Abstract
Vascular remodeling, a pathogenic hallmark in pulmonary hypertension, is mainly driven by a dysbalance between proliferation and apoptosis of human pulmonary artery smooth muscle cells. It has previously been shown that microRNAs are involved in the pathogenesis of pulmonary hypertension. However, the role of long noncoding RNAs has not been evaluated. long noncoding RNA expression was quantified in human pulmonary artery smooth muscle cells using PCR arrays and quantitative PCR. Knockdown of genes was performed by transfection of siRNA or GapmeR. Proliferation and migration were measured using BrdU incorporation and wound healing assays. The mouse model of hypoxia-induced PH was used to determine the physiological meaning of identified long noncoding RNAs. The expression of 84 selected long noncoding RNAs was assessed in hypoxic human pulmonary artery smooth muscle cells and the levels of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) were significantly increased. Depletion of hypoxia-inducible factor 1α abolished the hypoxia-induced upregulation of metastasis-associated lung adenocarcinoma transcript 1 expression. Silencing of MALAT1 significantly decreased proliferation and migration of human pulmonary artery smooth muscle cells. In vivo, MALAT1 expression was significantly increased in lungs of hypoxic mice. Of note, targeting of MALAT1 by GapmeR ameliorated heart hypertrophy in mice with pulmonary hypertension. This is the first report on functional characterization of MALAT1 in the pulmonary vasculature. Our data provide evidence that MALAT1 expression is significantly increased by hypoxia, probably by hypoxia-inducible factor 1α. Intervention experiments confirmed that MALAT1 regulates the proliferative phenotype of smooth muscle cells and silencing of MALAT1 reduced heart hypertrophy in mice with pulmonary hypertension. These data indicate a potential role of MALAT1 in the pathogenesis of pulmonary hypertension.
Impact statement
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a long noncoding RNA that mediates several biological processes. In the context of vascular biology, MALAT1 has been shown to be inducible by hypoxia and to control cell proliferation. These processes are of major importance for the pathophysiology of hypoxia-induced pulmonary hypertension (PH). Until now, the physiological role of MALAT1 in PH remains unclear. By using smooth muscle cells and by employing an established PH mouse model, we provide evidence that hypoxia induces MALAT1 expression. Moreover, depletion of MALAT1 inhibited migration and proliferation of smooth muscle cells, probably by the induction of cyclin-dependent kinase inhibitors. Of note, MALAT1 was significantly increased in mice exposed to hypoxia and silencing of MALAT1 ameliorated heart hypertrophy in mice with hypoxia-induced PH. Since vascular remodeling and right heart failure as a consequence of pulmonary pressure overload is a major problem in PH, these data have implications for our pathogenetic understanding.
Keywords: Metastasis-associated lung adenocarcinoma transcript 1, long noncoding RNA, Hypoxia, pulmonary vascular biology, remodeling, proliferation, pulmonary hypertension
Introduction
The process of pulmonary vascular remodeling is mainly driven by neoplastic-like alterations including abnormal proliferation, altered apoptosis, and increased migratory potential of vascular smooth muscle and endothelial cells. These events lead to media hypertrophy, intimal thickening and, in pulmonary arterial hypertension (PAH), to plexiform lesions.1 Several molecular pathways related to vascular remodeling have been identified. However, the pathogenesis of vascular remodeling remains ill-defined. Although PH is a disease of various origins,2,3 chronic hypoxia is considered to be a major trigger.4
By focusing on small noncoding RNAs (e.g. microRNAs, miRNAs), several novel factors that influence the vascular cell phenotype and thus are implicated in vascular remodeling have been identified and characterized recently.5–9 However, this part of gene regulation by noncoding RNAs neglects the potential contribution of long noncoding RNAs (lncRNAs), which are another emerging class of noncoding RNAs. LncRNAs are endogenously expressed RNA transcripts of >200 nt length that are not translated into proteins and, as such, lack a protein-coding function.10 Of note, despite this limitation, lncRNAs are versatile and biological active molecules that are involved in many cellular processes such as protein coding gene regulation.11
In vascular biology, lncRNAs were shown to be enriched in vascular cells to contribute to the contractile phenotype of smooth muscle cells,12 and, moreover, to control vascular smooth muscle cell proliferation and apoptosis.13 Of interest, Michalik et al.14 identified metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) as one of the most highly expressed lncRNA in vascular cells.14 MALAT1 was further shown to be regulated by hypoxia and to modulate sprouting, migration, and proliferation of endothelial cells.14 Together, these reports suggest a relevant role of lncRNAs in the context of hypoxia and vascular remodeling. However, a direct link of lncRNAs to pulmonary vascular biology and, in this context, to the pathogenesis of PH is yet to be made.
The present study is designed to investigate hypoxia-inducible lncRNAs in pulmonary vascular cells and to characterize identified lncRNAs in vascular cells with a special emphasis on apoptosis, migration, and proliferation.
Materials and methods
Animal experiments
Adult male mice (C57BL/6) used for the hypoxia-induced PH model were obtained from Charles River. The animals were randomized to a normoxic control group (room air, 21% O2) and to a hypoxic group (10% O2). Hypoxic condition was provided as described previously.8 Mice employed during initial screening for expression of lncRNAs (Figure 6) were exposed to 10% O2 for three or five weeks. After hypoxia, mice were anaesthetized with 5% isoflurane in oxygen and right ventricular pressure (RVP) was performed by right heart catheterization as reported elsewhere.15 Subsequently, mice were euthanized by cervical dislocation and lungs and hearts were collected for further analysis.
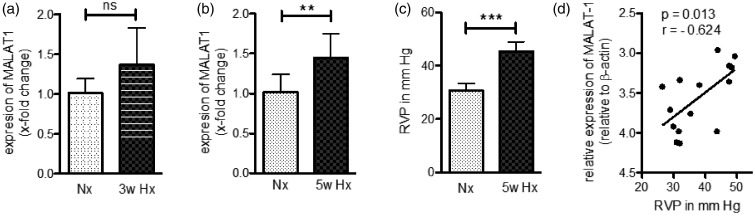
Figure 6.
Expression of MALAT1 is increased in the mouse model of hypoxia-induced PH. (a) Analysis of MALAT1 expression by qPCR demonstrated increased expression levels in total lung tissue of mice exposed for three weeks to hypoxia (Hx, 10% O2) as compared to normoxic (Nx) control mice. (b) Long-term exposure (five weeks) to Hx resulted in significant elevation of MALAT1 in total lung tissue of Hx mice. (c) Right ventricular pressure (RVP) was significantly elevated after five weeks of Hx indicating hypoxia-induced PH. (d) A significant positive correlation was found when comparing the expression levels of MALAT1 of each mouse with the corresponding RVP. n = 7–8 for all experiments. Statistical analysis by unpaired Student’s t-test (a–c) and Pearson’s correlation (d) (**P < 0.01, ***P < 0.001)
Mice used for the intervention study (Figure 7) were exposed to hypoxia for five weeks. After three weeks of hypoxic exposure (10% O2), mice were treated every four days by intraperitoneal (i.p.) injections with antisense locked nucleic acid (LNA) enhanced anti-sense oligonucleotides (GapmeRs, 10 mg/kg, solved in sterile 0.9% NaCl) targeting MALAT1 (5′ – TCA CAA TGC ATT CTA – 3′).14 As a negative control, a non-specific sequence was used (5′ – GAC TAA TGC ATT ATC – 3′). Two weeks later, RVP measurement was performed as stated above. Afterwards, animals were euthanized and lungs were harvested as described above. All animal experiments were approved by the Cantonal Veterinary Office Zurich (approval numbers 151/2012 and 212/2014).
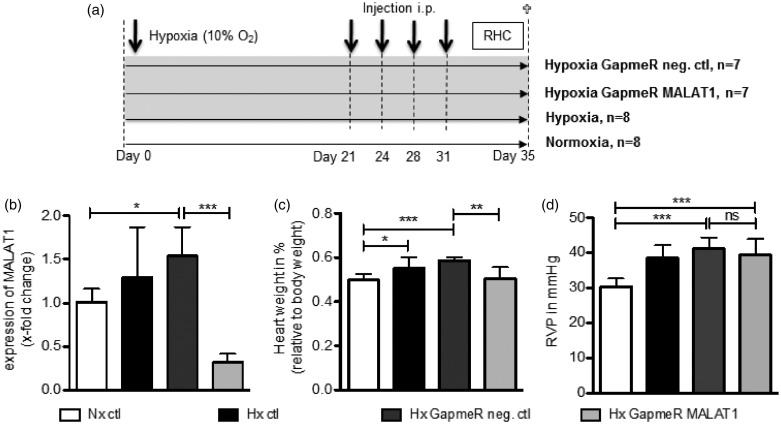
Figure 7.
Targeting of MALAT1 in the mouse model of hypoxia-induced PH. (a) Study design of the in vivo experiments. (b) As evaluated by qPCR, MALAT1 expression was significantly increased in the lungs of hypoxic GapmeR control mice, whereas the treatment with GapmeR MALAT1 significantly reduced the expression of MALAT1. (c) The relative heart weight of each mouse was measured demonstrating that GapmeR control mice developed heart hypertrophy, whereas mice treated with GapmeR MALAT1 had a significantly reduced relative heart weight. (d) The RVP measurement by right heart catheterization (RHC) showed significantly increased RVP in hypoxic control mice indicating the development of PH in these mice. However, the RVP was found to be unchanged between GapmeR control and GapmeR MALAT1 treated mice. Statistical analysis by one-way ANOVA with Tukey post hoc test (b–d) (*P < 0.05, **P < 0.01, ***P < 0.001)
Cell culture
Human pulmonary artery smooth muscle (HPASMC) and human pulmonary artery endothelial (HPAEC) cells were purchased (ScienCell) and cultivated according to the manufacturer’s instructions. Cells were incubated in a humidified atmosphere at 37℃ and 5% CO2. In all experiments, passage number between two and eight was used. Hypoxic exposure (1% O2) was carried out over different time points in an O2 adjustable incubator (Binder) maintained at 37℃ with 5% CO2.
Transient transfection of primary cells
For manipulation of endogenous levels of MALAT1, HPASMC were transfected with GapmeR negative control or GapmeR targeting MALAT1 (final concentration of 25 nM) using Lipofectamine 2000 transfection reagent according to the manufacturer’s protocol (Thermo Fisher Scientific). Cell culture grade GapmeR inhibitors were obtained from Exiqon. Following an incubation period of 24 or 48 h, cells either were harvested for gene expression analysis or prepared for performing functional assays.
RNA isolation and cDNA synthesis
Total RNA of cultured cells or murine lung tissue samples were purified using the Quick-RNA MiniPrep kit (Zymo Research) or the miRNeasy Mini kit (Qiagen), respectively. Quality of isolated RNA was assessed by spectrophotometric analysis (Nanodrop); 250 ng of purified RNA was reverse-transcribed using random hexamers and GoScript reverse transcriptase (both from Promega).
Subcellular fractionation
To detect the presence of lncRNAs in the nucleus and cytoplasm, subcellular fractioning of HPAEC and HPASMC exposed to hypoxia (1% O2) was performed as described elsewhere.16 In brief, HPAEC and HPASMC were seeded in petri dishes at a density of 500,000 cells and exposed to hypoxia for 24 h. Subsequently, cells were trypsinized, lysed with hypotonic lysis buffer (HLB), and centrifuged. The obtained pellet (nuclei) was washed twice with HLB, centrifuged, and lysed with 700 µl Qiazol (Qiagen). RNA preparation of the cytoplasmic fraction (supernatant of the first centrifugation step) was performed as indicated.16 As a last step, precipitated RNA of the cytoplasm was lysed in 700 µl Qiazol. Subsequent purification of the obtained fractionated RNA was carried out using the miRNeasy Mini kit (Qiagen).
Quantitative real-time PCR (qPCR) analysis
SYBR Green qPCR was carried out to quantify specific gene transcripts using the StepOnePlus system (Applied Biosystem, Thermo Fisher Scientific). Sequences of primers used for gene expression analysis are shown in Supplementary Table 1. Specific amplification was verified performing melt curve analysis. The expression of β-actin was used to normalize the obtained expression levels of genes of interest. Differential gene expression was calculated using the threshold cycle (Ct) method.17 The human lncFinder lncRNA PCR array was performed as recommended by the manufacturer (Qiagen).
Western blotting
For protein extraction, harvested cells were lysed using sample loading buffer (62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulphate (SDS), 10% glycerol, 5 mM β-mercaptoethanol, bromophenol blue). Whole-cell lysates were separated by 12% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were transferred by electroblotting to a nitrocellulose membrane (Whatman). Membranes were blocked with 5% dry milk in Tris-buffered saline containing 0.1% Tween20 (TBS-T) and incubated overnight at 4℃. The following primary antibodies were used: anti-CDKN1A (p21, rabbit monoclonal IgG, #2947, Cell Signaling Technology), anti-CDKN1B (p27, rabbit monoclonal IgG, #3686, Cell Signaling Technology), anti-CDKN2C (p18, rabbit monoclonal IgG, #ab192239, Abcam), anti-hypoxia-inducible factor 1 alpha (HIF1α, rabbit polyclonal IgG, #NB100-479, Novus Biologicals), anti-hypoxia-inducible factor 2 alpha (HIF2α, rabbit polyclonal IgG, #PAB12124, Abnova), and anti-β-actin (mouse monoclonal IgG, #A5441, Sigma-Aldrich). β-actin was used as a loading control. Bands were detected using species-specific secondary antibodies conjugated to horseradish peroxidase (Dako Agilent Technologies). Calculation of the expression of proteins was performed using Adobe Photoshop CS5.1 software (Adobe Systems Incorporated) via pixel quantification of the electronic image.
Migration assay
Migration of HPASMC was employed in a modified in vitro wound-healing assay. Briefly, 30,000 cells were seeded on each side of an Ibidi culture insert μ-dish (Ibidi GmbH) and transfected with GapmeR negative control or GapmeR targeting MALAT1 (final concentration of 25 nM) as described above; 24 h after transfection, inserts were removed to create a gap of approximately 500 µm width. The migration rate of HPASMC was quantitatively assessed at various time points after being photographed at 4× magnification (Olympus CKX41). The area of the initial gap was compared to the area of the healing wound at three different time points (3, 5, and 7 h).
Proliferation assay
To determine the proliferation rate, HPASMC were seeded in a 96-well plate at a density of 5000 cells per well and transfected with GapmeR negative control or GapmeR targeting MALAT1 (final concentration of 25 nM) as described above. After 24 h, 5-bromo-2-deoxyuridine (BrdU) was added to each well and cells were incubated for an additional 24 h. Incorporated BrdU was detected using the colorimetric BrdU assay from Roche (Roche Diagnostics).
Statistics
All data are presented as mean ± standard deviation (SD). Parametric or non-parametric distribution of data was determined using the Kolmogorov–Smirnov test. Data comparison was performed using independent Student’s t-test for parametric distribution as well as Mann–Whitney U test for nonparametric distribution. Correlation analysis was carried out with Pearson’s calculations. For comparing more than two sample groups, the one-way analysis of variance (ANOVA) with Tukey post hoc test for parametric samples and the Kruskal–Wallis test with Dunns post hoc test for nonparametric samples was used. In all statistical analyses, two-sided tests were applied. Values of P < 0.05 were considered to be statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001). The n number indicates independent experiments. All statistical calculations were performed using the software package GraphPad Prism Version 5.0 (GraphPad Software).
Results
The expression of several lncRNAs is influenced by hypoxia in human pulmonary artery smooth muscle cells
In order to determine hypoxia-inducible lncRNAs in vascular cells, a PCR array including 84 lncRNAs was performed. In detail, total RNA obtained from healthy human pulmonary artery smooth muscle (HPASMC) exposed to hypoxia for 24 h was purified and analyzed for lncRNA expression. As demonstrated in Figure 1, the expression levels of several lncRNAs were found to be downregulated (IGF2-AS, OIP5-AS1, TERC) and upregulated (LUCAT1, MALAT1, MIAT, NEAT1, ST7-AS1, ST7-AS2) when compared to normoxic control cells. Taking the clear induction of MALAT1 expression by hypoxia as well as its high basal expression levels in HPASMC (data not shown) into account, the focus of the further study was set on MALAT1.
Figure 1.
LncRNA PCR array in human smooth muscle cells exposed to hypoxia. Human pulmonary artery smooth muscle cells (HPASMC) from healthy donors were exposed to hypoxia (Hx, 1% O2, 24 h) and RNA was quantified for lncRNA expression by using human lncFinder lncRNA PCR array (Qiagen). The mean fold change of the expression of selected lncRNAs that were affected by exposure to Hx is shown (n = 2). Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was found to be two-fold induced by Hx. The expression levels of normoxic controls were set to 1 (shown as dashed line). The lncRNA PCR array was performed twice with two different HPASMC donors
MALAT1 expression is increased by hypoxia in pulmonary vascular cells
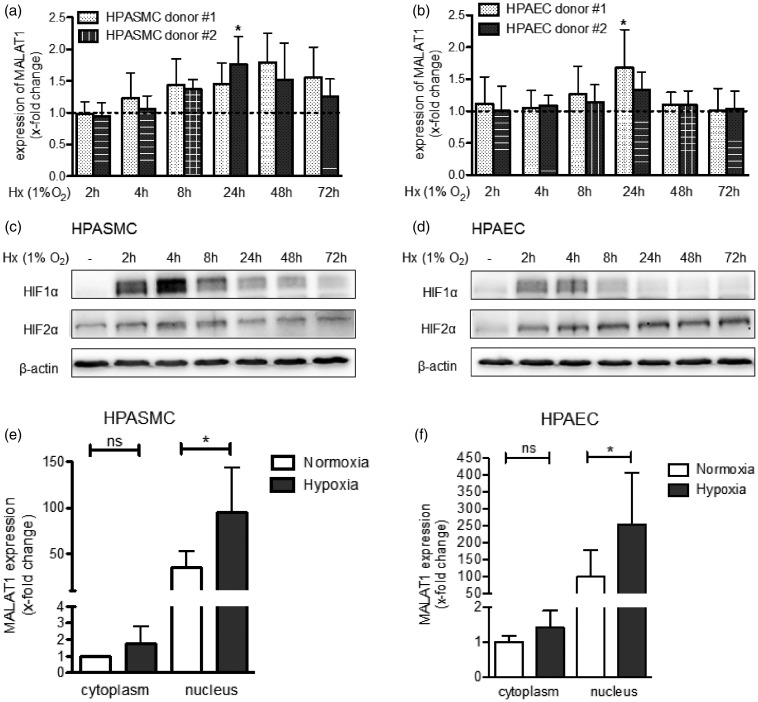
To further validate the effect of oxygen deprivation on the expression of MALAT1 in vascular cells, HPASMC and HPAEC from two healthy donors were exposed to hypoxia over different time points (1% O2 for 2–72 h). In HPASMC, exposure to hypoxia increased the levels of MALAT1 with the highest peak of induction at 24 h (donor#2: 1.77 ± 0.44 fold change, P < 0.05) or 48 h of exposure (donor#1: 1.80 ± 0.46 fold change, not significant) as indicated in Figure 2(a). In HPAEC, a similar expression pattern was observed showing a significant induction of MALAT1 by hypoxia at 24 h (Figure 2(b)). Successful application of the hypoxic condition was confirmed by Western blotting showing a characteristic stabilization pattern of HIF1α (Figure 2(c) and (d)). Of interest, the induction of MALAT1 expression followed the stabilization of HIF1α with a delay of several hours, whereas no association of MALAT1 expression with HIF2α stabilization was observed (Figure 2(c) and (d)).
Figure 2.
Hypoxia increases the expression of MALAT1 in human pulmonary vascular cells. (a) Human pulmonary artery smooth muscle cells (HPASMC) from healthy donors were exposed to hypoxia (Hx, 1% O2) and, as shown by qPCR analysis, the levels of MALAT1 were found to be induced after 24 h of Hx. (b) Similarly, human pulmonary artery endothelial cells (HPAEC) from healthy donors showed higher expression levels of MALAT1 when exposed to Hx (1% O2) with a significant induction of MALAT1 after 24 h of Hx. (c) To confirm successful application of Hx in HPASMC, Western blot analysis was performed that demonstrated stabilization of HIF1α but not of HIF2α after exposure of cells to Hx. (d) Along that line, activation of HIF signalling in HPAEC was demonstrated by Western blot analysis. (e) The subcellular localization of MALAT1 in HPASMC was assessed. As demonstrated by qPCR analysis, MALAT1 is mainly localized in the nucleus when compared to the cytoplasmic fraction. Levels of MALAT1 could be further induced by Hx (24 h, 1% O2). (f) The same observation was made when the subcellular localization of MALAT1 was investigated in HPAEC. n = 4–6 for all experiments. Statistical analysis by one-way ANOVA with Tukey post hoc test (a, b, e, and f) and representative Western blot are shown (c and d) (*P < 0.05)
Upon processing, lncRNAs are either transported into the cytoplasm or remain in the nucleus.18 Thus, we evaluated whether exposure to hypoxia has an effect on the subcellular localization of MALAT1. In this regard, pulmonary vascular cells were exposed to hypoxia for 24 h, nuclear and cytoplasmic RNA was purified and MALAT1 expression was analyzed by qPCR. As demonstrated in Figure 2(e) and (f), MALAT1 was found mainly localized in the nucleus of both cell types under normoxic as well as hypoxic conditions suggesting no redistribution of MALAT1 by oxygen deprivation. Of note, the expression of MALAT1 was further increased by exposure to hypoxia in both fractions; however, a statistical significant induction of MALAT1 expression was observed in the nuclear fraction only. These data confirmed that MALAT1 expression is driven by hypoxia in vascular cells with a more pronounced effect observed in smooth muscle cells and nuclear fractions.
HIF1α drives MALAT1 expression in pulmonary vascular cells
As a next step, the effect of hypoxia on MALAT1 expression was studied in more detail. HPAEC and HPASMC were treated with hypoxia-mimetic agents that are known to stabilize HIFs under normoxic conditions, i.e. deferoxamine (DFO) and cobalt chloride (CoCl2),19 and expression of MALAT1 was assessed using qPCR. Treatment with DFO for 72 h significantly induced MALAT1 expression in both cell types assessed (HPASMC: 4.04 ± 3.69 fold change, P < 0.05, Figure 3(a); HPAEC: 4.3 ± 2.14 fold change, P < 0.01, Figure 3(b)). Consistently, the second HIF stabilizer used in this experiment, CoCl2, was capable of inducing MALAT1 expression in both vascular cell types, however, without reaching significance (Figure 3(a) and (b)). These data demonstrate that stabilization of HIFs in normoxia drives MALAT1 expression.
Figure 3.
MALAT1 expression is mainly driven by HIF1α. (a) Human pulmonary artery smooth muscle cells (HPASMC) from healthy donors were treated with hypoxia-mimetic agents deferoxamine (DFO) and cobalt chloride (CoCl2) for different time points. The expression of MALAT1 was significantly upregulated after treating HPASMC with DFO for 72 h (n = 4). (b) Consistently, MALAT1 expression levels were significantly increased in human pulmonary artery endothelial cells (HPAEC) from healthy donors treated with DFO for 72 h (n = 4). (c) HPASMC were transfected with small interfering RNA (siRNA) targeting HIF1α, HIF2α or both factors concomitantly. After exposure to Hx (1% O2, 24 h), the expression of MALAT1 was analyzed by qPCR and showed significantly decreased MALAT1 levels in hypoxic samples when both HIF1α and HIF2α were repressed (n = 5). (d) Confirmation of knockdown of HIF1α and HIF2α was performed by qPCR analysis (n = 4). Statistical analysis by one-way ANOVA with Tukey post hoc test (a–d) (*P < 0.05, **P < 0.01, ***P < 0.001)
To further elucidate the impact of HIFs on the regulation of MALAT1 expression, we performed siRNA experiments to silence HIF1α, HIF2α, and both factors concomitantly in HPASMC exposed for 24 h to hypoxia. As presented in Figure 3(c), knockdown of HIF2α had no effect on the hypoxia-induced MALAT1 expression, whereas silencing of HIF1α reduced MALAT1 levels when compared to hypoxic siRNA control cells. However, a statistical significant effect on MALAT1 expression was achieved only when both factors were silenced. Successful inhibition of HIF expression was confirmed by qPCR analysis as shown in Figure 3(d). Taken together, these experiments demonstrate that MALAT1 expression is mainly driven by HIF1α under hypoxic conditions.
Depletion of MALAT1 affects the phenotype of smooth muscle cells
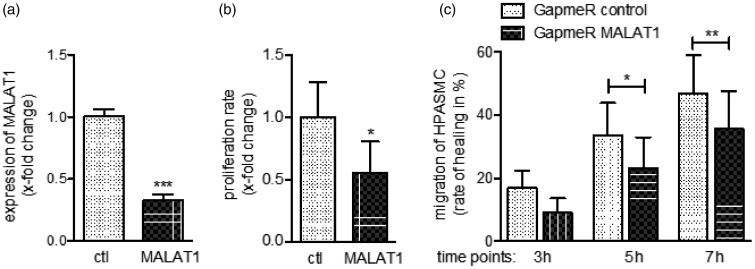
Until now our data demonstrated a significant effect of hypoxia on MALAT1 expression with a more pronounced effect in vascular smooth muscle cells. Thus, MALAT1 emerges as an interesting target in pulmonary vascular biology in PH. To investigate the function of MALAT1 we performed, as a first step, knockdown experiments in HPASMC. Since MALAT1 is mainly localized in the nucleus, we decided to use a GapmeR instead of a siRNA approach to target MALAT1. Quantification of MALAT1 expression in transfected HPASMC confirmed successful design and application of the inhibitor (reduction of MALAT1 levels by 67 ± 5%, P < 0.001) as presented in Figure 4(a). Of interest, MALAT1 depleted cells showed a strong reduction of the proliferation (reduction to 0.57 ± 0.27, P = 0.048, Figure 4(b)) as well as migration rate (e.g. after 7 h wound healed in control cells: 47 ± 12% vs. GapmeR MALAT1 cells: 36 ± 12%, P < 0.01, Figure 4(c)) when compared to control transfection. These results indicate a substantial impact of MALAT1 on the proliferative and migratory potential of smooth muscle cells.
Figure 4.
Silencing of MALAT1 affects the phenotype of pulmonary smooth muscle cells. (a) Transfection of GapmeR directed against MALAT1 significantly decreased the expression of MALAT1 as assessed by qPCR analysis (n = 5). As assessed by BrdU incorporation and wound-healing assays, HPASMC transfected with GapmeR MALAT1 showed significantly less (b) proliferation (n = 5) as well as (c) migration (n = 6) compared to GapmeR negative control transfected cells. Statistical analysis by unpaired Student’s t-test (a and b) and one-way ANOVA with Tukey post hoc test (c) (*P < 0.05, **P < 0.01, ***P < 0.001)
MALAT1 controls the expression of cell cycle regulators
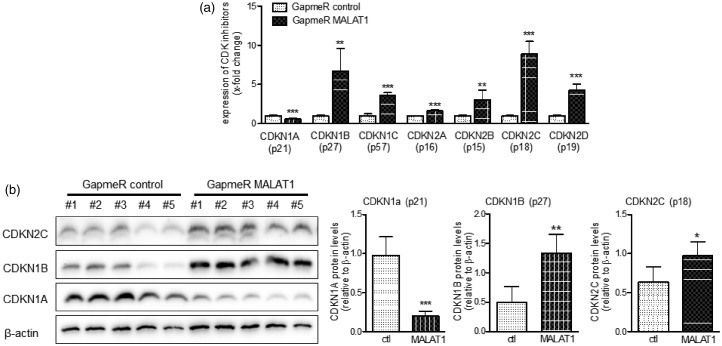
Our group recently demonstrated that proliferation of pulmonary vascular smooth muscle cells is regulated by the expression of CDK inhibitors.8 To test whether MALAT1 might regulate proliferation of smooth muscle cells by targeting CDK inhibitors, HPASMC were transfected with GapmeR MALAT1 and the mRNA levels of all relevant CDK inhibitors (CDKN1A /−1B /−1C /−2A /−2B /−2C and −2D) were quantified by qPCR. As summarized in Figure 5(a), the expression levels of all CDK inhibitors were found significantly changed when MALAT1 was repressed in HPASMC. In detail, the mRNA levels of CDKN1B (+672%), CDKN1C (+361%), CDKN2A (+165%), CDKN2B (+305%), CDKN2C (+889%), and CDKN2D (+426%) were significantly increased, whereas the mRNA levels of CDKN1A (−43%) were significantly decreased. Next, we confirmed these changes on protein level by Western blotting and densitometric analysis as demonstrated in Figure 5(b). Taken together, these data demonstrate that MALAT1 controls proliferation of HPASMC by modulating the expression of CDK inhibitors.
Figure 5.
MALAT1 controls the expression of cyclin-dependent kinase (CDK) inhibitors in vascular smooth muscle cells. (a) Upon transfection with GapmeR targeting MALAT1, the mRNA levels of all relevant CDK inhibitors were found to be significantly changed compared to GapmeR negative control transfected cells (n = 5). (b) Additionally, the observed mRNA levels of the genes CDKN1A (p21), CDKN1B (p27), and CDKN2C (p18) were confirmed on the protein level by Western blotting (n = 5). Statistical analysis by unpaired (a and b) student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001)
The expression of MALAT1 is significantly increased by hypoxia in vivo
To further prove the physiological meaning of MALAT1 in vascular biology, we measured the expression of MALAT1 in lungs of mice exposed to hypoxia (10% O2). As presented in Figure 6(a), expression of MALAT1 in mice kept under hypoxic conditions for three weeks was found to be upregulated when compared to control mice (by 36 ± 47%), however, without reaching statistical significance. Long-term exposure (five weeks) to hypoxia further increased MALAT1 expression resulting in a significant elevation of MALAT1 in murine lungs when compared to normoxic control mice (by 45 ± 30%, P < 0.01, Figure 6(b)). After five weeks of hypoxia, mice developed PH as indicated by an elevated RVP (Figure 6(c)) and, of interest, a significant association between the levels of MALAT1 and the RVP was found (Figure 6(d)). These data demonstrate that the expression of MALAT1 is inducible by hypoxia in vivo and is associated with PH.
Targeting of MALAT1 in mice
Next, the physiological consequence of MALAT1 was addressed in vivo by intervention experiments. To deplete MALAT1, hypoxic mice were treated with intraperitoneal injections of GapmeR directed against MALAT1 or negative control oligonucleotides (Figure 7(a)). In lung tissue collected from these mice, MALAT1 levels were significantly decreased by the application of GapmeR MALAT1 when compared to normoxic control mice (reduction to 31.9 ± 9.8%, P < 0.01, Figure 7(b)). Next, the phenotype of hypoxic mice depleted for MALAT1 was studied in more detail. In this regard, relative heart weight and RVP of mice were measured. As demonstrated in Figure 7(c), hypoxic GapmeR control mice developed heart hypertrophy when compared to normoxic mice (relative heart weight in GapmeR control mice: 0.59 ± 0.01% vs. normoxic mice: 0.5 ± 0.03%, P < 0.001), whereas mice treated with GapmeR MALAT1 had a significantly reduced relative heart weight (GapmeR MALAT1: 0.51 ± 0.05% vs. GapmeR control mice: 0.59 ± 0.01%, P < 0.01). However, RVP measured in these mice was found to be unchanged (Figure 7(d)). These data demonstrate a successful application of GapmeR MALAT1 in mice resulting in reduced MALAT1 levels and, moreover, to reduced heart weight.
Discussion
In this experimental study we show that (i) several lncRNAs are dysregulated by hypoxia of which MALAT1 was identified as the most promising candidate in the context of pulmonary vascular biology; (ii) the expression of MALAT1 is driven by HIF1a; (iii) MALAT1, largely by controlling cell cycle regulators, defines the phenotypic characterization of pulmonary artery smooth muscle cells; and that (iv) silencing MALAT1 by GapmeRs reduces heart hypertrophy in mice that developed hypoxia-induced PH.
Within the emerging group of noncoding RNAs that are believed to control a substantial part of the human genome, the expression and function of lncRNA are not well defined. As such, our study provides the first report on the functional characterization of these longer transcripts for the pulmonary vasculature in the context of hypoxic conditions.
Our analysis relies on a screening approach that employed an array of predefined lncRNAs. Using pulmonary artery smooth muscle cells, several lncRNAs were found differentially expressed between cells exposed to hypoxia and normoxic controls in vitro. Although not much data exist on the lncRNA signature in such a setting, our findings are along the line of a recently published report. Upon exposure to hypoxia, the expression profile was found largely dysregulated in rats.20 These findings support the assumption that hypoxia provides an important driving factor in the expression pattern of lncRNAs. Such interaction would make lncRNA to interesting pathogenetic transcripts in the lung and the pulmonary vasculature that strongly responds to alterations of oxygen pressure.
HIF1α is one of the master effectors of hypoxia that impacts several downstream signalling pathways. Our experiments here provide indirect evidence that the expression of MALAT1 is driven by HIF1α and, however to a lesser extent, HIF2α. By using compounds known to stabilize these factors and, as such, mimicking the effects of hypoxia, induction of MALAT1 expression could also be achieved in cells under normoxic conditions. These findings are of particular interest in the context of the molecular dysregulations observed in pulmonary vascular cells obtained from rats that spontaneously develop pulmonary hypertension (PH) and from patients with idiopathic PAH.21 These translational experiments suggested that, in PAH, HIF1α is active even in the absence of hypoxia. Such alterations of oxygen-sensing mechanisms and consecutive auto-enhancement of HIF1α-dependent mechanisms highlight the functional importance of MALAT1 and its expression upon stabilization of HIF1α as observed in our experiments.
Some lncRNAs are localized exclusively in the nuclear fraction of cells without concomitant expression in the cytoplasm. In general, a nuclear lncRNA localization indicates a potential role of these lncRNAs in the process of chromatin modification and transcriptional regulation, whereas cytoplasmic lncRNAs are potentially involved in gene regulation by targeting mRNAs, proteins, or other lncRNAs.18 In this context, it was previously shown that MALAT1 is mainly localized in the nucleus and, in contrast, its enzymatic processing product MALAT1-associated small cytoplasmic RNA (mascRNA) is expressed in the cytoplasm only.22,23 In this study, we confirmed the predominant nuclear expression of MALAT1 in cultured vascular endothelial and smooth muscle cells. However, we were not able to detect mascRNA by qPCR analysis in the cytoplasm (data not shown), therefore it remains speculative whether the cytoplasmic component of MALAT1, i.e. mascRNA, is expressed and functional active in vascular cells. In conclusion, MALAT1 is likely to function in the nucleus exclusively, probably by binding to specific chromatin sites,24 by interacting with transcription factors (e.g. EZH2)25 or by modulating the splicing of target mRNAs.26
Previous studies have identified MALAT1 as an important factor for angiogenesis that switches endothelial cells from a proliferative to a migratory phenotype.14 While this report described MALAT1 in the context of the systemic vasculature and exclusively in endothelial cells, our experiments were focused on the pulmonary vasculature and found similar expression levels of MALAT1 both in pulmonary artery endothelial and smooth muscle cells. However, due to a more pronounced response of MALAT1 expression by hypoxia, the focus of subsequent functional experiments was on smooth muscle cells. Pharmacological depletion of MALAT1 by using GapmeRs resulted in significant effects on both proliferation and migration indicating that MALAT1 has an important role for the functional phenotype of pulmonary vascular smooth muscle cells. These results have further implications for our understanding of the development of neoplastic-like lesions observed in the pulmonary vasculature from patients with PH and animal models mimicking the disease. Of interest, the expression of all CDK inhibitors except for CDKN1A was massively induced by MALAT1 depletion highlighting the pivotal role of MALAT1 in controlling cell cycle progression. The discrepancy of CDKN1A gene regulation by MALAT1 was reported previously27 and demonstrates that the regulatory network of cell cycle components is complex and multifactorial.
The differences between previous findings and our results, mostly due to the fact that we observed parallel alterations in migration and proliferation while, conversely, Michalik et al.14 described a switch from a proliferative to a migratory phenotype upon silencing of MALAT114 are not fully elaborated. However, such differences might be due to cell-specific effects and the distinct properties of the pulmonary vasculature that is known to react paradoxically to several physiopathological stimuli including hypoxia. Moreover, migration is the first functional step observed in cells exposed to hypoxia followed by proliferation.28 This model might explain why endothelial cells, acting as early sensors of alterations in oxygen levels, respond predominantly by the regulation of migration, whereas smooth muscle cells, as the main effectors of vascular remodeling, act through both migration and proliferation.
Limitations of this study include the lack of experimental proof for a direct interaction between HIF1α and MALAT1, and between MALAT1 and further downstream signaling events, respectively. Although the animal model used in this study indicates a physiological role of MALAT1 as shown by the decrease of relative heart weight in MALAT1 depleted hypoxic mice, our experiments do not provide direct evidence for an effect of MALAT1 on the proliferation of vascular smooth muscle cells in vivo.
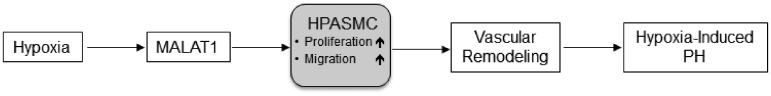
Addressing the expression of CDK inhibitors upon the application of GapmeRs targeting MALAT1 did not provide evidence for a regulatory effect on this pathway (data not shown). As such, the data from the animal model used here should be extrapolated with caution to process in vascular remodeling in patients with PH. In the light of the in vitro data discussed above and, moreover, the well-established link of MALAT1 on proliferation in other in vivo models,24,29 a pathogenetic role of MALAT1 in PH is highly likely. In this context, our study provides the first report on the functional characterization of the lncRNA MALAT1 in the pulmonary vasculature and, as such, is of pathogenetic relevance. Our data emphasize that MALAT1 expression is regulated by hypoxia, probably by the action of HIF1α. Intervention experiments confirmed that MALAT1 regulates the proliferative and migratory phenotype of smooth muscle cells and silencing of MALAT1 in vivo reduced heart hypertrophy in animals that developed hypoxia-induced PH (summarized in Figure 8). These data indicate a potential role of MALAT1 in the pathobiology of the pulmonary vasculature.
Figure 8.
Mechanism of vascular remodeling mediated by MALAT1. The proposed MALAT1-mediated mechanism of vascular remodeling in the context of hypoxia-induced PH is shown
Supplementary Material
Acknowledgements
The authors thank Rudolf Speich for his continuous support of their research, his commitment and enthusiasm to pulmonary hypertension and many invaluable inputs. The authors are deeply saddened by his unexpected death. This work was supported by the Swiss National Science Foundation (SNF grant 31003A_144212), the Olga Mayenfisch Foundation, and the Novartis Foundation for medical-biological Research (16B086).
Author’s contributions
MB and LCH participated in the design of the study. MB, CS, CL, CB, TJH, and JV carried out the data acquisition. MB, SU, MG, MK, and LCH participated in the writing of the article. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
Supplementary material for this paper can be found at http://ebm.sagepub.com/content/by/supplemental-data.
References
- 1.Pullamsetti SS, Schermuly R, Ghofrani A, Weissmann N, Grimminger F, Seeger W. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med 2014; 189: 394–400. [DOI] [PubMed] [Google Scholar]
- 2.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: Pulmonary arterial hypertension. Nat Rev Cardiol 2011; 8: 443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Davis M, Cornwell W. Pulmonary arterial hypertension. Curr Probl Cardiol 2011; 36: 461–517. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med 2011; 365: 537–47. [DOI] [PubMed] [Google Scholar]
- 5.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011; 208: 535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, Huber LC. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J 2014; 35: 3203–11. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, Erzurum SC, Chun HJ. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med 2013; 19: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock M, Haider TJ, Vogel J, Gassmann M, Speich R, Trenkmann M, Ulrich S, Kohler M, Huber LC. The hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell proliferation by directly targeting CDKN1A. Int J Biochem Cell Biol 2015; 61: 129–37. [DOI] [PubMed] [Google Scholar]
- 9.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, Stenmark K, White K, Seto AG, Morrell NW, Bradshaw AC, MacLean MR, Baker AH. MicroRNA-143 Activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res 2015; 117: 870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013; 152: 1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paralkar VR, Weiss MJ. Long noncoding RNAs in biology and hematopoiesis. Blood 2013; 121: 4842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell RD, Long X, Lin M, Bergmann JH, Nanda V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D, Miano JM. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol 2014; 34: 1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, Zeng C. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 2014; 130: 1452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, Dimmeler S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 2014; 114: 1389–97. [DOI] [PubMed] [Google Scholar]
- 15.Huber LC, Ulrich S, Leuenberger C, Gassmann M, Vogel J, von Blotzheim LG, Speich R, Kohler M, Brock M. microRNA-125a in pulmonary hypertension: Regulator of a proliferative phenotype of endothelial cells. Exp Biol Med 2015; 240: 1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagnon KT, Li L, Janowski BA, Corey DR. Analysis of nuclear RNA interference in human cells by subcellular fractionation and Argonaute loading. Nat Protoc 2014; 9: 2045–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–8. [DOI] [PubMed] [Google Scholar]
- 18.Thum T, Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res 2015; 116: 751–62. [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt S, Al-Ahmad AJ, Gassmann M, Ogunshola OO. Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J Cell Physiol 2014; 229: 1096–105. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Yan C, Xu X, Dong L, Su H, Hu Y, Zhang R, Ying K. Long noncoding RNA expression profiles of hypoxic pulmonary hypertension rat model. Gene 2016; 579: 23–8. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to human pulmonary arterial hypertension. Circulation 2006; 113: 2630–41. [DOI] [PubMed] [Google Scholar]
- 22.Wilusz JE, Freier SM, Spector DL. 3’ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 2008; 135: 919–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gast M, Schroen B, Voigt A, Haas J, Kuehl U, Lassner D, Skurk C, Escher F, Wang X, Kratzer A, Michalik K, Papageorgiou A, Peters T, Loebel M, Wilk S, Althof N, Prasanth KV, Katus H, Meder B, Nakagawa S, Scheibenbogen C, Schultheiss HP, Landmesser U, Dimmeler S, Heymans S, Poller W. Long noncoding RNA MALAT1-derived mascRNA is involved in cardiovascular innate immunity. J Mol Cell Biol 2016; 8: 178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West JA, Davis CP, Sunwoo H, Simon MD, Sadreyev RI, Wang PI, Tolstorukov MY, Kingston RE. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol Cell 2014; 55: 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res 2015; 75: 1322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arun G, Diermeier S, Akerman M, Chang KC, Wilkinson JE, Hearn S, Kim Y, MacLeod AR, Krainer AR, Norton L, Brogi E, Egeblad M, Spector DL. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev 2016; 30: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Li M, Wang Z, Han S, Tang X, Ge Y, Zhou L, Zhou C, Yuan Q, Yang M. Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. J Biol Chem 2015; 290: 3925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheikh AQ, Lighthouse JK, Greif DM. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Rep 2014; 6: 809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao H, Tang K, Liu P, Chen K, Hu J, Zeng J, Xiao W, Yu G, Yao W, Zhou H, Li H, Pan Y, Li A, Ye Z, Wang J, Xu H, Huang Q. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget 2015; 6: 38005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.