Summary
Circadian rhythms are 24-hour oscillations that control a variety of biological processes in living systems, including two hallmarks of cancer, cell division and metabolism. Circadian rhythm disruption by shift-work is associated with greater risk for cancer development and poor prognosis, suggesting a putative tumor suppressive role for circadian rhythm homeostasis. Using a genetically-engineered mouse model (GEMM) of lung adenocarcinoma, we have characterized the effects of circadian rhythm disruption on lung tumorigenesis. We demonstrate that both physiologic perturbation (jet lag) and genetic mutation of the central circadian clock components decreased survival and promoted lung tumor growth and progression. The core circadian genes Per2 and Bmal1 were shown to have cell-autonomous tumor suppressive roles in transformation and lung tumor progression. Loss of the central clock components led to increased c-Myc expression, enhanced proliferation and metabolic dysregulation. Our findings demonstrate that both systemic and somatic disruption of circadian rhythms contribute to cancer progression.
Keywords: Lung Cancer, Circadian Rhythms, Jet Lag, physiology, CRISPR
Introduction
Lung cancer is the leading cause of cancer deaths worldwide (Siegel et al., 2016). Despite the extensive characterization of the somatic events that contribute to tumor initiation and progression, little is known about the role of physiologic risk factors, such as circadian rhythm disruption by shift-work, in lung tumorigenesis. Epidemiological studies have revealed that individuals who work night-shifts are at higher risk for developing cancer (Hansen, 2001; Kloog et al., 2009; Megdal et al., 2005; Schernhammer et al., 2006; Schernhammer et al., 2001; Viswanathan et al., 2007) or display poorer cancer prognosis (Lis et al., 2003). The mammalian circadian machinery consists of a transcription-translation autoregulatory feedback loop composed of the transcriptional activators circadian locomotor output cycles kaput (Clock) and aryl hydrocarbon receptor nuclear translocator–like (Arntl, also known as Bmal1) and their target genes Period (Per) and Cryptochrome (Cry), which rhythmically accumulate and form a repressor complex that interacts with Clock/Bmal1 to inhibit their own transcription (Bass and Takahashi, 2010; Sahar and Sassone-Corsi, 2009). Systemic and tissue-specific disruption of the circadian machinery leads to changes in cellular functions such as cell division and metabolism, both highly relevant to cancer (Bass and Takahashi, 2010; Hanahan and Weinberg, 2011; Masri et al., 2016; Sahar and Sassone-Corsi, 2009). Emerging evidence in several different cellular and in vivo models points to an important role of the core circadian genes in carcinogenesis (Filipski et al., 2005; Fu et al., 2002; Gery et al., 2006; Janich et al., 2011; Kettner et al., 2014; Lee et al., 2010; Puram et al., 2016; Sahar and Sassone-Corsi, 2009; Van Dycke et al., 2015; Wood et al., 2008).
Despite several lines of evidence implicating circadian rhythm disruption in cancer, the underlying mechanism that contributes to disease development remains elusive. It is unclear how oncogenic events may cooperate with circadian clock disruption during cancer initiation and progression. Our laboratory has established an autochthonous mouse model of human lung adenoma and adenocarcinoma. In these GEMMs, lung tumors are induced in K-rasLSL-G12D/+;p53flox/flox (KP) or K-rasLSL-G12D/+ (K) mice after intratracheal administration of viral vectors expressing Cre-recombinase, which activate a K-rasG12D allele alone or concomitantly delete the tumor suppressor p53 in lung epithelial cells (Jackson et al., 2005). KRAS and P53 are mutated in 30% and 50% of human lung adenocarcinoma patients respectively, making these GEMMs highly relevant to a large subset of human lung cancers (Cancer Genome Atlas Research, 2014). In this study we used these GEMMs to model circadian rhythm disruption and determined that both genetic and physiologic disruption of circadian rhythms promoted lung tumorigenesis.
Results
Physiologic disruption of circadian rhythms by jet-lag accelerates lung tumorigenesis
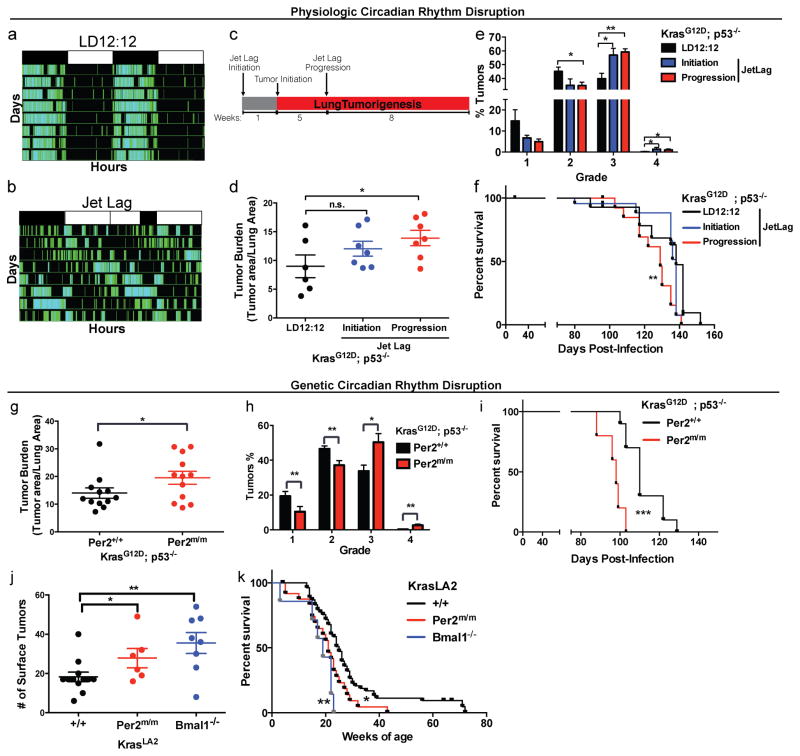
To investigate the role of circadian rhythm disruption in tumorigenesis, we used pre-clinical GEMMs of human lung adenoma and adenocarcinoma. Physiologic disruption of circadian rhythm was examined by placing KP animals in normal 12hr light/12hr dark schedule (LD12:12) or a previously described jet lag schedule of 8 hours light advance every 2–3 days, which mimics the circadian disturbance that humans undergo during shift-work (Lee et al., 2010; Thaiss et al., 2014). By measuring physical activity, we confirmed that jet lag disrupted the circadian behavior of animals (Figure 1a–b); this protocol has been shown to disrupt the oscillations of the core circadian genes in multiple tissues (Filipski et al., 2005; Lee et al., 2010; Thaiss et al., 2014). Animals were placed in jet lag either a week prior to tumor-initiation (jet lag initiation) or 5 weeks after lung tumor initiation (jet lag progression) and remained under jet lag conditions until the end of the study 13 weeks post tumor initiation (Figure 1c). Animals placed in jet lag during tumor progression for 8-weeks post-infection had a significant increase in the lung tumor burden (p<0.05; Figure 1d) and a shift in the tumor grades, with fewer grade 2 and more grade 3 and 4 tumors (grade 2 p<0.05; grade 3 p<0.01; Figure 1e) when compared to LD12:12 animals. Furthermore, animals placed in jet lag post-tumor initiation (jet lag progression) conditions showed decreased survival (9 days median survival) (p<0.01; Figure 1f). Jet lag starting a week prior to tumor initiation (jet lag initiation) led to a modest, but non-significant increase in tumor burden (Figure 1d); however, we did observe a significant increase in the percentage of high-grade tumors in this cohort compared to LD12:12 animals (grade 3 and 4; p<0.05; Figure 1e). Overall, our data demonstrate that physiologic disruption of circadian rhythms by simply altering the light cycle accelerates lung tumorigenesis in the context of Kras and p53 mutations.
Figure 1. Physiologic and genetic disruption of circadian rhythms accelerates lung tumorigenesis.
Representative double-plotted actogram of KP mice subjected to (A) regular LD12:12 (left) and (B) an 8 hour phase advance by Jet lag (right). (C) Schematic of the timeline of experiment to assess the effects of jet lag on lung tumorigenesis. Histological assessment of lung tumor burden (D) and grade (E) in KP mice placed in normal LD12:12 (n=6) or jet lag conditions at initiation (n=7) or tumor progression (n=7). (F) Kaplan-Meier survival analysis for KP animals placed in LD12:12 (n=15) or Jet lag initiation (n=13) and progression (n=13) conditions. Histological analysis of lung tumor burden (G) and grade (H) in KP mice with WT (Per2+/+) (n=12) or germline mutant (Per2m/m) Per2 (n=12). (I) Kaplan-Meier survival analysis for KP animals with WT (Per2+/+) (n=10) or mutant (Per2m/m) Per2 (n=5). (J) Surface tumor number in KrasLA2/+ WT (+/+) animals (n=12), systemic loss of Per2 (Per2m/m) (n=6) and Bmal1 loss (Bmal1−/−) (n=8). (K) Kaplan-Meier survival analysis for KrasLA2/+ animals with WT (+/+) (n=50), Per2m/m (n=31) and Bmal1−/− (n=7). Note: n.s. = not significant, * = p<0.05, ** = p<0.01, *** = p<0.001, obtained from two-sided Student’s t-test. All error bars denote s.e.m.
Whole-animal loss of Per2 and Bmal1 accelerates lung tumorigenesis
Physiologic disruption of circadian rhythms by jet lag may contribute to enhanced tumorigenesis in multiple ways, including effects other hormones, feeding behavior, metabolic alterations, inflammation, as well as desynchronization of the circadian machinery. To further dissect the role of circadian rhythm disruption in lung tumorigenesis, we performed genetic disruption of the circadian machinery by whole-animal clock disruption under normal LD12:12 conditions. We used a germline mutant allele of Per2 (Per2m/m) (Zheng et al., 1999) and generated K; Per2m/m and KP; Per2m/m animals. Upon tumor initiation by intratracheal infection with viral Cre, both K (Figure S1A and S1B) and KP (Figure 1G and 1H) animals that had systemic Per2 loss-of-function exhibited increased tumor burden and grade. Consistent with these findings, both K; Per2m/m (p<0.01; Figure S1c) and KP; Per2m/m (p<0.001; Figure 1I) animals also had decreased survival compared to wild-type (Per2+/+) controls.
To further validate our finding from the Cre/LoxP-based mouse model, we utilized an alternative autochthonous Kras-driven GEMM (KrasLA2/+) that gives rise to sporadic non-viral induced tumors in various tissues (e.g. lymphoma, papilloma and lung) and 100% tumor penetrance in the lung (Johnson et al., 2001). We assessed the effects of systemic circadian rhythm disruption by both germline Per2 (Per2m/m) and Bmal1 mutation (Bmal1−/−)(Bunger et al., 2000). We observed that both KrasLA2/+; Per2m/m and KrasLA2/+; Bmal1−/− animals had an increased lung tumor number (p<0.05 and p<0.01; Figure 1J) and exhibited decreased survival (p<0.05 and p<0.01; Figure 1K). We did not observe increased tumorigenesis in other sporadic Kras-driven tumors in this model (data not shown). Interestingly, KrasLA2/+; Bmal1−/− animals displayed a trend of developing more lung tumors and poorer survival than KrasLA2/+; Per2m/m animals, which may be explained by Bmal1 loss having a greater impact on circadian rhythm disruption (Bunger et al., 2000; Xu et al., 2007; Zheng et al., 1999). These data further support the conclusion that systemic circadian rhythm disruption by genetic loss-of-function enhances lung tumorigenesis in two independent Kras-driven GEMMs.
Tumor cell-autonomous role of circadian machinery in lung cancer
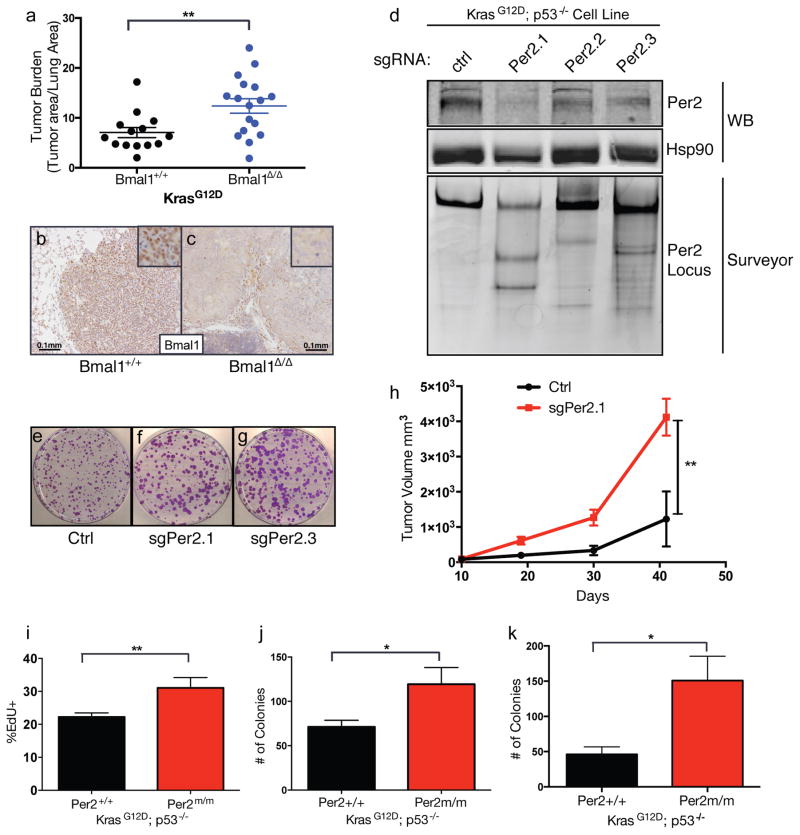
To assess whether the increase in tumorigenesis upon circadian rhythm disruption could be attributed to cancer cell autonomous mechanisms, we utilized a conditional allele of Bmal1 (Bmal1flox/flox) (Storch et al., 2007) to delete Bmal1 specifically in cancer cells in the K and KP models. Loss of Bmal1 (Bmal1Δ/Δ) in tumors accelerated lung tumor progression in the K model (Figure 2A, 2B and 2C). Interestingly however, there was no increase in tumor burden in KP tumors with conditional Bmal1 (Bmal1Δ/Δ) loss (Figure S2A), suggesting a possible p53-dependent role for Bmal1 as has been suggested by a prior study (Mullenders et al., 2009).
Figure 2. Cell autonomous loss of circadian genes enhances transformation and tumorigenesis.
(A) Graph of histological assessment of lung tumor burden tumor burden in K animals with tumor-specific loss of Bmal1 (Bmal1Δ/Δ) (n=17) compared to WT controls (Bmal1+/+) (n=14). Immunohistochemical staining for Bmal1 in Bmal1+/+ (B) and Bmal1Δ/Δ (C) K tumors, insets represent high magnification images, scale bar=0.1mm. (D) Western blot (WB) and Surveyor analysis of Per2 protein and the Per2 locus, respectively, in KP cell lines where Per2 was target by three different short-guide RNAs (sgRNAs) using the CRISPR/Cas9 system. Representative of three independent replicate low-density clonogenicity assays in Ctrl (E), sgPer2.1 (F) and sgPer2.3 (G) targeted KP cells. (H) Tumor volume measurements of subcutaneous transplanted CRISPR/Cas9-targeted KP murine lung cancer cells. (I) Flow cytometry measurements of EdU incorporation in KP MEFs with WT (+/+) or mutant (Per2m/m) Per2. (J) Colony numbers from low-density clonogenicity assays in WT (+/+) or mutant (Per2m/m) Per2 KP cells. (K) Colony numbers from 3D agar assays of WT (+/+) or mutant (Per2m/m) Per2 KP cells. All experiments in (E–K) represent data from three independent experimental replicates from 3 independent MEF lines. Note: * = p<0.05, ** = p<0.01, obtained from two-sided Student’s t-test. All error bars denote s.e.m.
To investigate the effects of Per2 loss in KP lung cancer cells growth, we generated multiple cell lines from WT and Per2m/m KP tumors of the same histological grade (Figure S2B). KP Per2m/m cells proliferated faster and had a greater clonogenic potential compared to WT KP cells (Figure S2C and S2D). To ensure that the increased growth of KP Per2m/m tumor-derived cells lines was not due to strain background, we confirmed the tumor suppressive role of Per2 by engineering loss-of-function mutations in Per2 in isogenic C57BL/6 KP murine lung cancer cells using CRISPR/Cas9-based editing (Figure S2E). We validated Cas9-based cutting and editing at the Per2 locus by immunoblotting and Surveyor assay a week after introduction of CRISPR/Cas9 system using three independent sgRNAs against Per2 (sgPer2.1, sgPer2.2 and sgPer2.3) or control non-targeting sgRNA (ctrl) (Figure 2D), according to standard procedures (Ran et al., 2013; Sanchez-Rivera and Jacks, 2015; Sayin and Papagiannakopoulos, 2016). Cells with CRISPR/Cas9-based knockout of Per2 (sgPer2.1 and sgPer2.3) displayed greater proliferation in low-density culture conditions (Figure 2E, 2F and 2G) and tumor formation following subcutaneous transplantation in congenic immunocompetent C57BL/6 animals (Figure 2H).
Tumor suppressive role of circadian machinery during cellular transformation
To assess whether Per2 loss promotes Kras-driven transformation, we derived mouse embryonic fibroblasts (MEFs) from K and KP animals that also harbor mutant (Per2m/m) or WT Per2 (Per2+/+). MEFs were infected with adenoviral-Cre to induce the KrasG12D/+ and KrasG12D/+; p53−/− mutations. We observed that K and KP Per2m/m MEFs proliferated faster than the Per2+/+ controls based on population doublings (Figure S3A and S3B) and EdU incorporation (Figure 2I). KP; Per2m/m cells also produced more colonies in low density (Figure 2J) and 3D agar (Figure 2K) assays, which assess the ability of cells to proliferate in limited paracrine signaling and anchorage-independent conditions, respectively. Furthermore, Kras mutant MEFs lacking Per2 function showed increased sensitivity to cellular transformation upon overexpression of c-Myc or adenovirus early region 1A (E1A) oncogenes compared to Per2+/+, based on low (Figure S3C and S3D) and high density (Figure S3F) growth assays as well as 3D agar growth assays (Figure S3E).
Increased proliferation and c-Myc levels in circadian mutants tumors
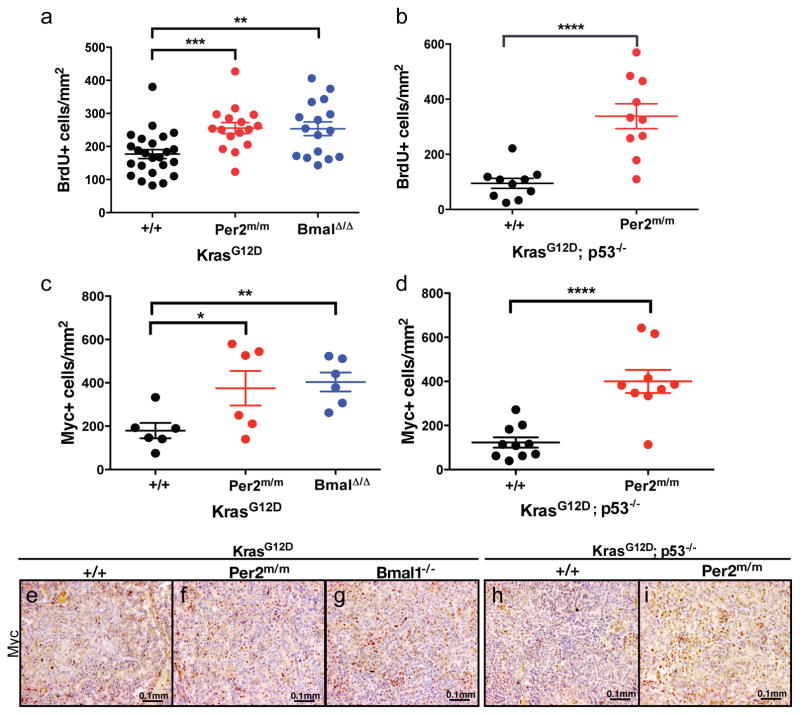
We next addressed whether the increased tumor burden in the content of circadian gene loss-of-function was due to increased proliferation. BrdU incorporation analysis revealed increased BrdU positive nuclei in K; Per2m/m (p<0.001; Figure 4A), K; Bmal1Δ/Δ tumors (p<0.01; Figure 3A) and KP; Per2m/m tumors (p<0.0001; Figure 3B) compared to controls. C-Myc function has been shown to be essential for lung tumor growth (Soucek et al., 2013) and has been previously implicated in circadian rhythm regulation (Altman et al., 2015; Fu et al., 2002; Lee et al., 2010). It has also been demonstrated that the core clock components can negatively regulate c-Myc expression (Fu et al., 2002). Therefore, we assayed the levels of c-Myc in autochthonous tumors by immunohistochemistry. As shown in Figure 3, K; Per2m/m (p<0.05; Figure 3C and 3F), K; Bmal1Δ/Δ (p<0.01; Figure 3C and 3G) and KP; Per2m/m (p<0.0001; Figure 3D and 3I) tumors showed a significant increase in the protein levels of c-Myc as compared to control samples K and KP tumors (Figure 3C, 3E and 3H).
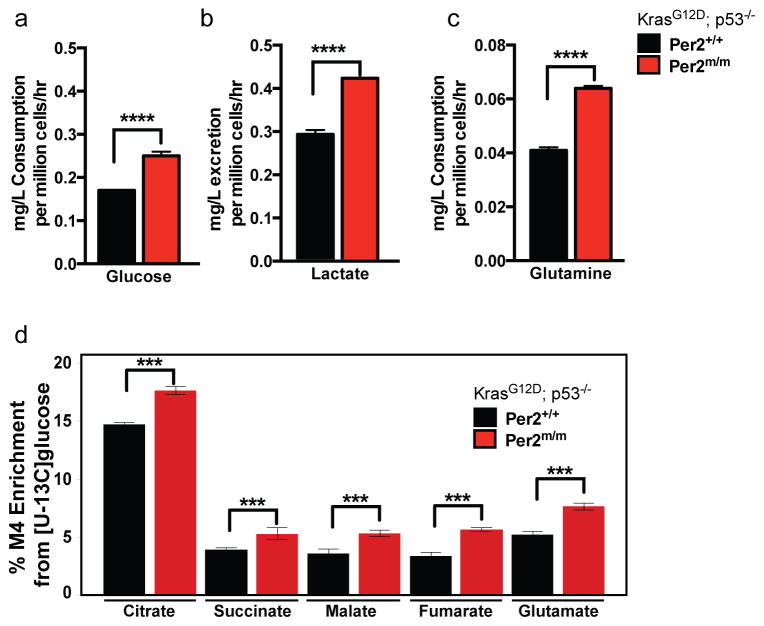
Figure 4. Altered glucose and glutamine metabolism in circadian mutant cells.
Measurements (mg/L per million cells/hour) of (A) glucose consumption and (B) lactate excretion, (C) glutamine consumption in KP WT (Per2+/+) or mutant (Per2m/m) cells. (D) Percent M4 carbon labeling enrichment in TCA cycle intermediates in in KP WT (Per2+/+) or mutant (Per2m/m) cells. Error bars denote standard deviation. Note: * = p<0.05, ** = p<0.01, **** = p<0.0001 obtained from two-sided Student’s t-test.
Figure 3. Enhanced proliferation and increased levels of c-Myc in circadian mutant tumors.
Immunohistochemical analysis of BrdU incorporation in tumors from (A) K animals with WT (+/+) (n=24), mutant Per2 (Per2m/m) (n=16) and Bmal1 mutant (Bmal1Δ/Δ) (n=16) tumors; (B) KP animals with WT (+/+) (n=10), mutant Per2 (Per2m/m) (n=10). Immunohistochemical staining of c-Myc (C) K animals with WT (+/+) (n=6), mutant Per2 (Per2m/m) (n=6) and Bmal1 mutant (Bmal1Δ/Δ) (n=6) tumors; (D) KP animals with WT (+/+) (n=10), mutant Per2 (Per2m/m) (n=10). Representative c-Myc immunohistochemical images of: K, (E) WT (+/+), (F) Per2 mutant (Per2m/m), (G) Bmal1 mutant (Bmal1Δ/Δ) and KP (H) WT (+/+), (I) Per2 mutant (Per2m/m). Scale bars 0.1mm. n= individual tumors. Note: * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001 obtained from two-sided Student’s t-test. All error bars denote s.e.m.
Metabolic reprograming of circadian mutant lung cancer cells
Circadian rhythms are known to control a number of metabolic processes, including oxidative phosphorylation (Peek et al., 2013), redox homeostasis (Pekovic-Vaughan et al., 2014) and lipid metabolism (Grimaldi et al., 2010). Recent work has demonstrated that c-Myc dampens circadian oscillations by opposing BMAL1 transcriptional oscillations and disrupting homeostatic glucose and glutamine oscillations (Altman et al., 2015). We hypothesized that the increased proliferation of circadian mutant tumor cells may be due to upregulated c-Myc driving glycolysis or glutaminolysis. Indeed, KP Per2 mutant cells had increased rates of glucose consumption and increased levels of lactate excretion compared to Per2+/+ cells (Figure 4A). Furthermore, we observed a significant increase in the levels of glutamine consumption in Per2 mutant cells, indicative of increased glutamine usage (Figure 4B). To determine whether the overall flux of glucose was differentially regulated in Per2 mutant cells with high levels of c-Myc, we performed tracing of isotope-labeled glucose (U-13Cglucose). Increased use of glucose for the TCA cycle was detected as determined by carbon 4 labeling (M4) of TCA cycle intermediates (Figure 4C). These data indicate that in addition to increased glycolysis by lactate production, there is increased utilization of glucose for the TCA cycle in the mitochondria. These data are in agreement with our recent findings demonstrating that lung tumors have increased glucose contribution to the TCA cycle relative to normal lung tissue (Davidson et al., 2016).
Altered expression of circadian machinery in human lung adenocarcinoma
Our data demonstrates a cell autonomous oncogenic role for circadian rhythm disruption in lung tumorigenesis in mouse models of the disease as well as in tissue culture models of cellular transformation. We next investigated whether the expression of the core circadian pathway genes (PER1, PER2, PER3, CRY1, CRY2, ARNTL and CLOCK) is altered in human lung adenocarcinomas. We examined the expression levels of these core circadian genes in normal lung samples (n=58) compared to either matched tumors (n=57) or all lung adenocarcinomas (n=431) in the TCGA RNAseq expression dataset (Cancer Genome Atlas Research, 2014). With the exception of CLOCK, whose expression was significantly increased in tumors, the transcripts of all the circadian genes were significantly decreased in matched tumors when compared to normal lung (Figure S4A). Furthermore, when we subdivided the tumors by grade (T1, T2 and T3) we observed that ARNTL, CRY2 and PER3 expression decreased significantly in higher grade (T3) compared to lower grade (T1) tumors (Figure S4A).
Increased MYC pathway activity in human lung adenocarcinomas with decreased PER2
Finally, we ranked KP lung tumors based on their expression of PER2 and used the top and bottom 25% of tumors (n=12) and derived a signature of differential gene expression between these sets. Subsequent gene set enrichment analysis (GSEA) of this signature revealed that tumors with low levels of PER2 were enriched in MYC signatures (MYC_UP.V1_UP, DANG_MYC_TARGETS_UP), suggesting that tumors with low PER2 may have increased MYC activity (Figure S4B). These results are consistent with our finding in the GEMM that loss of Per2 leads to an increase in c-Myc activity.
Discussion
Circadian rhythm disruption through shift-work has been linked to increased cancer risk in multiple types of cancer (Hansen, 2001; Kloog et al., 2009; Schernhammer et al., 2006; Schernhammer et al., 2001). The study of physiologic risk factors, such as circadian rhythm disruption, has been largely hindered by the lack of rigorous in vivo experimental systems. The data presented here demonstrate that circadian rhythm disruption can promote lung tumorigenesis and support an important tumor suppressive role for circadian homeostasis. Our study illustrates that physiologic or genetic (Bmal1/Per2) circadian can cooperate with Kras and p53 to promote lung tumorigenesis. Systemic loss of Per2 and Bmal1 has differential effects on circadian behavior of animals (Bunger et al., 2000; Zheng et al., 1999). Despite these differences on whole-animal behavior, loss of Per2 or Bmal1 accelerated lung cancer.
Earlier reports using germline mutations in circadian components have highlighted a role for circadian rhythm disruption in tumor predisposition (Filipski et al., 2005; Fu et al., 2002; Gery et al., 2006; Janich et al., 2011; Kettner et al., 2014; Lee et al., 2010; Sahar and Sassone-Corsi, 2009; Van Dycke et al., 2015; Wood et al., 2008). We demonstrate through several in vitro and in vivo systems that tumor cell-specific deletion of core clock components leads to increased proliferation, c-Myc levels/activation and metabolic activity. Our results highlight the importance of cell-autonomous circadian control of fundamental cellular processes that are known hallmarks of cancer and promote cellular transformation and tumor progression. Our results suggest that increased tumor incidence in circadian mutant animals may be a result of tumor cell-autonomous loss of circadian regulation which eliminates an additional barrier to transformation and tumor progression. Cell autonomous loss of circadian rhythms in human malignancies may occur through both genetic and epigenetic mechanisms (Alhopuro et al., 2010; Chen et al., 2005; Kettner et al., 2014; Taniguchi et al., 2009). We present evidence for decreased expression of most circadian genes in human lung adenocarcinoma patient tumors as compared to matched lung tissue. Furthermore, tumors with decreased PER2 gene expression show a significant enrichment in c-MYC target expression, further supporting our finding in the GEMMs, where both Per2 and Bmal1 mutant lung tumors have increased levels of c-Myc. We postulate that as part of a homeostatic circadian clock, Per2 and Bmal1 converge in suppressing c-Myc during lung tumorigenesis. Therefore loss of Per2 or Bmal1 can independently lead to increased c-Myc transcriptional output. Our results are in line with prior studies revealing the competition between both Per2 and Bmal1 with oncogenic c-MYC (Altman et al., 2015; Fu et al., 2002; Lee et al., 2010). Ebert et al. recently demonstrated that loss of Bmal1 and Clock suppressed leukemogenesis, suggesting that circadian disruption can have context specific effects in different types of cancers (Puram et al., 2016).
There are likely many cell-autonomous and non-autonomous mechanisms that are altered in response to circadian disruption that can influence tumorigenesis. In this study we developed new animal models that enabled us to comprehensively characterize the tumorigenic role of systemic and cell-autonomous circadian rhythm disruption. We anticipate our findings will provide the critical framework for future research that will further elucidate the mechanisms of organismal and cellular control of circadian oscillations on cancer initiation and progression.
Experimental Procedures
Animal Experiments
All animal studies were approved by the MIT IACUC. KrasLSL-G12D/+;p53flox/flox (KP), KrasLSL-G12D/+ (K) and KrasLA2/+ mice have already been described (DuPage et al., 2009; Jackson et al., 2005; Jackson et al., 2001). All animals with circadian mutant alleles were maintained on a mixed C57BL/6J x 129SvJ genetic background. For jet lag experiments we utilized pure 129SvJ background. We utilized several existing circadian mutant alleles which are available from Jackson Laboratories for germline mutant Per2 (Per2tm1Brd/J) (Zheng et al., 1999), germline mutant (Arntltm1Bra/J) (Bunger et al., 2000) and conditional (Arntltm1Weit/J) (Storch et al., 2007) Bmal1. These mutants were crossed to K, KP or KLA2 animals. Both male and female mice were infected intratracheally with adenoviruses as described (DuPage et al., 2009). Animals were injected intraperitoneally with 30ug of BrdU per gram of body weight 4 hours prior to sacrificing animals.
Jet lag conditions and activity monitoring
Randomization was used for mice placed into LD12:12 or jet lag. For jet lag, animals were placed in altered light cycle conditions with 8 hours light advance every 2–3 days (Filipski et al., 2005; Lee et al., 2010). Jet lag at ‘Initiation’ was performed by placing animals in altered light conditions a week before tumor initiation and kept under those conditions for 13 weeks. For jet lag at ‘Progression’, animals were placed in altered light conditions at five weeks post-tumor initiation and kept in jet lag conditions for 8 weeks. Activity monitoring of animals was recorded using wireless running wheels and actogram data analysis was performed using wheel analysis software (MED associates inc).
Statistics
P-values were determined by Student’s t-test for all measurements of tumor burden and IHC quantifications except for contingency tables, in which Fisher’s exact test or Chi-square test were used. All error bars denote standard error mean s.e.m.
Additional experimental procedures can be found in the Supplemental Information under Supplemental Experimental Procedures
Supplementary Material
Acknowledgments
We thank David G. McFadden, Francisco J. Sánchez-Rivera, Tuomas Tammela, Volkan Sayin and Sarah Leboeuf for critical reading of the manuscript. We would also like to thank Roderick Bronson for assistance in histopathological analysis of tumors. This work was supported by the NCI Cancer Center Support Core Grant, the Lung Cancer Research Foundation and the Koch Institute Frontier Fund. T.P. was supported by the American Cancer Society and Hope Funds for Cancer Research. T.J. is a Howard Hughes Medical Institute Investigator, the David H. Koch Professor of Biology, and a Daniel K. Ludwig Scholar. The authors have no conflict of interest to disclose.
Footnotes
Author Contributions
T.P. and T.J. designed this study; T.P., M.B., S.D., M.H., L.S., J.B. performed experiments; S.D. and M.V.H designed metabolomics experiments and provided conceptual advice, S.D. analyzed metabolomics data; A.B. performed bioinformatics analyses; T.P. and T.J. wrote the manuscript.
References
- Alhopuro P, Bjorklund M, Sammalkorpi H, Turunen M, Tuupanen S, Bistrom M, Niittymaki I, Lehtonen HJ, Kivioja T, Launonen V, et al. Mutations in the circadian gene CLOCK in colorectal cancer. Mol Cancer Res. 2010;8:952–960. doi: 10.1158/1541-7786.MCR-10-0086. [DOI] [PubMed] [Google Scholar]
- Altman BJ, Hsieh AL, Sengupta A, Krishnanaiah SY, Stine ZE, Walton ZE, Gouw AM, Venkataraman A, Li B, Goraksha-Hicks P, et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell metabolism. 2015;22:1009–1019. doi: 10.1016/j.cmet.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26:1241–1246. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, Bauer MR, Jha AK, O’Brien JP, Pierce KA, et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell metabolism. 2016;23:517–528. doi: 10.1016/j.cmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protocols. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipski E, Innominato PF, Wu M, Li XM, Iacobelli S, Xian LJ, Levi F. Effects of light and food schedules on liver and tumor molecular clocks in mice. J Natl Cancer Inst. 2005;97:507–517. doi: 10.1093/jnci/dji083. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Molecular cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell metabolism. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The Differential Effects of Mutant p53 Alleles on Advanced Murine Lung Cancer. Cancer Research. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Gene Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janich P, Pascual G, Merlos-Suarez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Kettner NM, Katchy CA, Fu L. Circadian gene variants in cancer. Annals of medicine. 2014;46:208–220. doi: 10.3109/07853890.2014.914808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Haim A, Stevens RG, Portnov BA. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int. 2009;26:108–125. doi: 10.1080/07420520802694020. [DOI] [PubMed] [Google Scholar]
- Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010;5:e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis CG, Grutsch JF, Wood P, You M, Rich I, Hrushesky WJ. Circadian timing in cancer treatment: the biological foundation for an integrative approach. Integr Cancer Ther. 2003;2:105–111. doi: 10.1177/1534735403002002002. [DOI] [PubMed] [Google Scholar]
- Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, Jacks T, Sassone-Corsi P. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell. 2016;165:896–909. doi: 10.1016/j.cell.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Mullenders J, Fabius AW, Madiredjo M, Bernards R, Beijersbergen RL. A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS One. 2009;4:e4798. doi: 10.1371/journal.pone.0004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28:548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, Tothova Z, Tejero H, Heckl D, Jaras M, et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016;165:303–316. doi: 10.1016/j.cell.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rivera FJ, Jacks T. Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev Cancer. 2015;15:387–395. doi: 10.1038/nrc3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin VI, Papagiannakopoulos T. Application of CRISPR-mediated genome engineering in cancer research. Cancer letters. 2016 doi: 10.1016/j.canlet.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17:108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Soucek L, Whitfield JR, Sodir NM, Masso-Valles D, Serrano E, Karnezis AN, Swigart LB, Evan GI. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes Dev. 2013;27:504–513. doi: 10.1101/gad.205542.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Fernandez AF, Setien F, Ropero S, Ballestar E, Villanueva A, Yamamoto H, Imai K, Shinomura Y, Esteller M. Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 2009;69:8447–8454. doi: 10.1158/0008-5472.CAN-09-0551. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Van Dycke KC, Rodenburg W, van Oostrom CT, van Kerkhof LW, Pennings JL, Roenneberg T, van Steeg H, van der Horst GT. Chronically Alternating Light Cycles Increase Breast Cancer Risk in Mice. Curr Biol. 2015;25:1932–1937. doi: 10.1016/j.cub.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- Wood PA, Yang XM, Taber A, Oh EY, Ansell C, Ayers SE, Al-Assaad Z, Carnevale K, Berger FG, Pena MMO, et al. Period 2 Mutation Accelerates Apc(Min/+) Tumorigenesis. Molecular Cancer Research. 2008;6:1786–1793. doi: 10.1158/1541-7786.MCR-08-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptacek LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128:59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.