Treatment worse than disease; Not a surgical candidate |
| Factors affecting physiological class | |
| Systemic factors (S) | Local factors (L) |
| Malnutrition Renal or hepatic failure Diabetes mellitus Chronic hypoxia Immune disease Extremes of age Immunosuppression Immune deficiency Tobacco abuse Alcohol abuse Malignancy |
Chronic lymphedema Venous stasis Major vessel compromise Arteritis Extensive scarring Radiation fibrosis Small-vessel disease Neuropathy |
Osteomyelitis can also be classified according to the mechanism of infection (pathogenesis), as exogenous or haematogenous.3 Most commonly, chronic osteomyelitis is secondary to direct inoculation of pathogens into the bone at the time of trauma, as a result of surgical trauma (i.e. following open reduction and internal fixation of fractures), from chronic overlying open wounds or contiguous soft tissue infections.3,9 In haematogenous osteomyelitis, the pathogens seed into the bone through the systemic circulation, even though this type is predominately encountered in paediatric populations.3,9 In adults, it typically occurs secondarily from a distal site of infection, often involving the vertebral bodies of the lower spine and can also be associated with inflammation of the adjacent intervertebral discs.2,12
Some authors have suggested the distinction of another mechanism of osteomyelitis which is secondary to vascular insufficiency, as this presents with several distinct clinical and pathophysiological features.2 It predominantly occurs in patients suffering from diabetes mellitus and it is usually the result of a soft tissue infection of the foot that spreads to the bone.2
Epidemiology
The incidence of haematogenous osteomyelitis and the mortality associated with it has dramatically reduced following the introduction of antibiotics in the 1940s.1 Nevertheless, the incidence of chronic osteomyelitis following contiguous focus of infection has apparently increased, especially in developed countries.3,13 Possible aetiological factors include the ageing of the population, the increased prevalence of trauma, the rising prevalence of diabetic foot infections and improvements in the diagnosis of the disease.3,14 Trauma-induced osteomyelitis remains the most common cause,15,16 with infection rates in open long bone fractures ranging between 4% and 64%, whereas recurrence rates following bony infection have been reported to be as high as 20% to 30%.16,17 On the other hand, prosthetic joint infections represent a relatively new entity of chronic osteomyelitis. Their incidence is reported to be as high as 1.5% to 2.5%, even though rates of up to 20% have been reported following revision surgery.4
Aetiopathogenesis
Compared with other types of tissue, bone is relatively resistant to the development of infection.4,12 Nonetheless, following a large inoculation of pathogens, or even a smaller number of particularly virulent bacteria, infection may occur.4,12 The type of pathogen isolated is highly dependent on patient-related factors such as age, immune status, history of trauma and geographical location.18 Generally, haematogenous osteomyelitis is monomicrobic in nature,12 in contrast to contiguous-focus osteomyelitis that is polymicrobic.3,10,12
In adult chronic osteomyelitis, the most commonly involved pathogen is by far Staphylococcus aureus (S. aureus).3,9 Methicillin-resistant S. aureus (MRSA) has also been increasingly isolated from chronic osteomyelitis lesions.9 Other causative pathogens include Staphylococcus epidermidis, Pseudomonas aeruginosa, Serratia marcescens and Escherichia coli.3,9 Mycobacterial and fungal infections are generally uncommon and are often associated with immunodeficiency.3,9
Following the introduction of pathogens such as S. aureus into the bone marrow cavity, regardless of the route of access, they adhere to membrane proteins such as fibronectin or collagen receptors, establishing an infection.3 Other microbial factors prevent access by host defences or penetration of the surrounding tissues.2 This is achieved by attacking several types of host cells and degrading the extracellular matrix.2 S. aureus has also been reported to survive within host cells, a mechanism also used by other pathogens.2
Consequently various pathogens produce a relatively impermeable polysaccharide/protein matrix (biofilm)8 that can be multi-layered and embedded within a glycocalyx or within a slime layer.12 Surrounded by the biofilm, these pathogens present with an altered phenotype with regards to growth, gene expression and protein production2 that protects them from the host’s defence mechanisms and the systemic effect of antibiotics.3,19 This is in contrast to the initial infectious phase where the bacteria are still in a planktonic phase, having a high metabolic and generational rate, a factor that increases their sensitivity to common antibiotics.19 The pathogens can remain in this state for long periods of time and can cause flare-ups many years after the initial inoculation.
The inflammatory factors produced by the pathogens, as well as by the host’s leucocytes, along with the compression and obliteration of the vascular network around the involved area, represent the main mechanisms of tissue necrosis and bone destruction.2 The resulting avascular area becomes an ideal harbour for bacteria, as neither inflammatory cells nor antibiotic agents can reach it. Around this avascular area, there is reactive hyperaemia and increased osteoclastic activity, which in turn results in localised bone loss and osteoporosis.2 At the same time, the osteoblasts deposit periosteal new bone.2
Predisposing factors
Several predisposing factors for development of chronic osteomyelitis have been reported. A history of trauma, open fractures and surgery are the most commonly encountered factors.20 Other factors include diabetes, peripheral vascular disease, malnutrition, hypotension, chronic steroid use, malignancy, alcoholism, smoking, systemic or local immunocompromise, intravenous drug use and development of decubitus ulcers.6,9,12,20
Nowadays, the presence of implants is one of the most important predisposing factors. Soon after implantation they become coated with the host’s proteins, an excellent source for attachment of pathogens.12 The biofilm they produce protects them from the host’s defence mechanisms so that they can re-activate months or years later.12
Clinical features
The clinical features of chronic osteomyelitis are usually not specific and therefore difficult to recognise. It can also be difficult to differentiate signs of osteomyelitis from soft tissue infection, especially in diabetic patients. A variety of symptoms have been reported, ranging from no skin lesions to open wounds over fractured bones. Chronic pain, an area of erythema around the affected bone, swelling and bone tenderness, impaired wound healing often associated with tissue necrosis, increased drainage or persistent sinus tracts, chills, low grade fever and general malaise are some of the most commonly reported clinical symptoms (Fig. 1).2,3,9,10,20 In neglected cases, patients typically report a cyclical pain that increases in severity, is associated with fever and subsides when pus breaks out through the fistula.18
Fig. 1.
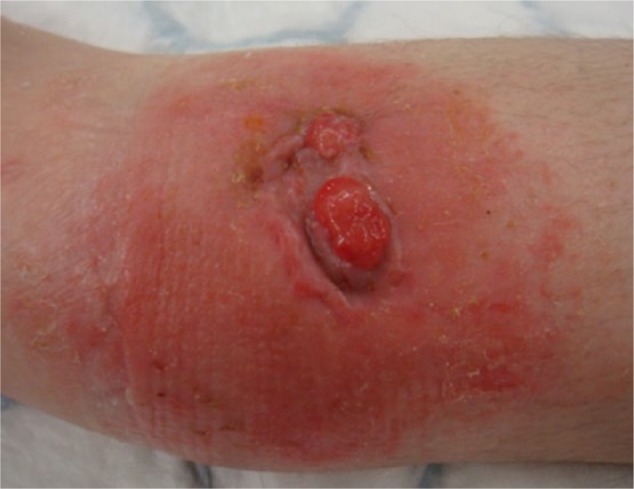
Patient presented with a discharging sinus and surrounding cellulitis over the distal tibia, 13 months following a closed distal tibia fracture that was surgically managed.
Imaging features
Imaging can help both in the characterisation and differential diagnosis of osteomyelitis. Plain radiography, a first line imaging modality, is of very low sensitivity and specificity. It is useful, however, to differentiate osteomyelitis from other pathologies such as fractures and malignancies (primary or metastatic).9 It can reveal soft tissue swelling, periosteal reaction, loss of definition, loss of bone density and osteolysis, as early as 10 to 21 days after the bone infection, but may not be detectable until there is a loss of 30% to 50% of the bone mineral content.2,9,12,20 Late signs include increased bone resorption, formation of sequestra and new bone formation in the periosteum or endosteum.20
CT provides the most detailed imaging of the cortical bone, being especially useful in the identification of sequestra and intra-osseous fistulae.2 It also demonstrates both the periosteal reaction and the bone marrow involvement, as well as demonstrating the soft tissue condition at an early stage.10,20 Even though in the presence of implants its quality degrades, it is routinely used for pre-operative planning and to guide biopsies.6,20
MRI has an advantage in assessing the bone marrow and the surrounding soft tissues, defining the associated oedema and hyperaemia that is present in the very early stages of the disease.2,21 It can differentiate bone from soft tissue infections and it can also be used as an adjunct in estimating the margins required for the debridement, or to assess the response to therapy.2,20 Nevertheless, it is of limited value in the presence of implants, scar tissue and recent operations.21
Routine bone scintigraphy has also been used in the diagnosis of chronic osteomyelitis. Nonetheless, it is associated with a limited specificity and false-positive results, especially in patients who have had diabetic arthropathy, gout, trauma and recent surgery. Therefore, its use is not recommended as a single imaging modality.2,21 Leucocyte scintigraphy, on the other hand is reported to be an accurate technique for diagnosing chronic osteomyelitis in the peripheral skeleton, but its diagnostic accuracy in the axial skeleton is significantly reduced.21 False-positive results are also reported in the presence of mechanically unstable nonunions, or peri-articular nonunions with associated post-traumatic arthropathy.20
Positron emission tomography (PET) has the highest sensitivity and specificity, delineating lesions with their concomitant inflammatory activity at very early stages.2,18 Its availability and associated costs, however, limit its routine use.6 However, a meta-analysis investigating the accuracy of diagnostic imaging for chronic osteomyelitis, showed that fluorodeoxyglucose PET has the highest diagnostic accuracy, both for confirming and excluding the diagnosis of chronic osteomyelitis, especially in the axial skeleton.21,22
Ultrasonography (US) is mainly used at the early stages for detecting purulent collections within the soft tissues.2 Some authors suggest that in some cases it can be diagnostic, but reliable estimates of its specificity and sensitivity are not available.18
Laboratory evaluation
A number of laboratory investigations can help with the diagnosis, even though they generally lack specificity for chronic osteomyelitis. The presence of inflammatory markers such as an increased C-reactive protein (CRP) level and increased erythrocyte sedimentation rate (ESR) may be used as an adjunct to the diagnosis and for monitoring clinical response to treatment.2,3,9,20 By contrast, in most cases, the presence of persistently normal CRP and ESR levels usually rules out osteomyelitis, even though in the presence of a discharging sinus or a background of diabetes, this may not be the case.9 Leukocytosis and elevated alpha-1 acid glycoprotein levels may also be present, but these are not reliable indicators.3,9 On the contrary, white cell count (WCC) can be within normal limits.10,20
Most importantly, for obtaining a definitive diagnosis in chronic osteomyelitis, the presence of positive microbial cultures from bone biopsies around areas of bone necrosis is considered essential.9 These should not be obtained from superficial wounds or fistulae, as these have been associated with low accuracy because of inclusion of non-pathogenic micro-organisms that colonise the wound.2,3 False-negative results are also reported, mainly because of the patchy distribution of the osteomyelitis lesions in the bone.2 Often, more than one organism is involved and these may include anaerobic, mycobacterial and fungal organisms so that specific cultures and microbiological testing may be necessary.9 Especially in cases of implant-related osteomyelitis, samples from up to five sites around the implant should be obtained to increase the diagnostic yield, and prolonged enrichment broth cultures are often necessary.2,20 It is very important that the cultures are obtained before commencing any antimicrobial treatment, to avoid false-negative results. Conventional blood cultures are generally useful only in cases of haematogenous osteomyelitis.
Histopathology of tissue specimens obtained during biopsy or debridement can also provide additional important information. Significant presence of neutrophils is indicative of infection, whereas positive special staining suggests the presence of pathogens earlier than the culture results.2
Diagnostic approach
The diagnosis of chronic osteomyelitis can often be challenging, but it is important to realise that an early diagnosis will lead to a more favourable outcome. A combination of high index of clinical suspicion and recognition of the clinical symptoms, along with imaging and laboratory investigations, can aid the diagnosis. Especially in patients with compromised peripheral vasculature, diagnosis of chronic osteomyelitis can be even more difficult, as the symptoms are usually subtle and systemic features are absent.12 The clinical examination should be focussed on identifying a possible nidus of infection.9 As mentioned, the most sensitive criterion is the presence of positive bacterial cultures from bone biopsies obtained from areas with bone necrosis.9
Management
The management of chronic osteomyelitis depends on the duration and severity of symptoms, as well as the presence of medical comorbidities. In most cases, the surrounding soft tissue envelope is compromised and the vascularisation of the area is poor, a factor that should be taken into account. The main goal of treatment is to eliminate the inflammatory process by removing all the pathogens and the devitalised tissue, and if healing has not occurred, to promote healing by optimising the mechanical and biological environment. This can be achieved with a combination of treatment with antibiotic agents, surgical debridement and management of the dead space.
Great care should also be taken in diabetic patients. Symptoms may not be clear in these cases, whereas concomitant vascular compromise and peripheral neuropathy can complicate the choice of treatment. A small but important percentage of these patients will require limb amputation.2
Antibiotic treatment
Initial empiric antibiotic treatment should be commenced as soon as the culture samples have been obtained. The antibiotic regimen should then be tailored to the results of the cultures and the sensitivities.6
Most authors recommend a four to six weeks’ duration of antibiotic therapy.3,5,23 This is based on the rationale that three to four weeks are required for the revascularisation of the bone, which gives a period of opportunity for the antimicrobial agents to infiltrate the inflamed area and attack the pathogens which are at that point susceptible to antibiotics.3,5 Nevertheless, no strong evidence supports this recommendation, or that prolonged antibiotic therapy reduces the rate of recurrence.5,23 In fact, prolonged antibiotic treatment is associated with increased risk of adverse events, costs and antibiotic resistance.23
The type of antibiotics and route of administration remain a matter of debate, with no clear evidence to guide practice. A recent Cochrane review by Conterno et al failed to show any difference between oral antibiotics compared with parenteral antibiotics in the rate of remission at the end of therapy and after 12 months or more of follow-up,3 a finding confirmed by other authors.5 The oral route of administration seems attractive, as if oral agents offer the same success with parenteral antibiotics, they have similar risks of adverse events but they are easier to administer and are associated with lower medical costs and reduced length of hospital stay.3,5
What is most important for the antibiotic agent used is the bone penetration it can achieve, as well as if it exceeds the minimum inhibitory concentrations for the isolated pathogen.5 The antibiotic regime used should also be based on the results of the cultures and sensitivities obtained by bone biopsies. In polymicrobial cases or in the presence of prosthetic infections, a combination of antibiotic agents is recommended as this has been reported to reduce the recurrence rate.5 Lastly, pathogens such as S. aureus have been reported to acquire resistance to a number of antibiotics, a facet that makes treatment choice even more difficult.12
Additionally, local antibiotics in the form of polymethylmethacrylate (PMMA) beads can be used to deliver high doses of antibiotics to the surrounding tissues.20,24 Several studies have supported their effectiveness, with the additional advantage of managing the dead space resulting from the debridement.24,25 The antibiotic agent selected for the mixture should be active against the targeted bacterial pathogen. In contrast to the PMMA beads, calcium sulfate beads provide a more rapid release of high concentrations of antibiotics, having the advantage of being biodegradable and therefore precluding the need for removal.26 Similarly, hydroxyapatite-ceramic beads and polylactide-polyglycolide co-polymer implants are also biodegradable and have been successfully used in the treatment of chronic osteomyelitis.26
With regard to the choice of antibiotics, our recommendation is to tailor treatment to each individual according to the type and extent of osteomyelitis, medical comorbidities, isolated organism(s) and whether this is the first presentation or a recurrence. For common micro-organisms like S. aureus, we recommend treatment with IV Nafcillin or Cefazolin in case of methicillin-susceptible S. aureus (MSSA), and treatment with IV Vancomycin for MRSA. Most commonly, at least six weeks of antimicrobial therapy is necessary, after which re-assessing the patient and further discussion with the microbiologists is advocated.
Surgical treatment
The cornerstone of the treatment of chronic osteomyelitis is surgical management (Table 2). This should include an adequate surgical debridement to remove all pathogens along with their biofilms and sequestra (dead bone) that act as a foreign material, reaching down to healthy and viable tissue (Fig. 2a). The local soft tissue envelope should also be debrided and reconstructed if indicated. In cases of significant extension of the osteomyelitis into the medullary canal, debridement with the reamer-irrigator-aspirator (RIA) technique and subsequent insertion of an antibiotic-impregnated intramedullary cement rod has been suggested.20 A more aggressive approach with reaming of the canal and, if involving a joint, the two adjacent canals, is advocated to decrease the risk of recurrence, as macroscopic determination of the extent of bone marrow infiltration is not reliable.
Table 2.
Different surgical techniques for treating chronic osteomyelitis
| Surgical technique | Advantages | Disadvantages |
|---|---|---|
| Conventional reaming of the IM canal | - Clearance of intramedullary sepsis | - Risk of fracture - Risk of bleeding - Need for fenestration of the distal diaphysis to allow drainage of the irrigation fluids |
| RIA technique | - Clearance of intramedullary sepsis - Less traumatic than convectional reaming |
- Risk of fracture - Risk of bleeding |
| Primary bone grafting / bone graft substitutes | - Single-stage procedure - Superior osteoconductivity and osteoinductivity of the bone graft |
- Confined to small defects / limited availability of bone graft - Risk of early resorption / highly depends on the soft tissue bed - Risk of relapse of infection - Graft incorporation is slow and unreliable - Donor site morbidity |
| Antibiotic-impregnated cement spacers / cement nails / antibiotic beads |
- Slow release of high concentrations of antibiotics, avoiding their systemic effects - Easy to mix - form into various shapes and sizes - Cement nails can provide some stability to associated fractures |
- Lack of biodegradability in some carriers / need for two-stage procedures - Concern that they can act as a foreign body, therefore harbouring infection - Increased risk of antibiotic resistance |
| Bioactive glass | - Anti-microbial, osteoconductive and angiogenic properties | - Depends on good soft-tissue coverage |
| Induced membrane (Masquelet) technique | - Combines the advantages of antibiotic-impregnated cement spacers with those of delayed bone grafting - The induced membrane is highly vascularised, rich in growth and osteoinductive factors - Offers a confined space for the application of the bone graft |
- Two-stage procedure - Increased risk of antibiotic resistance - Limited availability of bone graft - Can be associated with prolonged healing and recovery time |
| Circular external fixation devices and bone transport | - Increased blood flow in the area of corticotomy - Minimally-invasive nature |
- Distraction is often limited because of the neurovascular bundle contracture - Can be associated with pain for distraction > 2 cm - Pin-site complications - Need for specialised equipment - Need for re-interventions |
| Local flaps | - Transfer of well-vascularised tissue that aids wound and bone healing | - Limited by pedicle length - Donor-site morbidity |
| Vascularised free flaps | - Transfer of well-vascularised tissue that aids wound and bone healing | - Donor-site morbidity - Need for microsurgical anastomoses - Limited by peripheral artery disease - Prolonged operating time - High risk of early complications / risk of graft failure |
| Megaprosthesis | - Restores limb function quickly - No need for harvesting bone - ‘One-shot’ surgery |
- Risk of residual infection and early loosening - Risk of dislocation - Risk of revision surgery |
| Amputation | - Early mobilisation - One shot surgery |
- Soft tissue reconstruction procedures - Compromised function - Regular revisions of the prosthetic limb |
IM: intramedullary
RIA: Reamer/Irrigator/Aspirator
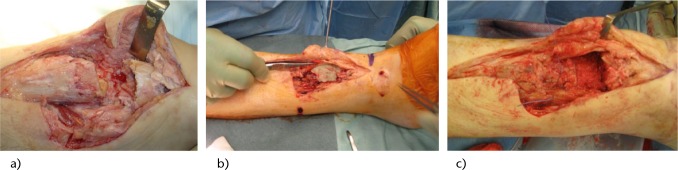
Fig. 2.
Following the excision of the sinus tract and radical surgical debridement of the impaired bone, a bone defect of 5 cm was formed. This was managed with a two-staged procedure (Masquelet technique). During the first stage, an antibiotic-loaded cement spacer was inserted, and the bone was stabilised with an external fixator. Two months later, the second stage involved incision of the induced membrane and removal of the cement spacer. The bone defect was subsequently filled with graft obtained from the ipsilateral femur using the RIA technique, mixed with BMP-7. Finally, the membrane was closed and the long bone was internally fixed. a) Radical debridement of the devitalised tissue and resulting bone defect; b) Induced membrane around the cement spacer, two months after the first stage procedure; c) Containment of the graft within the membrane.
Adequate debridement should not be limited by any concerns of resulting osseous and/or soft-tissue defects,20 as inadequate debridement has been associated with high incidence of recurrence.3 Following debridement, samples from the involved bone, sinus tract and surrounding tissues should be sent for pathological examination to ensure there are no malignant changes.20
Even though the need for surgical debridement in chronic osteomyelitis is unquestionable, many believe that it alone cannot sustain remission and that combination with antibiotics offers a better outcome.20 Though, one should bear in mind that not all cases of chronic osteomyelitis require surgical intervention, as the health state of the patient and the associated comorbidities that might be present could be a contraindication to operative intervention, especially in the spine. In these cases suppressive treatment with appropriate antibiotics can be considered.
Management of dead space
Following an aggressive debridement that may be required to remove all devitalised tissue, a large bone defect (dead space) may be formed.3 This space needs appropriate management for the eradication of the infection and subsequent implantation of graft materials to allow bone regeneration.
In general terms, the choice of the reconstruction technique depends on the characteristics of the lesion following the debridement and the physiological grading of the host. Primary bone grafting procedures are often not associated with good success rates because of the resorption of the bone graft due to ongoing inflammation and/or infection.2 Antibiotic-impregnated cement spacers and antibiotic beads can be used in cases of two-stage procedures, temporarily filling the dead space until reconstruction is performed. The induced membrane (Masquelet) technique has also been used with encouraging results (Figs 2b, 2c and 3),27 and circular external fixation devices and bone transport is another option in managing critical size bone defects.27 Local flaps including muscle flaps, pedicled muscle flaps, myocutaneous flaps and osseous flaps have been used to optimise the impaired soft tissue envelope, with good results.6,19,20,28. Vascularised free flaps have also been used to cover large defects where the local tissues are impaired.6,19,20
Fig. 3.
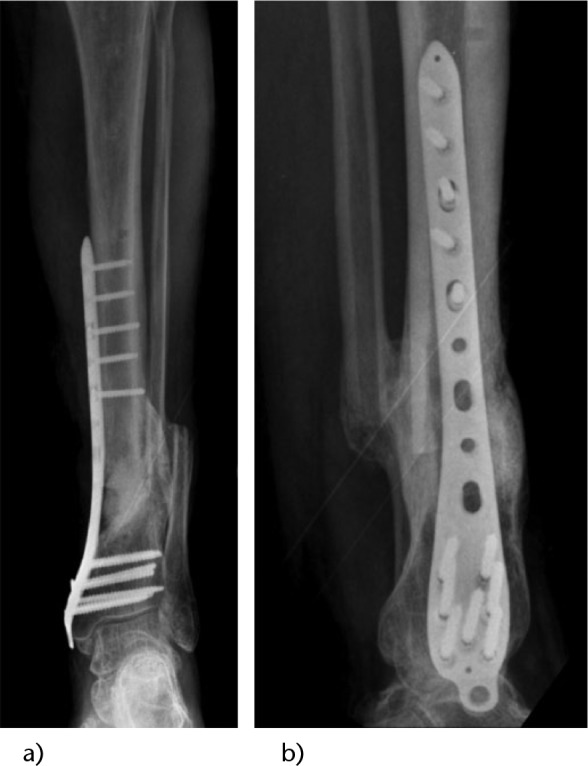
Radiographs taken nine months post-revision surgery, showing good incorporation of the graft and continuity of the tibia. a) Anteroposterior (AP) radiograph; b) lateral (LAT) radiograph.
Recently, the use of materials such as bioactive glass, in concurrence with antibiotic therapy, has been reported as safe and effective as a bone substitute in the presence of infection.29 Bioactive glass is a synthetic, biocompatible material that combines osteoconductive, angiogenic and antimicrobial properties, resulting in its integration into bone and soft tissues, thus becoming a potentially useful adjunct in the management of dead space.29
Complications
Numerous complications may arise due to the chronic inflammation and infective process. Abscess formation, sinus tracts and extension to adjacent structures are some of the most commonly encountered complications. Nonetheless, the most important, easily missed complication is that of malignant transformation of chronic osteomyelitis, also referred to as Marjolin’s ulcer.19 The incidence of Marjolin’s ulcer is higher in developing countries with limited medical resources, whereas it occurs in 1.6% to 23% of all patients with chronic osteomyelitis.30-32 Marjolin’s ulcers mainly involve aggressive squamous cell carcinomas (SCC), with a latent period of 27 to 30 years from onset of osteomyelitis to malignant transformation. The long duration of chronic osteomyelitis is the single most important predictive factor.30,31,33
Summary and conclusions
Chronic osteomyelitis continues to be a serious health problem worldwide, while representing an economic burden to any health system. Its occurrence, type, severity and prognosis depend on various factors, including the characteristics and virulence of the infecting pathogen, the physiological class of the host and the mechanism (source) of the infection. Before the initiation of treatment, it is very important that the causal host comorbidities, such as diabetes and peripheral vascular disease, are addressed. On the other hand, prevention of osteomyelitis in the form of focussed antibiotic prophylaxis in surgical and traumatic wounds, as well in prosthetic surgery, is of paramount importance.
The ‘gold standard’ for the diagnosis of chronic osteomyelitis is the presence of positive bone cultures and histopathological examination of the bone. Fluorodeoxy-glucose PET is the imaging technique with the highest diagnostic accuracy, but because of its limited availability, leucocyte scintigraphy can be used as an alternative in the peripheral skeleton.
The management of chronic osteomyelitis is challenging to the treating physician, complicated by the presence of infection, sequestra and impaired local vascularity with a compromised tissue envelope. A multidisciplinary approach involving radiologists, microbiologists with expertise in infectious diseases, orthopaedic surgeons and plastic-reconstructive surgeons is advocated. The treating physician should individualise treatment according to the patient’s severity and duration of symptoms, as well as the clinical and radiological response to treatment. A combined antimicrobial and surgical treatment should be considered in all cases, as well as appropriate dead space management and later skeletal reconstruction. Even following long periods of antibiotic treatment and recurrent surgical debridement, exacerbations can occur for many years. The follow-up is still a matter of debate, but most experts agree that this should be as long as five years, as incidence of relapse remains high.
A sound understanding of the aetiology, mechanisms of infection and pathophysiology of the chronicity of chronic osteomyelitis can aid the treating physician in individualising treatment for each patient. Further research on the biokinetics of the different pathogens, including the biofilm properties, can help in the development of novel therapies for treating chronic osteomyelitis.
Footnotes
Conflict of Interest: PG has received financial support outside of the current work in the form of consultancy fees and grants from Deput Synthes, Stryker and Zimmer Biomet, and royalties from Zimmer Biomet.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Klenerman L. A history of osteomyelitis from the Journal of Bone and Joint Surgery: 1948 to 2006. J Bone Joint Surg [Br] 2007;89:667-70. [DOI] [PubMed] [Google Scholar]
- 2. Lew DP, Waldvogel FA. Osteomyelitis. Lancet 2004;364:369-79. [DOI] [PubMed] [Google Scholar]
- 3. Conterno LO, Turchi MD. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev 2013;9:CD004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jorge LS, Chueire AG, Rossit AR. Osteomyelitis: a current challenge. Braz J Infect Dis 2010;14:310-315. [PubMed] [Google Scholar]
- 5. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012;54:393-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parsons B, Strauss E. Surgical management of chronic osteomyelitis. Am J Surg 2004;188(Suppl):57-66. [DOI] [PubMed] [Google Scholar]
- 7. Conterno LO, da Silva Filho CR. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev 2009;3:CD004439. [DOI] [PubMed] [Google Scholar]
- 8. Forsberg JA, Potter BK, Cierny G, 3rd, Webb L. Diagnosis and management of chronic infection. J Am Acad Orthop Surg 2011;19:S8-S19. [DOI] [PubMed] [Google Scholar]
- 9. Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. Am Fam Physician 2011;84:1027-33. [PubMed] [Google Scholar]
- 10. Sia IG, Berbari EF. Infection and musculoskeletal conditions: osteomyelitis. Best Pract Res Clin Rheumatol 2006;20:1065-81. [DOI] [PubMed] [Google Scholar]
- 11. Cierny G, 3rd, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res 2003;(414):7-24. [DOI] [PubMed] [Google Scholar]
- 12. Brady RA, Leid JG, Costerton JW, Shirtliff ME. Osteomyelitis: clinical overview and mechanisms of infection persistence. Clin Microbiol Newsl 2006;28:65-72. [Google Scholar]
- 13. Sanders J, Mauffrey C. Long bone osteomyelitis in adults: fundamental concepts and current techniques. Orthopedics 2013;36:368-75. [DOI] [PubMed] [Google Scholar]
- 14. Bradshaw L, Wasiak J, Cleland H. Is operative management of fractures safe in the collocated burn and fracture injury? Injury 2015;46:1145-9. [DOI] [PubMed] [Google Scholar]
- 15. McGrory JE, Pritchard DJ, Unni KK, Ilstrup D, Rowland CM. Malignant lesions arising in chronic osteomyelitis. Clin Orthop Relat Res 1999;(362):181-9. [PubMed] [Google Scholar]
- 16. Mathews JA, Ward J, Chapman TW, Khan UM, Kelly MB. Single-stage orthoplastic reconstruction of Gustilo-Anderson Grade III open tibial fractures greatly reduces infection rates. Injury 2015;46:2263-6. [DOI] [PubMed] [Google Scholar]
- 17. Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg [Am] 2004;86-A:2305-18. [DOI] [PubMed] [Google Scholar]
- 18. Chihara S, Segreti J. Osteomyelitis. Dis Mon 2010;56:5-31. [DOI] [PubMed] [Google Scholar]
- 19. Panteli M, Puttaswamaiah R, Lowenberg DW, Giannoudis PV. Malignant transformation in chronic osteomyelitis: recognition and principles of management. J Am Acad Orthop Surg 2014;22:586-94. [DOI] [PubMed] [Google Scholar]
- 20. Mouzopoulos G, Kanakaris NK, Kontakis G, et al. Management of bone infections in adults: the surgeon’s and microbiologist’s perspectives. Injury 2011;42:S18-S23. [DOI] [PubMed] [Google Scholar]
- 21. Termaat MF, Raijmakers PG, Scholten HJ, et al. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg [Am] 2005;87:2464-71. [DOI] [PubMed] [Google Scholar]
- 22. Shemesh S, Kosashvili Y, Groshar D, et al. The value of 18-FDG PET/CT in the diagnosis and management of implant-related infections of the tibia: a case series. Injury 2015;46:1377-82. [DOI] [PubMed] [Google Scholar]
- 23. Bernard L, Dinh A, Ghout I, et al. ; Duration of Treatment for Spondylodiscitis (DTS) Study Group. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385:875-82. [DOI] [PubMed] [Google Scholar]
- 24. Hake ME, Young H, Hak DJ, et al. Local antibiotic therapy strategies in orthopaedic trauma: practical tips and tricks and review of the literature. Injury 2015;46:1447-56. [DOI] [PubMed] [Google Scholar]
- 25. Barth RE, Vogely HC, Hoepelman AI, Peters EJ. ‘To bead or not to bead?’ Treatment of osteomyelitis and prosthetic joint-associated infections with gentamicin bead chains. Int J Antimicrob Agents 2011;38:371-5. [DOI] [PubMed] [Google Scholar]
- 26. Rao N, Ziran BH, Lipsky BA. Treating osteomyelitis: antibiotics and surgery. Plast Reconstr Surg 2011;127:177S-187S. [DOI] [PubMed] [Google Scholar]
- 27. Marais LC, Ferreira N. Bone transport through an induced membrane in the management of tibial bone defects resulting from chronic osteomyelitis. Strategies Trauma Limb Reconstr 2015;10:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caterson EJ, Singh M, Turko A, Weaver MJ, Talbot S. The medial femoral condyle free osteocutaneous flap for osteomyelitis in pilon fractures. Injury 2015;46:414-8. [DOI] [PubMed] [Google Scholar]
- 29. Aurégan JC, Bégué T. Bioactive glass for long bone infection: a systematic review. Injury 2015;46:S3-S7. [DOI] [PubMed] [Google Scholar]
- 30. Altay M, Arikan M, Yildiz Y, Saglik Y. Squamous cell carcinoma arising in chronic osteomyelitis in foot and ankle. Foot Ankle Int 2004;25:805-9. [DOI] [PubMed] [Google Scholar]
- 31. Kerr-Valentic MA, Samimi K, Rohlen BH, Agarwal JP, Rockwell WB. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg 2009;123:184-91. [DOI] [PubMed] [Google Scholar]
- 32. Onah II, Olaitan PB, Ogbonnaya IS, Onuigbo WI. Marjolin’s ulcer (correction of ucler) at a Nigerian hospital (1993-2003). J Plast Reconstr Aesthet Surg 2006;59:565-6. [DOI] [PubMed] [Google Scholar]
- 33. Ogawa B, Chen M, Margolis J, Schiller FJ, Schnall SB. Marjolin’s ulcer arising at the elbow: a case report and literature review. Hand (N Y) 2006;1:89-93. [DOI] [PMC free article] [PubMed] [Google Scholar]