Abstract
Serum or plasma proteases have been associated with various diseases including cancer, inflammation, or reno-cardiovascular diseases. We aimed to investigate whether the enzymatic activities of serum proteases are associated with the estimated glomerular filtration rate (eGFR) in patients with different stages of chronic kidney disease (CKD). Our study population comprised 268 participants of the “Greifswald Approach to Individualized Medicine” (GANI_MED) cohort. Enzymatic activity of aminopeptidase A, aminopeptidase B, alanyl (membrane) aminopeptidase, insulin-regulated aminopeptidase, puromycin-sensitive aminopeptidase, leucine aminopeptidase 3, prolyl-endopeptidase (PEP), dipeptidyl peptidase 4 (DPP4), angiotensin I-converting enzyme, and angiotensin I-converting enzyme 2 (ACE2) proteases was measured in serum. Linear regression of the respective protease was performed on kidney function adjusted for age and sex. Kidney function was modeled either by the continuous Modification of Diet in Renal Disease (MDRD)-based eGFR or dichotomized by eGFR < 15 mL/min/1.73 m2 or <45 mL/min/1.73 m2, respectively. Results with a false discovery rate below 0.05 were deemed statistically significant. Among the 10 proteases investigated, only the activities of ACE2 and DPP4 were correlated with eGFR. Patients with lowest eGFR exhibited highest DPP4 and ACE2 activities. DPP4 and PEP were correlated with age, but all other serum protease activities showed no associations with age or sex. Our data indicate that ACE2 and DPP4 enzymatic activity are associated with the eGFR in patients with CKD. This finding distinguishes ACE2 and DPP4 from other serum peptidases analyzed and clearly indicates that further analyses are warranted to identify the precise role of these serum ectopeptidases in the pathogenesis of CKD and to fully elucidate underlying molecular mechanisms.
Impact statement
• Renal and cardiac diseases are very common and often occur concomitantly, resulting in increased morbidity and mortality. Understanding of molecular mechanisms linking both diseases is limited, available fragmentary data point to a role of the renin–angiotensin system (RAS) and, in particular, Ras-related peptidases.
• Here, a comprehensive analysis of serum peptidase activities in patients with different stages of chronic kidney disease (CKD) is presented, with special emphasis given to RAS peptidases
• The serum activities of the peptidases angiotensin I-converting enzyme 2 and dipeptidyl peptidase 4 were identified as closely associated with kidney function, specifically with the estimated glomerular filtration rate. The findings are discussed in the context of available data suggesting protective roles for both enzymes in reno-cardiac diseases.
• The data add to our understanding of pathomechanisms underlying development and progression of CKD and indicate that both enzymes might represent potential pharmacological targets for the preservation of renal function.
Keywords: Protease activity, angiotensin-converting enzyme 2, dipeptidyl-peptidase IV, renin–angiotensin system, chronic kidney disease, estimated glomerular filtration rate
Introduction
In the last decade, membrane-bound proteases/peptidases (ectopeptidases) have gained considerable research interest due to their contribution in the formation, competitive metabolism, and degradation of a broad variety of peptide hormones that function as key regulators for intercellular communication. Pathophysiological conditions and, in particular, cardio-renal diseases greatly affect the release (shedding) of ectopeptidases into extracellular compartments including blood plasma. Members of the A Disintegrin And Metalloprotease family are prototype “sheddases” implicated in this process. Soluble peptidases/proteases shed into the plasma may represent a possible source of biomarkers. Thus, in this study a comprehensive analysis of serum ectopeptidases activities in patients with chronic kidney disease (CKD) at different disease stages is performed. Special emphasis is given to peptidases/proteases of the renin–angiotensin system (RAS), as dysregulation of the activity of both the classical and alternative axes of the RAS has been associated with cardiac and renal diseases. Both, cardiac and renal diseases, are very common and often occur concomitantly, resulting in increased morbidity and mortality.1 To date, 5–10% of the world population is affected by CKD.2 In the next decades, CKD prevalence will further increase due to rising prevalence of major risk factors for CKD including diabetes, hypertension, and cardiovascular disorders and the increasingly older population.3,4 CKD is defined by structural pathology (or kidney transplant), a decline in estimated glomerular filtration rate (eGFR) (<60 mL/min/1.73 m2) over three-month time with clinical implications or albuminuria.2
Experimental and clinical data indicate that the administration of inhibitors of the angiotensin-converting enzyme (ACEi) or angiotensin II type 1 receptor (AT1R) blockers can limit the progression of kidney injury (albuminuria, damage of podocytes, decrease of eGFR) due to chronic volume overload.5,6 Angiotensin II (AngII) promotes the production of reactive oxygen species and renal fibrosis via direct (activation of NADPH oxidase) and indirect (increased aldosterone plasma concentrations) mechanisms.7 Accordingly, aldosterone receptor antagonists have been also shown to diminish histological signs of kidney injury, creatinine serum concentrations, and proteinuria.8 In the SOLVD study, enalapril reduced proteinuria in diabetic patients.9 Captopril stabilized the GFR in postinfarct patients with heart failure.10 However, the CONSENSUS study showed a 10–15% increase in serum creatinine in patients treated with enalapril11 and a slight decrease in the GFR was observed for valsartan in the VALHEFT study. Furthermore, candesartan was not able to reduce proteinuria in the CHARM-Added study.12 In summary, available studies do not provide a simple answer to the question what the precise role of the RAS and, in particular, of its different axes, in the development and progression of CKD actually is. A better understanding might be hampered by the extensive heterogeneity among patients with CKD with respect to underlying pathomechanisms and concomitant diseases. However, any disease-, gender-, or age-dependent dysregulation of typical RAS proteases might be partly compensated by modulating the expression/activity of other ectoproteases. In support of this view, it was shown previously that an age-dependent decrease in ACE activity in the pericardial fluid of elder women is compensated by increased activities of membrane alanyl-aminopeptidase (APN, CD13) and dipeptidyl-peptidase IV (DPPIV, DPP4, CD26).13 Advanced age and ACEi treatment could increase the pathophysiological relevance of non-RAS proteases.14,15 In this study, we determined enzymatic activities of the RAS-related ectopeptidases ACE, ACE2, aminopeptidase A (APA), aminopeptidase B (APB), APN, and insulin-regulated aminopeptidase (IRAP) as well as of DPP4, puromycin-sensitive aminopeptidase (PSA), leucine aminopeptidase 3 (LAP), and prolyl-endopeptidase (PEP) in serum samples from patients with different stages of CKD (eGFR groups 1–5). Protease characteristics are summarized in Table 1.
Table 1.
Characteristics of proteases analyzed
|
Protease
|
Substrate
|
Inhibitor
|
||||||
|---|---|---|---|---|---|---|---|---|
| Name | Gene ID | CD number | EC number | Name | Concentration (mM) | Source | Name (concentration (M)) | Ref. |
| Leucine aminopeptidase 3 (LAP) | LAP3 | 3.4.11.1 | H-Leu-pNA-HCl | 4.375 | Sigma | – | ||
| Alanyl (membrane) aminopeptidase (APN) | ANPEP | CD13 | 3.4.11.2 | H-Ala-pNA-HCl | 2.925 | Bachem | RB3014 [1 × 10−7] | Chen et al.62 |
| Leucyl/cystinyl aminopeptidase (insulin-regulated aminopeptidase, IRAP) | LNPEP | 3.4.11.3 | H-Leu-pNA-HCl | 4.375 | Sigma | RB3014 [10−7] | Aroor et al.16 | |
| Arginyl aminopeptidase (aminopeptidase B) (APB) | RNPEP | 3.4.11.6 | H-Arg-pNA-2HCl | 2.5 | Bachem | – | ||
| Glutamyl aminopeptidase (aminopeptidase A) (APA) | ENPEP | CD249 | 3.4.11.7 | H-Glu-pNA | 5.0 | Bachem | – | |
| Puromycin-sensitive aminopeptidase (PSA) | NPEPPS | 3.4.11.14 | H-Ala-pNA-HCl | 2.925 | Bachem | RB3014 [1 × 10−7] | Riera et al.17 | |
| Dipeptidyl-peptidase 4 (DPPIV, DPP4) | DPP4 | CD26 | 3.4.14.5 | H-Gly-Pro-pNA-HCl | 1.65 | Bachem | Lys-Z-NO-thiazolidid [1 × 10−4] | Ansorge et al.63, Schön et al.64 |
| Angiotensin I-converting enzyme (ACE) | ACE | CD143 | 3.4.15.1 | ACE color-Kit | MAST-DIAGNOSTICA-GmbH | – | ||
| Angiotensin I-converting enzyme 2 (ACE2) | ACE2 | 3.4.17.23 | ACE color-Kit | MAST-DIAGNOSTICA-GmbH | Lisinopril [0.3 × 10−6] | Lehmann et al.65 | ||
| Prolyl endopeptidase (PEP) | PREP | 3.4.21.26 | H-Gly-Pro-pNA-HCl | 1.65 | Bachem | Lys-Z-NO-thiazolidid [1 × 10−4] | Ansorge et al.63, Schön et al.64 | |
Materials and methods
Study design and patients
Patients were selected from the “Greifswald Approach to Individualized Medicine” (GANI_MED) project. GANI_MED aims at implementing individualized diagnostic and therapeutic measures in patient cohorts with common cardiovascular, cerebrovascular, or metabolic conditions, with pulmonary disease, sepsis, and in patients with adverse medication effects. All GANI_MED patients were recruited in the university hospital or from special dialysis treatment centers. Patients are subjected to highly standardized anamnestic, somatometric, basic laboratory diagnostic analyses, which also include determination of eGFR. For cohort profile and detailed study procedures, please refer to Grabe et al.21 GANI_MED was approved by the ethics committee of the Medical Faculty of the University of Greifswald. All participants provided informed written consent.
In the present study, data from 244 GANI_MED patients enrolled between July 2011 and December 2013 in the “renal and renovascular disease cohort” were analyzed. None of these patients underwent a renal transplantation before inclusion of the study. All patients consented to blood sampling and subsequent measurements of serum protease activities and plasma creatinine concentrations were performed. The 244 patients were assigned to the different CKD stages (1–5) according to their eGFR: group 1 (CKD 1 + 2; eGFR ≥ 60 mL/min/1.73 m2), group 2 (CKD 3A; eGFR > 45–60 mL/min/1.73 m2), group 3 (CKD 3B; eGFR > 30–45 ml/min/1.73 m2), group 4 (CKD 4; eGFR > 16–30 mL/min/1.73 m2), group 5 (CKD 5; eGFR ≤ 15 mL/min/1.73 m2). As the number of patients in the CKD stages 1–3 was naturally low in the renal cohort patients, 24 age- and sex-matched patients from the GANI_MED “cardiovascular cohort” were selected to fill up these stages, so that each group comprised more than 25 patients.
Laboratory testing
Blood sampling
Eight milliliters of blood was drawn from a cubital vein using an 18-gauge needle (Becton Dickinson Diagnostic Systems, USA) and a SST II Advance, vacutainer® (Becton Dickinson Diagnostic Systems, USA) tube for serum samples. Blood samples rested 30 min to undergo coagulation which was followed by centrifugation at 2800 × g for 10 min. The supernatant was filled into 1.5 mL sample tubes (Eppendorf, Germany) and stored at −80℃ until analysis. Determination of protease activities and plasma creatinine was performed from separate collection tubes that were, however, obtained during one blood draw.
Determination of protease activity
Enzymatic activity of ACE, ACE2, APA, APB, APN, LAP, PEP, PSA, IRAP, and DPP4 was determined from serum samples after one freeze–thaw cycle using the substrates and inhibitors specified in Table 1.
The assays for determination of APA, APB, APN, LAP, PEP, PSA, IRAP, and DPP4 enzymatic activity were carried out in 50 mM Hepes buffer containing 200 mM NaCl, 10 µM ZnCl2, and 1% DMSO pH 6.8. Enzyme reactions were performed in flat-bottom 96-well microtiter plates (Greiner Bio-One, Frickenhausen, Germany). The total reaction volume amounting to 100 µL consisted of 65 µL buffer, 10 µL serum and 25 µL substrate. The reaction was started by addition of the substrate. When appropriate, inhibitors were used to increase the specificity of the assays, e.g. RB3014 was applied to discriminate between APN (sensitive) and PSA (insensitive) Ala-pNA-hydrolyzing activity as well as between IRAP (sensitive) and LAP (insensitive) Leu-pNA-hydrolyzing activity. Lys-Z-NO-thiazolidid was used to distinguish DPP4 (sensitive) from PEP (insensitive). When an inhibitor was applied, a preincubation with the inhibitor was performed for 30 min at 37℃ before addition of the substrate. The optical density at the starting point was used as blank sample. After incubation at 37℃ for 1 h in the dark, the optical density was measured at 405 nm with 620 nm reference wavelength by a microtiter plate reader infinite M200 (Tecan, Crailsheim, Germany). The enzymatic activity of ACE and ACE2 was determined using the ACE color-Kit (MAST-DIAGNOSTICA-GmbH, Reinfeld, Germany) according to the manufacturer’s instructions adapted to 96-well plates, with the lisinopril-insensitive fraction being considered as ACE2 activity.
All samples were analyzed in duplicate. Reanalysis was indicated when values differed by >5%. The enzymatic activities are given in nkat/L and were calculated as follows: A = (ΔE Vt)/(t ɛ d Vs)/L.
Creatinine measurement and eGFR
Plasma creatinine concentrations in Gani_Med were measured at the Institute of Clinical Chemistry and Laboratory Medicine in the University Medicine Greifswald (Greifswald, Germany). Gani_Med was started in 2011, when creatinine measurements were performed with a modified kinetic Jaffé method on the Siemens Dimension Vista (Siemens Healthcare Diagnostics, Eschborn, Germany). In November 2012 a new, enzymatic method for creatinine measurement on the Siemens Dimension Vista was introduced in our lab. All subsequent Gani_Med samples were measured with this method. As method changes may lead to uncomparable values, a method comparison was performed. For this comparison, 327 plasma samples (0–700 µmol/L creatinine) were measured with each method. The comparison of the respective pairs revealed that the enzymatic method measures lower creatinine concentrations (difference based on the median: −13.4%) than the modified kinetic Jaffé method. Therefore, it was considered necessary to convert the creatinine concentrations measured with the Jaffé method to the newer enzymatic method. Passing–Bablok regression (r = 0.993) yielded the coefficients for the following formula: Creatinine enzymatic µmol/L = Creatinine Jaffé µmol/L *0.915 – 3.61. This formula was applied to convert the 142 measurements obtained by the Jaffé method to the enzymatic method and all analyses are based on the converted data. The eGFR was calculated using the four-variable MDRD equation.22 The MDRD formula was chosen, as about 90% of subjects included in the present study have an eGFR < 60 mL/min/1.73 m2, values at which the MDRD formula has a high accuracy.23
As mentioned above, for eGFR groups 1–3 only very few samples were available in the CKD cohort. By design, samples from the cardiovascular cohort were selected randomly and matched by age and sex. In detail, two, 10, and 12 samples were added to eGFR groups 1, 2, and 3, respectively. The sample sizes per group after successful measurement of protease activities are presented in Table 2.
Table 2.
Characterization of eGFR groups
| eGFR group (eGFR range (mL/min/1.73 m2)) | CKD stage | N samples | N men (%) | Mean age (SD) | N medication (%) |
|---|---|---|---|---|---|
| 1 (≥60) | 1 + 2 | 27 | 19 (70.4) | 60.6 (13.2) | 21 (84.0) |
| 2 (45 – <60) | 3A | 29 | 23 (79.3) | 68.5 (10.4) | 21 (72.4) |
| 3 (30 – <45) | 3B | 31 | 19 (61.3) | 70.8 (11.6) | 16 (51.6) |
| 4 (15 – <30) | 4 | 52 | 30 (57.7) | 72.5 (10.5) | 30 (61.2) |
| 5 (<15) | 5 | 129 | 75 (58.1) | 67.8 (14.0) | 67 (54.0) |
| Total | 268 | 166 (61.9) | 68.4 (13.0) | 155 (60.1) |
ATC code C9: agents acting on the renin–angiotensin system; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; SD: standard deviation.
Statistical analyses
For all analyses, a linear regression of the respective protease activity on kidney function adjusted for age, in years, and sex was calculated. Kidney function was modeled either using the continuous eGFR values (eGFRcrea_MDRD), the groups, or dichotomized by eGFR < 15 mL/min/1.73 m2 (egfr_group15) or <45 mL/min/1.73 m2 (egfr_group45), respectively.
The dichotomizations and the calculations based on groups were performed as sensitivity analyses and to address potential non-linear relationships between the eGFR values and the protease activities.
The results were adjusted for multiple testing by applying the false discovery rate (FDR)24 and claimed significant having an FDR < 0.05.
All analyses were carried out in R v3.1.3 (www.r-project.org).
Results
A total of 268 patients were included in the study. The classification of the patients in the eGFR groups is given in Table 2. Multivariable regression analysis revealed a significant association of patients’ eGFR with the serum enzymatic activities of ACE2 and DPP4. These associations remained significant after adjusting for age and sex (Table 3). For the other proteases analyzed, no association of their enzymatic activity with eGFR was detected (Table 3).
Table 3.
Associations between protease activity and eGFR.
| Protease | Beta | SE | p | FDR | n |
|---|---|---|---|---|---|
| ACE2 | −0.135 | 0.040 | 8.00E-04 | 0.008 | 268 |
| DPP4 | −0.744 | 0.252 | 0.003 | 0.017 | 268 |
| ACE | −0.846 | 0.378 | 0.026 | 0.065 | 268 |
| PREP | −0.231 | 0.102 | 0.024 | 0.065 | 268 |
| ENPEP | −0.148 | 0.083 | 0.075 | 0.150 | 268 |
| LNPEP | −0.164 | 0.170 | 0.334 | 0.477 | 268 |
| NPEPPS | 0.173 | 0.179 | 0.334 | 0.477 | 268 |
| LAP3 | 0.365 | 0.485 | 0.452 | 0.503 | 268 |
| RNPEP | 0.350 | 0.460 | 0.447 | 0.503 | 268 |
| ANPEP | 0.504 | 0.770 | 0.513 | 0.513 | 268 |
ACE. angiotensin I-converting enzyme; ACE2: angiotensin I-converting enzyme 2; ANPEP: memnrane alanyl-aminopeptidase (APN); DPP4: dipeptidyl peptidase 4; ENPEP: glutamyl-aminopeptidase (APA); LAP3: leucine aminopeptidase 3; LNPEP: leucyl/cystinyl aminopeptidase; NPEPPS: aminopeptidase puromycine sensitive (PSA); PREP: prolyl endopeptidase (PEP); RNPEP: arginyl aminopeptidase (APB); eGFR: estimated glomerular filtration rate; FDR: false discovery rate (statistically significant values are shown in bold); SE: standard error.
Results from linear regression models adjusted for age in years and sex. Results were adjusted for multiple testing by applying the FDR.
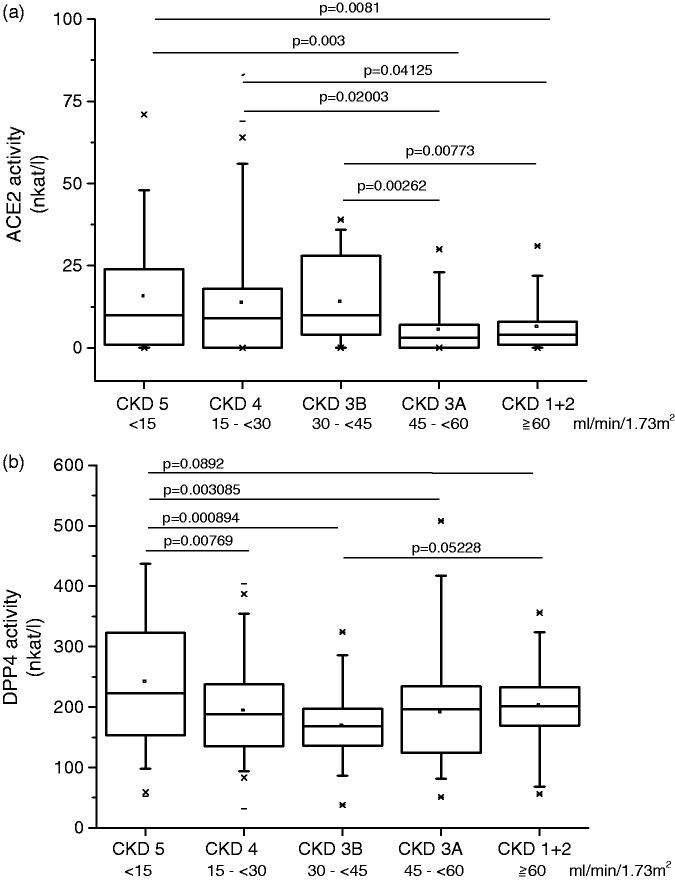
Categorical analysis of eGFR groups confirmed the significant associations of ACE2 and DPP4 serum activities with the eGFR group (overall P values DPP4: P = 0.000487; ACE2: P = 0.0043; Figure 1). As shown in Figure 1(a), of all eGFR groups, the groups 1 and 2 showed the lowest ACE2 activities. Thus, a dichotomous analysis was performed to compare ACE2 protease activity in patients with eGFR < 45 mL/min/1.73 m2 to that in patients with eGFR ≥ 45 mL/min/1.73 m2. The result show a significantly higher ACE2 activity in patients with eGFR ≥ 45 mL/min/1.73 m2 (P < 0.00016) (Supplementary Table S1). For DPP4, the eGFR group 5 exhibited the highest serum activity levels of all groups (Figure 1(b)). Dichotomous analysis of DPP4 activity in patients with eGFR < 15 mL/min/1.73 m2 versus ≥ 15 mL/min/1.73 m2 confirmed significantly higher DPP4 serum activities in patients with eGFR < 15 mL/min/1.73 m2 compared to patients with higher eGFR (p = 3.73 × 10−5) (Supplementary Table S2). Of note, ACE, ENPEP, and NPEPPS passed the significance threshold in the analyses in patients with eGFR < 45 and <15 mL/min/1.73 m2, respectively, but in no other analysis.
Figure 1.
Serum ACE2 and DPP4 activities according to eGFR groups. (a) ACE2 serum activity was inversely correlated with eGFR. Patients with well-preserved eGFR (eGFR groups 1 and 2; >45 mL/min/1.73 m2) had lower ACE2 activities than groups 3–5 (P < 0.00016). (b) DPP4 activity according to eGFR groups. Significantly higher DPP4 serum activities were observed in patients with eGFR < 15 mL/min/1.73 m2 compared to serum activity of patients with eGFR ≥ 15 mL/min/1.73 m2 (p = 3.73 × 10−5).
ACE2: angiotensin I-converting enzyme 2; DPP4: dipeptidyl-peptidase 4; eGFR: estimated glomerular filtration rate
DPP4 and PEP were inversely correlated with age (Table 4), whereas none of the others proteases studied here showed significant age- or sex-dependent changes in serum activity (Supplementary Tables S3 and S4). Among the 268 patients included in the study, 80 had atrial fibrillation (AF) in their medical history. In agreement with the established fact that AF prevalence increases with age, in our study AF was positively correlated with age (r = 0.27, p = 1.02 × 10−5). AF was not correlated to protease activity (Supplementary Table S4).
Table 4.
Associations between protease activity and age.
| Protease | Beta | SE | p | FDR | n |
|---|---|---|---|---|---|
| PREP | –0.790 | 0.191 | 4.706E-05 | 4.706E-04 | 268 |
| DPP4 | –1.368 | 0.474 | 0.004 | 0.021 | 268 |
| ENPEP | –0.318 | 0.155 | 0.041 | 0.136 | 268 |
| LAP3 | –1.829 | 0.899 | 0.043 | 0.107 | 268 |
| ANPEP | –2.571 | 1.425 | 0.072 | 0.145 | 268 |
| NPEPPS | –0.467 | 0.332 | 0.161 | 0.268 | 268 |
| RNPEP | –0.980 | 0.852 | 0.251 | 0.359 | 268 |
| LNPEP | –0.358 | 0.315 | 0.256 | 0.320 | 268 |
| ACE | –0.666 | 0.706 | 0.346 | 0.385 | 268 |
| ACE2 | –0.021 | 0.075 | 0.779 | 0779 | 268 |
ACE. angiotensin I-converting enzyme; ACE2: angiotensin I-converting enzyme 2; ANPEP: memnrane alanyl-aminopeptidase (APN); DPP4: dipeptidyl peptidase 4; ENPEP: glutamyl-aminopeptidase (APA); LAP3: leucine aminopeptidase 3; LNPEP: leucyl/cystinyl aminopeptidase; NPEPPS: aminopeptidase puromycine sensitive (PSA); PREP: prolyl endopeptidase (PEP); RNPEP: arginyl aminopeptidase (APB); FDR: false discovery rate (statistically significant values are shown in bold); SE: standard error.
Results were adjusted for multiple testing by applying the FDR.
ACE activity was influenced by medication (ATC code C9; agents acting on the RAS). However, the significant association between eGFR and DPP4 or ACE2 activity, respectively, remained after adjusting for medication intake.
Discussion
We performed a comprehensive analysis of serum protease activity to assess their potential association with CKD. Of the 10 protease activities studied, that of ACE2 and DPP4 were found to be associated with eGFR.
ACE2 is fairly well characterized as part of the alternative ACE2/Ang-(1–7)/Mas axis of the RAS. The kidney is among the tissues with highest ACE2 expression levels,25,26 and in polarized kidney cells ACE2 appears to be preferentially expressed on the apical surface.27 ACE2 is proteolytically cleaved from the apical surface to release a soluble form.27,28 In human plasma, ACE2 enzymatic activity is rather low and, dependent on the assay applied and the clinical setting, might even be too low to be measured.29 Nevertheless, plasma or serum enzymatic activity of ACE2 exerts significant protective effects due to its ability to convert AngII, the major effector peptide of the classical ACE/AngII/AT1R RAS axis, to Ang-(1–7). Such protective activity of Ang-(1–7) has been observed in animal models of Xu et al.30 and patients with CKD.31 Accordingly, loss of ACE2 was shown to exacerbate experimental renal ischemia–reperfusion injury32 and pharmacological inhibition of ACE2 aggravated albuminuria in (5/6) nephrectomy mice; the latter effect could be prevented by the AT1R antagonist, losartan.33
The mutual influence of the classical AngII/ACE/AT1R and alternative Ang-(1–7)/ACE2/Mas axis of the RAS is also emphasized by the interesting data of Reich et al.,34 who found that ACE2 mRNA levels were reduced whereas ACE mRNA levels were increased in glomerular and proximal tubular cells of diabetic patients. Another study revealed no differences in renal expression of ACE2 between patients with diverse primary and secondary renal diseases, including patients with diabetic nephropathy.35 Podocytes were shown to preferentially express the components of an alternative RAS axis, neprilysin, aminopeptidase A, ACE2, and renin, which leads to the primarily formation of Ang-(1–7) and simultaneous degradation of AngII.36
In our patient cohort, direct serum ACE2 activity (without removal of any endogenous inhibitor prior to activity measurement) was inversely associated with eGFR. Lowest ACE2 activities were found in patient groups exhibiting an eGFR ≥ 45 mL/min/1.73 m2. It has been shown previously that plasma ACE2 levels are low in healthy subjects, but substantially increase under pathologic conditions including cardiac disease or diabetes.37–39 Higher serum ACE2 activity in patients with lower eGFR as observed here could represent a similar compensatory and protective mechanism aimed at counteracting disease-associated induction of the classical AngII/ACE/AT1R RAS axis. Elevated AngII levels have been associated previously with various cardiovascular or cardio-renal risk factors including diabetes, hypertension, obesity, and hyperlipidemia.
In a recent study, however, Roberts et al.40 report lower ACE2 plasma activities in patients on hemodialysis when compared to CKD patients who do not require hemodialysis (stage 3–4; eGFR ≥ 15 – <60 mL/min/1.73 m2). This is particularly true for the ACE2 activity after inhibitor removal (indirect activity; about 2.5-fold decrease), whereas the direct ACE2 activity was not significantly affected (1.25-fold lower). The latter finding might partly explain the discrepant results. It should be emphasized, however, that the ACE2 activities measured in our study are in the range of those previously reported.39,40 Notably, the direct ACE2 enzymatic activities of healthy controls (8.3 nkat/L in Wang et al.39) were closest to that of patients with well-preserved eGFR (≥60 mL/min/1.73 m2) in our study (6.25 nkat/L). A recent study observed lower circulating ACE2 activity in CKD patients without concomitant cardiovascular disease (stages 3–5 and 5/dialysis) when compared to controls.41 Urinary ACE2 levels were found to be increased in diabetic patients and in patients with CKD stage 4 (compared to CKD1-3).42 Urinary ACE2 seems to reflect glomerular damage as it was associated with albuminuria and urinary liver-type fatty acid binding protein levels.
Dipeptidyl peptidase 4 (DPP4, CD26, EC3.4.14.5) is a 110 kDa type II membrane protein. In the plasma membrane it occurs as a 220–240 kDa homodimeric peptidase. The enzyme is ubiquitously expressed with highest expression levels in kidney, spleen, and lung.43 Furthermore, it has been established as a T-cell activation marker44,45 and implicated in T-cell function.46,47 Various diseases, in particular cancer, are associated with altered expression/activity of DPP4.15,48 Bone marrow-derived cells but not the kidney have been identified as a source of soluble DPP4.43 Serum DPP4 levels are subject to change; altered activities have been associated with various cancers49–51 and activity increased after oral glucose load in healthy subjects.52 The postproline cleaving substrate specificity makes DPP4 relatively unique among other proteases.53,54 It preferentially removes X-Pro, but also X-Ala dipeptides from the N-terminus of substrates. GLP-1 is among the established DPP4 substrates and, therefore, DPP4 inhibitors (gliptins) emerged as antidiabetic compounds with increasing clinical use.55 DPP4 has been implicated in the preservation of renal function in streptozotocin-induced diabetic rats56 and in the non-obese diabetic mouse.17 In the latter, insulin improved renal function as well as circulating and renal ACE2 activity.17 However, inhibition of DPP4 appears to be nephroprotective by mechanisms independent of changes in blood glucose, too.57 This view is supported by a recent study demonstrating in two different animal models of renal injury (db/db mice and adriamycin-induced nephropathy) that the DPP4 inhibitor, DA-1229, improved proteinuria, renal fibrosis, and urinary nephrin loss without affecting glycemic control.58 Similarily, gemigliptin suppressed albuminuria and reduced apoptosis of podocytes independent of its glucose-lowering effects.59 Furthermore, inhibition of DPP4 by vildagliptin was shown to restore urinary flow, GFR, and proteinuria in a rat model of heart failure-induced renal dysfunction.60 Recent data provide mechanistic insight into the role of DPP4 by demonstrating that linagliptin prevents TGF-β1/Smad2/3-signaling in a mannose-6-phosphate receptor-dependent manner in a model of diabetic nephropathy.61 Furthermore, the DPP4 inhibitor, MK0626, normalized the AngII-dependent decrease in the expression of megalin, an endocytic receptor of the proximal tubule.16 This emphasizes the important contribution of an inappropriately activated RAS (due to, e.g. hypertension, AF, obesity, hyperlipidemia, or hyperglycemia) to CKD.16,58 DPP4 inhibitors have been shown to reduce fibrotic and inflammatory markers with NF-κB, TGF-β1/Smad, AngII, and mitogen-activated kinase Erk1/2 being possible mediators identified.16,18,61
In this study we observed a negative association between eGFR and serum DPP4 activity. Highest activities (240 ± 113 nkat/L; mean ± SD) were found in patients with eGFR < 15 mL/min/1.73 m2. This activity range is about 50% lower than of previously published reference values.19 Of note, this previous work did not consider the contribution of other members of the DPP4 family to the Gly-Pro-pNA-hydrolyzing activity that was used for measuring serum/plasma activities in the assay. In our study, DPP4 activity was defined as the fraction of Gly-Pro-pNA-hydrolyzing activity that could be inhibited by Lys-Z-nitro-thiazolidid, thereby excluding the non-inhibited fraction that rather represents PEP activity. Whereas Durinx et al.19 observed a decline of serum DPP4 activity with growing age and higher activities in men than in women, these effects could be observed here only for age. Again, the apparent discrepancy might be due to the application of a specific DPP4 inhibitor in our study. An age-dependent increase in Gly-Pro-pNA-hydrolyzing activity has been observed previously in the pericardial fluid of elder female patients (> 80 years) with coronary artery disease, whereas elder male patients showed a tendency toward decreased DPP4 activities.13 In another study, where DPP4 activity was higher in patients with appropriate implantable cardioverter/defibrillator (ICD) intervention than in patients without ICD intervention, DPP4 activity was negatively correlated with age.20 This finding suggests that the specific response to diverse pathophysiological conditions may depend on age and sex. Although ACE, ENPEP, and NPEPPS passed the significance threshold in one of the dichotomous analyses, further studies are needed to test whether these enzymatic activities are truly associated with the eGFR, since both associations were close to the significance level only in one specific dichotomous analysis and not in the other tests, and thus giving inconclusive results.
In summary, our data indicate that ACE2 and DPP4 enzymatic activities but not the enzymatic activities of several other serum peptidases are associated with the eGFR in patients with CKD. It remains to be established if and to what extent altered protease activities may serve as useful markers for monitoring development and progression of CKD.
However, our findings clearly show that further analyses are warranted to identify the precise role of serum ectopeptidases in the pathogenesis of CKD and to fully elucidate underlying molecular mechanisms.
Supplementary Material
Acknowledgements
We thank Ines Schultz and Manja Möller for excellent technical assistance. This work is part of the research project Greifswald Approach to Individualized Medicine (GANI_MED), which is funded by the Federal Ministry of Education and Research and the Ministry of Cultural Affairs of the Federal State of Mecklenburg-West Pomerania (03IS2061A). The results presented in this paper have not been published previously in whole or part.
Authors’ contributions
All authors participated in the design of the study, interpretation of data, and review of the manuscript. AH and AT performed statistical analyses. AH, CW, UL performed the laboratory experiments. BF, KE, NE, RR, SA, SBF, and SS contributed data/materials/analysis tools. CW and UL wrote the manuscript. AH and RR checked and polished the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010; 31: 703–11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011; 80: 17–28. [DOI] [PubMed] [Google Scholar]
- 3.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010; 375: 1296–309. [DOI] [PubMed] [Google Scholar]
- 4.Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, Mendelssohn D, Finkelstein FO. Health-related quality of life in CKD Patients: correlates and evolution over time. Clin J Am Soc Nephrol 2009; 4: 1293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, McCullough PA. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contribut Nephrol 2013; 182: 117–36. [DOI] [PubMed] [Google Scholar]
- 6.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 2012; 125: 1402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remuzzi G, Cattaneo D, Perico N. The aggravating mechanisms of aldosterone on kidney fibrosis. J Am Soc Nephrol 2008; 19: 1459–62. [DOI] [PubMed] [Google Scholar]
- 8.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrol Dial Transplant 2007; 22: 1314–22. [DOI] [PubMed] [Google Scholar]
- 9.Capes SE, Gerstein HC, Negassa A, Yusuf S. Enalapril prevents clinical proteinuria in diabetic patients with low ejection fraction. Diabetes Care 2000; 23: 377–80. [DOI] [PubMed] [Google Scholar]
- 10.Hillege HL, van Gilst WH, van Veldhuisen DJ, Navis G, Grobbee DE, de Graeff PA, de Zeeuw D, Trial CR. Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ACE inhibition: the CATS randomized trial. Eur Heart J 2003; 24: 412–412. [DOI] [PubMed] [Google Scholar]
- 11.Ljungman S, Kjekshus J, Swedberg K. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study [CONSENSUS] Trial). Am J Cardiol 1992; 70: 479–87. [DOI] [PubMed] [Google Scholar]
- 12.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ. CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374: 543–50. [DOI] [PubMed] [Google Scholar]
- 13.Bechtloff R, Goette A, Bukowska A, Kähne T, Peters B, Huth C, Wolke C, Lendeckel U. Gender and age-dependent differences in the bradykinin-degradation within the pericardial fluid of patients with coronary artery disease. Int J Cardiol 2011; 146: 164–70. [DOI] [PubMed] [Google Scholar]
- 14.Blais C, Jr, Drapeau G, Raymond P, Lamontagne D, Gervais N, Venneman I, Adam A. Contribution of angiotensin-converting enzyme to the cardiac metabolism of bradykinin: an interspecies study. Am J Physiol 1997; 273: H2263–71. [DOI] [PubMed] [Google Scholar]
- 15.Lendeckel U, Arndt M, Wrenger S, Nepple K, Huth C, Ansorge S, Klein HU, Goette A. Expression and activity of ectopeptidases in fibrillating human atria. J Mol Cell Cardiol 2001; 33: 1273–81. [DOI] [PubMed] [Google Scholar]
- 16.Aroor A, Zuberek M, Duta C, Meuth A, Sowers JR, Whaley-Connell A, Nistala R. Angiotensin II stimulation of DPP4 activity regulates megalin in the proximal tubules. Int J Mol Sci 2016; 17: pii:E780–pii:E780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riera M, Marquez E, Clotet S, Gimeno J, Roca-Ho H, Lloreta J, Juanpere N, Batlle D, Pascual J, Soler MJ. Effect of insulin on ACE2 activity and kidney function in the non-obese diabetic mouse. PloS One 2014; 9: e84683–e84683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangadharan Komala M, Gross S, Zaky A, Pollock C, Panchapakesan U. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology 2016; 21: 423–31. [DOI] [PubMed] [Google Scholar]
- 19.Durinx C, Neels H, Van der Auwera JC, Naelaerts K, Scharpe S, De Meester I. Reference values for plasma dipeptidyl-peptidase IV activity and their association with other laboratory parameters. Clin Chem Lab Med 2001; 39: 155–9. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann HI, Wolke C, Malenke W, Röhl FW, Hammwöhner M, Bukowska A, Lendeckel U, Goette A. Enzymatic activity of DPIV and renin-angiotensin system (RAS) proteases in patients with left ventricular dysfunction and primary prevention implantable cardioverter/defibrillator (ICD). Int J Cardiol 2013; 168: 255–60. [DOI] [PubMed] [Google Scholar]
- 21.Grabe HJ, Assel H, Bahls T, Dorr M, Endlich K, Endlich N, Erdmann P, Ewert R, Felix SB, Fiene B, Fischer T, Flessa S, Friedrich N, Gadebusch-Bondio M, Salazar MG, Hammer E, Haring R, Havemann C, Hecker M, Hoffmann W, Holtfreter B, Kacprowski T, Klein K, Kocher T, Kock H, Krafczyk J, Kuhn J, Langanke M, Lendeckel U, Lerch MM, Lieb W, Lorbeer R, Mayerle J, Meissner K, zu Schwabedissen HM, Nauck M, Ott K, Rathmann W, Rettig R, Richardt C, Salje K, Schminke U, Schulz A, Schwab M, Siegmund W, Stracke S, Suhre K, Ueffing M, Ungerer S, Volker U, Volzke H, Wallaschofski H, Werner V, Zygmunt MT, Kroemer HK. Cohort profile: Greifswald approach to individualized medicine (GANI_MED). J Transl Med 2014; 12: 144–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–70. [DOI] [PubMed] [Google Scholar]
- 23.Delanaye P, Pottel H, Botev R, Inker LA, Levey AS. Con: should we abandon the use of the MDRD equation in favour of the CKD-EPI equation? Nephrol Dial Transplant 2013; 28: 1396–403. discussion 1403. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological) 1995; 57: 289–300. [Google Scholar]
- 25.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000; 275: 33238–43. [DOI] [PubMed] [Google Scholar]
- 26.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002; 532: 107–10. [DOI] [PubMed] [Google Scholar]
- 27.Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem 2005; 280: 39353–62. [DOI] [PubMed] [Google Scholar]
- 28.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 2005; 280: 30113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol 2016; 310: H137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C, Ding W, Zhang M, Gu Y. Protective effects of angiotensin-(1–7) administrated with an angiotensin-receptor blocker in a rat model of chronic kidney disease. Nephrology 2013; 18: 761–9. [DOI] [PubMed] [Google Scholar]
- 31.Abe M, Oikawa O, Okada K, Soma M. Urinary angiotensin-converting enzyme 2 increases in diabetic nephropathy by angiotensin II type 1 receptor blocker olmesartan. JRAAS 2015; 16: 159–64. [DOI] [PubMed] [Google Scholar]
- 32.Fang F, Liu GC, Zhou X, Yang S, Reich HN, Williams V, Hu A, Pan J, Konvalinka A, Oudit GY, Scholey JW, John R. Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PloS One 2013; 8: e71433–e71433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dilauro M, Zimpelmann J, Robertson SJ, Genest D, Burns KD. Effect of ACE2 and angiotensin-(1–7) in a mouse model of early chronic kidney disease. Am J Physiol 2010; 298: F1523–32. [DOI] [PubMed] [Google Scholar]
- 34.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 2008; 74: 1610–6. [DOI] [PubMed] [Google Scholar]
- 35.Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol 2004; 204: 587–93. [DOI] [PubMed] [Google Scholar]
- 36.Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol 2007; 293: F398–407. [DOI] [PubMed] [Google Scholar]
- 37.Chong CP, Lim WK, Velkoska E, van Gaal WJ, Ryan JE, Savige J, Burrell LM. N-terminal pro-brain natriuretic peptide and angiotensin-converting enzyme-2 levels and their association with postoperative cardiac complications after emergency orthopedic surgery. Am J Cardiol 2012; 109: 1365–73. [DOI] [PubMed] [Google Scholar]
- 38.Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH, FinnDiane Study G. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 2012; 30: 375–83. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Moreira Mda C, Heringer-Walther S, Ebermann L, Schultheiss HP, Wessel N, Siems WE, Walther T. Plasma ACE2 activity is an independent prognostic marker in Chagas’ disease and equally potent as BNP. J Cardiac Fail 2010; 16: 157–63. [DOI] [PubMed] [Google Scholar]
- 40.Roberts MA, Velkoska E, Ierino FL, Burrell LM. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol Dial Transplant 2013; 28: 2287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anguiano L, Riera M, Pascual J, Valdivielso JM, Barrios C, Betriu A, Mojal S, Fernandez E, Soler MJ, study N. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant 2015; 30: 1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abe M, Maruyama N, Oikawa O, Maruyama T, Okada K, Soma M. Urinary ACE2 is associated with urinary L-FABP and albuminuria in patients with chronic kidney disease. Scand J Clin Lab Invest 2015; 75: 421–7. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Grigo C, Steinbeck J, von Horsten S, Amann K, Daniel C. Soluble DPP4 originates in part from bone marrow cells and not from the kidney. Peptides 2014; 57: 109–17. [DOI] [PubMed] [Google Scholar]
- 44.Ansorge S, Schön E. Dipeptidyl peptidase IV (DP IV), a functional marker of the T lymphocyte system. Acta Histochem 1987; 82: 41–46. [DOI] [PubMed] [Google Scholar]
- 45.Schön E, Ansorge S. Dipeptidyl peptidase IV in the immune system. Cytofluorometric evidence for induction of the enzyme on activated T lymphocytes. Biol Chem Hoppe-Seyler 1990; 371: 699–705. [DOI] [PubMed] [Google Scholar]
- 46.Lendeckel U, Kähne T, Riemann D, Neubert K, Arndt M, Reinhold D. Review: the role of membrane peptidases in immune functions. Adv ExpMed Biol 2000; 477: 1–24. [DOI] [PubMed] [Google Scholar]
- 47.Reinhold D, Bank U, Täger M, Ansorge S, Wrenger S, Thielitz A, Lendeckel U, Faust J, Neubert K, Brocke S. DP IV/CD26, APN/CD13 and related enzymes as regulators of T cell immunity: implications for experimental encephalomyelitis and multiple sclerosis. Front Biosci 2008; 13: 2356–63. [DOI] [PubMed] [Google Scholar]
- 48.Carl-McGrath S, Lendeckel U, Ebert M, Wolter AB, Roessner A, Röcken C. The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int J Oncol 2004; 25: 1223–32. [PubMed] [Google Scholar]
- 49.Boccardi V, Marano L, Rossetti RR, Rizzo MR, di Martino N, Paolisso G. Serum CD26 levels in patients with gastric cancer: a novel potential diagnostic marker. BMC Cancer 2015; 15: 703–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cordero OJ, Imbernon M, Chiara LD, Martinez-Zorzano VS, Ayude D, de la Cadena MP, Rodriguez-Berrocal FJ. Potential of soluble CD26 as a serum marker for colorectal cancer detection. World J Clin Oncol 2011; 2: 245–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother 2009; 58: 1723–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aso Y, Terasawa T, Kato K, Jojima T, Suzuki K, Iijima T, Kawagoe Y, Mikami S, Kubota Y, Inukai T, Kasai K. The serum level of soluble CD26/dipeptidyl peptidase 4 increases in response to acute hyperglycemia after an oral glucose load in healthy subjects: association with high-molecular weight adiponectin and hepatic enzymes. J Lab Clin Med 2013; 162: 309–16. [DOI] [PubMed] [Google Scholar]
- 53.Gorrell MD, Wang XM, Park J, Ajami K, Yu DM, Knott H, Seth D, McCaughan GW. Structure and function in dipeptidyl peptidase IV and related proteins. Adv Exp Med Biol 2006; 575: 45–54. [DOI] [PubMed] [Google Scholar]
- 54.Kähne T, Lendeckel U, Wrenger S, Neubert K, Ansorge S, Reinhold D. Dipeptidyl peptidase IV: a cell surface peptidase involved in regulating T cell growth (review). Int J Mol Med 1999; 4: 3–15. [DOI] [PubMed] [Google Scholar]
- 55.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 2005; 48: 612–5. [DOI] [PubMed] [Google Scholar]
- 56.Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K, Nakahori Y. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: a streptozotocin-induced model using wild-type and DPP4-deficient rats. J Endocrinol 2009; 200: 53–61. [DOI] [PubMed] [Google Scholar]
- 57.Vallon V, Docherty NG. Intestinal regulation of urinary sodium excretion and the pathophysiology of diabetic kidney disease: a focus on glucagon-like peptide 1 and dipeptidyl peptidase 4. Exp Physiol 2014; 99: 1140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eun Lee J, Kim JE, Lee MH, Song HK, Ghee JY, Kang YS, Min HS, Kim HW, Cha JJ, Han JY, Han SY, Cha DR. DA-1229, a dipeptidyl peptidase IV inhibitor, protects against renal injury by preventing podocyte damage in an animal model of progressive renal injury. Lab Invest 2016; 96: 547–60. [DOI] [PubMed] [Google Scholar]
- 59.Jung E, Kim J, Ho Kim S, Kim S, Cho MH. Gemigliptin improves renal function and attenuates podocyte injury in mice with diabetic nephropathy. Eur J Pharmacol 2015; 761: 116–24. [DOI] [PubMed] [Google Scholar]
- 60.Arruda-Junior DF, Martins FL, Dariolli R, Jensen L, Antonio EL, Dos Santos L, Tucci PJ, Girardi AC. Dipeptidyl peptidase IV inhibition exerts renoprotective effects in rats with established heart failure. Front Physiol 2016; 7: 293–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gangadharan Komala M, Gross S, Zaky A, Pollock C, Panchapakesan U. Linagliptin limits high glucose induced conversion of latent to active TGFss through interaction with CIM6PR and limits renal tubulointerstitial fibronectin. PloS One 2015; 10: e0141143–e0141143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H, Roques BP, Fournie-Zaluski MC. Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett 1999; 9: 1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ansorge S, Bank U, Heimburg A, Helmuth M, Koch G, Tadje J, Lendeckel U, Wolke C, Neubert K, Faust J, Fuchs P, Reinhold D, Thielitz A, Täger M. Recent insights into the role of dipeptidyl aminopeptidase IV (DPIV) and aminopeptidase N (APN) families in immune functions. Clin Chem Lab Med 2009; 47: 253–61. [DOI] [PubMed] [Google Scholar]
- 64.Schön E, Born I, Demuth HU, Faust J, Neubert K, Steinmetzer T, Barth A, Ansorge S. Dipeptidyl peptidase IV in the immune system. Effects of specific enzyme inhibitors on activity of dipeptidyl peptidase IV and proliferation of human lymphocytes. Biol Chem Hoppe-Seyler 1991; 372: 305–11. [DOI] [PubMed] [Google Scholar]
- 65.Alves MF, Araujo MC, Juliano MA, Oliveira EM, Krieger JE, Casarini DE, Juliano L, Carmona AK. A continuous fluorescent assay for the determination of plasma and tissue angiotensin I-converting enzyme activity. Braz J Med Biol Res 2005; 38: 861–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.