Abstract
While treatment of patients with attention-deficit hyperactivity disorder (ADHD) is based on a multimodal approach that combines medication with specific psychological interventions, pharmacotherapy alone is generally considered an essential and cost-effective element. This paper aims to comprehensively and critically review factors involved in prescribing and medication use in individuals diagnosed with ADHD, focusing on the difficulties facing patients with ADHD seeking treatment, as well as the safety and tolerability aspects of ADHD pharmacotherapies, with particular attention on the cardiovascular adverse events and the potential risk of misuse or diversion of ADHD medications. A comprehensive and systematic literature search of PubMed/MEDLINE database was conducted to identify studies published in peer-reviewed journals until 1 August 2016. Children, adolescents and adults often encounter significant difficulties in the process of accessing specialist assessment and treatment for ADHD as a consequence of disparities in service organization and available treatment provision. Despite the well-established efficacy and overall safety profile, ADHD medications are not exempt from adverse events. The cardiovascular safety of pharmacotherapies used for treating individuals with ADHD has raised particular concerns; however there is little evidence of serious cardiovascular adverse events, including no serious corrected QT (QTc) abnormalities associated with stimulants, atomoxetine or α2-adrenergic receptor agonists. Although the abuse of prescription stimulant drugs, particularly, short-acting stimulants is a prevalent and growing problem, nonmedical use of prescription stimulants within the clinical context is very limited. In addition, nonstimulant ADHD medications lack any reinforcing effects and consequently any abuse potential.
Keywords: attention-deficit hyperactivity disorder, cardiovascular events, drug diversion, pharmacotherapy, systematic review
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a complex and multifactorial neurodevelopmental disorder characterized by a persistent pattern of age-inappropriate symptoms of inattention, hyperactivity or impulsivity [American Psychiatric Association, 2013; Faraone et al. 2015]. In addition, ADHD is associated with functional impairment, as well as significant personal, medical and social complications in children, adolescents and adults [American Psychiatric Association, 2013; Faraone et al. 2015; Martinez-Raga et al. 2013a]. As shown in longitudinal studies, symptoms of ADHD can persist beyond childhood in at least two-thirds of patients [Turgay et al. 2012]. ADHD is a common disorder; it is estimated that ADHD affects approximately 5–8% of children and 2.5–4% of adults worldwide [Polanczyk et al. 2014; Willcutt, 2012]. Along the lifespan, individuals with ADHD have high rates of other comorbid psychiatric disorders, including oppositional defiant disorder and other conduct disorders, anxiety and mood disorders, bipolar disorder, personality disorders or substance use disorders [Connor et al. 2010; Martinez-Raga et al. 2013b; Meinzer et al. 2014; Torres et al. 2015].
Treatment of ADHD has proven to be effective in improving symptoms, emotional lability, and patient functioning, generally leading to favorable outcomes, such as improved academic or work performance, or reduced criminality [Kooij et al. 2010; Lichtenstein et al. 2012]. However, partly due to methodological difficulties inherent in comparing effect sizes across treatment trials with divergent inclusion criteria, efficacy measures, and designs, ADHD is an underdiagnosed and undertreated condition, particularly in adults [Ginsberg et al. 2014a]. The treatment of patients with ADHD is based on a multimodal approach that combines medication with specific psychological interventions [Kooij et al. 2010; National Collaborating Centre for Mental Health, 2009]. While psychotherapeutic and psychosocial interventions are effective strategies for patients with ADHD, pharmacotherapy alone is generally considered an essential element in the management of children, adolescents and adults with ADHD [Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA), 2011; Kooij et al. 2010; National Collaborating Centre for Mental Health, 2009]. Indeed, a substantial body of evidence has suggested that pharmacotherapy is cost-effective compared with no treatment or behavioral therapy alone among children and adolescents with ADHD [National Collaborating Centre for Mental Health, 2009; Wu et al. 2012]. In addition, medication has been found to be cost-effective when compared with the combination of medication plus behavioral treatment in the overall ADHD population [Wu et al. 2012].
Stimulant medications, including amphetamine derivatives and methylphenidate formulations have been widely used for decades and remain as first-line pharmacotherapies for individuals with ADHD, but in the last 15 years several well-tolerated and effective nonstimulant pharmacotherapies have been approved for the treatment of this disorder, including the selective noradrenaline reuptake inhibitor atomoxetine, and the α2-adrenergic receptor agonists guanfacine and clonidine, both in extended-release (XR) formulations. Despite the well-established efficacy and safety profiles of the different medications approved for the treatment of patients with ADHD, some patients may exhibit a poor tolerability or a lack of response to medication [Peterson et al. 2008]. In addition, other aspects may emerge when considering medication for patients with ADHD, such that some patients or their caregivers may prefer not to take pharmacological treatment [Gajria et al. 2014], In some instances there may be contraindications for the different medications [Graham and Coghill, 2008], or there may be concerns regarding drug diversion [Martinez-Raga et al. 2013b].
This paper aims to comprehensively and critically review some of the factors involved in prescribing, along with medication use in children, adolescents and adults diagnosed with ADHD. We focus on the barriers and difficulties facing patients with ADHD seeking treatment, as well as the safety and tolerability aspects of ADHD pharmacotherapies, with particular attention to the cardiovascular adverse events and the potential risk of misuse or diversion of ADHD medications.
Methods
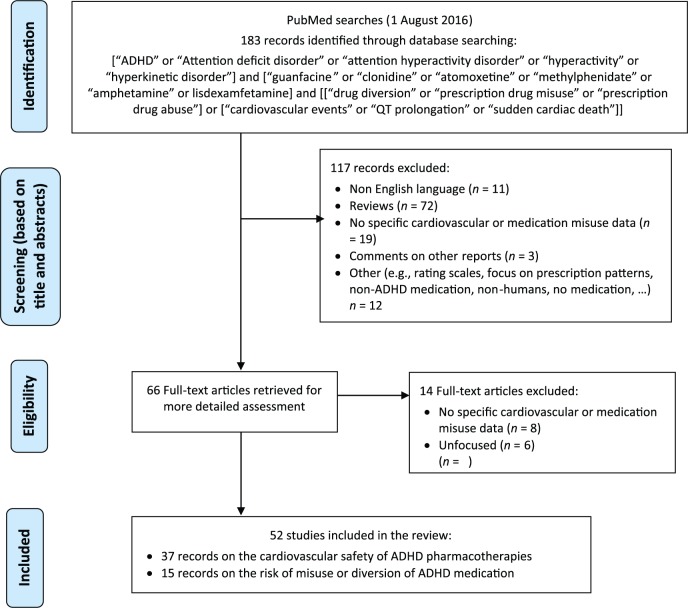
A comprehensive and systematic literature search of the PubMed/MEDLINE database was conducted to identify potentially relevant studies published in English in peer-reviewed journals up to 1 August 2016, using the following search terms: [“ADHD” or “attention deficit disorder” or “attention hyperactivity disorder” or “hyperactivity” or “hyperkinetic disorder”] and [“guanfacine” or “clonidine” or “atomoxetine” or “methylphenidate” or “amphetamine” or lisdexamfetamine] and [“drug diversion” or “prescription drug misuse” or “prescription drug abuse”] or [“cardiovascular events” or “QT prolongation” or “sudden cardiac death”]. The literature search included clinical studies with children, adolescents or adults as participants. The search was supplemented by manually reviewing reference lists from the identified publications. There were no restrictions on the identification or inclusion of studies in terms of publication status, type of publication and design type. However, abstracts of presentations to specialist meetings and conferences were not included. Titles and abstracts were screened for inclusion/exclusion, and appropriateness, and full text versions were retrieved. A flow chart of the output generated by the literature search is shown in Figure 1.
Figure 1.
Systematic review flow chart to identify the cardiovascular safety outcomes and the potential risk of misuse or diversion of ADHD medications.
ADHD, attention-deficit hyperactivity disorder.
The long and winding road to treatment
Partly due to differences in cultural beliefs and resource prioritization there are marked geographical disparities in service provision, as well as in the availability and access to adequate specialist care for individuals seeking assessment and treatment for ADHD [Ginsberg et al. 2014b]. However, ADHD is a costly disease associated with a substantial economic burden to the individual, the society and the healthcare system [Erskine et al. 2014; Doshi et al. 2012; Wittchen et al. 2011]. As highlighted in a systematic review of 19 United States-based studies on costs of children/adolescents and adults with ADHD, adequate treatment of patients with ADHD is very cost-effective, in children and adolescents, as well in adults [Doshi et al. 2012]. The largest cost category for adults appears to be productivity and income losses, while for children the largest cost categories were related with health care and education [Doshi et al. 2012]. Similar findings were reported in another systematic review of studies conducted in Europe [Le et al. 2014]. Furthermore, another European study, using data from the Danish National Register, suggests that children diagnosed with ADHD have fewer hospital contacts if receiving pharmacological treatment and that treatment, at least to some extent, protects against criminal behavior [Dalsgaard et al. 2014a]. The authors of this report highlighted the potential benefits of investing in improving the diagnosis and management of ADHD in the European public health care and insurance-based systems, because this may offset costs in sectors like education and the justice system, as well as productivity loss in the workforce [Dalsgaard et al. 2014a]. Indeed, if focusing solely on adult individuals, ADHD is associated with increased morbidity, as well as higher divorce rates, more traffic violations, and negative occupational, economic, and psychosocial functioning and higher rates of concomitant psychiatric disorders [Kessler et al. 2005].
The importance of ensuring adequate treatment provision for individuals of all ages with ADHD is further underscored by the results from a large cohort study that revealed that a diagnosis of ADHD was associated with significantly increased mortality rates [Dalsgaard et al. 2015]. This study, with over 32 years of follow up, included 1,922,248 individuals from the Danish National Registry, 32,061 of them with a diagnosis of ADHD. In this report, mortality rates for any cause was 5.85 per 10,000 person-years in individuals with ADHD, compared with 2.21 for those without the disorder. The higher mortality in ADHD was primarily related with deaths from unnatural causes, especially accidents. Moreover, a delayed diagnosis was associated with an increased mortality rate, as individuals diagnosed with ADHD in adulthood had higher mortality rates than those diagnosed during childhood or adolescence [Dalsgaard et al. 2015].
Despite vast geographical differences in treatment provision for individuals with ADHD, these patients face common obstacles and share similar unmet needs. Certain factors such as economic status, sex and ethnic background are related with the probability of seeking help or receiving treatment for ADHD. Boys are up to five times more likely than girls, and Whites are twice as likely as African Americans of being assessed and diagnosed, or receiving treatment for ADHD [Bussing et al. 2003; Eiraldi et al. 2006]. Moreover, not only many ADHD cases go undiagnosed and untreated, but ADHD may also be mistaken for other mental health problems or even missed in the presence of psychiatric comorbidities [Ginsberg et al. 2014b]. In addition, patients, particularly adults, often present poor adherence and experience a sense of abandonment from healthcare providers [Matheson et al. 2013].
Another barrier that young patients with ADHD may encounter relates with the often inadequate and uncoordinated transition from pediatric or neuropediatric care, or from child and adolescent mental health care to adult mental health services [Swift et al. 2014; Young et al. 2011]. The persistence of ADHD symptoms into adulthood, as it is the case in a majority of cases, poses several difficulties for adult psychiatric care in view of the frequent lack of awareness and expertise for diagnostic assessment, limited treatment options and specialized facilities across Europe and in other parts of the world [Kooij et al. 2010]. In addition, successful transition to adult psychiatric care often fails due to the limited number of treatment resources for the management of adult patients with ADHD, and the restrictions and prohibitive prescribing practices of approved ADHD medications for adult individuals established by regulatory agencies [Ginsberg et al. 2014b; Swift et al. 2014; Young et al. 2011]. As a consequence of a lack of adequate continuity of care, compliance with prescribed medical interventions is low, and subsequently adolescents and young adults with ADHD are at a greater risk of experiencing unsafe driving, delinquency or substance abuse, as well as academic, social, and vocational difficulties [Kooij et al. 2010].
Overall, amphetamine derivatives, methylphenidate formulations and atomoxetine are all considered first-line treatments for patients with ADHD [National Collaborating Centre for Mental Health, 2009; CADDRA, 2011]. While the decision about which pharmacological alternative ought be prioritized for each patient should be evidenced-based and rely on treatment guidelines, in common clinical practice such a decision is often taken by trial and error. Nonetheless, there are several factors that generally influence and should be considered when choosing the adequate pharmacological options, including the need for a rapid onset of action, the patient’s age, the predominant ADHD core symptoms, psychiatric comorbidity, the patient’s or the parental expectations, attitudes and preferences towards treatment, the risk for potentially severe adverse effects, the concerns about medication diversion or misuse, risk of drug-to-drug interactions, the cost of the medication for the patient, and of course the individual variability in the response to treatment or adverse events [CADDRA, 2011]. Thus, adults and older adolescents may require a longer duration of medication effects throughout the day than children, while short-acting stimulants are more likely to result in poorer adherence and have a higher risk for diversion or abuse [Newcorn et al. 2007].
Safety and tolerability considerations of ADHD medications
Medications used for the treatment of ADHD are well-established and effective, and generally safe and well-tolerated. However, although commonly mild, time-limited and manageable with dose adjustment, without the need to stop treatment, ADHD pharmacotherapies are not exempt from treatment emergent adverse events (TEAEs), as evidenced in randomized controlled trials [Aagaard and Hansen, 2011; Adler et al. 2009; Graham and Coghill, 2008; Upadhyaya et al. 2015]. Indeed, treatment discontinuation rates due to adverse events with any of the approved medications for ADHD are generally low [Clavenna and Bonati, 2014; Michelson et al. 2003; Sayer et al. 2016; Weisler et al. 2006].
The most common TEAEs associated with stimulant medications (including both methylphenidate formulations and amphetamine derivatives), generally observed early in the course of treatment, include difficulty falling asleep, decreased appetite, gastrointestinal pain, headache, and dizziness [Wolraich et al. 2007]. The most common TEAEs of atomoxetine appear to resemble those of stimulant medications and generally emerge early in the course of treatment and are frequently transient, as is the case with stimulant medications [Clavenna and Bonati, 2014; Upadhyaya et al. 2015; Wietecha et al. 2013]. Children, adolescents and adults taking atomoxetine may experience headache, dry mouth, dizziness, reduced appetite, sweating, and gastrointestinal tract symptoms, including abdominal pain, constipation, nausea and vomiting [Garnock-Jones and Keating, 2009; Michelson et al. 2003; Wietecha et al. 2013], while adult patients are more prone to experience dysuria or sexual problems [Upadhyaya et al. 2015; Wietecha et al. 2013]. While, atomoxetine overall is less likely than stimulants to worsen sleep problems, initial somnolence can be observed, particularly if the dosage is increased too rapidly [Wolraich et al. 2007]; however insomnia may also be experienced by adult patients taking atomoxetine [Wietecha et al. 2013]. Likewise, the most common TEAEs associated with the α2-adrenergic receptor agonists guanfacine-XR (GXR) and clonidine-XR (CLON-XR) are somnolence, headache, upper abdominal pain, fatigue, and sedation [Hirota et al. 2014; Martinez-Raga et al. 2015; Ruggiero et al. 2014]. In the long-term prospective trials of GXR and CLON-XR the most commonly reported TEAEs are somnolence, headache, fatigue and abdominal pain [Martinez-Raga et al. 2015].
Cardiovascular safety of ADHD pharmacotherapies
The cardiovascular safety of medications used for treating patients diagnosed with ADHD, including the risk of severe forms of arrhythmias and sudden cardiac death has raised a lot of attention by regulatory agencies, scientific societies, as well as by patients and clinicians. Indeed, concerns about the emergence of severe cardiovascular complications or sudden unexplained death in children, adolescents and adults receiving any of the available pharmacological treatment for ADHD cannot be dismissed. Nonetheless, the published evidence arising from case series reports, open uncontrolled studies, randomized controlled studies and population-based studies suggest that serious cardiovascular events that may lead to sudden unexplained cardiac death associated with ADHD medications are extremely rare, and benefits of treating individual patients with ADHD, after an adequate assessment, outweigh the risks [Aagaard and Hansen, 2011; Adler et al. 2009; Cooper et al. 2011; Dalsgaard et al. 2014b; Graham et al. 2011; Habel et al. 2011; Hammerness et al. 2009; Martinez-Raga et al. 2013c; McCarthy et al. 2009; McCracken et al. 2016; Michelson et al. 2003; Weisler et al. 2006; Winterstein, 2013]. Great caution is advised when considering medication for children, adolescents or adults with ADHD in the presence of risk factors for cardiovascular problems, particularly those with a personal or family history of severe cardiovascular or cerebrovascular disease, or when co-prescription with other drugs that may increase the risk of cardiac adverse events [CADDRA, 2011].
Stimulant medications, including methylphenidate formulations and amphetamine derivatives, have been associated with statistically significant, albeit small and generally minor and of little or no clinical significance, increases in either systolic and diastolic blood pressure (BP, 1–4 mmHg) and heart rate (HR, 1–6 bpm) in children and adolescents, [Arcieri et al. 2012; Donner et al. 2007; Hammerness et al. 2009; Martinez-Raga et al. 2013c], as well as in adults with ADHD [Adler et al. 2009; Martinez-Raga et al. 2013c; Mick et al. 2013; Weisler et al. 2005; Weisler et al. 2006]. Similarly, atomoxetine, like other noradrenergic enhancing drugs, may also cause minor but statistically significant increases in HR and BP both in children and adolescents [Arcieri et al. 2012; Michelson et al. 2001] and in adults with ADHD [Michelson et al. 2003; Upadhyaya et al. 2015]. Increases in HR and diastolic BP during atomoxetine treatment are larger in poor metabolizers of this medication [Michelson et al. 2007]. The increases in HR and BP, reported with stimulants and atomoxetine, generally are observed early in treatment, more commonly during the initial titration phase, and stabilize over time [Hammerness et al. 2009; Martinez-Raga et al. 2013c; Upadhyaya et al. 2015]. In contrast, treatment with the α2-adrenergic receptor agonists guanfacine and clonidine, either as monotherapy or as add-on to psychostimulants, can induce small-to-modest and dose-dependent decreases in diastolic and systolic BP and HR, ranging between −3 and −5 mmHg and −3 to −6 bpm, respectively [Martinez-Raga et al. 2015]. Nonetheless, in some instances these drugs, particularly in their immediate release (IR) formulations, may be associated with significant hypotension, including the risk of orthostatic hypotension, and marked bradycardia [Connor et al. 1999]. Tolerance to the hypotensive effects usually develops in the course of long-term treatment with α2-adrenergic agonists [Sallee et al. 2013].
Drug-induced sudden death is most commonly attributed to a relatively rare form of potentially life-threatening polymorphic ventricular tachycardia, torsades de pointes (TdP), that frequently develops with a QT-interval prolongation. Drug-induced prolongation of the corrected QT (QTc) interval and TdP are relatively rare adverse events from a wide range of drugs commonly used in clinical practice [Roden, 2004]. As suggested by large population-based studies that enrolled several million patients, at therapeutic doses, prescription stimulants or atomoxetine are not associated with an increased risk of developing ventricular arrhythmia, or other severe cardiovascular adverse events, including myocardial infarction or stroke, as well as sudden cardiac death in children, adolescents or adults with ADHD [Cooper et al. 2011; Habel et al. 2011; McCarthy et al. 2009; Olfson et al. 2012; Schelleman et al. 2011; Winterstein et al. 2012]. In contrast, a recent self-controlled case series analysis with 1224 patients aged 17 or younger from a health insurance database has suggested that the relative risk of myocardial infarction and arrhythmias is increased in the early period after the start of methylphenidate treatment for ADHD [Shin et al. 2016]. To date, the only report of significant QT prolongation associated with atomoxetine is a case report of an 11-year-old Japanese boy who had been treated for more than 2 years with this medication [Yamaguchi et al. 2014]. Likewise, the case of an 11-year-old child is the only published report to date of myocardial infarction associated with methylphenidate exposure [Munk et al. 2015].
There are no reports of serious cardiovascular adverse events, including no serious QTc abnormalities associated with guanfacine or clonidine, alone or in combination with psychostimulants, in the different multisite studies [Daviss et al. 2008; McCracken et al. 2016; Ruggiero et al. 2014; Wilens et al. 2016]. This is of particular relevance, as both α2-adrenergic agonists have been approved by the United States Food and Drug Administration for the treatment of ADHD in children and adolescents, as monotherapy or as adjunctive therapy in combination with stimulants for those patients who failed to have an adequate response with stimulants alone. Indeed, in a comprehensive double-blind, cross-over QT study with 83 healthy adults therapeutic and supra-therapeutic doses (4 mg and 8 mg, respectively) of guanfacine-IR revealed that neither doses of this α2-adrenergic receptor agonist prolonged the QTc interval through 12 h after drug administration [Martin et al. 2015]. In addition, a recent double-blind randomized trial assessed the acute and long-term cardiovascular effects of stimulants (d-methylphenidate-XR), guanfacine-IR and their combination in 207 children and adolescents with ADHD, aged 7–14 years [Sayer et al. 2016]. The study showed that combination therapy might attenuate the small but statistically significant changes associated with either single treatment. Thus, while guanfacine caused a short-term decrease in HR, systolic BP, and diastolic BP, and dexmethylphenidate a short-term increase in HR, systolic BP, diastolic BP, and QTc interval during acute titration phase, combination of these two medications only was associated with an increase in diastolic BP, but had no effects on HR, systolic BP, or QTc [McCracken et al. 2016; Sayer et al. 2016].
Cardiovascular adverse events of ADHD medications overdose
Several reports have documented the effects of an overdose with ADHD medications. Mild hypertension and tachycardia that did not require additional treatment was described in an 8-year-old girl following an overdose of 210 mg (8.75 mg/kg) of methylphenidate-IR [Fettahoglu et al. 2009]. Likewise, a transient tachycardia (132 bpm) was the only abnormal finding following the ingestion of 270 mg of osmotic-controlled release oral delivery (OROS) methylphenidate by a 17-year-old girl [Ozdemir et al. 2010]. Furthermore, in a case-control study conducted to explore the effects of methylphenidate overdose on the QT interval in a group of 23 adults with methylphenidate overdose (median reported dose 120 mg, range 40–1500 mg) and a matched group of patients with overdose of a well-established noncardiotoxic substance, not only were no arrhythmias recorded, but there were also no significant differences between the two groups in mean HR, in mean QRS, or mean QT intervals [Hill et al. 2010].
There is also limited evidence of the effects of atomoxetine overdose. Transient tachycardia was the only cardiovascular complication associated with supra-therapeutic ingestion (up to 1200 mg) atomoxetine in a systematic chart review from poison center encounters with a series of 17 patients, aged 9 months to 28 years old [Lovecchio and Kashani, 2006]. A short-term increase in HR (106 bpm) with normal BP (118/74 mmHg) was observed in a 12-year-old boy who mistakenly was given 180 mg of atomoxetine instead of dextroamphetamine [Cantrell and Nestor, 2005]. Similarly, transient sinus tachycardia (110 bpm), but no further electrocardiograph abnormalities were reported in a 17-year-old female after an overdose of 2840 mg of atomoxetine [Kashani and Ruha, 2007]. There are no reports of QTc prolongation associated with atomoxetine overdose.
There are scattered reports of overdoses with α2-adrenergic receptor agonist medications used for ADHD. Depressed level of consciousness, bradycardia and hypotension are the most common clinical symptoms associated with clonidine overdose as observed in an 11-year-old boy who took an overdose of clonidine [Gitter and Cox, 2000], and in a series of 16 clonidine overdoses during an 8-year period in children aged 13 months to 8 years of age [Kappagoda et al. 1998]. The unusual presentation of an extremely protracted course of hypertension followed by prolonged symptomatic hypotension has been documented in a 12-year-old boy after ingesting three times his usual dose of GXR [Fein et al. 2013]. Similar findings were seen in a 16-year-old female who also presented with a prolonged QTc interval of 593 ms after taking 25 mg of guanfacine [Minns et al. 2010].
The risk of misuse or diversion of ADHD medication
The potential risk of medication abuse or misuse is one of the most common recurrent worries for both patients and practitioners when considering pharmacological treatment for children, adolescents or adults with ADHD. Indeed, both the diversion and misuse of stimulant medications, that is, using stimulants without a prescription, or using higher or more frequent doses of stimulant drugs than prescribed, is a prevalent and growing problem among different populations of adolescents and young adults, particularly among college students [Benson et al. 2015; McCabe et al. 2014]. A growing body of evidence, including a systematic review [Wilens et al. 2008] and a meta-analysis [Benson et al. 2015], suggests the increasing misuse of stimulant medications by secondary education and university students, both with and without ADHD. Previous year stimulant misuse rates have been reported to be of 5–9% among secondary education students and of 5–35% by university students [Wilens et al. 2008; Benson et al. 2015]. While stimulant medication abuse has risen in recent years in all age groups along with increase in prescriptions for treating individuals diagnosed with ADHD [Setlik et al. 2009], it is more prevalent among those aged 12–25 years [Kroutil et al. 2006]. Nonetheless, nonmedical use and diversion of ADHD prescription stimulants is commonly low compared with other prescription medications, such as benzodiazepines or opioid analgesics [Cassidy et al. 2015; Novak et al. 2016]. The most frequent source of diverted prescription stimulants is given by a friend or relative [McNiel et al. 2011; Novak et al. 2007; Novak et al. 2016]
The abuse liability of prescription psychostimulants is mediated by several factors, such as the dose, pharmacokinetic characteristics and route of administration of the formulations, certain individual personality and character traits, as well as the context, expectations and motivation of drug consumption [Clemow and Walker, 2014; Jardin et al. 2011; Martinez-Raga et al. 2013b; Swanson et al. 2011; Volkow and Swanson, 2003; Wilens et al. 2016]. Several studies have shown that both among college students and among older adults, abuse of prescription stimulants is more common among individuals taking other psychiatric medication or with comorbid conduct or other psychiatric disorders, as well as among those with other concurrent illicit drug use [Darredeau et al. 2007; Frauger et al. 2011; Jardin et al. 2011; Wilens et al. 2016].
Overall, particularly in high-risk populations, short-acting stimulants should be best avoided, considering that prescription drugs more commonly abused are those with pharmacologic and pharmacokinetic characteristics that provide a rapid high [Bright, 2008]. Furthermore, long-acting stimulant formulations should be used with caution in patients at risk of stimulant abuse or diversion, and atomoxetine or α2-adrenergic receptor agonists, whenever available, may be considered as first-line ADHD medications in these cases [National Collaborating Centre for Mental Health, 2009]. However, nonmedical use of prescription stimulants may occur in individuals with and without a diagnosis of ADHD, but the majority of students with ADHD generally use their prescribed medication as instructed and abuse of psychostimulants in the clinical context is very limited [Rabiner et al. 2009]. It has been hypothesized that nonmedical use and diversion of prescription stimulants may be associated with unrecognized or untreated ADHD [Poulin, 2007]. While methylphenidate and amphetamines may be self-administered by laboratory animals and have shown subjective and reinforcing effects in humans [Kollins et al. 2009], nonstimulant medications approved for the treatment of ADHD, including, atomoxetine, guanfacine or clonidine, lack any reinforcing effects and consequently any abuse potential [Jasinski et al. 2008; Martinez-Raga et al. 2015]. However, a recent report suggested that, although to a lesser extent than methylphenidate, atomoxetine is used by adults for nonmedical purposes, including recreational use [Jensen et al. 2015].
Conclusion
Despite the major progress in the understanding and management of ADHD along the lifespan over the last two decades, adult patients with symptoms of ADHD continue to experience frequent barriers in the access to specialist assessment and treatment [Ginsberg et al. 2014b; Kooij et al. 2010]. Considering the physical and psychiatric comorbidities, as well as the individual, societal and health costs associated with ADHD [Doshi et al. 2012; Dalsgaard et al. 2015], it is highly important to improve training and raise awareness of clinicians about ADHD symptoms when assessing individuals seeking treatment for various disorders, particularly in adolescents and adults not previously diagnosed. Likewise, it is crucial to encourage managers and healthcare organizations to develop wider services for patients of all ages with ADHD and improve transition from child and adolescent to adult treatment services.
Although ADHD medications have a well-established efficacy and overall safety profile, these drugs are not exempt from adverse events. While the cardiovascular safety of ADHD pharmacotherapies has raised concern, there is little evidence of serious cardiovascular adverse events, including no significant QTc abnormalities associated with stimulants, atomoxetine or α2-adrenergic receptor agonists in children, adolescents and adults [Martinez-Raga et al. 2013c; Nahshoni et al. 2012; Schelleman et al. 2011; Winterstein, 2013]. In addition, although the abuse liability of prescription stimulant drugs, particularly, short-acting stimulants is a prevalent and growing problem, nonmedical use of prescription stimulants within the clinical context is limited. Nonstimulant ADHD medications lack any reinforcing effects and consequently any abuse potential [Benson et al. 2015; Wilens et al. 2008]. Overall, ADHD medications should solely be avoided in patients with symptomatic cardiac disease, in those with moderate to severe hypertension, as well as in individuals with advanced arteriosclerosis or hyperthyroidism, and they should be used with caution in patients with known structural cardiac abnormalities, or in those with a family history of severe cardiovascular problems [CADDRA, 2011]. Likewise, caution is advised when considering stimulant medication in patients with current or past substance use disorders.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jose Martinez-Raga has received honoraria for being a speaker and on advisory boards from Shire and Janssen Pharmaceuticals, Madrid, Spain. Amparo Ferreros, Carlos Knecht, Raquel de Alvaro and Eloisa Carabal report no conflicts of interest that are directly relevant to the content of this manuscript. No sources of funding were used in the preparation, review or approval of this manuscript.
Contributor Information
Jose Martinez-Raga, Teaching Unit of Psychiatry and Clinical Psychology, University of Valencia, University Cardenal Herrera CEU and Hospital Universitario Doctor Peset, Avda. Gaspar Aguilar, 90, 46017 Valencia, Spain.
Amparo Ferreros, University Hospital Doctor Peset, Valencia, Spain.
Carlos Knecht, Mental Health Area, Hospital Padre Jofré, Valencia, Spain.
Raquel de Alvaro, Hospital General de Castellón, Consorcio Hospitalario Provincial, Castellón, Spain.
Eloisa Carabal, Teaching Unit of Psychiatry and Clinical Psychology, University Hospital Doctor Peset, Valencia, Spain University Cardenal Herrera CEU, Valencia, Spain.
References
- Aagaard L., Hansen E. (2011) The occurrence of adverse drug reactions reported for attention deficit hyperactivity disorder (ADHD) medications in the pediatric population: a qualitative review of empirical studies. Neuropsychiatr Dis Treat 7: 729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L., Weisler R., Goodman D., Hamdani M., Niebler G. (2009) Short-term effects of lisdexamfetamine dimesylate on cardiovascular parameters in a 4-week clinical trial in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 70: 1652–1661. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Arcieri R., Germinario E., Bonati M., Masi G., Zuddas A., Vella S., et al. (2012) Cardiovascular measures in children and adolescents with attention-deficit/hyperactivity disorder who are new users of methylphenidate and atomoxetine. J Child Adolesc Psychopharmacol 22: 423–431. [DOI] [PubMed] [Google Scholar]
- Benson K., Flory K., Humphreys K., Lee S. (2015) Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev 18: 50–76. [DOI] [PubMed] [Google Scholar]
- Bright G. (2008) Abuse of medications employed for the treatment of ADHD: results from a large-scale community survey. Medscape J Med 10: 111. [PMC free article] [PubMed] [Google Scholar]
- Bussing R., Zima B., Gary F., Garvan C. (2003) Barriers to detection, help-seeking, and service use for children with ADHD symptoms. J Behav Health Serv Res 30: 176–189. [DOI] [PubMed] [Google Scholar]
- Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA) (2011) Canadian ADHD Practice Guidelines, 3rd ed. Toronto, ON: CADDRA. [Google Scholar]
- Cantrell F., Nestor M. (2005) Benign clinical course following atomoxetine overdose. Clin Toxicol (Phila) 43: 57. [DOI] [PubMed] [Google Scholar]
- Cassidy T., Varughese S., Russo L., Budman S., Eaton T., Butler S. (2015) Nonmedical use and diversion of ADHD stimulants among U.S. adults ages 18–49: a national internet survey. J Atten Disord 19: 630–640. [DOI] [PubMed] [Google Scholar]
- Clavenna A., Bonati M. (2014) Safety of medicines used for ADHD in children: a review of published prospective clinical trials. Arch Dis Child 99: 866–872. [DOI] [PubMed] [Google Scholar]
- Clemow D., Walker D. (2014) The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med 126: 64–81. [DOI] [PubMed] [Google Scholar]
- Connor D., Fletcher K., Swanson J. (1999) A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 38: 1551–1559. [DOI] [PubMed] [Google Scholar]
- Connor D., Steeber J., McBurnett K. (2010) A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr 31: 427–440. [DOI] [PubMed] [Google Scholar]
- Cooper W., Habel L., Sox C., Chan K., Arbogast P., Cheetham T., et al. (2011) ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med 365: 1896–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S., Kvist A., Leckman J., Nielsen H., Simonsen M. (2014b) Cardiovascular safety of stimulants in children with attention-deficit/hyperactivity disorder: a nationwide prospective cohort study. J Child Adolesc Psychopharmacol 24: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S., Nielsen H., Simonsen M. (2014a) Consequences of ADHD medication use for children’s outcomes. J Health Econ 37: 137–151. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S., Østergaard S., Leckman J., Mortensen P., Pedersen M. (2015) Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet 385: 2190–2196. [DOI] [PubMed] [Google Scholar]
- Darredeau C., Barrett S., Jardin B., Pihl R. (2007) Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol 22: 529–536. [DOI] [PubMed] [Google Scholar]
- Daviss W., Patel N., Robb A., McDermott M., Bukstein O., Pelham W., et al. (2008) Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry 47: 189–198. [DOI] [PubMed] [Google Scholar]
- Donner R., Michaels M., Ambrosini P. (2007) Cardiovascular effects of mixed amphetamine salts extended release in the treatment of school-aged children with attention-deficit/hyperactivity disorder. Biol Psychiatry 61: 706–712. [DOI] [PubMed] [Google Scholar]
- Doshi J., Hodgkins P., Kahle J., Sikirica V., Cangelosi M., Setyawan J., et al. (2012) Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 51: 990–1002. [DOI] [PubMed] [Google Scholar]
- Eiraldi R., Mazzuca L., Clarke A., Power T. (2006) Service utilization among ethnic minority children with ADHD: a model of help-seeking behavior. Adm Policy Ment Health 33: 607–622. [DOI] [PubMed] [Google Scholar]
- Erskine H., Ferrari A., Polanczyk G., Moffitt T., Murray C., Vos T., et al. (2014) The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. J Child Psychol Psychiatry 55: 328–336. [DOI] [PubMed] [Google Scholar]
- Faraone S., Asherson P., Banaschewski T., Biederman J., Buitelaar J., Ramos-Quiroga J., et al. (2015) Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 1: 15020. [DOI] [PubMed] [Google Scholar]
- Fein D., Hafeez Z., Cavagnaro C. (2013) An overdose of extended-release guanfacine. Pediatr Emerg Care 29: 929–931. [DOI] [PubMed] [Google Scholar]
- Fettahoglu E., Satilmis A., Gokcen C., Ozatalay E. (2009) Oral megadose methylphenidate ingestion for suicide attempt. Pediatr Int 51: 844–845. [DOI] [PubMed] [Google Scholar]
- Frauger E., Pauly V., Natali F., Pradel V., Reggio P., Coudert H., et al. (2011) Patterns of methylphenidate use and assessment of its abuse and diversion in two French administrative areas using a proxy of deviant behavior determined from a reimbursement database: main trends from 2005 to 2008. CNS Drugs 25: 415–424. [DOI] [PubMed] [Google Scholar]
- Gajria K., Lu M., Sikirica V., Greven P., Zhong Y., Qin P., et al. (2014) Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat 10: 1543–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnock-Jones K., Keating G. (2009) Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs 11: 203–226. [DOI] [PubMed] [Google Scholar]
- Ginsberg Y., Beusterien K., Amos K., Jousselin C., Asherson P. (2014b) The unmet needs of all adults with ADHD are not the same: a focus on Europe. Expert Rev Neurother 14: 799–812. [DOI] [PubMed] [Google Scholar]
- Ginsberg Y., Quintero J., Anand E., Casillas M., Upadhyaya H. (2014a) Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature. Prim Care Companion CNS Disord 16: PCC.13r01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitter M., Cox R. (2000) Clonidine toxicity in an adolescent patient. J Miss State Med Assoc 41: 757–759. [PubMed] [Google Scholar]
- Graham J., Coghill D. (2008) Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs 22: 213–237. [DOI] [PubMed] [Google Scholar]
- Graham J., Banaschewski T., Buitelaar J., Coghill D., Danckaerts M., Dittmann R., et al. (2011) European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry 20: 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel L., Cooper W., Sox C., Chan K., Fireman B., Arbogast P., et al. (2011) ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA 306: 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerness P., Wilens T., Mick E., Spencer T., Doyle R., McCreary M., et al. (2009) Cardiovascular effects of longer-term, high-dose OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr 155: 84–89. [DOI] [PubMed] [Google Scholar]
- Hill S., El-Khayat R., Sandilands E., Thomas S. (2010) Electrocardiographic effects of methylphenidate overdose. Clin Toxicol (Phila) 48: 342–346. [DOI] [PubMed] [Google Scholar]
- Hirota T., Schwartz S., Correll C. (2014) Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 53: 153–173. [DOI] [PubMed] [Google Scholar]
- Jardin B., Looby A., Earleywine M. (2011) Characteristics of college students with attention-deficit hyperactivity disorder symptoms who misuse their medications. J Am Coll Health 59: 373–377. [DOI] [PubMed] [Google Scholar]
- Jasinski D., Faries D., Moore R., Schuh L., Allen A. (2008) Abuse liability assessment of atomoxetine in a drug-abusing population. Drug Alcohol Depend 95: 140–146. [DOI] [PubMed] [Google Scholar]
- Jensen L., Pagsberg A., Dalhoff K. (2015) Differences in abuse potential of ADHD drugs measured by contrasting poison centre and therapeutic use data. Clin Toxicol (Phila) 53: 210–214. [DOI] [PubMed] [Google Scholar]
- Kappagoda C., Schell D., Hanson R., Hutchins P. (1998) Clonidine overdose in childhood: implications of increased prescribing. J Paediatr Child Health 34: 508–512. [DOI] [PubMed] [Google Scholar]
- Kashani J., Ruha A. (2007) Isolated atomoxetine overdose resulting in seizure. J Emerg Med 32: 175–178. [DOI] [PubMed] [Google Scholar]
- Kessler R., Adler L., Ames M., Barkley R., Birnbaum H., Greenberg P., et al. (2005) The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med 47: 565–572. [DOI] [PubMed] [Google Scholar]
- Kollins S., English J., Robinson R., Hallyburton M., Chrisman A. (2009) Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD). Psychopharmacology (Berl) 204: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij S., Bejerot S., Blackwell A., Caci H., Casas-Brugué M., Carpentier P., et al. (2010) European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroutil L., Van Brunt D., Herman-Stahl M., Heller D., Bray R., Penne M. (2006) Nonmedical use of prescription stimulants in the United States. Drug Alcohol Depend 84: 135–143. [DOI] [PubMed] [Google Scholar]
- Le H., Hodgkins P., Postma M., Kahle J., Sikirica V., Setyawan J., et al. (2014) Economic impact of childhood/adolescent ADHD in a European setting: the Netherlands as a reference case. Eur Child Adolesc Psychiatry 23: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P., Halldner L., Zetterqvist J., Sjölander A., Serlachius E., Fazel S., et al. (2012) Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med 367: 2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovecchio F., Kashani J. (2006) Isolated atomoxetine (Strattera) ingestions commonly result in toxicity. J Emerg Med 31: 267–268. [DOI] [PubMed] [Google Scholar]
- Martin P., Satin L., Kahn R., Robinson A., Corcoran M., Purkayastha J., et al. (2015) A thorough QT study of guanfacine. Int J Clin Pharmacol Ther 53: 301–316. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J., Knecht C., de Alvaro R. (2015) Profile of guanfacine extended-release and its potential in the treatment of attention deficit hyperactivity disorder. Neuropsychiatr Dis Treat 11: 1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Raga J., Knecht C., de Alvaro R., Szerman N., Ruiz P. (2013b) Addressing dual diagnosis patients suffering from attention deficit hyperactivity disorders and comorbid substance use disorders: a review of treatment considerations. Addict Dis Treat 12: 213–230. [Google Scholar]
- Martinez-Raga J., Knecht C., Szerman N., Martinez M. (2013c) Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs 27: 15–30. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J., Szerman N., Knecht C., de Alvaro R. (2013a) Attention deficit hyperactivity disorder and dual disorders: educational needs for an underdiagnosed condition. Int J Adolesc Med Health 25: 231–243. [DOI] [PubMed] [Google Scholar]
- Matheson L., Asherson P., Wong I., Hodgkins P., Setyawan J., Sasane R., et al. (2013) Adult ADHD patient experiences of impairment, service provision and clinical management in England: a qualitative study. BMC Health Serv Res 13: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe S., West B., Teter C., Boyd C. (2014) Trends in medical use, diversion, and nonmedical use of prescription medications among college students from 2003 to 2013: connecting the dots. Addict Behav 39: 1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S., Cranswick N., Potts L., Taylor E., Wong I. (2009) Mortality associated with attention-deficit hyperactivity disorder (ADHD) drug treatment: a retrospective cohort study of children, adolescents and young adults using the general practice research database. Drug Saf 32: 1089–1096. [DOI] [PubMed] [Google Scholar]
- McCracken J., McGough J., Loo S., Levitt J., Del’Homme M., Cowen J., et al. (2016) Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: a controlled, comparative study. J Am Acad Child Adolesc Psychiatry 55: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiel A., Muzzin K., DeWald J., McCann A., Schneiderman E., Scofield J., et al. (2011) The nonmedical use of prescription stimulants among dental and dental hygiene students. J Dent Educ 75: 365–376. [PubMed] [Google Scholar]
- Meinzer M., Pettit J., Viswesvaran C. (2014) The co-occurrence of attention-deficit/hyperactivity disorder and unipolar depression in children and adolescents: a meta-analytic review. Clin Psychol Rev 34: 595–607. [DOI] [PubMed] [Google Scholar]
- Michelson D., Adler L., Spencer T., Reimherr F., West S., Allen A., et al. (2003) Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 53: 112–120. [DOI] [PubMed] [Google Scholar]
- Michelson D., Faries D., Wernicke J., Kelsey D., Kendrick K., Sallee F., et al. (2001) Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 108: e83. [DOI] [PubMed] [Google Scholar]
- Michelson D., Read H., Ruff D., Witcher J., Zhang S., McCracken J. (2007) CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry 46: 242–251. [DOI] [PubMed] [Google Scholar]
- Mick E., McManus D., Goldberg R. (2013) Meta-analysis of increased heart rate and blood pressure associated with CNS stimulant treatment of ADHD in adults. Eur Neuropsychopharmacol 23: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns A., Clark R., Schneir A. (2010) Guanfacine overdose resulting in initial hypertension and subsequent delayed, persistent orthostatic hypotension. Clin Toxicol (Phila) 48: 146–148. [DOI] [PubMed] [Google Scholar]
- Munk K., Gormsen L., Kim W., Andersen N. (2015) Cardiac arrest following a myocardial infarction in a child treated with methylphenidate. Case Rep Pediatr 2015: 905097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nahshoni E., Golubchik P., Glazer J., Sever J., Strasberg B., Imbar S., et al. (2012) Late potentials in the signal-averaged electrocardiogram in pre-pubertal children with ADHD, before and after methylphenidate treatment. Eur Child Adolesc Psychiatry 21: 75–78. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health. (2009) Attention Deficit Hyperactivity Disorder. The NICE Guideline on diagnosis and management of ADHD in children, young people and adults. National Clinical Practice Guideline Number 72. London, UK: Alden Press. [Google Scholar]
- Newcorn J., Weiss M., Stein M. (2007) The complexity of ADHD: diagnosis and treatment of the adult patient with comorbidities. CNS Spectr 12(Suppl. 12): 1–14. [DOI] [PubMed] [Google Scholar]
- Novak S., Håkansson A., Martinez-Raga J., Reimer J., Krotki K., Varughese S. (2016) Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 16: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak S., Kroutil L., Williams R., Van Brunt D. (2007) The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., Huang C., Gerhard T., Winterstein A., Crystal S., Allison P., et al. (2012) Stimulants and cardiovascular events in youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir E., Karaman M., Yurteri N., Erdogan A. (2010) A case of suicide attempt with long-acting methylphenidate (Concerta). Atten Defic Hyperact Disord 2: 103–105. [DOI] [PubMed] [Google Scholar]
- Peterson K., McDonagh M., Fu R. (2008) Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl) 197: 1–11. [DOI] [PubMed] [Google Scholar]
- Polanczyk G., Willcutt E., Salum G., Kieling C., Rohde L. (2014) ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 43: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin C. (2007) From attention-deficit/hyperactivity disorder to medical stimulant use to the diversion of prescribed stimulants to non-medical stimulant use: connecting the dots. Addiction 102: 740–751. [DOI] [PubMed] [Google Scholar]
- Rabiner D., Anastopoulos A., Costello E., Hoyle R., McCabe S., Swartzwelder H. (2009) The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord 13: 144–153. [DOI] [PubMed] [Google Scholar]
- Roden D. (2004) Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022. [DOI] [PubMed] [Google Scholar]
- Ruggiero S., Clavenna A., Reale L., Capuano A., Rossi F., Bonati M. (2014) Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol 24: 1578–1590. [DOI] [PubMed] [Google Scholar]
- Sallee F., Connor D., Newcorn J. (2013) A review of the rationale and clinical utilization of α2-adrenoceptor agonists for the treatment of attention-deficit/hyperactivity and related disorders. J Child Adolesc Psychopharmacol 23: 308–319. [DOI] [PubMed] [Google Scholar]
- Sayer G., McGough J., Levitt J., Cowen J., Sturm A., Castelo E., et al. (2016) Acute and long-term cardiovascular effects of stimulant, guanfacine, and combination therapy for attention-deficit/hyperactivity Disorder. J Child Adolesc Psychopharmacol 26: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelleman H., Bilker W., Strom B., Kimmel S., Newcomb C., Guevara J., et al. (2011) Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics 127: 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlik J., Bond G., Ho M. (2009) Adolescent prescription ADHD medication abuse is rising along with prescriptions for these medications. Pediatrics 124: 875–880. [DOI] [PubMed] [Google Scholar]
- Shin J., Roughead E., Park B., Pratt N. (2016) Cardiovascular safety of methylphenidate among children and young people with attention-deficit/hyperactivity disorder (ADHD): nationwide self-controlled case series study. BMJ 353: i2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Wigal T., Volkow N. (2011) Contrast of medical and nonmedical use of stimulant drugs, basis for the distinction, and risk of addiction: comment on Smith and Farah (2011). Psychol Bull 137: 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift K., Sayal K., Hollis C. (2014) ADHD and transitions to adult mental health services: a scoping review. Child Care Health Dev 40: 775–786. [DOI] [PubMed] [Google Scholar]
- Torres I., Gómez N., Colom F., Jiménez E., Bosch R., Bonnín C., et al. (2015) Bipolar disorder with comorbid attention-deficit and hyperactivity disorder: main clinical features and clues for an accurate diagnosis. Acta Psychiatr Scand 132: 389–399. [DOI] [PubMed] [Google Scholar]
- Turgay A., Goodman D., Asherson P., Lasser R., Babcock T., Pucci M., et al. (2012) Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry 73: 192–201. [DOI] [PubMed] [Google Scholar]
- Upadhyaya H., Tanaka Y., Lipsius S., Kryzhanovskaya L., Lane J., Escobar R., et al. (2015) Time-to-onset and -resolution of adverse events before/after atomoxetine discontinuation in adult patients with ADHD. Postgrad Med 127: 677–685. [DOI] [PubMed] [Google Scholar]
- Volkow N., Swanson J. (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160: 1909–1918. [DOI] [PubMed] [Google Scholar]
- Weisler R., Biederman J., Spencer T., Wilens T. (2005) Long-term cardiovascular effects of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr 10(12 Suppl. 20): 35–43. [DOI] [PubMed] [Google Scholar]
- Weisler R., Biederman J., Spencer T., Wilens T., Faraone S., Chrisman A., et al. (2006) Mixed amphetamine salts extended-release in the treatment of adult ADHD: a randomized, controlled trial. CNS Spectr 11: 625–639. [DOI] [PubMed] [Google Scholar]
- Wietecha L., Ruff D., Allen A., Greenhill L., Newcorn J. (2013) Atomoxetine tolerability in pediatric and adult patients receiving different dosing strategies. J Clin Psychiatry 74: 1217–1223. [DOI] [PubMed] [Google Scholar]
- Wilens T., Adler L., Adams J., Sgambati S., Rotrosen J., Sawtelle R., et al. (2008) Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry 47: 21–31. [DOI] [PubMed] [Google Scholar]
- Wilens T., Bukstein O., Brams M., Cutler A., Childress A., Rugino T., et al. (2012) A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51: 74–85. [DOI] [PubMed] [Google Scholar]
- Wilens T., Zulauf C., Martelon M., Morrison N., Simon A., Carrellas N., et al. (2016) Nonmedical stimulant use in college students: association with attention-deficit/hyperactivity disorder and other disorders. J Clin Psychiatry 77: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E. (2012) The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 9: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterstein A. (2013) Cardiovascular safety of stimulants in children: findings from recent population-based cohort studies. Curr Psychiatry Rep 15: 379. [DOI] [PubMed] [Google Scholar]
- Winterstein A., Gerhard T., Kubilis P., Saidi A., Linden S., Crystal S., et al. (2012) Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ 345: e4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H., Jacobi F., Rehm J., Gustavsson A., Svensson M., Jönsson B., et al. (2011) The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: 655–679. [DOI] [PubMed] [Google Scholar]
- Wolraich M., McGuinn L., Doffing M. (2007) Treatment of attention deficit hyperactivity disorder in children and adolescents: safety considerations. Drug Saf 30: 17–26. [DOI] [PubMed] [Google Scholar]
- Wu E., Hodgkins P., Ben-Hamadi R., Setyawan J., Xie J., Sikirica V., et al. (2012) Cost effectiveness of pharmacotherapies for attention-deficit hyperactivity disorder: a systematic literature review. CNS Drugs 26: 581–600. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nagumo K., Nakashima T., Kinugawa Y., Kumaki S. (2014) Life-threatening QT prolongation in a boy with attention-deficit/hyperactivity disorder on atomoxetine. Eur J Pediatr 173: 1631–1634. [DOI] [PubMed] [Google Scholar]
- Young S., Murphy C., Coghill D. (2011) Avoiding the ‘twilight zone’: recommendations for the transition of services from adolescence to adulthood for young people with ADHD. BMC Psychiatry 11: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]