Abstract
Aryl hydrocarbon receptor (AhR) has been increasingly recognized to play a crucial role in normal physiological homeostasis. Additionally, disrupted AhR signaling leads to several pathological states in the lung and liver. AhR activation transcriptionally induces detoxifying enzymes such as cytochrome P450 (CYP) 1A and NAD(P)H quinone dehydrogenase 1 (NQO1). The toxicity profiles of the classical AhR ligands such as 3-methylcholanthrene and dioxins limit their use as a therapeutic agent in humans. Hence, there is a need to identify nontoxic AhR ligands to develop AhR as a clinically relevant druggable target. Recently, we demonstrated that leflunomide, a FDA approved drug, used to treat rheumatoid arthritis in humans, induces CYP1A enzymes in adult mice via the AhR. However, the mechanisms by which this drug induces NQO1 in vivo are unknown. Therefore, we tested the hypothesis that leflunomide will induce pulmonary and hepatic NQO1 enzyme in neonatal mice via AhR-dependent mechanism(s). Leflunomide elicited significant induction of pulmonary CYP1A1 and NQO1 expression in neonatal mice. Interestingly, the dose at which leflunomide increased NQO1 was significantly higher than that required to induce CYP1A1 enzyme. Likewise, it also enhanced hepatic CYP1A1, 1A2 and NQO1 expression in WT mice. In contrast, leflunomide failed to induce these enzymes in AhR-null mice. Our results indicate that leflunomide induces pulmonary and hepatic CYP1A and NQO1 enzymes via the AhR in neonatal mice. These findings have important implications to prevent and/or treat disorders such as bronchopulmonary dysplasia in human infants where AhR may play a crucial role in the disease pathogenesis.
Keywords: Leflunomide, Aryl hydrocarbon Receptor, Cytochrome P450 1A enzymes, NAD(P)H quinone dehydrogenase 1, and Neonates
Introduction
The aryl hydrocarbon receptor (AhR) is a member of basic - helix – loop – helix/PER – ARNT – SIM family of transcriptional regulators [1]. The non-ligand bound AhR is predominantly cytosolic. Ligand-induced AhR activation results in a conformational change of the cytosolic AhR complex and release of XAP2 that exposes the nuclear localization sequence, resulting in translocation of this complex into the nucleus [2, 3]. In the nucleus, the AhR dissociates from the core complex, dimerizes with the AhR nuclear translocator, and initiates transcription of many phase I and phase II enzymes such as cytochrome P450 (CYP) 1A1 and NAD(P)H quinone dehydrogenase 1 (NQO1) by binding to the xenobiotic-response elements (XREs)/AhR-response elements (AhREs) motifs in the promoter region of these genes [4–7].
The well-established function of the AhR is to facilitate the metabolism of xenobiotics via induction of phase I and II enzymes [8, 9]. The phase I enzymes such as cytochrome P450 (CYP) monooxygenases introduce reactive and polar groups to their xenobiotic substrates to facilitate excretion. In phase II reactions, enzymes such as NQO1 conjugate the activated substrates with glutathione, sulfate, glycine, or glucuronic acid to detoxify the substrates and make them more polar so that they can be actively transported. Together, phase I and II enzymes detoxify toxic compounds and metabolites. The AhR is of particular interest to toxicologists and extensive research has been conducted on its role in the bioactivation of polycyclic and aromatic hydrocarbons and carcinogenesis [10]. However, the creation of knockout and transgenic mice has provided mechanistic insights into the potential role(s) that AhR might play in normal physiological homeostasis [11–14]. Hence, search for novel and nontoxic AhR agonists is of paramount importance to understand the role of AhR and its downstream target genes, such as CYP1A and NQO1 enzymes, in physiological and abnormal disease states. To this end, we chose leflunomide; to test the hypothesis that leflunomide will induce pulmonary and hepatic CYP1A and NQO1 enzymes via AhR-dependent mechanism(s) in neonatal mice.
Leflunomide or N- (4-trifluoromethylphenyl) – 5 – methylisoxazol - 4 – carboxamide) is an immunomodulatory drug that is used to treat rheumatoid arthritis in humans [15]. Several studies indicate that leflunomide activates AhR and its downstream target genes such as CYP1A enzymes, mainly in vitro [16–19]. Hu et. al. [20] have shown that leflunomide directly binds to and activates AhR in vitro and induces hepatic CYP1A mRNA in vivo in rats. Recently, our studies in adult mice showed that leflunomide induces pulmonary and hepatic CYP1A enzymes in wild type, but not in AhR-null mice, indicating that leflunomide induces CYP1A enzymes via the AhR [21]. Whether leflunomide exerts similar effects in neonatal mice is not known. Also, whether leflunomide induces the phase II enzyme, NQO1, in the neonatal period is unknown. Addressing these knowledge gaps are important to improve therapies of human neonatal disorders such as bronchopulmonary dysplasia (BPD), where AhR may play a crucial role in the pathogenesis of the disorder. Thus, the goals of this study were to investigate whether leflunomide regulates the expression of pulmonary and hepatic CYP1A and NQO1 enzymes in neonatal mice. In addition to neonatal wild type mice, we chose AhR-null mice for our studies to determine the mechanisms through which leflunomide regulates these enzymes.
Materials and Methods
Animals
This study was approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine (Protocol number: AN-5631). The C57BL/6J wild type (WT) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Dr. Christopher A. Bradfield (University of Wisconsin, Madison) kindly provided us the AhR-null mice on a C57BL/6J background. Timed-pregnant mice raised in our animal facility were used for the experiments.
Chemicals
Polyethylene glycol (PEG) and leflunomide were purchased from Sigma-Aldrich (St. Louis, MO). The primary monoclonal antibodies to CYP1A1 (which cross reacts with CYP1A2) and CYP1A2 were generous gifts from P.E. Thomas (Rutgers University, Piscataway, NJ), whereas anti-NQO1, anti-78 kDa glucose-regulated protein (GRP78) and anti-β-actin antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA).
Experiment design
One-day-old WT mice were injected intraperitoneally (i.p.) with 1 (L 1), 5 (L 5) or 15 (L 15) mg/kg of leflunomide or the vehicle, PEG (control), once daily for 4 d. Similarly, one-day-old AhR-null mice were injected intraperitoneally with either the vehicle, PEG, or with 15 mg/kg (L 15) of leflunomide once daily for 4 d. Following the injections, the lung and liver tissues were harvested to determine the expression of CYP 1A1/A2 and NQO1 enzymes.
Real-time RT-PCR assays
Total mRNA was isolated and reverse transcribed to cDNA [22]. Real-time RT-PCR analysis was performed using iTaq Universal SYBR Green Supermix (Biorad, Hercules, CA; 1725121). The sequences of the primer pairs were mCYP1a1: 5′-GGT TAA CCA TGA CCG GGA ACT-3′ and 5′-TGC CCA AAC CAA AGA GAG TGA-3′; mCYP1a2: 5′-TGG AGC TGG CTT TGA CAC AG-3′ and 5′-CGT TAG GCC ATG TCA CAA GTA GC-3′; mNQO1: 5′-GGA AGC TGC AGA CCT GGT GA-3′ and 5′-CCT TTC AGA ATG GCT GGC A-3′; and mβ-actin: 5′-TAT TGG CAA CGA GCG GTT CC-3′ and 5′-GGC ATA GAG GTC TTT ACG GAT GTC-3′. β-actin was used as the reference gene. Following an RT hold for 10 minutes at 95°C, the samples were denatured at 95°C for 10 minutes. The thermal cycling step was for 40 cycles at 95°C for 15 s, and 40 cycles at 60°C for 1 minute [23].
Preparation of microsomes and cytosolic proteins
Lung cytosolic proteins were extracted as described before [24]. Briefly, a mortar and pestle was used to homogenize the lung tissue in a buffer containing 50mM Tris-HCL (pH 7.5), 0.5M KCL, 1M MgCL, and 0.5M EDTA. The homogenate was centrifuged at 2400 g for 5 min at 4°C. The supernatant (cytoplasmic fraction) was stored at − 80°C until further use. Liver microsomes and cytosolic proteins were isolated by calcium chloride precipitation method as described previously [25, 26].
Western blotting
Lung and liver proteins were subjected to SDS polyacrylamide gel electrophoresis. The lung cytosolic and liver microsomal proteins were used to determine the expression of CYP1A enzymes, whereas the cytosolic proteins of the lung and liver were used to determine NQO1 expression. β-actin and GRP 78 were used as reference proteins for the cytoplasmic and microsomal fractions, respectively. The separated proteins on the gels were transferred to polyvinylidene difluoride membranes. The membranes were then incubated overnight at 4°C with the following primary antibodies: anti-CYP1A1, a specific anti-CYP1A2 (dilution 1:1500), anti-NQO1 antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-16464, dilution 1:500), anti-GRP 78 (Santa Cruz Biotechnologies, Santa Cruz, CA;; sc-13968, dilution 1:500), and anti-β-actin (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-47778, dilution 1:2000). The primary antibodies were detected by incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies.
Analyses of data
The results were analyzed by GraphPad Prism 5 software. The data are expressed as means ± SEM. One-way ANOVA was used to determine the effect of treatment (leflunomide, dose-response studies) on enzyme (CYP1A and NQO1) expression in WT mice, while two-way ANOVA was used to determine the effects of treatment (Leflunomide) and gene (AhR), and their associated interactions for the outcome variables (CYP1A and NQO1 expression). Post hoc multiple t-tests with Dunnett’s corrections were performed if a statistical difference for the dose-response studies was noted by one-way ANOVA, whereas post hoc multiple t-tests with Bonferroni corrections were performed if statistical significance of either variable or interaction was noted by two-way ANOVA. A p value of <0.05 was considered significant.
Results and Discussion
This study demonstrates that leflunomide induces the expression of the enzymes, CYP1A1/A2 and NQO1, via the AhR. In neonatal WT mice in vivo, leflunomide induced CYP1A1/A2 and NQO1 expression, whereas in neonatal AhR-null mice, the lack of leflunomide-mediated induction of CYP1A1/A2 and NQO1 enzymes correlated with the absence of a functional AhR gene.
The AhR is a versatile transcription factor that has important physiological functions in addition to its widely established role in xenobiotic metabolism. Studies from our and other laboratories have reported that the AhR regulates pulmonary and hepatic oxidant stress and inflammation by inducing several detoxifying enzymes or via “cross-talk” with other signal transduction pathways [24, 26–30]. However, the prototypical inducers such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 3-methylcholanthrene (MC) are unsuitable for clinical use because of their well known toxicities. Hence, identification of novel non-toxic AhR ligands is important for developing the AhR as a clinically relevant therapeutic target in oxidant injury- and inflammation-mediated lung and liver disorders. Therefore, we conducted in vivo studies with a novel FDA approved drug, leflunomide, to determine whether it induces the expression of the AhR-regulated pulmonary and hepatic CYP1A and NQO1 enzymes.
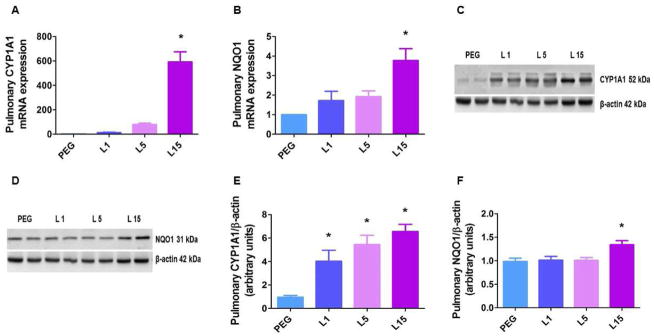
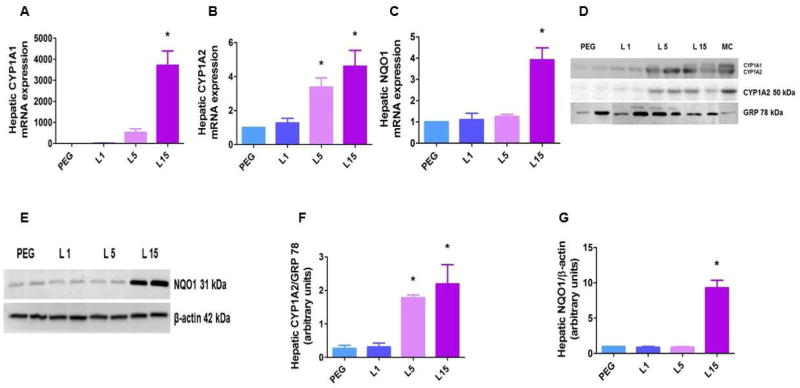
To examine whether leflunomide activates pulmonary AhR in the neonatal period, we treated newborn mice with incremental doses of leflunomide (1 to 15 mg/kg/d i.p.) for 4 d. The dose of leflunomide used in this study was comparable to that used in previous rodent studies [20, 31], and we did not observe any toxic effects of leflunomide in our experiments. Functional activation of AhR results in the transcriptional activation of various target genes referred to as AhR gene battery, of which the best studied example is CYP1A1 gene [32]. Therefore, we initially analyzed the expression of CYP1A enzymes in leflunomide-treated neonatal WT mice. Based on our prior study suggesting that CYP1A1, but not CYP1A2, is induced in the lungs, whereas both CYP1A1 and 1A2 are induced in the liver [21], we analyzed pulmonary CYP1A1 and hepatic CYP1A1/A2 expression in this study. Consistent with our prior study [21] and those of others [20, 33], leflunomide transcriptionally induced pulmonary CYP1A1 (Figs. 1A, C, and E) and hepatic CYP1A1/A2 (Figs. 2A, B, D, and F) enzymes in a dose-dependent manner. However, the knowledge gaps of the effects of leflunomide on pulmonary and hepatic NQO1 expression and the mechanism by which leflunomide induces these enzymes in vivo are addressed in this current investigation.
Figure 1. Leflunomide up-regulates pulmonary CYP1A1 and NQO1 expression in neonatal WT mice.
The lung tissues of neonatal WT mice treated i.p. with the vehicle, polyethylene glycol (PEG), or 1 (L 1), 5 (L 5), or 15 (L 15) mg/kg of leflunomide daily for 4 d were harvested to determine pulmonary CYP1A1 mRNA (A), NQO1 mRNA (B), CYP1A1 protein (C), and NQO1 (D) protein expression. Densitometric analyses wherein CYP1A1 (E) and NQO (F) band intensities were normalized to β-actin. Values are means ± SEM (n=3). One-way ANOVA showed an effect of L 15 for CYP1A1 and NQO1 mRNA expression and NQO1 protein expression, and an effect of L1, L5, or L15 for CYP1A1 protein expression. Significant differences between PEG- and leflunomide-treated animals are indicated by *, p < 0.05.
Figure 2. Leflunomide up-regulates hepatic CYP1A1/A2 and NQO1 expression in neonatal WT mice.
The liver tissues of neonatal WT mice treated as mentioned in Figure 1 were harvested to determine hepatic CYP1A1 (A), CYP1A2 (B), and NQO1 (C) mRNA expression, and CYP1A1/A2 (D), and NQO1 (E) protein expression. Densitometric analyses wherein CYP1A2 (F) and NQO1 (G) band intensities were normalized to GRP 78 and β-actin, respectively. Values are means ± SEM (n=3). One-way ANOVA showed an effect of L15 for CYP1A1 and NQO1 expression, and an effect of L5 and L15 for CYP1A2 expression. Significant differences between PEG- and leflunomide-treated animals are indicated by *, p < 0.05.
NQO1 is a flavoprotein enzyme, which catalyzes the two-electron reduction of quinoid compounds into their reduced form, such as hydroquinones, and prevents the one electron reduction of quinones that results in increased levels of radical species [34]. NQO1 is mainly a cytosolic enzyme that is highly expressed in the lungs and liver [35, 36], and is readily inducible by stressful stimuli such as oxidative stress [37]. The NQO1 promotor region contains both antioxidant-response elements (AREs) and XREs; therefore, NQO1 expression is transcriptionally regulated by both AhR and nuclear factor erythroid 2–related factor 2 (Nrf2)/Kelch-like ECH-associated protein 1 pathways [38–40]. O’Donnell et al., have shown that leflunomide induces NQO1 expression in human and mouse hepatic cells in vitro. Similarly, Hu et al., demonstrated that leflunomide increases hepatic NQO1 mRNA in a dose-dependent manner in adult rats. Our studies further complement these observations by demonstrating that leflunomide also induces NQO1 mRNA and protein in the lung (Figs. 1B, D, and F) and liver (Figs. 2C, E, and G) of neonatal mice. Interestingly, we observed that dose of leflunomide required to induce pulmonary NQO1 was higher than that required to induce pulmonary CYP1A1 enzyme, with the minimum effective dose being 15 mg/kg/d. The reasons for this observation in unknown at this time and needs further investigation.
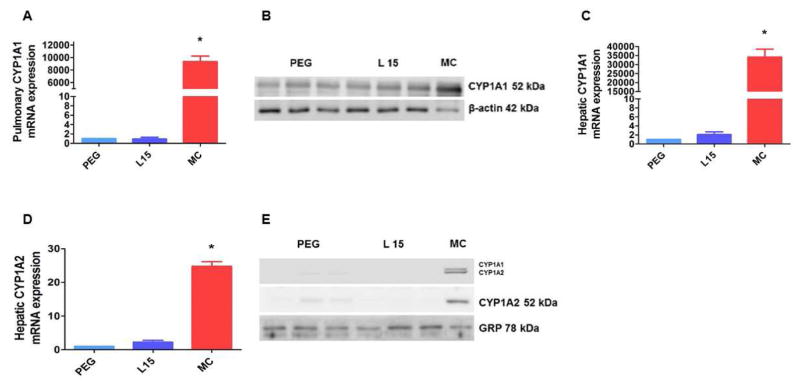
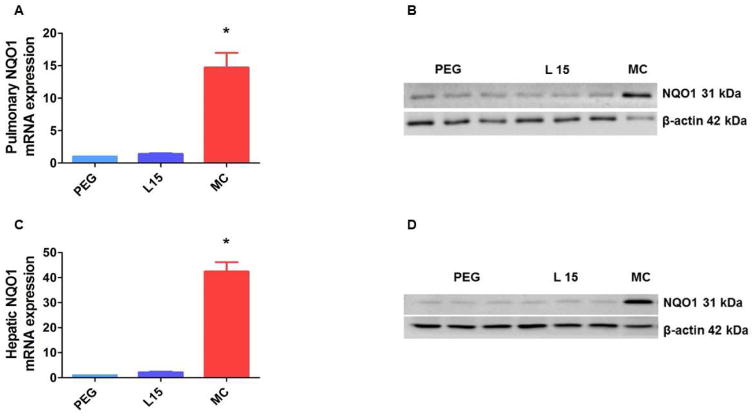
The mechanistic role of AhR in the induction of CYP1A by prototypical inducers, MC and TCDD has been extensively studied. Whether leflunomide induces CYP1A and NQO1 enzymes via AhR in neonatal mice in vivo is unknown. Therefore, we conducted experiments with leflunomide in neonatal AhR-null mice. We injected AhR-null neonatal mice with the vehicle, PEG, or leflunomide for 4 days, following which we determined the expression of CYP1A1 and NQO1 in the lungs and liver, and CYP1A2 in the liver. In AhR-null neonatal mice, leflunomide failed to increase the mRNA and protein levels of pulmonary CYP1A1 and hepatic CYP1A1/A2 enzymes (Fig. 3). Similarly, leflunomide failed to induce NQO1 in the lung and liver of these mice (Fig. 4). These results supports the hypothesis that induction of these enzymes by leflunomide is mediated via AhR-dependent mechanism(s). To the best of our knowledge, this is the first in vivo study to demonstrate that AhR is necessary for leflunomide-mediated up-regulation of pulmonary and hepatic CYP1A and NQO1 enzymes in neonatal mice.
Figure 3. Leflunomide fails to induce pulmonary and hepatic CYP1A enzymes in neonatal AhR-null mice.
The lung and liver tissues of neonatal AhR-null mice treated with PEG or 15 (L 15) mg/kg of leflunomide i.p. daily for 4 d were harvested to determine pulmonary and hepatic CYP1A1 mRNA (A and C) and protein (B and E) expression, respectively, and hepatic CYP1A2 mRNA (D) and protein (E) expression. Additionally, neonatal WT mice were injected i.p. with 26 mg/kg/d of 3-methylcholanthrene (MC) (n=3), following which the lung and liver tissues of these animals were harvested for analysis of CYP1A induction. MC-treated mice were used as positive controls. Significant differences between PEG- and MC-treated animals are indicated by *, p < 0.05.
Figure 4. Leflunomide fails to induce pulmonary and hepatic NQO1 enzyme in neonatal AhR-null mice.
The lung and liver tissues of neonatal AhR-null mice treated as mentioned in Figure 3 were harvested to determine pulmonary and hepatic NQO1 mRNA (A and C) and protein (B and D) expression. Additionally, the lung and liver tissues of neonatal WT mice treated with 3-methylcholanthrene (MC) (n=3) as mentioned in Figure 3 were harvested for analysis of NQO1 induction. MC-treated mice were used as positive controls. Significant differences between PEG- and MC-treated animals are indicated by *, p < 0.05.
In summary, we provide evidence that leflunomide is an AhR agonist in neonatal mice because it induces pulmonary and hepatic CYP1A1 and NQO1, and hepatic CYP1A2 enzymes via an AhR-mediated mechanism. The protective effects of CYP1A enzymes against hyperoxia-induced lung injury in rodents have been extensively documented [26, 41–43]. In addition, NQO1 has been shown to protect cells and tissues against oxidant injury induced by various toxic chemicals [44] and oxygen [45]. The protective mechanisms of these enzymes have been attributed to their ability to conjugate and scavenge the reactive electrophiles and lipid peroxidation products generated by an oxidant injury [46]. Thus, our results suggest that leflunomide can be used to investigate AhR biology in the lung and liver, which can lead to the discovery of novel therapies in the prevention and treatment of oxidative stress- and inflammation-induced disorders like BPD, liver disorders, and necrotizing enterocolitis in human preterm infants.
Supplementary Material
Highlights.
Leflunomide induces CYP1A and NQO1 enzymes in neonatal mice.
Leflunomide is a more potent inducer of CYP1A than NQO1 enzyme.
AhR deficiency abrogates leflunomide-mediated induction of CYP1A and NQO1 enzymes.
Acknowledgments
This work was supported by grants from National Institutes of Health [K08 HD073323 to B.S. and R01 grants ES009132, HL112516, ES019689, and HL129794 to B.M.]; American Heart Association [BGIA 20190008]; and American Lung Association [RG 349917] to B.S.
Abbreviations
- AhR
aryl hydrocarbon receptor
- AhREs
AhR-response elements
- AREs
antioxidant-response elements
- BPD
bronchopulmonary dysplasia
- CYP
cytochrome P450
- GRP 78
78 kDa glucose-regulated protein
- L
leflunomide (PubChem CID: 3899)
- L 1
leflunomide 1 mg/kg/d
- L 5
leflunomide 5 mg/kg/d
- L 15
leflunomide 15 mg/kg/d
- MC
3-methylcholanthrene (PubChem CID: 1674)
- Nrf2
nuclear factor erythroid 2–related factor 2
- NQO1
NAD(P)H quinone dehydrogenase 1
- PEG
polyethylene glycol
- ROS
reactive oxygen species
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- XREs
xenobiotic-response elements
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollenz RS, Sattler CA, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Molecular pharmacology. 1994;45:428–438. [PubMed] [Google Scholar]
- 3.Hord NG, Perdew GH. Physicochemical and immunocytochemical analysis of the aryl hydrocarbon receptor nuclear translocator: characterization of two monoclonal antibodies to the aryl hydrocarbon receptor nuclear translocator. Molecular pharmacology. 1994;46:618–626. [PubMed] [Google Scholar]
- 4.Emi Y, Ikushiro S, Iyanagi T. Xenobiotic responsive element-mediated transcriptional activation in the UDP-glucuronosyltransferase family 1 gene complex. J Biol Chem. 1996;271:3952–3958. doi: 10.1074/jbc.271.7.3952. [DOI] [PubMed] [Google Scholar]
- 5.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 6.Fujisawa-Sehara A, Sogawa K, Yamane M, Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic acids research. 1987;15:4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrenk D. Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo- and xenobiotics. Biochemical pharmacology. 1998;55:1155–1162. doi: 10.1016/s0006-2952(97)00591-1. [DOI] [PubMed] [Google Scholar]
- 9.Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Critical reviews in toxicology. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 10.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 11.Bock KW, Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem. 2009;390:1225–1235. doi: 10.1515/BC.2009.138. [DOI] [PubMed] [Google Scholar]
- 12.Fujii-Kuriyama Y, Kawajiri K. Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:40–53. doi: 10.2183/pjab.86.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauzeau V, Carvajal-Gonzalez JM, Riolobos AS, Sevilla MA, Menacho-Marquez M, Roman AC, Abad A, Montero MJ, Fernandez-Salguero P, Bustelo XR. Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J Biol Chem. 2011;286:2896–2909. doi: 10.1074/jbc.M110.187534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey S, Papoutsakis ET. The evolving role of the aryl hydrocarbon receptor (AHR) in the normophysiology of hematopoiesis. Stem cell reviews. 2012;8:1223–1235. doi: 10.1007/s12015-012-9384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto P, Dougados M. Leflunomide in clinical practice. Acta reumatologica portuguesa. 2006;31:215–224. [PubMed] [Google Scholar]
- 16.Jin UH, Lee SO, Pfent C, Safe S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC cancer. 2014;14:498. doi: 10.1186/1471-2407-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin UH, Lee SO, Safe S. Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. The Journal of pharmacology and experimental therapeutics. 2012;343:333–341. doi: 10.1124/jpet.112.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell EF, Kopparapu PR, Koch DC, Jang HS, Phillips JL, Tanguay RL, Kerkvliet NI, Kolluri SK. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PloS one. 2012;7:e40926. doi: 10.1371/journal.pone.0040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell EF, Saili KS, Koch DC, Kopparapu PR, Farrer D, Bisson WH, Mathew LK, Sengupta S, Kerkvliet NI, Tanguay RL, Kolluri SK. The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PloS one. 2010;5:e13128. doi: 10.1371/journal.pone.0013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Molecular pharmacology. 2007;71:1475–1486. doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, Zhang S, Paramahamsa M, Jiang W, Wang L, Moorthy B, Shivanna B. Leflunomide Induces Pulmonary and Hepatic CYP1A Enzymes via Aryl Hydrocarbon Receptor. Drug metabolism and disposition: the biological fate of chemicals. 2015;43:1966–1970. doi: 10.1124/dmd.115.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivanna B, Chu C, Welty SE, Jiang W, Wang L, Couroucli XI, Moorthy B. Omeprazole attenuates hyperoxic injury in H441 cells via the aryl hydrocarbon receptor. Free radical biology & medicine. 2011;51:1910–1917. doi: 10.1016/j.freeradbiomed.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anwar-Mohamed A, Abdelhamid G, Amara IE, El-Kadi AO. Differential modulation of aryl hydrocarbon receptor regulated enzymes by arsenite in the kidney, lung, and heart of C57BL/6 mice. Archives of toxicology. 2012;86:897–910. doi: 10.1007/s00204-012-0855-x. [DOI] [PubMed] [Google Scholar]
- 24.Shivanna B, Zhang W, Jiang W, Welty SE, Couroucli XI, Wang L, Moorthy B. Functional deficiency of aryl hydrocarbon receptor augments oxygen toxicity-induced alveolar simplification in newborn mice. Toxicology and applied pharmacology. 2013;267:209–217. doi: 10.1016/j.taap.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. The Journal of pharmacology and experimental therapeutics. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- 27.Shivanna B, Jiang W, Wang L, Couroucli XI, Moorthy B. Omeprazole attenuates hyperoxic lung injury in mice via aryl hydrocarbon receptor activation and is associated with increased expression of cytochrome P4501A enzymes. The Journal of pharmacology and experimental therapeutics. 2011;339:106–114. doi: 10.1124/jpet.111.182980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. The Journal of biological chemistry. 2008;283:28944–28957. doi: 10.1074/jbc.M800685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rico de Souza A, Zago M, Pollock SJ, Sime PJ, Phipps RP, Baglole CJ. Genetic ablation of the aryl hydrocarbon receptor causes cigarette smoke-induced mitochondrial dysfunction and apoptosis. The Journal of biological chemistry. 2011;286:43214–43228. doi: 10.1074/jbc.M111.258764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. The American journal of pathology. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baban B, Liu JY, Mozaffari MS. Aryl hydrocarbon receptor agonist, leflunomide, protects the ischemic-reperfused kidney: role of Tregs and stem cells. American journal of physiology. Regulatory integrative and comparative physiology. 2012;303:R1136–1146. doi: 10.1152/ajpregu.00315.2012. [DOI] [PubMed] [Google Scholar]
- 32.Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell EF, Saili KS, Koch DC, Kopparapu PR, Farrer D, Bisson WH, Mathew LK, Sengupta S, Kerkvliet NI, Tanguay RL, Kolluri SK. The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PloS one. 2010;5 doi: 10.1371/journal.pone.0013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lind C, Cadenas E, Hochstein P, Ernster L. DT-diaphorase: purification, properties, and function. Methods in enzymology. 1990;186:287–301. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- 35.Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free radical biology & medicine. 2000;29:246–253. doi: 10.1016/s0891-5849(00)00310-5. [DOI] [PubMed] [Google Scholar]
- 36.Prochaska HJ, Talalay P. Purification and characterization of two isofunctional forms of NAD(P)H: quinone reductase from mouse liver. The Journal of biological chemistry. 1986;261:1372–1378. [PubMed] [Google Scholar]
- 37.Boothman DA, Meyers M, Fukunaga N, Lee SW. Isolation of x-ray-inducible transcripts from radioresistant human melanoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7200–7204. doi: 10.1073/pnas.90.15.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiswal AK. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free radical biology & medicine. 2000;29:254–262. doi: 10.1016/s0891-5849(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 39.Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chemico-biological interactions. 2000;129:77–97. doi: 10.1016/s0009-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 40.Bloom D, Dhakshinamoorthy S, Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2002;21:2191–2200. doi: 10.1038/sj.onc.1205288. [DOI] [PubMed] [Google Scholar]
- 41.Couroucli XI, Liang YH, Jiang W, Wang L, Barrios R, Yang P, Moorthy B. Prenatal administration of the cytochrome P4501A inducer, Beta-naphthoflavone (BNF), attenuates hyperoxic lung injury in newborn mice: implications for bronchopulmonary dysplasia (BPD) in premature infants. Toxicol Appl Pharmacol. 2011;256:83–94. doi: 10.1016/j.taap.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansour H, Levacher M, Azoulay-Dupuis E, Moreau J, Marquetty C, Gougerot-Pocidalo MA. Genetic differences in response to pulmonary cytochrome P-450 inducers and oxygen toxicity. J Appl Physiol. 1988;64:1376–1381. doi: 10.1152/jappl.1988.64.4.1376. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Lingappan K, Jiang W, Couroucli XI, Welty SE, Shivanna B, Barrios R, Wang G, Firoze Khan M, Gonzalez FJ, Jackson Roberts L, Moorthy B. Disruption of cytochrome P4501A2 in mice leads to increased susceptibility to hyperoxic lung injury. Free radical biology & medicine. 2015;82:147–159. doi: 10.1016/j.freeradbiomed.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien PJ. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- 45.Das A, Kole L, Wang L, Barrios R, Moorthy B, Jaiswal AK. BALT development and augmentation of hyperoxic lung injury in mice deficient in NQO1 and NQO2. Free Radic Biol Med. 2006;40:1843–1856. doi: 10.1016/j.freeradbiomed.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.