Abstract
Background:
Administration of intravenous immunoglobulins (IVIgs) is established for long-term treatment of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Prevention of secondary axonal loss going along with permanent clinical disability and muscular atrophy is a major aim in CIDP therapy. To assess long-term clinical efficacy of IVIg treatment despite heterogenous disease course and variable complaints reported by the patients, long-term electrophysiological monitoring was performed for systematic evaluation of therapeutic efficacy of IVIg.
Methods:
A total of 21 patients with CIDP treated with IVIg 1 g/kg bodyweight every 3–6 weeks were examined electrophysiologically every 12 months over a period of 2 years.
Results:
Assessment of clinical symptoms, using the Inflammatory Neuropathy Cause and Treatment (INCAT) and Hughes functional grading score (F-score) revealed improvement of motor and sensory symptoms over a period of 2 years. As electrophysiological results remained stable, IVIg treatment seems to be suitable to prevent axonal loss in CIDP.
Conclusions:
This study confirms efficacy of IVIg as firstline therapy in CIDP. Doses and frequency of IVIg application should be adapted based on clinical evaluation and analysis of long-term electrophysiological findings.
Keywords: chronic inflammatory demyelinating polyradiculoneuropathy, INCAT, intravenous immunoglobulins, long-term treatment, nerve conduction study
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an immune-mediated chronic disorder of the peripheral nervous system with a prevalence of 1.0–8.9 patients per 100,000 [Laughlin et al. 2009]. The disease course is heterogenous and can be monophasic, relapsing or progressive [Yoon et al. 2011]. There is a wide spectrum of clinical presentation reaching from pure sensory deficits to severe tetraparesis with predominant distal or proximal weakness, symmetric or multifocal distribution and autonomic deficits. Thus, clinical and electrophysiological aspects are most important while other components [cerebral spinal fluid (CSF), magnetic resonance imaging, sural biopsy, therapeutic response] are supportive but not essential for diagnosis (Table 1) [Sander and Latov, 2003; Joint Task Force of the EFNS and the PNS, 2010]. CIDP patients often need long-term treatment and at the same time, it is a challenge to follow therapeutic efficacy in these patients. Due to the nature of the disease early diagnosis and treatment are crucial for prevention of progression of CIDP and outcome [Bouchard et al. 1999].
Table 1.
Diagnostic criteria for CIDP based on European Federation of Neurological Societies (EFNS)/Peripheral Nerve Society (PNS) guidelines [Joint Task Force of the EFNS and the PNS, 2010].
| Clinical | Clinical involvement time course reflexes |
Motor or sensory dysfunction in ⩾1 limb ⩾2 months areflexia or hyporeflexia |
|
Electrophysiological
EFNS |
Definite (always in ⩾2 nerves) |
⩾1 of the following • motor distal latency ⩾ 50% above ULN • reduction of motor conduction velocity ⩾ 30% below LLN • prolongation of F-wave latency in 2 nerves if these have distal negative peak CMAP amplitudes ⩾20% of LLN + ⩾1 other demyelinating parameter in ⩾1 nerve • partial motor conduction block: ⩾50% amplitude reduction • abnormal temporal dispersion (>30% duration increase) • distal CMAP duration increase in ⩾1 nerve + ⩾1 other demyelinating parameter |
| Probable | ⩾30% amplitude reduction of CMAP in ⩾2 nerves | |
| Possible | As in ‘definite’ but in only 1 nerve | |
| Pathologic | Sural nerve biopsy | Demyelination and remyelination supportive: subperineurial or endoneurial edema mononuclear cell infiltration ‘onion-bulb’ formation |
| Laboratory | Cerebrospinal fluid | Cell count <10/mm3
VDRL negative supportive: elevated protein |
CMAP, compound muscle action potential; LLN, lower limit of normal; ULN, upper limit of normal; VDRL, Venereal disease research laboratory.
Corticosteroids with starting dose of 1–1.5 mg/kg/day are still accepted as a mainstay of long-term treatment [Dyck et al. 1982].
Immunoglobulins with intravenous (IVIgs) application [Markvardsen et al. 2013] have the highest recommendation level. Based on a Cochrane review, disability is reduced in 54% of CIDP patients within the first 6 weeks after IVIg therapy [Eftimov et al. 2013]. Several trials and case series have demonstrated a response rate of even 60% during a short observation period over 24 weeks of IVIg treatment [Dyck et al. 1982; Vermeulen et al. 1993; Waniewski et al. 1994; Hahn et al. 1996b; Mendell et al. 2001; Mehndiratta and Hughes, 2002; Hughes et al. 2008b; Frauger et al. 2011].
Data for long-term efficacy beyond 48 weeks are rare [Choudhary and Hughes, 1995; Gorson et al. 1997; Briellmann et al. 1998; Kuwabara et al. 2006; Cocito et al. 2010]. Hughes and colleagues suggested that IVIgs were beneficial in both short and long-term CIDP treatment [Hughes et al. 2008b]. There are two retrospective case series studies that showed remission in 26% and stable disease in 65% of patients after long-term IVIg treatment [Kuwabara et al. 2006; Querol et al. 2013].
A more invasive approach with a higher incidence of relapse is plasma exchange; also an established and evidence-based supported method for treating CIDP [Hahn et al. 1996a]. Alternative therapeutic options are immunosuppressive (IS) drugs including azathioprine [Dyck et al. 1985], mycophenolate mofetil [Gorson et al. 2004], cyclosporine A [Matsuda et al. 2004], cyclophosphamide [Gladstone et al. 2005], and rituximab [Benedetti et al. 2011] that have recently been analyzed in a Cochrane review [Mahdi-Rogers et al. 2013]. Data of the listed drugs are based on case series or uncontrolled trials and therefore not evidence-based.
Our objective was to evaluate the long-term efficacy after early initiation of IVIg treatment in 21 CIDP patients over a period of 2 years.
Methods
Patients
Clinical and electrophysiological data of 21 patients diagnosed with CIDP according to European Federation of Neurological Societies (EFNS) criteria were analyzed retrospectively for a period under review of 24 months [Joint Task Force of the EFNS and the PNS, 2010; Van Den Bergh et al. 2010]. We included patients that were regularly treated in our hospital as inpatients or outpatients within the last 10 years. Data of patients, who continued therapy with their local physician after several infusions or who missed follow up were not included although they met EFNS criteria.
Clinical disease course was assessed by the Inflammatory Neuropathy Cause and Treatment (INCAT) score with upper and lower limbs analyzed separately (Table 2) [Merkies et al. 2003] and Hughes score (F-score) [Hughes et al. 1978] that were both done at baseline and at follow ups after 12 and 24 months. In the Hughes functional grading score (F-score) the range is 0–6: grade 0 = no sign or symptom, grade 1 = minor signs or symptoms of neuropathy but capable of running, grade 2 = able to walk without support for a minimum of 10 meters (m), incapable of running, grade 3 = able to walk with a cane, appliance or support for 10 m, grade 4 = confined to bed or chair-bound, grade 5 = requiring assisted ventilation, and grade 6 = dead.
Table 2.
INCAT disability scale.
| Arm disability | Leg disability |
|---|---|
| 0 = no upper limb problems | 0 = walking not affected |
| 1 = symptoms, in 1 or both arms, not affecting the ability to perform any of the following functions: doing all zips and buttons; washing or brushing hair, using a knife or fork together and handling small coins | 1 = walking affected, but walks independently outdoors |
| 2 = symptoms in 1 or both arms, affecting but not preventing any of the above mentioned functions | 2 = usually uses unilateral support (stick, single crutch,1 arm) to walk outdoors |
| 3 = symptoms, in 1 or both arms, preventing 1 or 2 of the above mentioned functions | 3 = usually uses bilateral support (sticks, crutches, frame, 2 arms) to walk outdoors |
| 4 = symptoms, in 1 or both arms, preventing 3 or all of the functions listed, but some purposeful movements still possible | 4 = usually uses wheelchair to travel outdoors, but able to stand and walk a few steps with help |
| 5 = instability to use either arm for any purposeful movement | 5 = restricted to wheelchair, unable to stand and walk a few steps with help |
INCAT, Inflammatory Neuropathy Cause and Treatment.
The study was approved by the ethics committee of the University of Bochum, Germany (registration number 16-5639).
Electrodiagnostic data
Nerve conduction studies (NCSs) were all performed by a board-certified neurologist with the same device in standardized conditions (Medtronic 4 canal electromyography device, Medtronic, Meerbusch, Germany). Sural, tibial and median nerves of both sides were examined at baseline with skin temperature at 36°C. As there were no constant data of both sides on follow up we used the more seriously affected side for analysis. Compound muscle action potential (CMAP) or sensory nerve action potential (SNAP) amplitudes, sensory and motor nerve conduction velocities, distal motor latency (DML), conduction block (CB), F-wave latencies and persistence were analyzed. CB was defined as either 30–50% or ⩾50% amplitude reduction of the proximal negative peak CMAP relative to distal [Joint Task Force of the EFNS and the PNS, 2010]. Values from Stöhr and colleagues were used as references [Stöhr and Pfister, 2014].
During routine clinical care we intend to obtain NCS in CIDP every 6 months during the first year after diagnosis. Afterwards, as clinical stability is confirmed, NCS is carried out once a year. Those standards have become more accurate within the last 5 years. Nevertheless, several patients missed follow up and intervals became irregular.
Statistics
Demographic data are provided as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) with Bonferroni post-hoc test and Student’s t-test were performed for statistical analysis of neurophysiological data. All analyses were done by Graph Pad Prism 5 (San Diego, CA, USA). A probability level of *p < 0.05, **p < 0.01, ***p < 0.001 was considered to be statistically significant for all tests.
Results
Demographic data
A total of 21 patients meeting the criteria of CIDP were included (Table 3). Disease duration was 8.4 years (SD 5.1 years) and time until diagnosis took in average 1.95 years (SD 3.17 years, maximum 14 years). CSF protein was elevated in 17 patients with on average up to about 800 mg/l.
Table 3.
Demographic features.
| Age at evaluation (years), mean (SD) | 64.9 (11.9) |
| Male (%) | 71 |
| Time since diagnosis (years), mean (SD) | 1.95 (3.17) |
| Disease duration (years), mean (SD) | 8.4 (5.1) |
| IVIg dosage (g/kg body weight), mean (SD) | 0.97 (0.24) |
| IVIg interval of application (weeks), mean (SD) | 6.9 (5) |
| Duration of IVIg therapy (months) | 26.2 (24.0) |
| CSF protein (mg/l), mean (SD) | 797.0 (340.7) |
| CB | |
| Median nerve baseline (%) | Yes (CB 30–50%) 17 Yes (CB > 50%) 5 No 78 |
| Median nerve 12 months (%) | Yes (CB 30–50%) 22 Yes (CB > 50%) 0 No 78 |
| Median nerve 24 months (%) | Yes (CB 30–50%) 43 Yes (CB > 50%) 0 No 57 |
| Tibial nerve baseline (%) | Yes (CB 30–50%) 13 Yes (CB > 50%) 60 No 27 |
| Tibial nerve 12 months (%) | Yes (CB 30–50%) 17 Yes (CB > 50%) 33 No 50 |
| Tibial nerve 24 months (%) | Yes (CB 30–50%) 33 Yes (CB > 50%) 33 No 67 |
| Nerve biopsy (%) (n) | None 38.1 (8) Positive 42.9 (9) Not clear 19.0 (4) |
| Time from baseline to first follow up, T1 (months), mean (SD) |
10.9 (3.1) |
| Time from T1 to second follow up, T2 (months), mean (SD) |
13.7 (1.8) |
CB, conduction block; CSF, cerebral spinal fluid; IVIg, intravenous immunoglobulin; SD, standard deviation.
In 13 patients sural nerve biopsy was performed and therefore diagnosis confirmed in 43%.
Treatment
Patients were treated for 26 months with a mean interval of application of around 7 weeks (SD 5 weeks) and a mean dosage of 0.97 g/kg body weight (SD 0.24). Starting dose was 1 g/kg body weight and interval 6–8 weeks at beginning. Dosage and interval was re-evaluated every 3 months and adapted based on clinical course and examination. Either dosage or interval was adapted. We increased dosage for 15–20% if symptoms objectively worsened or NCS changed: decline of CMAP amplitude of >1 mV, prolongation of DML of >1 ms or increase of conduction velocity (CV) and F-wave latencies of >20%. A total of 38% had stable IVIg, 9% had decreased IVIg and 53% had increased IVIg during the 2-year observation period.
In cases of clinical and electrophysiological stability for >9 months we extended the interval by weekly steps up to a maximum of the doubling interval or reduced dosage slowly for a maximal decrease of 20%. Relevant concomitant medication was documented (Figure 1).
Figure 1.
Concomitant treatment at baseline and after two-years-treatment. Six patients had two or more substances for pre-treatment (A) which explains the n- number > 21. One patient had a rapid progression of disease and treatment was adjusted several times also using off-label therapies like fumarates. In the end, this patient died one year after observation period.
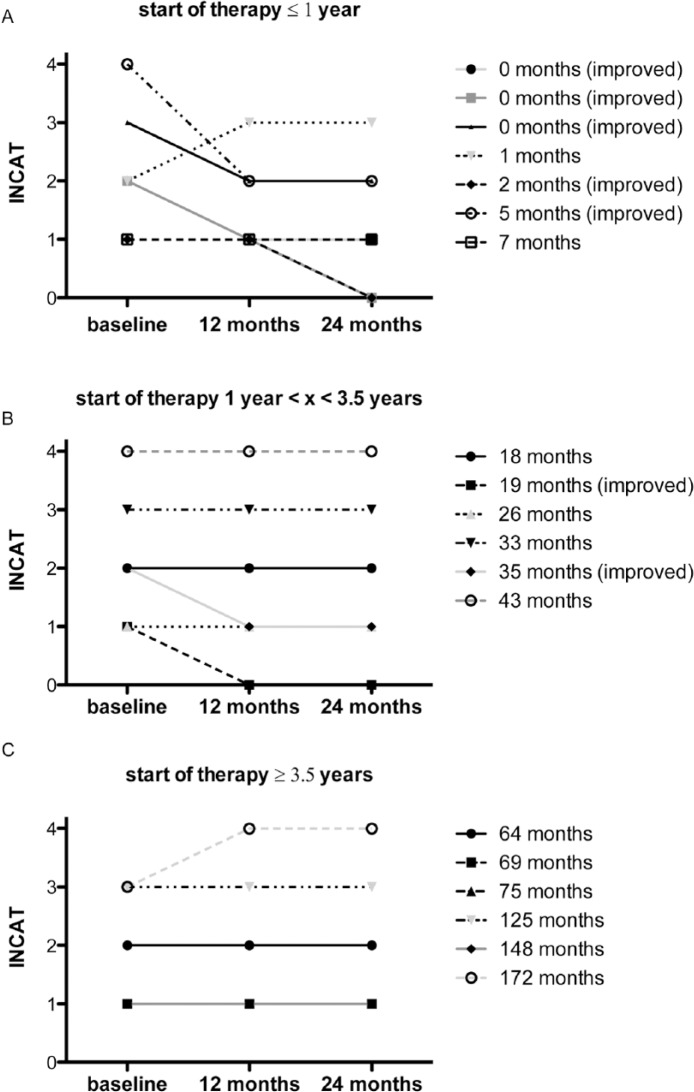
Focusing time-point of treatment, especially early start of long-term IVIg treatment within 1 year after manifestation of first symptoms led not only to stability of symptoms, measured by the INCAT score, but five out of seven patients even showed improvement (Figure 2A). If IVIgs were administered with a latency of more than 3.5 years after clinical disease onset, the INCAT score remained stable but the clinical course did not improve (Figure 2C).
Figure 2.
Start of treatment after first symptoms. We analyzed the latency (in months) until IVIg were initially applied after first manifestation of disease. Based on this, patients were divided into three groups (initiation of IVIg treatment within one year (A), lately after 3.5 years (B) and ⩾ 3.5 years (C). INCAT for lower extremity (LE) was evaluated over time (baseline, after 12 months and 24 months).
Clinical outcomes
Clinical impairment (reflected by INCAT scale of the upper and lower extremities as well as by the Hughes score) significantly improved, in terms of the Hughes score, from baseline to follow up after 2 years (*p < 0.05, t-test). This improvement was confirmed, although not significantly, by the INCAT score within 2 years (Figure 3).
Figure 3.

Clinical course. All patients (n=21) were examined at baseline and after 12 and 24 months. INCAT disability score was evaluated for the upper extremity. Scale range is from 0 (=no upper limp problems/ walking not affected) to 5 (inability to use either arm for any purposeful movement/restricted to wheelchair). In our cohort the most affected patients had a score of 4. Patients also underwent Hughes functional grading score (n=21); LE, lower extremity; UE, upper extremity.
Nerve conduction studies
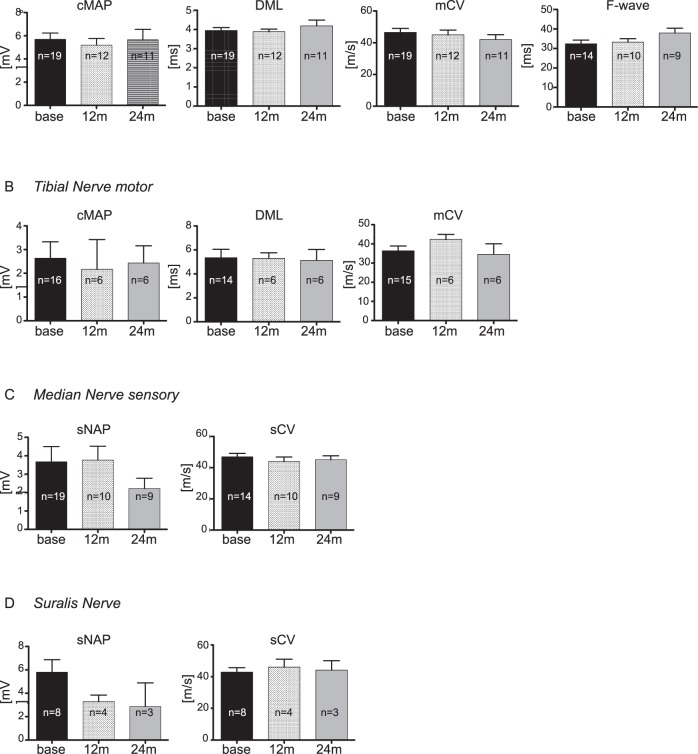
Results of NCSs are summarized in Figure 4 presenting median, tibial and sural nerves with either CMAP, SNAP, DML, motor/sensory CV, F-waves or all parameters (Figure 4). There were no significant differences over time at 12 and 24 months between all of the parameters assessed.
Figure 4.
Electrophysiological data. Median (A,C), tibial (B) and sural nerve (D) were analyzed at three time points: baseline, follow-ups after 12 months and after 24 months. Number of values differs indicated by (n=) in each bar as some patients reject single measurements; CMAP, compound motor action potential; DML, distal motor latency; mCV, motor conduction velocity; SNAP, sensory nerve action potential; sCV, sensory conduction velocity.
CB was present in 73% of tibial nerves at baseline. After 12 and 24 months, CB >50% was noted approximately half as often (33%) (Table 3). In general, for technical reasons the presence and course of tibial nerve CB must be interpreted with caution. But also in median nerve analysis, the percentage of CB over 50% decreased during the 2 year observation period (Table 3).
The most common finding was the stability of the different parameters over a 2-year period. A decrease in SNAP within 2 years was only observed in the sural nerve. Sural nerve CV remained unchanged over time.
For all items, we analyzed patients who received IVIg monotherapy and separated them from those who received concomitant IS substances during the study. There were no significant differences between these groups (data not shown).
Discussion
First, we focused on clinical course and subclinical axonal loss as shown by neurophysiological parameters including CMAP amplitudes in CIDP patients and demonstrated several positive effects after long-term IVIg therapy.
The INCAT scale used in this study is accepted to be the most suitable tool for rating CIDP dependent symptoms. The scale meets the criteria of high responsiveness, high feasibility, good reliability and high validity [Breiner et al. 2014]
NCSs are objective and reproducible parameters. In our study, both, sensory and motor nerve analysis remained stable over time but did not reflect clinical improvement. These discrepancies are common in CIDP studies.
The changes of NCS parameters highly depend both on the intensity of the inflammatory demyelinating process and the duration of disease: early disease stage is characterized by predominant and sometimes fulminant demyelination and chronic stage is associated with secondary axonal pathology and permanent impairment [Kerasnoudis et al. 2015]. In our cohort, mean duration from first symptoms to date of diagnosis were 1.95 years with a maximum of 14 years. Thus, we included patients at different stages of the disease at both ends of the spectrum.
In our patients we adapted therapy during disease course by extending infusion intervals or reducing IVIg doses as described above. In particular, we did not increase the dose or shorten infusion intervals on the basis of reported (subjective) neuropathic symptoms, but on objective progression of sensory or motor deficits as revealed by thorough neurological examination and electrophysiology. Overall, 91% of the patients needed stable IVIg dosages, and most of them even needed increased dosages. This strengthens the assumption that these patients had active disease.
Both, clinical improvement/stability and stability of neurophysiological parameters in active CIDP patients suggest that long-term IVIg reduces immune response, influences processes in the nodal or paranodal regions and promotes remyelination and preservation of axonal function allowing for (collateral) reinnervation.
Secondly, an early initiation of treatment is assumed to prevent disease progression and also to improve the clinical course of disease. We are aware of the rather small number of patients but data suggest this hypothesis to be true. This might be explained by the anti-inflammatory effects of IVIg and mechanisms of IVIg efficacy in CIDP. The Fc fragment of the IgG molecule interferes with autoantibody-triggered inflammation [Samuelsson et al. 2001; Bruhns et al. 2009] by activating the complement pathway on the one hand and crosslinking Fc receptors specific for IgG (FcγRS) on the other [Tackenberg et al. 2010]. FcγRIIB, as an inhibitory subtype, is among others expressed on B-cells that are blocked becoming IgG-positive plasma cells [Nimmerjahn and Ravetch, 2008]. Impaired expression of FcγRIIB in CIDP can be effectively restored by early IVIg treatment [Tackenberg et al. 2010]. Unfortunately, IgG dependency is not analyzed in this study as it is based on retrospective data and regular measurements with meaningful results are not available.
Thirdly, similar to previous long-term studies, we showed the maintained efficacy of IVIg over time [Kuwabara et al. 2006; Hughes et al. 2008b; Querol et al. 2013]. As we could see a reversal in CB and other stable neurophysiological parameters, as described earlier, we assume IVIg mediates remyelination through immunomodulatory effects. In line with further studies these effects are suspected to act on humoral and cellular levels and directly at myelin sheath levels [van Doorn et al. 1990; Frank et al. 1992; van Engelen et al. 1994; Miyagi et al. 1997; Vucic et al. 2007]. Other studies suggest that the main target for immunomodulatory effects might be within the nodal or paranodal regions because clinical improvement within days after IVIg treatment could otherwise not be explained by rapid remyelination [Dalakas and Medscape, 2011; Pollard and Armati, 2011; Dalakas, 2015]. Humoral factors are assumed to block molecules that influence saltatory conduction at the nodes of Ranvier. In long-term treatment, both mechanisms presumably play an important role.
In conclusion, the present study, to our knowledge is one of the longest observational studies in CIDP treatment, and has demonstrated that long-term IVIg is a very effective, well tolerated therapy in early-diagnosed CIDP. More limited efficacy becomes obvious in the latter and severe stages of the disease. Although very challenging and mainly trial and error, it seems to be very important to individualize treatment concerning doses and frequency of infusion [Adrichem et al. 2016]. In contrast with the ICE trial [Hughes et al. 2008b] with a fixed therapy regimen, ‘over-treatment’ could therefore be avoided.
Unfortunately, this retrospective study lacks a control group in which therapy was stopped in patients who achieved stabilization or improvement for a distinct time period. Due to the design of the study, primary and secondary endpoints had not been defined. The lack of such criteria implicates a potential risk of over-treatment and a bias to attribute stabilization to continuous application of IVIgs.
Therefore, further studies in which IVIg therapy is stopped according to distinct clinical or electrophysiological criteria defining definite or probable stabilization are warranted. Guidelines derived from such long-term studies could support decision making in clinical practice in order to adjust therapy adequately and to prevent side effects as well as to save costs.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Gisa Ellrichmann, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Gudrunstrasse 56, D-44791 Bochum, Germany.
Ralf Gold, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Ilya Ayzenberg, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Min-Suk Yoon, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Christiane Schneider-Gold, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
References
- Adrichem M., Eftimov F., van Schaik I. (2016) Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyradiculoneuropathy, a time to start and a time to stop. J Peripher Nerv Syst 21: 121–127. [DOI] [PubMed] [Google Scholar]
- Benedetti L., Briani C., Franciotta D., Fazio R., Paolasso I., Comi C., et al. (2011) Rituximab in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a report of 13 cases and review of the literature. J Neurol Neurosurg Psychiatry 82: 306–308. [DOI] [PubMed] [Google Scholar]
- Bouchard C., Lacroix C., Plante V., Adams D., Chedru F., Guglielmi J., et al. (1999) Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology 52: 498–503. [DOI] [PubMed] [Google Scholar]
- Breiner A., Barnett C., Bril V. (2014) INCAT disability score: a critical analysis of its measurement properties. Muscle Nerve 50: 164–169. [DOI] [PubMed] [Google Scholar]
- Briellmann R., Nydegger U., Sturzenegger M., Fierz L., Hess C., Hauser S. (1998) Long-term treatment of chronic relapsing inflammatory demyelinating polyradiculoneuropathy: combination of corticosteroids, plasma exchange, and intravenous immunoglobulins. Eur Neurol 39: 190–191. [PubMed] [Google Scholar]
- Bruhns P., Iannascoli B., England P., Mancardi D., Fernandez N., Jorieux S., et al. (2009) Specificity and affinity of human Fc gamma receptors and their polymorphic variants for human IgG subclasses. Blood 113: 3716–3725. [DOI] [PubMed] [Google Scholar]
- Choudhary P., Hughes R. (1995) Long-term treatment of chronic inflammatory demyelinating polyradiculoneuropathy with plasma exchange or intravenous immunoglobulin. QJM 88: 493–502. [PubMed] [Google Scholar]
- Cocito D., Paolasso I., Antonini G., Benedetti L., Briani C., Comi C., et al. (2010) A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol 17: 289–294. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2015) Pathogenesis of immune-mediated neuropathies. Biochim Biophys Acta 1852: 658–666. [DOI] [PubMed] [Google Scholar]
- Dalakas M., Medscape (2011) Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol 7: 507–517. [DOI] [PubMed] [Google Scholar]
- Dyck P., O’Brien P., Oviatt K., Dinapoli R., Daube J., Bartleson J., et al. (1982) Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol 11: 136–141. [DOI] [PubMed] [Google Scholar]
- Dyck P., O’Brien P., Swanson C., Low P., Daube J. (1985) Combined azathioprine and prednisone in chronic inflammatory-demyelinating polyneuropathy. Neurology 35: 1173–1176. [DOI] [PubMed] [Google Scholar]
- Eftimov F., Winer J., Vermeulen M., de Haan R., van Schaik I. (2013) Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 12: CD001797. doi: 10.1002/14651858.CD001797 [DOI] [PubMed] [Google Scholar]
- Frank M., Basta M., Fries L. (1992) The effects of intravenous immune globulin on complement-dependent immune damage of cells and tissues. Clin Immunol Immunopathol 62: S82–S86. [DOI] [PubMed] [Google Scholar]
- Frauger E., Grassi J., Pradel V., Bornet C., Rouby F., Delorme J., et al. (2011) Use of intravenous immunoglobulins in clinical practice: data from three French university hospitals. Fundam Clin Pharmacol 25: 753–761. [DOI] [PubMed] [Google Scholar]
- Gladstone D., Prestrud A., Brannagan T. (2005) High-dose cyclophosphamide results in long-term disease remission with restoration of a normal quality of life in patients with severe refractory chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 10: 11–16. [DOI] [PubMed] [Google Scholar]
- Gorson K., Allam G., Ropper A. (1997) Chronic inflammatory demyelinating polyneuropathy: clinical features and response to treatment in 67 consecutive patients with and without a monoclonal gammopathy. Neurology 48: 321–328. [DOI] [PubMed] [Google Scholar]
- Gorson K., Amato A., Ropper A. (2004) Efficacy of mycophenolate mofetil in patients with chronic immune demyelinating polyneuropathy. Neurology 63: 715–717. [DOI] [PubMed] [Google Scholar]
- Hahn A., Bolton C., Pillay N., Chalk C., Benstead T., Bril V., et al. (1996a) Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy: a double-blind, sham-controlled, cross-over study. Brain 119(Pt 4): 1055–1066. [DOI] [PubMed] [Google Scholar]
- Hahn A., Bolton C., Zochodne D., Feasby T. (1996b) Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double-blind, placebo-controlled, cross-over study. Brain 119(Pt 4): 1067–1077. [DOI] [PubMed] [Google Scholar]
- Hughes R., Donofrio P., Bril V., Dalakas M., Deng C., Hanna K., et al. (2008) Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 7: 136–144. [DOI] [PubMed] [Google Scholar]
- Hughes R., Newsom-Davis J., Perkin G., Pierce J. (1978) Controlled trial prednisolone in acute polyneuropathy. Lancet 2: 750–753. [DOI] [PubMed] [Google Scholar]
- Joint Task Force of the EFNS and the PNS (2010) European Federation of Neurological Societies (EFNS)/Peripheral Nerve Society (PNS) guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society, first revision. J Peripher Nerv Syst 15: 1–9. [DOI] [PubMed] [Google Scholar]
- Kerasnoudis A., Pitarokoili K., Gold R., Yoon M. (2015) Nerve ultrasound and electrophysiology for therapy monitoring in chronic inflammatory demyelinating polyneuropathy. J Neuroimaging 25: 931–939. [DOI] [PubMed] [Google Scholar]
- Kuwabara S., Misawa S., Mori M., Tamura N., Kubota M., Hattori T. (2006) Long term prognosis of chronic inflammatory demyelinating polyneuropathy: a five year follow up of 38 cases. J Neurol Neurosurg Psychiatry 77: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin R., Dyck P., Melton L., III, Leibson C., Ransom J., Dyck P. (2009) Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology 73: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi-Rogers M., van Doorn P., Hughes R. (2013) Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013. June 14(6): CD003280. doi: 10.1002/14651858.CD003280.pub4. Review. PMID:23771584. [DOI] [PubMed] [Google Scholar]
- Markvardsen L., Debost J., Harbo T., Sindrup S., Andersen H., Christiansen I., et al. (2013) Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol 20: 836–842. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Hoshi K., Gono T., Morita H., Ikeda S. (2004) Cyclosporin A in treatment of refractory patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci 224: 29–35. [DOI] [PubMed] [Google Scholar]
- Mehndiratta M., Hughes R. (2002) Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 1: CD002062. Review. PMID: 11869620 [DOI] [PubMed] [Google Scholar]
- Mendell J., Barohn R., Freimer M., Kissel J., King W., Nagaraja H., et al. (2001) Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 56: 445–449. [DOI] [PubMed] [Google Scholar]
- Merkies I., Schmitz P., van Der Meché F., Samijn J., van Doorn P. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. et al. (2003) Connecting impairment, disability, and handicap in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry 74: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi F., Horiuchi H., Nagata I., Kitahara S., Kiyoki M., Komoriya K., et al. (1997) Fc portion of intravenous immunoglobulin suppresses the induction of experimental allergic neuritis. J Neuroimmunol 78: 127–131. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J. (2008) Fc gamma receptors as regulators of immune responses. Nat Rev Immunol 8: 34–47. [DOI] [PubMed] [Google Scholar]
- Pollard J., Armati P. (2011) CIDP - the relevance of recent advances in Schwann cell/axonal neurobiology. J Peripher Nerv Syst 16: 15–23. [DOI] [PubMed] [Google Scholar]
- Querol L., Rojas-Garcia R., Casasnovas C., Sedano M., Munoz-Blanco J., Alberti M., et al. (2013) Long-term outcome in chronic inflammatory demyelinating polyneuropathy patients treated with intravenous immunoglobulin: a retrospective study. Muscle Nerve 48: 870–876. [DOI] [PubMed] [Google Scholar]
- Samuelsson A., Towers T., Ravetch J. (2001) Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291: 484–486. [DOI] [PubMed] [Google Scholar]
- Sander H., Latov N. (2003) Research criteria for defining patients with CIDP. Neurology 60: S8–S15. [DOI] [PubMed] [Google Scholar]
- Stöhr M., Pfister R. (2014) Klinische Elektromyographie und Neurographie - Lehrbuch und Atlas, 6th Edition. Kohlhammer Verlag, ISBN 317028374X, 9783170283749. [Google Scholar]
- Tackenberg B., Nimmerjahn F., Lunemann J. (2010) Mechanisms of IVIg efficacy in chronic inflammatory demyelinating polyneuropathy. J Clin Immunol 30(Suppl. 1): S65–S69. [DOI] [PubMed] [Google Scholar]
- Van den Bergh P., Hadden R., Bouche P., Cornblath D., Hahn A., Illa I., et al. (2010) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol 17: 356–363. [DOI] [PubMed] [Google Scholar]
- Van Doorn P., Rossi F., Brand A., Van Lint M., Vermeulen M., Kazatchkine M. (1990) On the mechanism of high-dose intravenous immunoglobulin treatment of patients with chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol 29: 57–64. [DOI] [PubMed] [Google Scholar]
- Van Engelen B., Miller D., Pavelko K., Hommes O., Rodriguez M. (1994) Promotion of remyelination by polyclonal immunoglobulin in Theiler’s virus-induced demyelination and in multiple sclerosis. J Neurol Neurosurg Psychiatry 57 Suppl: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen M., van Doorn P., Brand A., Strengers P., Jennekens F., Busch H. (1993) Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: a double blind, placebo controlled study. J Neurol Neurosurg Psychiatry 56: 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S., Black K., Baldassari L., Tick Chong P., Dawson K., Cros D. (2007) Long-term effects of intravenous immunoglobulin in CIDP. Clin Neurophysiol 118: 1980–1984. [DOI] [PubMed] [Google Scholar]
- Waniewski J., Gardulf A., Hammarstrom L. (1994) Bioavailability of gamma-globulin after subcutaneous infusions in patients with common variable immunodeficiency. J Clin Immunol 14: 90–97. [DOI] [PubMed] [Google Scholar]
- Yoon M., Chan A., Gold R. (2011) Standard and escalating treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Ther Adv Neurol Disord 4: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]