Abstract
Autoantibodies are a hallmark of autoimmune diseases such as lupus and have the potential to be used as biomarkers for diverse diseases, including immunodeficiency, infectious disease, and cancer. More precise detection of antibodies to specific targets is needed to improve diagnosis of such diseases. Here, we report the development of reusable peptide microarrays, based on giant magnetoresistive (GMR) nanosensors optimized for sensitively detecting magnetic nanoparticle labels, for the detection of antibodies with a resolution of a single post-translationally modified amino acid. We have also developed a chemical regeneration scheme to perform multiplex assays with a high level of reproducibility, resulting in greatly reduced experimental costs. In addition, we show that peptides synthesized directly on the nanosensors are approximately two times more sensitive than directly spotted peptides. Reusable peptide nanosensor microarrays enable precise detection of autoantibodies with high resolution and sensitivity and show promise for investigating antibody-mediated immune responses to autoantigens, vaccines, and pathogen-derived antigens as well as other fundamental peptide–protein interactions.
Keywords: nanosensors, peptide microarray, regeneration, giant magnetoresistance, autoantibody, lupus
Graphical abstract
Autoantibodies, or antibodies that bind to self-antigens, are associated with pathogenesis of autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).1–3 Autoantibodies predate clinical onset of SLE and RA and are used in their diagnosis.4,5 Interestingly, autoantibodies have also been identified in immunodeficiency,6 infectious disease,7,8 and multiple types of cancer9–12 and have potential for use as early stage biomarkers to diagnose cancer.10–12 With this increased understanding of the significance of autoantibodies and following on the revolutionizing success of the use of DNA microarrays for analyzing gene expression,13,14 protein microarrays have been recognized as a high-performance technique for the multiplexed detection of autoantibodies in autoimmune diseases15,16 and cancer.10,17 Unlike nucleic acids, however, protein replication or amplification in the sample being studied is not possible with existing tools; thus, analysis of proteins requires high binding capacity and high sensitivity of protein microarrays.18,19 In addition, protein libraries immobilized on microarrays are produced through costly processes such as expression of recombinant DNAs and purification of their products. Furthermore, proteins can be degraded during microarray production processes such as purification, printing, and immobilization. To overcome these problems, one approach implements the entire process on the microarray by printing complementary DNAs and translating them into their corresponding proteins.20 Another approach is peptide microarrays, which can be synthesized in situ or off-chip.19 While peptide microarrays are limited to incorporating linear antigens or epitopes (i.e., binding sites), they have a number of advantages compared to proteins, including lower cost and greater resolution. Importantly, the greater stability of peptides opens up the possibility of regeneration of the microarrays under severe conditions that often denature proteins on the microarrays. Microarray regeneration would reduce experimental cost, duration, and variability, especially intermicroarray variation. Additionally, peptide microarrays enable the precise examination of specific post-translational modifications.
Our group has developed GMR nanosensors capable of detecting multiple target proteins in the format of sandwich assays at femtomolar sensitivities.21,22 The GMR nanosensors employ the effect of giant magnetoresistance, rooted in quantum mechanics, to measure changes in the resistance of the sensor induced by a stray field from magnetic nanoparticles (MNPs) bound to the surface of the sensor via biological complexes.23 In the current study, we have developed reusable peptide GMR nanosensor microarrays to detect antibodies at a resolution of a single post-translationally modified amino acid. Using digital micromirror device (DMD)-based photolithography,24,25 peptides have been synthesized in situ on the GMR nanosensor microarray. To the best of our knowledge, in situ synthesis of peptides on GMR nanosensors has not been demonstrated prior to this work.
RESULTS AND DISCUSSION
Development of Peptide Nanosensor Microarrays
To create the microarrays, peptides (Figure 1a) were spotted on the nanosensors of a GMR nanosensor chip. Antibody-containing samples were added to probe the microarray, allowing the antibodies to bind to target peptides. The bound antibodies were labeled with biotinylated, species-specific secondary antibodies, and streptavidin-coated MNPs were used as labeling tags (Figure 1b). The stray field from the bound MNPs disturbs spin-dependent scattering of electrons passing through the nanoscale GMR nanosensor, which results in changes in electrical resistance. The resistance changes are monitored as GMR sensor signals in real time using the double modulation scheme, as described previously.26,27
Figure 1.
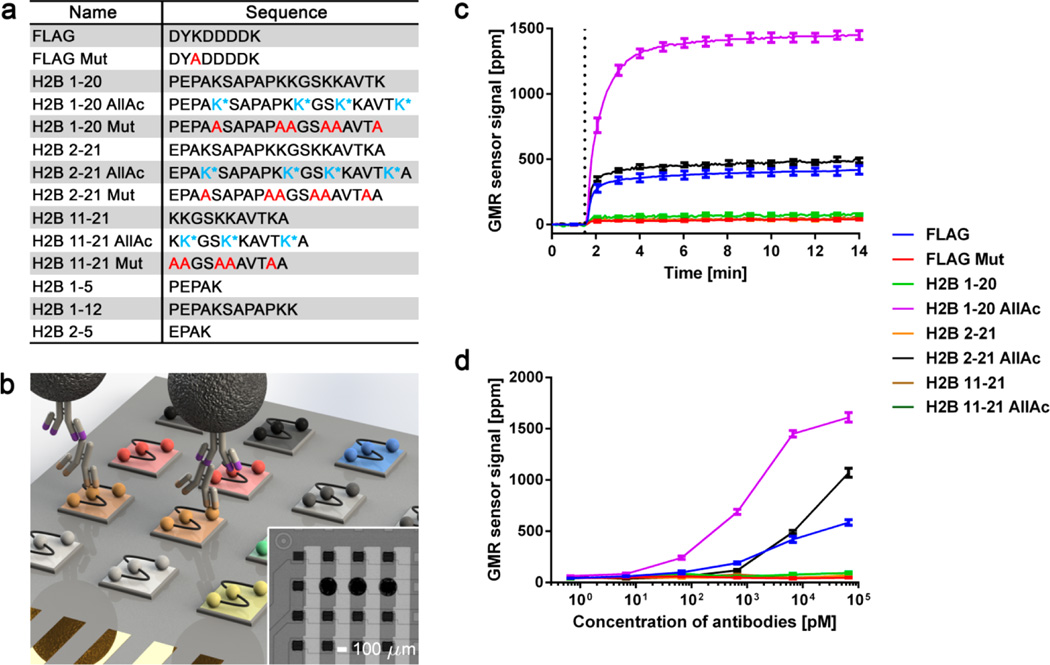
Development and validation of GMR nanosensor peptide microarrays. (a) List of peptides used in GMR nanosensor microarray. Acetylated lysine (K*) and alanine substitution (A) are represented in blue and red, respectively. (b) Schematic of GMR nanosensor peptide microarray (not to scale). A panel of peptides (indicated by different colors) was spotted on a GMR nanosensor chip. Antibody-containing samples were used to probe the microarrays, combined with biotinylated secondary antibodies (illustrated with purple tips) and streptavidin-coated MNPs. The stray field from the bound MNPs was detected by the nanosensors underneath. Inset: 16 sensors from an 8 × 8 GMR nanosensor chip (peptides were spotted on 3 sensors in the second row from the top). (c) Real-time signals obtained from the GMR nanosensor peptide microarray probed with anti-FLAG and anti-K5Ac antibodies. MNPs were added to the microarray at ~1.5 min as indicated by the dotted line. (d) Titration curves of anti-FLAG and anti-K5Ac antibodies measured with the microarrays. Error bars represent standard deviations of 4 identical sensor signals.
Our peptide library consisted of the FLAG octapeptide (DYKDDDDK) and a mutated control as well as peptides corresponding to post-translationally modified forms of the N-terminal tail of histone H2B peptides. The FLAG octapeptide is an engineered tag used in protein purification and was selected because a well-characterized anti-FLAG monoclonal antibody (M2 clone) is commercially available and known to bind the first four amino acid (DYKD) of FLAG.28 Histone H2B functions in packaging genomic DNA into nucleosomes, the primary component of chromatin. The N-terminal tail of H2B is naturally linear and undergoes post-translational modification, such as acetylation and methylation, which alters chromatin structure and the accessibility of DNA to transcriptional machinery.29 In addition, the N-terminal tail of H2B is a known autoantigen in autoimmune diseases,15,25 and post-translational modification has been proposed as a mechanism by which immune tolerance to self-proteins is lost.30,31 To detect autoantibodies to such post-translationally modified H2B and different segments of H2B, acetylated, mutated, or unmodified H2B peptides with different segments or lengths as well as their respective negative controls were included in our peptide library (Figure 1a).
To test the sensitivity and specificity of GMR nanosensor microarrays, we probed them with anti-FLAG and anti-K5Ac antibodies that specifically bind to FLAG and H2B with an acetylated lysine on the fifth sequence from the N-terminus (K5Ac), respectively. After incubation with secondary antibodies, the microarray was inserted into a reader station, and MNPs were added to the microarray at ~1.5 min (Figure 1c). The results show that anti-FLAG antibodies bound to the FLAG octapeptide, and not to a mutant peptide with a K3A substitution (DYADDDDK). Similarly, anti-K5Ac antibodies bound to H2B peptides with K5Ac (H2B 1–20 AllAc and H2B 2–21 AllAc), and not to unmodified forms of the same peptides (H2B 1–20 and H2B 2–21). These assays were run in duplex to measure titration curves, as anti-FLAG and anti-K5Ac antibodies were found to be highly specific (Supporting Information, Figure S1). Titration curves of anti-FLAG and anti-K5Ac antibodies showed that the microarrays were sensitive within the 1–100 pM range, depending on the antibodies (Figure 1d). Taken together, these results demonstrate that GMR nanosensor microarrays are capable of highly sensitive and specific detection of antibodies, including detection of reactivity to peptides that differ by only a post-translational modification at a single residue.
Analysis of Serum Autoantibodies
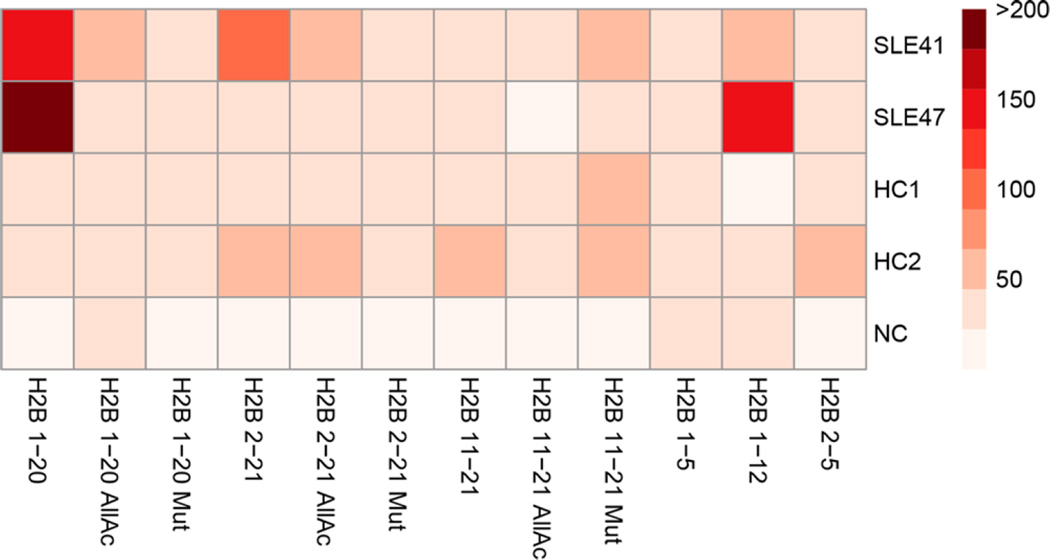
To investigate the ability of the GMR nanosensor microarrays to detect autoantibodies in clinical samples, we measured sera from two SLE patients with H2B autoantibodies and two healthy controls. The measurements revealed that the individuals with SLE had serum autoantibodies that bind to the H2B N-terminal tail (Figure 2). In agreement with these findings, ELISA measurements showed that both patients’ sera contained IgG reactive to the H2B N-terminal tail, while healthy controls did not (Supporting Information, Figure S2). Differences in the reactivity between patients were likely due to the use of slightly longer peptides (21mers) for the ELISA analysis. Interestingly, patient SLE47’s serum IgG reactivity to the H2B N-terminal tail was critically dependent on the N-terminal proline residue of H2B, while patient SLE41’s reactivity was not and appeared to have critical residues near the N-terminal tail. These results demonstrate that the microarrays can be used to measure and characterize IgG autoantibodies present in clinical samples with single amino acid resolution.
Figure 2.
GMR nanosensor peptide microarrays identify IgG autoantibodies to H2B in the sera of individuals with SLE. Sera from two H2B positive individuals with SLE (SLE41 and SLE47) and two healthy controls (HC1 and 2) were measured with the microarrays. Buffer solution without sera was used as a negative control (NC). The signal intensity of each peptide feature is the average of 4 identical sensor signals.
Development of Regeneration Scheme
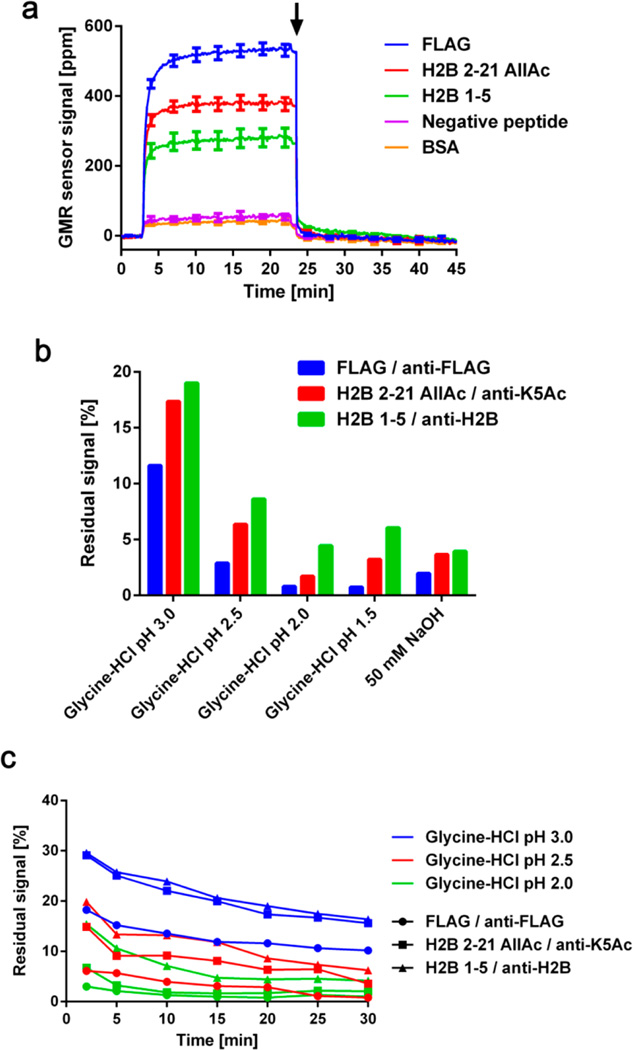
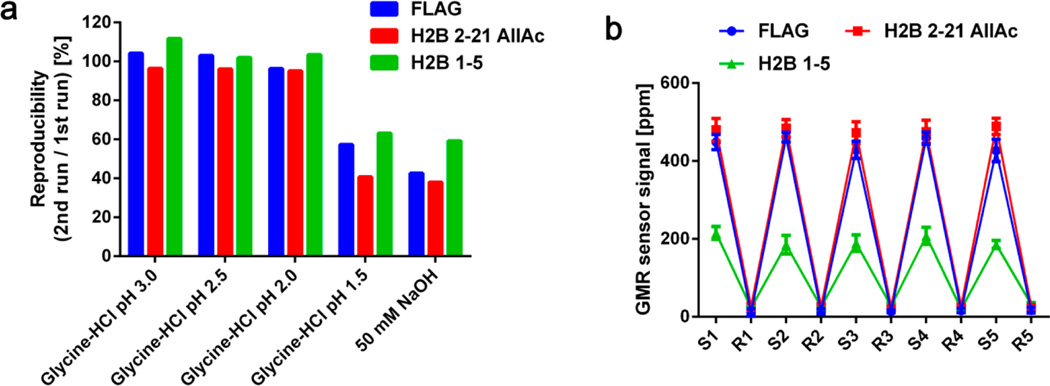
Regeneration of the microarrays, allowing multiple measurements with the same microarray, would substantially reduce material costs, experiment duration, and intermicroarray variation.32,33 Although these aspects are helpful in all analytical tools, they can be more advantageous for calibration of the tools and point-of-care (POC) measurements because elimination of intermicroarray variation and reliance on single-use test cartridge allows for precise detection and repeating measurements with a single device in remote areas, respectively. However, regeneration typically involves treatment with strong acidic or basic solutions, which often denatures proteins on the microarray. GMR nanosensor microarrays have multiple advantages that make regeneration feasible, including the stability of covalently attached peptides and relative pH-insensitivity of GMR nanosensor signal.22 Most importantly, the GMR microarrays allow continuous monitoring of signals during regeneration to ensure that bound MNPs are completely removed prior to subsequent measurements. To illustrate the process, we probed a GMR nanosensor microarray with a mixture of anti-FLAG, anti-K5Ac, and anti-H2B (binding to the N-terminal tail of H2B, Supporting Information, Figure S3) antibodies and then added a regeneration solution of glycine-HCl pH 2.0 at ~23 min after signals had plateaued (Figure 3a). We found the signals returned to near baseline levels (before addition of MNPs, 0 to 3 min). With this scheme, we monitored the sensor signals during regeneration with five commercially available surface plasmon resonance (SPR) regeneration solutions. The results show that more than 10% of MNPs still remained on the microarray after a 20 min treatment with glycine-HCl pH 3.0, while glycine-HCl pH 2.0 reduced the signals below 5% of the initial binding signals within 20 min (Figure 3b). We found that a few sensors from the microarrays treated with glycine-HCl pH 1.5 and 50 mM NaOH showed evidence of corrosion (usually large changes in the nominal resistance). These regeneration solutions were excluded from further tests, and Figure 3b only includes signals from intact sensors. We then tested the remaining solutions over a longer (up to 30 min) regeneration time course and found that most of MNPs were removed within the first 2 min, followed by a gradual decrease in signal (Figure 3c). Importantly, the reductions in signal were due to disruption of antibody complexes, as the regeneration solution (glycine-HCl pH 2.0) did not interfere with the interaction between biotin and streptavidin (Supporting Information, Figure S4).
Figure 3.
(a) Real-time signals obtained during the probing and regeneration. Anti-FLAG (1 µg/mL), anti-K5Ac (0.5 µg/mL), and anti-H2B (5 µg/mL) antibodies were incubated with the microarray. The MNPs were added at ~3 min, and the signals reached their plateaus at ~20 min. Glycine-HCl pH 2.0 was added to the microarray at ~23 min, as indicated by the arrow. Bovine serum albumin (BSA) and peptide AIYAAPFK were used as negative controls. Error bars represent standard deviations of 4 identical sensor signals. (b) Residual signals for each peptide-antibody pair were calculated after 20 min of regeneration with 5 different regeneration solutions. Residual signals were calculated with respect to their plateau signals. (c) Residual signals for each peptide–antibody complex were monitored for 30 min after regeneration solutions were added. All signals were referenced to BSA signals.
To investigate whether the binding capacity of the microarrays was affected by regeneration, we measured the same sample containing anti-FLAG, anti-K5Ac, and anti-H2B antibodies twice with the same microarray before and after 1 h of regeneration with 5 different regeneration solutions. We found that the signals were reproduced with <±5% error when microarrays were regenerated with glycine-HCl pH 3.0, 2.5, and 2.0 (Figure 4a). There was a slight increase in signal for H2B 1–5 sensors after regeneration with glycine-HCl pH 3.0, which could have been caused by some antibody carry-over from the first run. Again, several sensors in the microarrays treated with glycine-HCl pH 1.5 and 50 mM NaOH were damaged during the regeneration. Based on these results, we concluded that glycine-HCl pH 2.0 is optimal for regeneration, and it was used for subsequent experiments.
Figure 4.
(a) Reproducibility of measurements with regeneration. A sample containing anti-FLAG (1 µg/mL), anti-K5Ac (0.5 µg/mL), and anti-H2B (5 µg/mL) antibodies was measured before and after 1 h of regeneration with 5 different regeneration solutions. The reproducibility is shown as the signals obtained in the second run as a percentage of those in the first run for each peptide–antibody complex (blue: FLAG–anti-FLAG, red: H2B 2–21 AllAc–anti-K5Ac, and green: H2B 1–5–anti-H2B). (b) Five cycles of probing with the same antibody mixture, followed by 1 h regeneration with glycine-HCl pH 2.0 were performed with a single chip. “S” indicates the plateau signals from the sample measurement, and “R” represents the signals at the end of regeneration. After each regeneration, the chip was neutralized with 1% BSA for 30 min. All signals were referenced to BSA signals. Error bars represent standard deviations of 4 identical sensor signals.
To further investigate the feasibility of regeneration, we performed 5 cycles of regeneration and measurements on anti-FLAG, anti-K5Ac, and anti-H2B antibodies with the same microarray. We found that the measurements were highly reproducible and the signals returned to baseline levels after each regeneration cycle (Figure 4b). Notably, the coefficients of variation (CVs) of the assays were <10% (anti-FLAG: 3.7%, anti-K5Ac: 1.4%, and anti-H2B: 7.1%), which is much better than typical criteria for intermicroarray CVs.34 To confirm that regeneration removed antibody complexes completely, we performed 3 cycles of alternating measurements on the sample containing antibodies and zero analyte, respectively, with regenerations between cycles. The signals from zero analyte measurement were as low as baseline levels, indicating that there was no antibody carry-over from previous cycles (Supporting Information, Figure S5).
In Situ Synthesis of Peptides on Nanosensor Microarrays
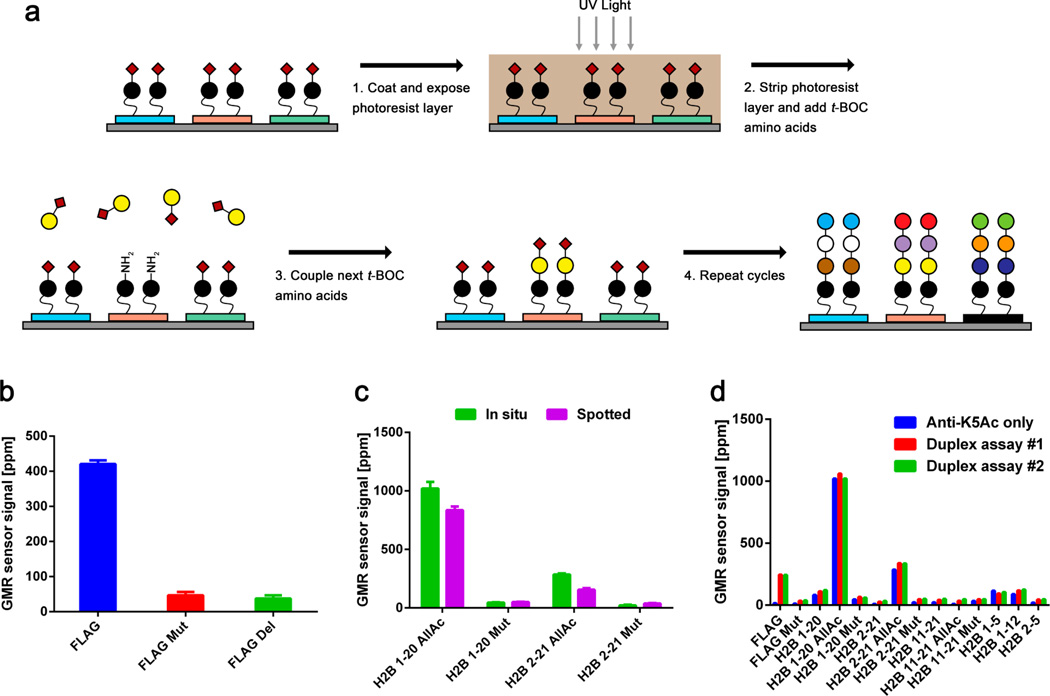
The sensitivity of protein microarrays is remarkably important for detection of low abundance antibodies because protein amplification is not possible with existing tools. Orientation of capture probes has been investigated in protein microarrays, and it has been reported that specifically oriented capture probes improved the sensitivity of the microarrays compared to randomly oriented ones.35 For peptide microarrays, there are two commonly used approaches to orienting peptides: spotting biotinylated peptides on a streptavidin-coated surface,36 or directly synthesizing peptides, covalently linked to the surface.25,37–39 As in situ synthesis of peptides is preferable for high-density microarrays,37 we synthesized peptides directly on the GMR nanosensors using maskless photolithography (Figure 5a). To our knowledge, this direct synthesis of peptides on nanosensors using photolithography has not been reported before (detailed method in MATERIALS AND METHODS). As a proof of concept, FLAG, K3A mutant (FLAG Mut), and FLAG with the M2 epitope (DYKD) deleted (FLAG Del) peptides were synthesized on the nanosensors. We then probed the microarrays with anti-FLAG antibodies and found that the antibodies specifically bound to FLAG, and not to mutant or deleted forms of FLAG (Figure 5b). This result was cross-validated with fluorescence measurement (Supporting Information, Figure S6).
Figure 5.
(a) Schematic of peptide synthesis directly on GMR nanosensor microarray using photolithography. After a seed layer is created on GMR nanosensors (blue, red, and green rectangles), a photoacid-generating photoresist layer is coated on the surface. The red diamonds represent t-BOC groups (tert-butyloxycarbonyl group), and the circles with different colors are amino acids. Maskless DMD technology is used to selectively expose the photoresist to UV light, deprotecting the t-BOC end groups via generation of photoacid. The deprotected amino acids are coupled with the next amino acids. (b) FLAG (blue), K3A mutant FLAG (FLAG Mut, red), and FLAG without the M2 epitope (FLAG Del, green) were synthesized on the microarrays and used to measure anti-FLAG antibodies at 1 µg/mL. (c) In situ synthesized and spotted peptide microarrays featuring four peptides (H2B 1–20 AllAc, H2B 1–20 Mut, H2B 2–21 AllAc, and H2B 2–21 Mut) were probed with anti-K5Ac antibodies at 0.2 µg/mL. (d) Three cycles of measurements and regeneration (glycine-HCl pH 2.0) were performed with an in situ synthesized GMR nanosensor peptide microarray. Cycle 1: anti-K5Ac antibodies at 0.2 µg/mL (blue bars). Cycles 2 and 3: anti-FLAG (0.5 µg/mL) and anti-K5Ac (0.2 µg/mL) antibodies (cycle 2: red, and cycle 3: green).
To compare performance between “in situ synthesized” and “spotted” peptide microarrays, we synthesized the peptides shown in Figure 1a on GMR nanosensor microarrays and probed them with anti-K5Ac antibodies (Figure 5c). We found that in situ synthesized microarrays generated higher signals than spotted microarrays. Compared to the titration curve shown in Figure 1d, in situ synthesized microarrays probed with anti-K5Ac at 0.2 µg/mL produced signals equivalent to spotted microarrays probed with twice the concentration, 0.4 µg/mL. In addition, in situ synthesized peptide microarrays were highly specific, as reactivity to mutated peptides (H2B 1–20 Mut and H2B 2–21 Mut) remained extremely low. The increased sensitivity appeared to be application specific, as anti-FLAG and anti-H2B antibodies had equivalent binding patterns to both in situ synthesized and spotted microarrays. This may be because the target peptides of anti-K5Ac antibodies are longer than those of anti-FLAG and anti-H2B antibodies and may be less accessible when randomly oriented.
To investigate whether in situ synthesized peptides could be regenerated, we performed three cycles of measurements and regeneration with the in situ synthesized peptide GMR nanosensor microarray (Figure 5d). In the first cycle, the microarray was used to measure only anti-K5Ac antibodies. Then the same microarray was probed with anti-K5Ac and anti-FLAG antibodies in the second and third cycles. The reactivity of anti-K5Ac antibodies to its target peptides (H2B 1–20 AllAc and H2B 2–21 AllAc) was observed in all cycles and was highly consistent. Similarly, the reactivity of anti-FLAG antibodies to the FLAG peptide was consistent in the second and third cycles.
CONCLUSIONS
We have demonstrated that reusable GMR nanosensor microarrays with in situ synthesized peptides are capable of detection of antibody binding to linear peptides with high sensitivity and resolution, including antibodies to specific post-translational modifications. Regeneration of the microarrays reduces variation across measurements and greatly reduces the cost of performing GMR nanosensor peptide microarray experiments. While the previous studies on in situ synthesized peptides with fluorescent techniques were not able to allow continuous monitoring of antibody binding,25,38,40 the GMR nanosensors added functionality to the microarrays, including kinetics monitoring and quantitative measurement. In addition, measurement with GMR nanosensors can be rapid and portable,41,42 with the use of a palm-sized device operated by a smartphone, as demonstrated previously.43 Faster measurement of antibodies could reduce turnaround time of clinical tests, allowing clinicians to initiate treatment more rapidly. Regeneration of microarrays combined with portability of the GMR nanosensor would further facilitate the measurement of antibodies in POC settings.
Limitations of the microarrays include the relatively small number of features on the microarrays and that they are only capable of detecting antibodies to linear epitopes, although use of constrained peptides could enable analysis of three-dimensional epitopes. Currently, the next generation of GMR nanosensor chips are being developed to include up to 256 sensors on a chip,44 which will allow researchers to measure antibody reactivity to 128 peptides with duplicates in a single measurement. In the future, measurement of longitudinal clinical samples will allow monitoring of the evolution of the immune response, through affinity maturation and epitope spreading. Moreover, the GMR nanosensor peptide microarrays can be used for all possible applications of current peptide microarrays such as studies on immunosignaturing,38,45 immune response upon vaccination,46–48 peptide–protein interactions,49–51 enzyme activities,52 and epitope mapping,25,53 providing higher sensitivity, resolution, and precision.
MATERIALS AND METHODS
GMR Nanosensor Chip
An 8 × 8 array of GMR nanosensors was fabricated on a 10 × 12 mm chip, with the geometry of each nanosensor optimized to sensitively detect MNP labels so that multiple (up to 64) analytes can be assayed independently and simultaneously. Each GMR nanosensor consists of multiple stripes of spin valves that have multilayers of InMr (8 nm)/CoFe (2 nm)/Ru (0.8 nm)/CoFe (2 nm)/Cu (2.3 nm)/CoFe (4.5 nm) on a thermally grown oxide layer and has an active sensing area of 100 × 100 µm.23 Each nanosensor was connected through a grid network of electrodes made of Ta/Au/Ta layers (300 nm). Each electrode was connected to an electrical contact pad on the chip, which is externally accessed by the reader station to read out the signals from individual sensors. Within the sensing area, a 30 nm oxide layer was deposited to protect the surface of the sensor, while a 300 nm oxide layer was deposited on the rest of the chip to passivate the electrodes. The changes in sensor resistance induced by the stray field from MNPs were monitored using the double modulation scheme.27 Correction methods to compensate variations in temperature and sensor resistances were also applied to the data acquisition.54
The reader station consists of 3 in. Helmholtz electromagnetic coil, power amplifier, in-house electronic circuit, and computer equipped with ADC/DAC boards (National Instruments) as described previously.27,55 Briefly, a customized LabView program drives the Helmholtz coil via the power amplifier (BOP 72-6 ML, KEPCO) to generate an AC magnetic field, and a GMR nanosensor chip is located in the middle of the coil. The in-house electronic circuit interfaces with the GMR nanosensor chips, applies an AC voltage to sensors, and delivers amplified signals from the sensors to the ADC board. The LabView program processes the signals using fast Fourier transform (FFT) and calculates GMR sensor signals.
Preparation of Peptide Microarrays
For spotted peptide microarrays, a GMR nanosensor chip was washed sequentially with acetone, methanol, and isopropanol. A reaction well was installed on the chip, and the chip was treated with oxygen plasma for 3 min. The chip was then treated with 1% poly(allylamine hydrochloride) (Sigma-Aldrich) for 5 min and washed with distilled water (Invitrogen). After the chip was cured on a hot plate at 120 °C for 1 h, it was treated with 2% poly(ethylene-alt-maleic anhydride) (Sigma-Aldrich) for 5 min.56 The chip was again washed with distilled water, and a mixture of hydroxysuccinimide (NHS, Sigma-Aldrich) and 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride (EDC, Thermo Scientific) was added to the chip. After 1 h incubation, the chip was washed with distilled water. Peptides listed in Figure 1a were presynthesized and spotted on the designated sensors using a robotic arrayer (sciFLEXARRAYER, Scienion). The spotted chips were then stored in a humid chamber at 4 °C.
For the fabrication of in situ synthesized peptide microarrays on the GMR nanosensor chip, previously reported wafer-scale processes25 were modified to be compatible with the GMR nanosensors. Briefly, without piranha cleaning, a GMR nanosensor chip was washed 3 times with ethanol and treated with 5% (3-aminopropyl)triethoxysilane (APTES, Sigma-Aldrich) in ethanol for 30 min, followed by sequential washes with ethanol and isopropanol. Next, the chip was cured on a hot plate at 100 °C for 1 h to promote silanol cross-linking. After silanation, a polyethylene glycol (PEG)6 linker was added to the chip to facilitate the addition of custom peptides. We used a spin coater to apply a photosensitive imaging solution containing a photoacid generator, a sensitizer, and 2.5% poly(methyl methacrylate) (PMMA, Sigma-Aldrich) in propylene glycol methyl ether acetate (PGMEA, Sigma-Aldrich).57 Patterned UV light exposure using a maskless photolithography tool (SFC-100, Intelligent Micro Patterning) resulted in the removal of protective t-BOC groups at specific array features via generation of photoacid, allowing for incorporation of an amino acid of interest at the deprotected sites during the subsequent coupling step. After UV light exposure, the GMR nanosensor chip was immersed in acetone to remove the photoresist layer, followed by rinsing with isopropanol. Then, the chip was treated with coupling solution containing t-BOC amino acid of interest, hydroxybenzotriazole (HOBt, CPC Scientific), and N,N′-diisopropylcarbodiimide (DIC, CPC Scientific) dissolved in N-methyl-2-pyrrolidone (NMP, Sigma-Aldrich) for 30 min at room temperature. Briefly rinsed with N,N-dimethylformamide (DMF, Sigma-Aldrich), the chip was treated with capping solution (10% acetic anhydride in DMF). This cycle of photolithography and amino acid coupling was repeated to achieve peptide microarrays with desired amino acid sequences.
Antibody Assay
Both spotted and in situ synthesized microarrays were blocked with 1% BSA (Sigma-Adrich) for 1 h before their use. After the arrays were washed with a rinsing buffer (PBS pH 7.4 with 0.1% BSA and 0.05% Tween-20), a sample of interest such as patient sample or commercial antibodies was added to the microarray and incubated for 1 h. The commercial antibodies included anti-FLAG (F1804, Sigma-Aldrich), anti-K5Ac (ab61227, Abcam), and anti-H2B (ab18977, Abcam). After incubation with the sample, the array was washed with rinsing buffer, and then biotinylated secondary antibodies (antihuman IgG at 100 ng/mL, antimouse IgG at 50 ng/mL, and antirabbit IgG at 100 ng/mL) were added. After incubation, the chip was washed with rinsing buffer and inserted into the reader station. After the initial measurement of baseline signals, MNPs were added to the chip, and real-time signals were monitored.
Patients
Serum samples from individuals with SLE (SLE41 and SLE47) and normal controls were collected as part of the Autoimmune Biomarkers Collaborative Network (ABCoN), with approval from the University of Minnesota Institutional Review Board (protocol 0110M09982). All individuals with SLE met American College of Rheumatology (ACR) revised criteria for classification of SLE.58 Written informed consent was obtained from all participants.
Optimization of Regeneration
After GMR nanosensor signals reached their plateaus for anti-FLAG (0.5 µg/mL), anti-K5Ac (0.5 µg/mL), and anti-H2B (5 µg/mL) antibodies, unbound MNPs were washed away. Then, 5 different regeneration solutions (glycine-HCl pH 3.0, 2.5, 2.0, and 1.5 and 50 mM NaOH, GE Healthcare) were added to different chips, respectively, and the signals were monitored for 30 min. The residual signal was calculated as a ratio of the signal after the regeneration solution was added with respect to the plateau signal.
Reproducibility Test
To test 5 different regeneration solutions, 5 chips were used to measure anti-FLAG (0.5 µg/mL), anti-K5Ac (0.5 µg/mL), and anti-H2B (5 µg/mL) antibodies. The chips were then treated with 5 different regeneration solutions for 1 h. After regeneration, the chips were neutralized with 1% BSA for 30 min. The same chips were used to measure the same sample. The ratios of signals in the second measurement to those in the first measurement were calculated as reproducibility.
In the experiments with multiple cycles of regeneration, glycine-HCl pH 2.0 was used for regeneration. A single chip was used to repeatedly measure the sample of interest, and the chip was treated with glycine-HCl pH 2.0 for 1 h and 1% BSA for 30 min sequentially between the measurements as well as before the first measurement to ensure each measurement experiences the identical prior condition. The signals after 1 h treatment of the regeneration solution were recorded as regenerated sensor signals.
Supplementary Material
Acknowledgments
We would like to thank the individuals with SLE and healthy volunteers who participated in this research. This work was supported in part by the National Institutes of Health Physical Science Oncology Center (U54CA143907), the Center for Cancer Nanotechnology Excellence (U54CA151459), a Sanofi BioSTAR seed grant (through Stanford Bio-X program), a grant from Intel Corp. (Santa Clara, CA), and the Autoimmunity Center of Excellence (ACE, 1U19AI110491) at Stanford. P. Utz is the recipient of a Donald E. and Delia B. Baxter Foundation Career Development Award, gifts from the Floren Family Trust and Ben May Charitable Trust, and support from the NHLBI (Proteomics contract 268201000034C), NIH (grant numbers T32GM007365, U19-AI082719, U19-AI110491, UH2-AR067676, UM2-AR067678, UM1-AI110498, and U19-AI090019), and the Alliance for Lupus Research (grant number 21858). The collection of the SLE patient samples was funded by NIAMS contract (N01-AR-1-2256) and the Hopkins Lupus Cohort by NIH AR-43727. D. Haddon was funded by the Canadian Institutes for Health Research (CIHR Fellowship). The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 261382, and Canadian Institutes of Health Research.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Cross-reactivity test, clinical sample measurement, titration curve of anti-H2B antibody, characteristics of biotin–streptavidin interaction, regeneration efficiency, and fluorescence measurement of anti-FLAG antibody (PDF)
The authors declare the following competing financial interest(s): D. Hall and S. Wang have related patents or patent applications assigned to Stanford University and out-licensed for potential commercialization. D. Hall and S. Wang have stock or stock options in MagArray, Inc., which has licensed relevant patents from Stanford University for commercialization of GMR nanosensor chips.
REFERENCES
- 1.Obermoser G, Pascual V. The Interferon-Alpha Signature of Systemic Lupus Erythematosus. Lupus. 2010;19:1012–1019. doi: 10.1177/0961203310371161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaniv G, Twig G, Shor DB, Furer A, Sherer Y, Mozes O, Komisar O, Slonimsky E, Kiang E, Lotan E, Welt M, Maraj I, Shina A, Amital H, Shoenfeld Y. A Volcanic Explosion of Autoantibodies in Systemic Lupus Erythematosus: a Diversity of 180 Different Antibodies Found in SLE Patients. Autoimmun. Rev. 2015;14:75–79. doi: 10.1016/j.autrev.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody Systems in Rheumatoid Arthritis: Specificity, Sensitivity and Diagnostic Value. Arthritis Res. 2002;4:87–93. doi: 10.1186/ar395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of Autoantibodies before the Clinical Onset of Systemic Lupus Erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 5.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific Autoantibodies Precede the Symptoms of Rheumatoid Arthritis: a Study of Serial Measurements in Blood Donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 6.Golding H, Shearer GM, Hillman K, Lucas P, Manischewitz J, Zajac RA, Clerici M, Gress RE, Boswell RN, Golding B. Common Epitope in Human Immunodeficiency Virus (HIV) I-GP41 and HLA Class II Elicits Immunosuppressive Autoantibodies Capable of Contributing to Immune Dysfunction in HIV I-Infected Individuals. J. Clin. Invest. 1989;83:1430–1435. doi: 10.1172/JCI114034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, Nicol M, Scholvinck E, Relman D, Waddell S, Langford P, Sheehan B, Semple L, Wilkinson KA, Wilkinson RJ, Ress S, Hibberd M, Levin M. Acquired Predisposition to Mycobacterial Disease due to Autoantibodies to IFN-Gamma. J. Clin. Invest. 2005;115:2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, Tobar A, Shoenfeld Y. Bacterial Induction of Autoantibodies to Beta2-Glycoprotein-I Accounts for the Infectious Etiology of Antiphospholipid Syndrome. J. Clin. Invest. 2002;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes AK, Cichacz Z, Scheck A, Coons SW, Johnston SA, Stafford P. Immunosignaturing Can Detect Products from Molecular Markers in Brain Cancer. PLoS One. 2012;7:e40201. doi: 10.1371/journal.pone.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang XJ, Yu JJ, Sreekumar A, Varambally S, Shen RL, Giacherio D, Mehra R, Montie JE, Pienta KJ, Sanda MG, Kantoff PW, Rubin MA, Wei JT, Ghosh D, Chinnaiyan AM. Autoantibody Signatures in Prostate Cancer. N. Engl. J. Med. 2005;353:1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 11.Pereira-Faca SR, Kuick R, Puravs E, Zhang Q, Krasnoselsky AL, Phanstiel D, Qiu J, Misek DE, Hinderer R, Tammemagi M, Landi MT, Caporaso N, Pfeiffer R, Edelstein C, Goodman G, Barnett M, Thornquist M, Brenner D, Hanash SM. Identification of 14–3-3 Theta as an Antigen That Induces a Humoral Response in Lung Cancer. Cancer Res. 2007;67:12000–12006. doi: 10.1158/0008-5472.CAN-07-2913. [DOI] [PubMed] [Google Scholar]
- 12.Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, Barnes A, Robertson J. Autoantibodies in Breast Cancer: Their Use as an Aid to Early Diagnosis. Ann. Oncol. 2007;18:868–873. doi: 10.1093/annonc/mdm007. [DOI] [PubMed] [Google Scholar]
- 13.Schena M, Shalon D, Davis RW, Brown PO. Quantitative Monitoring of Gene Expression Patterns with a Complementary DNA Microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 14.Lapuk A, Marr H, Jakkula L, Pedro H, Bhattacharya S, Purdom E, Hu Z, Simpson K, Pachter L, Durinck S, Wang N, Parvin B, Fontenay G, Speed T, Garbe J, Stampfer M, Bayandorian H, Dorton S, Clark TA, Schweitzer A, et al. Exon-Level Microarray Analyses Identify Alternative Splicing Programs in Breast Cancer. Mol. Cancer Res. 2010;8:961–974. doi: 10.1158/1541-7786.MCR-09-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, Fournel S, Fong D, Genovese MC, de Vegvar HEN, Skriner K, Hirschberg DL, Morris RI, Muller S, Pruijn GJ, van Venrooij WJ, Smolen JS, Brown PO, Steinman L, Utz PJ. Autoantigen Microarrays for Multiplex Characterization of Autoantibody Responses. Nat. Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 16.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, Sonderstrup G, Monach P, Drijfhout JW, van Venrooij WJ, Utz PJ, Genovese MC, Robinson WH. Antigen Microarray Profiling of Autoantibodies in Rheumatoid Arthritis. Arthritis Rheum. 2005;52:2645–2655. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KS, Sibani S, Wallstrom G, Qiu J, Mendoza EA, Raphael J, Hainsworth E, Montor WR, Wong J, Park JG, Lokko N, Logvinenko T, Ramachandran N, Godwin AK, Marks J, Engstrom P, Labaer J. Protein Microarray Signature of Autoantibody Biomarkers for the Early Detection of Breast Cancer. J. Proteome Res. 2011;10:85–96. doi: 10.1021/pr100686b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angenendt P. Progress in Protein and Antibody Microarray Technology. Drug Discovery Today. 2005;10:503–511. doi: 10.1016/S1359-6446(05)03392-1. [DOI] [PubMed] [Google Scholar]
- 19.Cretich M, Damin F, Pirri G, Chiari M. Protein and Peptide arrays: Recent Trends and New Directions. Biomol. Eng. 2006;23:77–88. doi: 10.1016/j.bioeng.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran N, Hainsworth E, Bhullar B, Eisenstein S, Rosen B, Lau AY, Walter JC, LaBaer J. Self-Assembling Protein Microarrays. Science. 2004;305:86–90. doi: 10.1126/science.1097639. [DOI] [PubMed] [Google Scholar]
- 21.Osterfeld SJ, Yu H, Gaster RS, Caramuta S, Xu L, Han SJ, Hall DA, Wilson RJ, Sun S, White RL, Davis RW, Pourmand N, Wang SX. Multiplex Protein Assays Based on Real-Time Magnetic Nanotag Sensing. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20637–20640. doi: 10.1073/pnas.0810822105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaster RS, Hall DA, Nielsen CH, Osterfeld SJ, Yu H, Mach KE, Wilson RJ, Murmann B, Liao JC, Gambhir SS, Wang SX. Matrix-Insensitive Protein Assays Push the Limits of Biosensors in Medicine. Nat. Med. 2009;15:1327–1332. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JR, Sato N, Bechstein DJ, Osterfeld SJ, Wang J, Gani AW, Hall DA, Wang SX. Experimental and Theoretical Investigation of the Precise Transduction Mechanism in Giant Magnetoresistive Biosensors. Sci. Rep. 2016;6:18692. doi: 10.1038/srep18692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh-Gasson S, Green RD, Yue Y, Nelson C, Blattner F, Sussman MR, Cerrina F. Maskless Fabrication of Light-Directed Oligonucleotide Microarrays Using a Digital Micromirror Array. Nat. Biotechnol. 1999;17:974–978. doi: 10.1038/13664. [DOI] [PubMed] [Google Scholar]
- 25.Price JV, Tangsombatvisit S, Xu G, Yu J, Levy D, Baechler EC, Gozani O, Varma M, Utz PJ, Liu CL. On Silico Peptide Microarrays for High-Resolution Mapping of Antibody Epitopes and Diverse Protein-Protein Interactions. Nat. Med. 2012;18:1434–1440. doi: 10.1038/nm.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Boer BM, Kahlman JA, Jansen TP, Duric H, Veen J. An Integrated and Sensitive Detection Platform for Magneto-Resistive Biosensors. Biosens. Bioelectron. 2007;22:2366–2370. doi: 10.1016/j.bios.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Hall DA, Gaster RS, Lin T, Osterfeld SJ, Han S, Murmann B, Wang SX. GMR Biosensor Arrays: a System Perspective. Biosens. Bioelectron. 2010;25:2051–2057. doi: 10.1016/j.bios.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roosild TP, Castronovo S, Choe S. Structure of Anti-FLAG M2 Fab Domain and Its Use in the Stabilization of Engineered Membrane Proteins. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun. 2006;62:835–839. doi: 10.1107/S1744309106029125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouzarides T. Chromatin Modifications and Their Function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Utz PJ, Anderson P. Posttranslational Protein Modifications, Apoptosis, and the Bypass of Tolerance to Autoantigens. Arthritis Rheum. 1998;41:1152–1160. doi: 10.1002/1529-0131(199807)41:7<1152::AID-ART3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Doyle HA, Yang ML, Raycroft MT, Gee RJ, Mamula MJ. Autoantigens: Novel Forms and Presentation to the Immune System. Autoimmunity. 2014;47:220–233. doi: 10.3109/08916934.2013.850495. [DOI] [PubMed] [Google Scholar]
- 32.Kirby R, Cho EJ, Gehrke B, Bayer T, Park YS, Neikirk DP, McDevitt JT, Ellington AD. Aptamer-Based Sensor Arrays for the Detection and Quantitation of Proteins. Anal. Chem. 2004;76:4066–4075. doi: 10.1021/ac049858n. [DOI] [PubMed] [Google Scholar]
- 33.Choi S, Chae J. Reusable Biosensors via in Situ Electrochemical Surface Regeneration in Microfluidic Applications. Biosens. Bioelectron. 2009;25:527–531. doi: 10.1016/j.bios.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Matson RS. Applying Genomic and Proteomic Microarray Technology in Drug Discovery. 2nd. Boca Raton, FL: CRC Press; 2013. p. 259. [Google Scholar]
- 35.Peluso P, Wilson DS, Do D, Tran H, Venkatasubbaiah M, Quincy D, Heidecker B, Poindexter K, Tolani N, Phelan M, Witte K, Jung LS, Wagner P, Nock S. Optimizing Antibody Immobilization Strategies for the Construction of Protein Microarrays. Anal. Biochem. 2003;312:113–124. doi: 10.1016/s0003-2697(02)00442-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Jarrell JA, Price JV, Tabakman SM, Li Y, Gong M, Hong G, Feng J, Utz PJ, Dai H. An Integrated Peptide-Antigen Microarray on Plasmonic Gold Films for Sensitive Human Antibody Profiling. PLoS One. 2013;8:e71043. doi: 10.1371/journal.pone.0071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min DH, Mrksich M. Peptide Arrays: towards Routine Implementation. Curr. Opin. Chem. Biol. 2004;8:554–558. doi: 10.1016/j.cbpa.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Legutki JB, Zhao ZG, Greving M, Woodbury N, Johnston SA, Stafford P. Scalable High-Density Peptide Arrays for Comprehensive Health Monitoring. Nat. Commun. 2014;5:4785. doi: 10.1038/ncomms5785. [DOI] [PubMed] [Google Scholar]
- 39.Stadler V, Felgenhauer T, Beyer M, Fernandez S, Leibe K, Guttler S, Groning M, Konig K, Torralba G, Hausmann M, Lindenstruth V, Nesterov A, Block I, Pipkorn R, Poustka A, Bischoff FR, Breitling F. Combinatorial Synthesis of Peptide Arrays with a Laser Printer. Angew. Chem., Int. Ed. 2008;47:7132–7135. doi: 10.1002/anie.200801616. [DOI] [PubMed] [Google Scholar]
- 40.Haddon DJ, Jarrell JA, Diep VK, Wand HE, Price JV, Tangsombatvisit S, Credo GM, Mackey S, Dekker CL, Baechler EC, Liu CL, Varma M, Utz PJ. Mapping Epitopes of U1–70K Autoantibodies at Single-Amino Acid Resolution. Autoimmunity. 2015;48:513–523. doi: 10.3109/08916934.2015.1077233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaster RS, Hall DA, Wang SX. NanoLAB: an Ultraportable, Handheld Diagnostic Laboratory for Global Health. Lab Chip. 2011;11:950–956. doi: 10.1039/c0lc00534g. [DOI] [PubMed] [Google Scholar]
- 42.Choi J, Gani AW, Bechstein DJ, Lee JR, Utz PJ, Wang SX. Portable, One-Step, and Rapid GMR Biosensor Platform with Smartphone Interface. Biosens. Bioelectron. 2016;85:1–7. doi: 10.1016/j.bios.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 43.Lee JR, Choi J, Shultz TO, Wang SX. Small Molecule Detection in Saliva Facilitates Portable Tests of Marijuana Abuse. Anal. Chem. 2016;88:7457–7461. doi: 10.1021/acs.analchem.6b01688. [DOI] [PubMed] [Google Scholar]
- 44.Hall DA, Gaster RS, Makinwa K, Wang SX, Murmann B. A 256 Pixel Magnetoresistive Biosensor Microarray in 0.18µm CMOS. IEEE J. Solid-State Circuits. 2013;48:1290–1301. doi: 10.1109/JSSC.2013.2245058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sykes KF, Legutki JB, Stafford P. Immunosignaturing: a Critical Review. Trends Biotechnol. 2013;31:45–51. doi: 10.1016/j.tibtech.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Legutki JB, Johnston SA. Immunosignatures Can Predict Vaccine Efficacy. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18614–18619. doi: 10.1073/pnas.1309390110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price JV, Jarrell JA, Furman D, Kattah NH, Newell E, Dekker CL, Davis MM, Utz PJ. Characterization of Influenza Vaccine Immunogenicity Using Influenza Antigen Microarrays. PLoS One. 2013;8:e64555. doi: 10.1371/journal.pone.0064555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts WK, Livingston PO, Agus DB, Pinilla-Ibarz J, Zelenetz A, Scheinberg DA. Vaccination with CD20 Peptides Induces a Biologically Active, Specific Immune Response in Mice. Blood. 2002;99:3748–3755. doi: 10.1182/blood.v99.10.3748. [DOI] [PubMed] [Google Scholar]
- 49.Falsey JR, Renil M, Park S, Li S, Lam KS. Peptide and Small Molecule Microarray for High Throughput Cell Adhesion and Functional Assays. Bioconjugate Chem. 2001;12:346–353. doi: 10.1021/bc000141q. [DOI] [PubMed] [Google Scholar]
- 50.Kuo AJ, Song J, Cheung P, Ishibe-Murakami S, Yamazoe S, Chen JK, Patel DJ, Gozani O. The BAH Domain of ORC1 Links H4K20me2 to DNA Replication Licensing and Meier-Gorlin Syndrome. Nature. 2012;484:115–119. doi: 10.1038/nature10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews AG, Kuo AJ, Ramon-Maiques S, Han S, Champagne KS, Ivanov D, Gallardo M, Carney D, Cheung P, Ciccone DN, Walter KL, Utz PJ, Shi Y, Kutateladze TG, Yang W, Gozani O, Oettinger MA. RAG2 PHD Finger Couples Histone H3 Lysine 4 Trimethylation with V(D)J Recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houseman BT, Huh JH, Kron SJ, Mrksich M. Peptide Chips for the Quantitative Evaluation of Protein Kinase Activity. Nat. Biotechnol. 2002;20:270–274. doi: 10.1038/nbt0302-270. [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Chang S, Mohan C. Molecular Signatures of Antinuclear Antibodies-Contributions of Heavy Chain CDR Residues. Mol. Immunol. 2002;39:333–347. doi: 10.1016/s0161-5890(02)00110-4. [DOI] [PubMed] [Google Scholar]
- 54.Hall DA, Gaster RS, Osterfeld SJ, Murmann B, Wang SX. GMR Biosensor Arrays: Correction Techniques for Reproducibility and Enhanced Sensitivity. Biosens. Bioelectron. 2010;25:2177–2181. doi: 10.1016/j.bios.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JR, Bechstein DJ, Ooi CC, Patel A, Gaster RS, Ng E, Gonzalez LC, Wang SX. Magneto-Nanosensor Platform for Probing Low-Affinity Protein-Protein Interactions and Identification of a Low-Affinity PD-L1/PD-L2 Interaction. Nat. Commun. 2016;7:12220. doi: 10.1038/ncomms12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D, Marchetti F, Chen Z, Zaric S, Wilson RJ, Hall DA, Gaster RS, Lee JR, Wang J, Osterfeld SJ, Yu H, White RM, Blakely WF, Peterson LE, Bhatnagar S, Mannion B, Tseng S, Roth K, Coleman M, Snijders AM, et al. Nanosensor Dosimetry of Mouse Blood Proteins after Exposure to Ionizing Radiation. Sci. Rep. 2013;3:2234. doi: 10.1038/srep02234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao X, Zhou X, Gulari E. Light Directed Massively Parallel On-Chip Synthesis of Peptide Arrays with t-Boc Chemistry. Proteomics. 2003;3:2135–2141. doi: 10.1002/pmic.200300597. [DOI] [PubMed] [Google Scholar]
- 58.Hochberg MC. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.