Abstract
The identification of factors capable of enhancing neurogenesis has great potential for cellular therapies in neurodegenerative diseases. Multiple studies have shown the neuroprotective effects of L-carnitine (LC). This study determined whether neuronal differentiation of rat adipose tissue-derived mesenchymal stem cells (ADSCs) can be activated by LC. In this study, protein kinase A (PKA) and Wnt/β-catenin pathways were detected to show if this activation was due to these pathways. The expression of LC-induced neurogenesis markers in ADSCs was characterized using real-time PCR. ELISA was conducted to assess the expression of cyclic adenosine monophosphate (cAMP) and PKA. The expression of β-catenin, reduced dickkopf1 (DKK1), low-density lipoprotein receptor-related protein 5 (LRP5), Wnt1, and Wnt3a genes as Wnt/β-catenin signaling members were used to detect the Wnt/β-catenin pathway. It was observed that LC could promote neurogenesis in ADSCs as well as expression of some neurogenic markers. Moreover, LC causes to increase the cAMP levels and PKA activity. Treatment of ADSCs with H-89 (dihydrochloride hydrate) as PKA inhibitor significantly inhibited the promotion of neurogenic markers, indicating that the PKA signaling pathway could be involved in neurogenesis induction. Analyses of real-time PCR data showed that the mRNA expressions of β-catenin, DKK1, LRP5c-myc, Wnt1, and Wnt3a were increased in the presence of LC. Therefore, the present study showed that LC promotes ADSCs neurogenesis and the LC-induced neurogenic markers could be due to both the PKA and Wnt/β-catenin signaling pathway.
Impact statement
Neural tissue has long been believed as incapable of regeneration and the identification of cell types and factors capable of neuronal differentiation has generated intense interest. Mesenchymal stem cells (MSCs) are considered as potential targets for stem cell-based therapy. L-carnitin (LC) as an antioxidant may have neuroprotective effects in oxidative damage and possibly in neurodegenerative disorders. We have tried to evaluate the effect of LC as an antioxidant on the neurogenic differentiation of ADSCs in order to further elucidate the simultaneous effects on the capability of the neural regeneration. In this study, PKA and Wnt/β-catenin signaling pathways were detected to see if LC could also activate these pathways. The results of this study showed that 200 µM LC promoted ADSCs neurogenic differentiation, and that it was correlated with the PKA and Wnt/β-catenin signaling pathways.
Keywords: L-carnitine, neurogenesis, adipose tissue-derived mesenchymal stem cells, protein kinase A, Wnt/β-catenin, neurogenesis markers
Introduction
Incapability of neural tissue to regeneration has long been believed and the potential recognition of cell types causing to neural differentiation has intensively increased. Mesenchymal stem cells (MSCs) are considered as potential targets for stem cell-based therapy.1,2 The in vitro differentiation of MSCs into several lineages such as fat, bone, cartilage, skeletal, hepatocytes, and neuronal cells is easily achieved using different induction protocols.3,4 Nevertheless, identification of the factors and pathways governing the proliferation, and differentiation potential of neural cell types, in addition to the stimulation of neurogenesis, is important in cell transplantation and other medical fields. L-carnitine (3-hydroxy-4-N-trimethylammonium-butyrate) (LC), an amino acid derivative that is essential for β-oxidation of fatty acids in the mitochondria for the production of adenosine triphosphate, also plays important roles in mitochondrial-related functions.5,6 With regard to its biological effects, LC may have neuroprotective effects on conditions of mitochondrial dysfunction, oxidative damage and possibly in neurodegenerative disorders.7 Another important characteristic of LC is their ability to neutralize toxic acyl-CoA production in the mitochondria, which correlates with numerous diseases of the central nervous system, including Alzheimer’s, Huntington’s, Parkinson’s disease, etc.8 As mentioned earlier, many neurobiological effects of LC have been shown. In a study by Hollister and Gruber,9 it was demonstrated that LC leads to a modest decrease in the rate of progression of Alzheimer’s disease. Farahzadi et al.10 reported that LC, as an antioxidant, could be used as a good candidate for extending the replicative life-spans of aged MSCs by increasing hTERT gene expression and telomere length. Kobayashi et al.11 showed that acetyl-L-carnitine improves learning capacity and age-related memory in aged rats via strengthening synaptic plasticity. Some researchers generally believed that changes in gene and protein expression may be associated with activation or suppression of the signaling pathway.12 Studies are required to fully elucidate the complexity and diversity of the signaling pathways involved in diseases. Wnt/β-catenin signaling is known as an important pathway in a number of developmental processes including cell proliferation, cell differentiation, cell migration, genetic stability, and cell apoptosis.13 Abnormal regulation of this pathway leading to some diseases such as cancer, fibrosis, bipolar disorder, heart failure, and neurodegeneration.12 So far, the effects of LC on the neurogenic differentiation of adipose tissue-derived mesenchymal stem cells (ADSCs) are yet to be reported. Therefore, the aim of this study was to evaluate the effects of LC on the neurogenesis of ADSCs, in order to further elucidate its simultaneous effects on the capability of the neural regeneration. As a first question, if LC could promote neurogenesis of ADSCs and neurogenic gene expression, and the second one is related to cyclic adenosine monophosphate–protein kinase A (cAMP–PKA) and Wnt/β-catenin pathways, was whether these pathways could be activated by LC.
Materials and methods
Isolation and culturing of ADSCs
Adipose tissue of epididymal region was obtained with consent ethical guidelines of University of Tabriz, Iran, from five male Rattus rats. Adipose tissues were washed with PBS and carefully dissected and minced. Tissues were then enzymatically dissociated for 30 min at 37℃ using 0.075% (w/v) collagenase type I (Invitrogen, UK). This solution neutralized by the addition of complete medium containing Dulbecco’s modified Eagle’s medium-low glucose, 10% (v/v) Fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin solution and centrifuged at 800 × g (2610 rpm) for 5 min. The cell pellet was re-suspended in complete medium.14 Cells were maintained at confluency in a 37℃ incubator with 5% CO2. ADSCs at passages 3–6 were used for the experiments described below.
Immunophenotyping of ADSCs
Approximately 106 cells were washed with PBS and were incubated with fluorescein isothiocyanate-conjugated primary antibody CD44, CD90, CD31, and CD34 (BD Phar-mingen, San Diego, CA, USA) (1 µg/106 cells) in PBS containing 1% FBS for 30 min on ice. ADSCs were washed in PBS containing 3% FBS and fluorescence activated cell sorter (Becton Dickinson Franklin Lakes, USA) was used for characterization of ADSCS. Data were analyzed using with the FlowJo software (version 6.2).
MTT cell proliferation assay of ADSCs in the presence of LC
ADSCs were trypsinized and seeded in 96-well culture plates at the density of 4 × 103 cells/wells. After 24 h of culture, cells were treated with 200, 400, and 600 µM LC for 14 days. After termination of LC treatment, the medium was replaced with MTT solution (5 mg/mL in PBS buffer) for 4 h at 37℃ in a 5% CO2 incubator. Finally, the MTT solution was removed, and dimethyl sulfoxide was added to dissolve formazan crystals. After 10 min, the optical density of each well was measured on an ELISA reader at 570 nm.15
Quantitative real-time PCR
ADSCs were seeded at a concentration of 50 × 103 cells per T-25 plastic flask in a neurogenic induction medium containing 40 µg bFGF, 400 µg EGF, 40 µg GDNF, and 2 mM L-ascorbic acid-2-phosphate in the absence and presence of 200 µM LC for 14 days. Total RNA from the ADSCs was isolated using Trizol reagent (Invitrogen), and cDNA synthesis was carried out using the RevertAid™ first strand cDNA synthesis kit (K1622; Fermentas, Germany). For real-time PCR analysis, all PCRs were performed using the Corbett Rotor-Gene™ 6000 HRM (Corbett Research, Australia). The mRNA expressions of target genes in the ADSCs included BDNF, NGF, nestin, β-catenin, dickkopf1 (DKK1), low-density lipoprotein receptor-related protein 5 (LRP5), Wnt1, and Wnt3a. GAPDH was selected as an endogenous housekeeping gene, and the relative mRNA expression of genes was calculated using 2−ΔΔCT method.16
Protein kinase A catalytic activity and cAMP assay
A cAMP enzyme immunoassay kit according to competitive enzyme immunoassay method (Sigma, USA) was used to measure intracellular cAMP levels according to the manufacturer’s guidelines. Briefly, ADSCs were seeded in a neurogenic induction medium for 5, 15, 30, 45, 60, 90, and 120 min in the presence and absence of 200 µM LC as test and control groups, respectively.17 The cells were rinsed with PBS, trypsinized with Trypsin-EDTA and lysed with lysis buffer. Following incubation of cells in the presence and absence of LC for abovementioned times, PKA activity was assayed in cell lysates using the Pep Tag assay for non-radioactive detection of PKA (Promega Corporation, USA).
Results and discussion
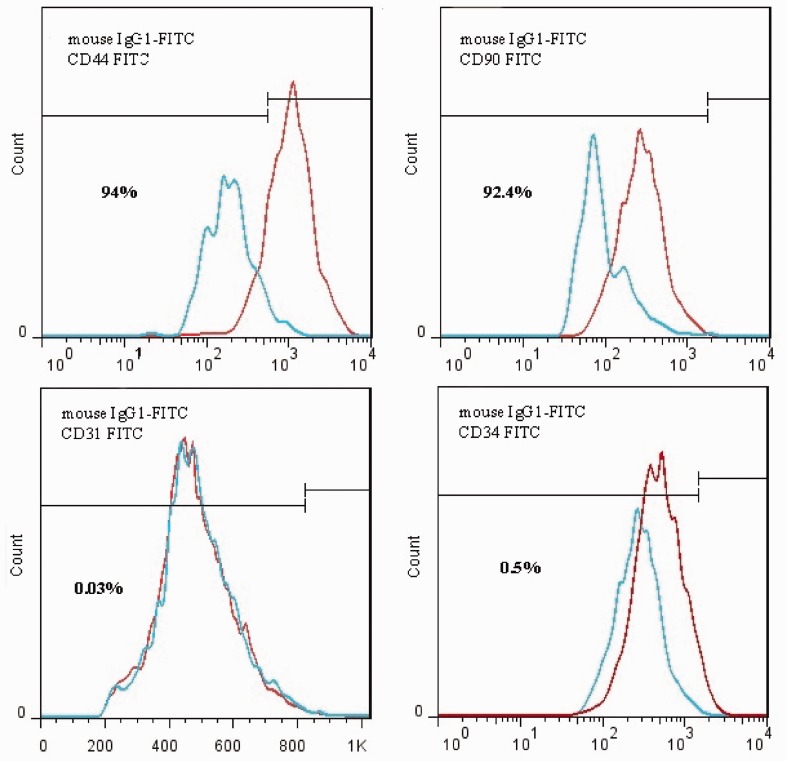
Although the role of LC in the increase of neurogenesis and neuroprotective effects in neurodegenerative disorders has been reported, the precise mechanism is still unclear. The results of the present research support the hypothesis that LC effectively increases neurogenesis in ADSCs. The results provide the hypothesis that this increase was due to both the PKA and Wnt/β-catenin pathways. To determine the effective concentration of LC under this study’s experimental conditions, MTT assay was used to evaluate cell viability. At a concentration of 200 µM, significant proliferation effect without cytotoxicity was recorded. Therefore, 200 µM LC was used in a complete culture medium to treat the cells. Flow cytometric analysis was conducted to identify MSCs markers. The ADSCs were consistently positive for CD44 (94%), CD90 (92.4%) and negative for hematopoietic cell lineage-specific antigens, such as CD31 (0.03%) and CD34 (0.5%) (Figure 1). To examine the effects of LC on neurogenic differentiation and Wnt/β-catenin pathway which is an important pathway of neurogenesis in ADSCs, quantitative real-time PCR for the detection of NGF, BDNF, nestin, β-catenin, Wnt1, Wnt3a, LRP5, and DKK1 expression in neurogenic differentiated ADSCs, was carried out.
Figure 1.
Flow cytomertic analysis of rat Adipose tissue-MSCs (rADSCs). rADSCs are stained with monoclonal antibodies directed against either CD44, CD90, CD31, and CD34 coupled to fluorescein isothiocyanate (FITC). rADSCs were positive for CD44 and CD90 and negative for CD31, CD34, and CD56. (A color version of this figure is available in the online journal.)
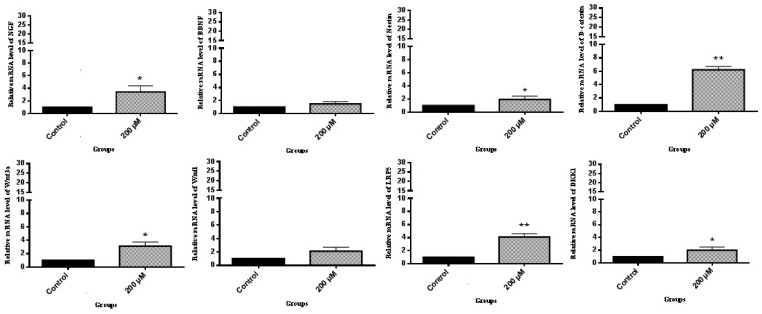
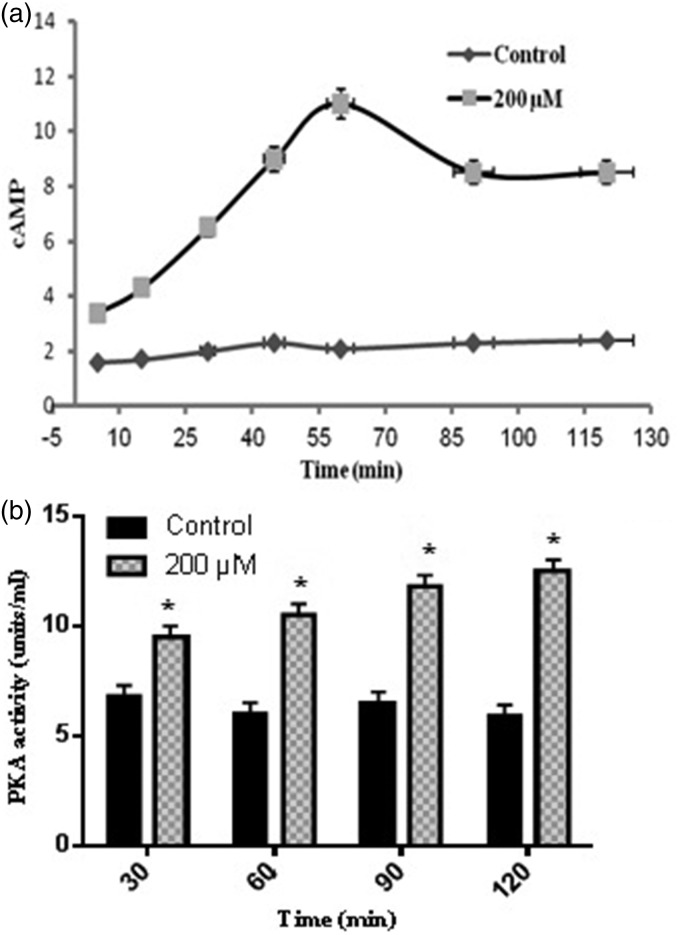
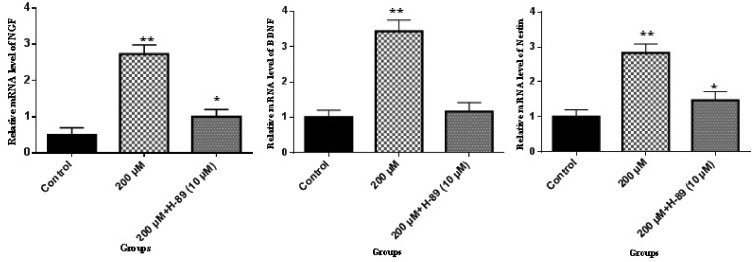
The results of this present work demonstrated that LC promoted the mRNA expression of NGF, BDNF, and nestin which are important transcription factors for neurogenesis.18 According to Figure 2, in the presence of LC, the expression of β-catenin, DKK1, LRP5, Wnt1, and Wnt3a genes was significantly increased, as compared to the control group. Thereafter, to check whether cAMP–PKA played a role in the activation of neurogenesis effected by 200 µM LC, ELISA was performed to evaluate the cAMP levels and PKA activity. According to Figure 3(a), cAMP levels were significantly elevated (11-fold) when ADSCs were exposed to 200 µM LC in 60 min as compared to the control group without LC treatment and decreased during the 60–90-min period. The cAMP level at 120 min was still about 8.4 at the tested concentration of the control group. The PKA activity was examined to further confirm the effect of LC on cAMP–PKA signaling pathway. According to Figure 3(b), PKA activity was significantly raised in the tested group in comparison to the control group at 30, 60, 90, and 120 min. At 120 min, the PKA activity in the tested group was three-fold when compared to the control. It was investigated whether ADSCs neurogenic differentiation promoted by 200 µM LC in the tested group was regulated by PKA. The effects of the specific PKA inhibitor (H-89 dihydrochloride hydrate) was assessed on ADSCs’ neurogenic differentiation induced in the tested group as mentioned earlier. To investigate the effect of PKA inhibitor (H-89 dihydrochloride hydrate) on NGF, BDNF, and nestin, cells were treated with 10 μM H-89 for 60 min at 37℃ before the treatment with 200 µM LC. According to Figure 4, the results indicated that the inductions of NGF, BDNF, and nestin by 200 µM LC in the tested group was inhibited by H89, demonstrating that these neurogenic markers were influenced by PKA signaling pathway. Up to now, the roles of cAMP/PKA and Wnt/β-catenin signaling pathways in the neurogenic differentiation are being disputed. In this study, PKA and Wnt/β-catenin signaling pathways were detected to see if LC could also activate these pathways. This research showed that 200 µM LC activated these pathways through increase in PKA activities, cAMP levels and expression of NGF, BDNF, and nestin as neurogenic differentiation genes, like the expression of β-catenin, Wnt1, Wnt3a, and LRP5 as Wnt/β-catenin pathway-related genes. To further demonstrate the correlation between the PKA signaling pathway and the neurogenic markers induced by the LC, the inhibitor of PKA pathway (H-89) was used to determine whether inhibiting it could also reduce the neurogenic markers of the ADSCs induced by the LC. This study showed that H-89 reduced the expression of ADSCs neurogenic markers, such as NGF, BDNF, and nestin. Previous studies also demonstrated that LC as an antioxidant may inhibit oxidation of lipids, reactive oxygen species production, oxidative stress and apoptotic death of neuronal cells through scavenging free radicals, which might be profitable in the treatment of neurodegenerative diseases.19 However, the findings of this study are consistent with the hypothesis that LC, can promote ADSCs’ neurogenic differentiation via the cAMP–PKA and Wnt/β-catenin pathways. Therefore, to determine the precise mechanism involved in the activation of Wnt/β-catenin and PKA signaling pathways by LC, further studies are required.
Figure 2.
LC effect on NGF, BDNF, nestin, β-catenin, Wnt1, Wnt3a, LRP5, and DKK1 mRNA expression in ADSCs. Cells were cultured in 6-well plates at a concentration of 30 × 105 cells/well. The RNA was extracted from ADSCs exposed to neurogenic culture medium in the absence and presence of 200 µM LC and was subjected to real-time PCR assay; mean ± SEM; n = 3; *P < 0.05, **P < 0.01
Figure 3.
cAMP and PKA activities affected by LC. Cells were exposed to neurogenic culture medium in the presence and absence of 200 µM LC for 5, 15, 30, 45, 60, 90, and 120 min periods for (a) cAMP levels measurement and 30, 60, 90, and 120 min periods for (b) PKA activity assay; mean ± SEM; n = 3; *P < 0.05
Figure 4.
NGF, BDNF, and nestin are regulated by PKA signaling pathway. The cells were subjected to 14 days of neurogenic culture medium treatment in the presence and absence of 200 µM LC for 30 min/day, with 10 μM H-89 added to the cells. The RNA was extracted from ADSCs exposed to neurogenic culture medium in the presence and absence of 200 µM LC was subjected to real-time PCR assay; mean ± SEM; n = 3; *P < 0.05, **P < 0.01
Conclusion
In conclusion, this research showed that 200 µM LC promoted ADSCs’ neurogenic differentiation, and it correlated with the PKA and Wnt/β-catenin signaling pathways.
Acknowledgments
This research was supported by a grant (No. S/27/3523-2, 08/02/1393) from the University of Tabriz.
Authors’ contributions
This work was carried out in collaboration between all authors. EF carried out in vitro experimental procedures, wrote the protocol as well as the draft of the manuscript. RF as a correspondence, designed the study, managed the literature and supervised the manuscript preparation. HNC prepared all equipment related to this research. All authors read and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M. A new multistep induction protocol for the transdifferentiation of bone marrow stromal stem cells into GABAergic neuron-like cells. Iran Biomed J 2013; 17: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fathi E, Farhzadi R. Survey on impact of trace elements (Cu, Se AND Zn) on veterinary and human mesenchymal stem cells. Rom J Biochem 2015; 52: 67–77. [Google Scholar]
- 3.Mohammad-Gharibani P, Tiraihi T, Arabkheradmand J. In vitro transdifferentiation of bone marrow stromal cells into GABAergic-like neurons. Iran Biomed J 2009; 13: 137–43. [PubMed] [Google Scholar]
- 4.Fathi E, Farhzadi R. Isolation, culturing, characterization and aging of adipose tissue-derived mesenchymal stem cells: a brief overview. Braz Arch Biol Technol 2016; 59: e16150383–e16150383. [Google Scholar]
- 5.Kim YJ, Kim SY, Sung DK, Chang YS, Park WS. Neuroprotective effects of L-carnitine against oxygen-glucose deprivation in rat primary cortical neurons. Korean J Pediatr 2012; 55: 238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fathi E, Farahzadi R. Application of L-carnitine as nutritional supplement in veterinary medicine. Rom J Biochem 2014; 51: 31–41. [Google Scholar]
- 7.Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr 2004; 36: 381–6. [DOI] [PubMed] [Google Scholar]
- 8.Vamos E, Voros K, Vecsei L, Klivenyi P. Neuroprotective effects of L-carnitine in a transgenic animal model of Huntington’s disease. Biomed Pharmacother 2010; 64: 282–6. [DOI] [PubMed] [Google Scholar]
- 9.Hollister L, Gruber N. Drug treatment of Alzheimer’s disease. Drugs Aging 1996; 8: 47–55. [DOI] [PubMed] [Google Scholar]
- 10.Farahzadi R, Mesbah-Namin SA, Zarghami N, Fathi E. L-carnitine effectively induces hTERT gene expression of human adipose tissue-derived mesenchymal stem cells obtained from the aged subjects. Int J Stem Cells 2016; 9: 107–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Iwamoto M, Kon K, Waki H, Ando S, Tanaka Y. Acetyl l carnitine improves aged brain function. Geriatr Gerontol Int 2010; 10: 99–106. [DOI] [PubMed] [Google Scholar]
- 12.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810. [DOI] [PubMed] [Google Scholar]
- 13.Teo JL, Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: a tale of two coactivators. Adv Drug Deliv Rev 2010; 62: 1149–55. [DOI] [PubMed] [Google Scholar]
- 14.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013; 15: 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ting Wang JCZ, Chen Y, Xiao PG, Yang MS. Effect of zinc ion on the osteogenic and adipogenic differentiation of mouse primary bone marrow stromal cells and the adipocytic trans-differentiation of mouse primary osteoblasts. J Trace Elem Med Biol 2007; 21: 84–91. [DOI] [PubMed] [Google Scholar]
- 16.Xiao C, Zhou H, Liu G, Zhang P, Fu Y, Gu P, Hou H, Tang T, Fan X. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed Mater 2011; 6: 015013–015013. [DOI] [PubMed] [Google Scholar]
- 17.Yong Y, Ming ZD, Feng L, Chun ZW, Hua W. Electromagnetic fields promote osteogenesis of rat mesenchymal stem cells through the PKA and ERK1/2 pathways. J Tissue Eng Regen Med 2016; 10: E537–45. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson KL, Slack RS. Growth factors: can they promote neurogenesis? Trends Neurosci 2003; 26: 283–5. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Ye J, Liu X, Han Y, Wang C. Protective effect of L-carnitine against H2O2-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. Neurolog Res 2011; 33: 708–16. [DOI] [PubMed] [Google Scholar]