Abstract
Background:
Therapeutic hypothermia (TH) improves survival and neurologic function in comatose survivors of cardiac arrest. Many medications used to support TH have altered pharmacokinetics and pharmacodynamics during this treatment. It is unknown if or at what frequency the medications used during TH cause adverse drug reactions (ADRs).
Methods:
A retrospective chart review was conducted for patients admitted to an intensive care unit (ICU) after cardiac arrest and treated with TH from January 2009 to June 2012 at two urban, university-affiliated, tertiary-care medical centres. Medications commonly used during TH were screened for association with significant ADRs (grade 3 or greater per Common Terminology Criteria for Adverse Events) using three published ADR detection instruments.
Results:
A total of 229 patients were included, the majority being males with median age of 62 presenting with an out-of-hospital cardiac arrest in pulseless electrical activity or asystole. The most common comorbidities were hypertension, coronary artery disease, and diabetes mellitus. There were 670 possible ADRs and 69 probable ADRs identified. Of the 670 possible ADRs, propofol, fentanyl, and acetaminophen were the most common drugs associated with ADRs. Whereas fentanyl, insulin, and propofol were the most common drugs associated with a probable ADR. Patients were managed with TH for a median of 22 hours, with 38% of patients surviving to hospital discharge.
Conclusions:
Patients undergoing TH after cardiac arrest frequently experience possible adverse reactions associated with medications and the corresponding laboratory abnormalities are significant. There is a need for judicious use and close monitoring of drugs in the setting of TH until recommendations for dose adjustments are available to help prevent ADRs.
Keywords: adverse drug reactions, cardiac arrest, critical care, drug-related side effects and adverse drug reactions, intensive care, pharmacovigilance, therapeutic hypothermia
Introduction
Annually, there are 356,500 out-of-hospital cardiac arrests in the US, with a median survival rate of 12% [Members et al. 2009]. Only a small proportion of those survivors will have a favourable neurologic recovery [Members et al. 2009]. In the post-resuscitative period, therapeutic hypothermia (TH) is thought to mitigate reperfusion injury by decreasing cerebral oxygen consumption and biochemical damage [The Hypothermia after Cardiac Arrest Study Group, 2002]. Two landmark trials published in 2002 demonstrated favourable neurologic outcomes, improved functional neurological status at discharge, and decreased mortality in patients treated with TH after cardiac arrest [Bernard et al. 2002; The Hypothermia after Cardiac Arrest Study Group, 2002]. As a result of these investigations, national guidelines from the American Heart Association recommend TH for survivors of both out-of-hospital and in-hospital cardiac arrest [Peberdy et al. 2010]. These practices have been reinforced by several professional societies supporting the use of TH after cardiac arrest to improve survival and neurologic outcomes [Nunnally et al. 2011].
Despite the benefits of TH, patients may experience physiologic aberrations or adverse drug reactions (ADRs) potentially diminishing the effects of TH. ADRs are described at a considerable frequency in the general intensive care unit (ICU) setting with serious consequences that can be potentially life-threatening [Rothschild et al. 2005; Kopp et al. 2006; Valentin et al. 2006; Wilmer et al. 2010]. Electrolyte abnormalities such as hypokalaemia, hypomagnesaemia, hypophosphatemia, hypo- and hyperglycaemia, as well as coagulopathies have been described during TH, specifically [Al-Senani et al. 2004; Arrich, 2007; Pichon et al. 2007; Nielsen et al. 2009, 2011; Xiao et al. 2013; MacLaren et al. 2014]. In the setting of TH, altered drug metabolism, changes in receptor affinity, and prolonged elimination may contribute to the alteration in pharmacokinetics of commonly used medications in the critically ill, leading to supratherapeutic concentrations and the possibility of resultant toxicity and adverse outcomes [Tortorici et al. 2006; Van Den Broek et al. 2010; Zhou et al. 2011a; Bell, 2012; De Haan et al. 2012; Bjelland et al. 2013, 2014]. The cytochrome P450 enzyme system is remarkably affected by TH, which likely alters the metabolism of any medication subjected to this pathway [Tortorici et al. 2006; Zhou et al. 2011a]. Alterations in cytochrome P450 metabolism resulted in rhabdomyolysis following high-dose simvastatin therapy during TH [Dearing and Norgard, 2010]. Also, sedatives such as propofol, fentanyl, morphine, dexmedetomidine, and midazolam exhibit altered pharmacokinetics, resulting in decreased clearance and increased serum concentrations at otherwise appropriate doses when used in the setting of TH [Bjelland et al. 2013, 2014]. It is imperative to account for these drug−therapy interactions when managing patients undergoing TH. While these changes in laboratory values, pharmacokinetics, and pharmacodynamics are known to occur, there is no comprehensive evaluation regarding the frequency of laboratory abnormalities due to drugs known as ADRs or clinical consequences of medications used during TH [Tortorici et al. 2007; Zhou et al. 2011b; Poloyac, 2012]. The purpose of this study is to evaluate the frequency of ADRs during and immediately after TH for cardiac arrest.
Methods
Setting
This study was a retrospective chart review that was conducted at two urban, university-affiliated, tertiary-care medical centres. The first site is an approximately 750-bed hospital with 90 critical care beds across six ICUs. The second site is an approximately 800-bed facility with over 120 critical care beds across eight ICUs. The study was approved by the Institutional Review Boards at each study site. The Institutional Review Board deemed that patient consent was not required; patient confidentiality was maintained throughout the study.
Patients
Patients admitted from 1 January 2009 to 30 June 2012 with an ICD-9 code for cardiac arrest (427.5) and either an order for a medication used to control shivering during TH (meperidine, buspirone, midazolam, propofol or vecuronium) or an order for a cooling blanket were screened for inclusion [Chamorro et al. 2010]. Patients were included successively if they were 18 years or older, admitted to an ICU, and were cooled to 33°C for at least 12 hours and rewarmed for at least 24 hours. Patients rewarmed for less than 24 hours were not included in an effort to increase homogeneity amongst patients and capture all ADRs throughout the cooling, hypothermia, and rewarming phases. There was no restriction on the type of ICU in which the patient was admitted for care.
Data collection
At the time of TH, the following baseline demographic information was collected: age, sex, comorbidities, initial rhythm leading to cardiac arrest, admitting ICU and device used for TH. Other data points were collected from the electronic medical record beginning 24 hours prior to TH through 48 hours after the discontinuation of TH: duration of TH, medications administered during TH, and pertinent physiologic and laboratory values (as seen in Table 1). Derangements in these physiologic and laboratory values were then graded using the US Department of Health and Human Services’ Common Terminology Criteria for Adverse Events. This grading system categorizes events on a scale from 1 to 5, with a grade 1 event representing a mild derangement requiring no intervention and a grade 5 event resulting in death. Only serious events including grade 3, 4 and 5, as described in Table 2, were evaluated in this study, which signifies the clinical significance of each event. Select medications commonly used for indications of sedation, analgesia, paralysis, shivering prevention, electrolyte replacement and glycaemic control in the setting of TH at each institution per protocol were cross-referenced with the deviations from normal laboratory or physiologic values based on published ADRs, as reported in the drug information resources (Table 1). This predetermined list of drugs and reactions allowed for consistency in data collection between sites and reviewers. These deviations were selected because of the possible association with drugs. Deviations from normal laboratory or physiological values are known to occur solely as a result of TH, however it is unknown if medications used during this therapy contribute to or increase the severity of these deviations from normal values. These occurrences were then assessed for the presence of an ADR.
Table 1.
Included medications and corresponding adverse drug reactions.
| Adverse reaction |
Hypokalaemia | Hyperkalaemia | Hypoglycaemia | Hyperglycaemia | Hypomagnesaemia | Hypophosphatemia | Elevated AST | Elevated ALT | Elevated INR | Bradycardia | Hypocalcaemia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Offending agent | |||||||||||
| Acetaminophen | X | X | X | ||||||||
| Calcium | X | ||||||||||
| Dexmedetomidine | X | X | |||||||||
| Dextrose | X | X | X | X | |||||||
| Fentanyl | X | X | X | X | X | X | X | ||||
| Fosphenytoin | X | ||||||||||
| Hydromorphone | X | X | X | X | |||||||
| Insulin | X | X | |||||||||
| Meperidine | X | ||||||||||
| Morphine | X | X | X | X | |||||||
| Phenytoin | X | X | X | ||||||||
| Phosphate | X | X | |||||||||
| Potassium | X | ||||||||||
| Propofol | X |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio.
Table 2.
Definitions of grade 3, 4, and 5 adverse events.
| Adverse event | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|
| Hypokalaemia (mEq/ml) | 2.5–3.0 | <2.5 | Death |
| Hyperkalaemia (mEq/ml) | 6–7.0 | >7.0 | Death |
| Hypoglycaemia (mg/dl) | 30–40 | <30 | Death |
| Hyperglycaemia (mg/dl) | 250–500 | >500 | Death |
| Hypocalcaemia (mg/dl) | 6.0–7.0 | <6.0 | Death |
| Hypomagnesaemia (mg/dl) | 0.7–0.9 | <0.7 | Death |
| Hypophosphatemia (mg/dl) | 1.0–2.0 | <1.0 | Death |
| AST (unit/l) | 5–20 × ULN | >20 × ULN | N/A |
| ALT (unit/l) | 5–20 × ULN | >20 × ULN | N/A |
| INR | >2.5 × ULN | N/A | N/A |
| Heart rate (beats per minute) |
Bradycardia < 60 and pharmacologic intervention | Bradycardia < 60 and pharmacologic intervention or cardiac arrest/pacing | Death |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ULN, upper limit of normal; INR, international normalized ratio.
Adverse drug reaction definition and determination instruments
An ADR was defined as an undesirable clinical manifestation that is consequent to and caused by the administration of a particular drug [Kramer et al. 1979]. Derangements were assessed using three objective, published ADR determination instruments: modified ADR scoring system, ADR probability scale, and the Jones algorithm [Naranjo et al. 1981; Jones, 1988; Goh, 1989]. The use of more than one instrument for ADR determination is a more rigorous approach, thus the inclusion of these three instruments was applied in this study in an attempt to overcome the challenge of differentiating the causality of a drug versus TH [Kane-Gill et al. 2012]. An event was considered an ADR when at least two out of three instruments had agreement of possible, probable or definite. The use of possible as inclusion for an ADR is a common criteria [Evans et al. 1991; Rivkin, 2007; Kane-Gill et al. 2009]. A sensitivity analysis was performed to report detection of ADR by just one ADR determination instrument as well as detection by all three instruments [Kane-Gill et al. 2011].
The modified ADR scoring system is a six-axis tool designed to assess possible ADRs based on documented evidence, presence of other possible causes, timing, laboratory data, resulting clinical status, and effect of rechallenging a possibly offending agent related to the ADR [Goh, 1989]. The ADR probability scale, also referred to as the Naranjo scale, is a ten-part tool in which ADRs are assessed based on documented evidence, timing, resulting clinical status, effect of rechallenging with a possibly offending agent, known or theoretical alternative causes, response to placebo, laboratory evidence, severity with higher and lower doses of a possibly offending agent, reaction to other drugs with known class-effects, and objectivity of evidence of ADR [Naranjo et al. 1981]. The Jones algorithm assesses ADRs based on timing, dechallenging of a possibly offending agent, reaction to dechallenging of a possibly offending agent, presence of rechallenging of a possibly offending agent, reaction of rechallenging a possibly offending agent, and presence of clinical conditions that could contribute to ADR [Jones, 1988]. These three scales are well established tools, and the concurrent use of each together has been suggested to yield a more definitive assessment of ADRs [Michel and Knodel, 1986; Kane-Gill et al. 2012].
Outcomes
The primary endpoint of this study was to determine the occurrence of possible ADRs occurring during and immediately after TH. Patient-centred outcomes (i.e. duration of TH, survival to discharge, and disposition at discharge) for patients with a suspected ADR are provided.
Results
A total of 492 patients were screened, resulting in the inclusion of 229 patients (Figure 1). A total of 157 patients were screened from site one and 70 patients were included; 335 patients were screened from site two and 159 patients were included (Figure 1). Baseline characteristics of the included patients are presented in Table 3. The mean age was 62 years and 58.1% of patients were male. The majority of patients experienced an out-of-hospital cardiac arrest presenting as asystole or pulseless electrical activity as the initial rhythm. The most common comorbidities were hypertension, coronary artery disease, and diabetes mellitus. The majority of patients were admitted to the coronary ICU or neurologic ICU for TH. The most commonly used cooling device was the Arctic Sun® system (Medivance, Louisville, CO, USA), although it was not possible to determine the method of cooling retrospectively from the majority of the electronic medical records.
Figure 1.
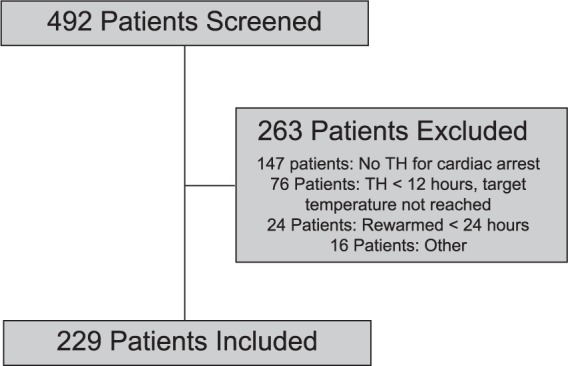
Patient Screening.
TH, therapeutic hypothermia.
Table 3.
Baseline demographics.
| n = 229 | |
|---|---|
| Age, years* | 62.0 (50.0–71.3) |
| Male | 133 (58.1) |
| Setting of cardiac arrest | |
| Out-of-hospital | 148 (64.6) |
| In-hospital, floor | 44 (19.2) |
| In-hospital, ICU | 21 (9.2) |
| Other | 16 (7.0) |
| Presenting rhythm | |
| Asystole/pulseless electrical activity | 160 (69.9) |
| Ventricular fibrillation/ventricular tachycardia | 69 (30.1) |
| Comorbidities | |
| Hypertension | 115 (50.2) |
| Coronary artery disease | 68 (29.7) |
| Diabetes mellitus | 67 (29.3) |
| Neurologic disease | 54 (23.6) |
| Chronic heart failure | 37 (16.2) |
| Chronic kidney disease | 32 (14.0) |
| ICU admission for TH | |
| Coronary ICU | 113 (49.3) |
| Neurologic ICU | 38 (16.6) |
| Medical ICU | 28 (12.2) |
| Surgical ICU | 23 (10.0) |
| Cardiothoracic ICU | 8 (3.5) |
| Transplant, neurosurgical, or surgical trauma ICU | 19 (8.3) |
| Cooling device | |
| Arctic Sun® Temperature Management System | 56 (24.5) |
| Alsius Thermogard™ System | 17 (7.4) |
| InnerCool Celsius Control System with Accutrol© | 3 (1.3) |
| Undetermined | 153 (66.8) |
ICU, intensive care unit; TH, therapeutic hypothermia.
All data presented as n (%) unless otherwise specified.
Median (interquartile range).
Of all physiologic or laboratory derangements identified via the Common Terminology Criteria for Adverse Events that required evaluation for the presence of an ADR, 739 grade 3 or grade 4 derangements were associated with a medication and were evaluated for the likelihood of the presence of an ADR. No grade 5 derangements were detected in this evaluation. The absence of grade 5 derangements may have been influenced by the inclusion criteria of rewarming for at least 24 hours. Evaluation of these derangements resulted in 670 possible ADRs and 69 probable ADRs. No definite ADRs were detected in this analysis.
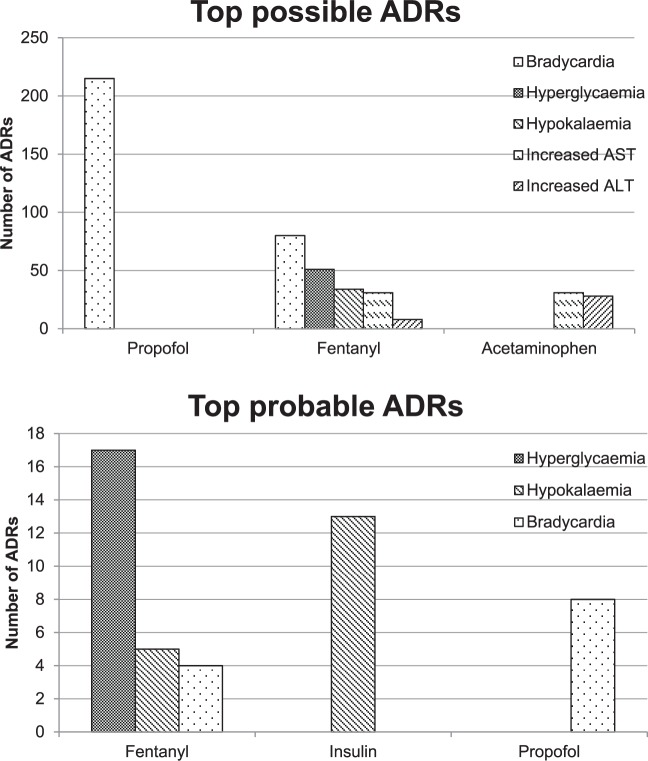
Medications associated with possible and probable ADRs can be found in Table 4. Of the 670 possible ADRs, propofol, fentanyl, and acetaminophen were the most common drugs associated with ADRs. Whereas fentanyl, insulin, and propofol were the most common drugs associated with a probable ADR (Figure 2). In the sensitivity analysis for possible ADRs, 670 ADRs were ranked as possible by at least one instrument, and 611 were ranked as possible or greater using all three for agreement instruments. Interestingly, in the sensitivity analysis for probable ADRs, 244 ADRs were ranked as probable on at least one instrument, and 38 were ranked as probable on all three instruments. A complete report of all evaluations can be found in Appendix A in the supplement.
Table 4.
Adverse drug reactions.
| Possible ADRs |
Probable ADRs |
||
|---|---|---|---|
| Drug | Number of ADRs | Drug | Total ADRs |
| Fentanyl | 220 | Fentanyl | 30 |
| Propofol | 211 | Insulin | 14 |
| Acetaminophen | 80 | Propofol | 8 |
| Insulin | 53 | Dextrose 50% | 6 |
| Phenytoin | 42 | Phenytoin | 3 |
| Calcium | 25 | Acetaminophen | 2 |
| Meperidine | 12 | Calcium | 2 |
| Dextrose 50% | 8 | Potassium | 2 |
| Morphine | 7 | Morphine | 1 |
| Dexmedetomidine | 5 | Fosphenytoin | 1 |
| Fosphenytoin | 4 | ||
| Potassium | 3 | ||
| Total | 670 | Total | 69 |
ADRs, adverse drug reactions.
Figure 2.
Top adverse drug reaction medications.
ADR, Adverse drug reaction; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Patients were managed with TH for a median time [interquartile range (IQR)] of 22 (16–25) hours. Survival to discharge in this population was 38.0%; of these, 59.8% of patients discharged to a skilled nursing facility, 33.3% to home, and 6.9% to a nursing home.
Discussion
In our sample of critically ill adult patients undergoing TH after cardiac arrest, the most frequently associated ADRs of the most common medications included bradycardia, hypokalaemia, transaminitis, and hyperglycaemia. A large, prospective trial evaluating the adverse reactions in patients during TH after cardiac arrest found similar results, reporting most commonly hyperglycaemia in 37% of patients, hypophosphatemia in 19% of patients, hypokalaemia in 18% of patients, hypomagnesaemia in 17% of patients, and bradycardia in 14% of patients [Nielsen et al. 2011]. These findings are consistent with results from a recent meta-analysis evaluating safety and outcomes in TH [Xiao et al. 2013]. Authors described hyperglycaemia occurring in up to 52.4% of patients being treated with TH after cardiac arrest. These comparisons are limited in that the definitions of these reported values and derangement vary and possible precipitating factors such as medical management are not reported [Nielsen et al. 2011; Xiao et al. 2013]. Though pharmacokinetic and pharmacodynamic pathways are significantly altered in TH, and it is understood that these abnormal laboratory values occur during TH, the multifactorial causes are not delineated [Tortorici et al. 2006; Van Den Broek et al. 2010; Zhou et al. 2011a; Bell, 2012; De Haan et al. 2012; Xiao et al. 2013]. Our study suggests that in many situations, drugs may be contributing to these abnormalities, and in approximately 10% (69/670) of cases, drugs are a probable cause.
Substantial changes in activated partial thromboplastin time (aPTT) with heparin therapy during and after TH are reported [Wahby et al. 2014]. Elevations in INR are not well reported in the literature, but bleeding events have been reported in up to 10.3% of patients being treated with TH after cardiac arrest [Nielsen et al. 2011; Xiao et al. 2013]. While we did not collect data on bleeding events, some of our patients developed an increase in INR during TH. Similarly, transaminitis is not well documented, but we found elevations in AST and ALT in our population. It is difficult, though, to discern transaminitis related to shock liver from cardiac arrest, the effects of TH or ADRs. Our evaluation with objective ADR-determination instruments takes into account temporal association of drug administration, dechallenges and rechallenges, suggesting drugs as a possible contributing factor. These challenges in ADR determination are no different than aminoglycoside- or vancomycin-induced nephrotoxicity in a septic patient. Despite the challenge, drugs should be considered as part of the differential diagnosis and safe drug administration (dose, route, and duration) should be considered.
It is clearly reported that there are physiologic alterations in vivo during TH, resulting in augmented pharmacokinetics and pharmacodynamics, and these alterations result in decreased clearance of medications with the possibility of toxicity and negative outcomes [Van Den Broek et al. 2010; Zhou et al. 2011b, 2011; Bell, 2012; De Haan et al. 2012]. What is not clearly elucidated is the impact these alterations have on the occurrence of ADRs in this setting. As demonstrated in this report of ADRs in critically ill patients undergoing TH, medications with known ADRs were associated with physiologic and biologic derangements. Most commonly, propofol, fentanyl, acetaminophen, and insulin were associated with possible or probable adverse reactions. As all three ADR detection tools used in this evaluation address the possibility of alternative aetiologies, and in every evaluation included in this study, TH exists as an alternative aetiology, there were no definite ADRs determined in this study. Based on these associations, we recommend cautious use of these agents in the setting of TH and increased vigilance for these possible ADRs as evaluated in this study.
Although data from two sites were included here, this study is limited by its retrospective nature and lack of a control group to assess additional outcomes. Our study is intended as a real world or effectiveness evaluation, thus we included all patients meeting the inclusion criteria, although one could question the use of TH for pulseless electrical activity and asystole and the application of these results to a ventricular tachycardia-/ventricular fibrillation-only population. Furthermore, the utility and applicability of the included ADR-determination instruments have limitations in routine clinical practice; however, these instruments still offer the benefit of a standardized approach to evaluation and are commonly used in ADR studies. This study is limited in that a targeted group of medications was included in the analysis, focusing on agents routinely used in the setting of TH. A dose association was not conducted to evaluate the severity of an ADR with the magnitude of the dose administered of a possibly offending agent. It is often challenging to discern drug causes from other causes associated with an event. Given this limitation, three independent ADR-determination instruments were included in this evaluation in an effort to make the determination of an ADR more robust [Kane-Gill et al. 2012]. Notably, these three tools are similar in their evaluation of ADRs, thus there exists the possibility that they could agree erroneously on the evaluation of an event (i.e. false positives). Nevertheless, this more rigorous approach has been described previously, though does not completely eliminate the possibility that the events evaluated could be attributable to TH alone [Kane-Gill et al. 2012]. Also, we acknowledge events could be considered normal physiology during TH. The premise of this study is that many of these events are known to occur with TH, and medications may either cause or contribute to these effects. Thus we designed this study to be as robust as possible an analysis of this question, realizing a prospective controlled study would provide more definitive results. Another contributor to fluctuating abnormal laboratory values possibly confounding our results is the cooling technique, which was indeterminable in most cases. Passive cooling (i.e. wet blankets, cold fluids) can lead to unstable temperatures and unstable physiology, thus influencing abnormal laboratory values. Additionally, this investigation was performed in patients with a target temperature for TH of between 32°C and 34°C. Emerging evidence that mild TH to 36°C may result in similar clinical outcomes to deeper TH may have implications on the incidence and severity of ADRs seen in the setting of different temperature targets for TH [Nielsen et al. 2013]. Findings from this study may not be translatable to a population being treated with milder TH. While these limitations exist, this study is the first comprehensive evaluation of drug-related adverse effects in critically ill patients receiving TH. Advancing from the strengths of this study, further investigation to account for the limitations noted above is warranted.
Conclusion
Patients experience a number of physiologic effects during TH, and many of these effects may be the result of drugs. We are just beginning to understand the pharmacokinetic alterations of drugs that occur during TH, and this study illustrates that the alterations may be significant, with the inclusion of grade 3 and 4 derangements. There is a need for judicious use and close monitoring of drugs in the setting of TH until recommendations on dosing are available to help prevent ADRs.
Supplementary Material
Appendix
Appendix A.
Adverse drug events by probability of association and specific adverse event.
| n | Bradycardia | Hyperglycemia | Hyperkalemia | Hypocalcemia | Hypoglycemia | Hypokalemia | Hypomagnesemia | Increased ALT | Increased AST | Increased INR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Possible ADRs | |||||||||||
| Acetaminophen | 80 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 35.0% | 52.5% | 12.5% |
| Calcium | 25 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Dexmedetomidine | 5 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Dextrose 50% | 8 | 0.0% | 75.0% | 0.0% | 0.0% | 0.0% | 25.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Fentanyl | 220 | 36.0% | 23.0% | 0.0% | 5.9% | 0.9% | 15.3% | 1.4% | 3.6% | 14.0% | 0.0% |
| Fosphenytoin | 4 | 0.0% | 25.0% | 0.0% | 0.0% | 0.0% | 75.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Insulin | 53 | 0.0% | 0.0% | 0.0% | 0.0% | 9.6% | 90.4% | 0.0% | 0.0% | 0.0% | 0.0% |
| Meperidine | 12 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Morphine | 7 | 71.4% | 0.0% | 0.0% | 0.0% | 0.0% | 28.6% | 0.0% | 0.0% | 0.0% | 0.0% |
| Phenytoin | 42 | 52.5% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 17.5% | 30.0% | 0.0% |
| Potassium | 3 | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Propofol | 211 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Probable ADRs | |||||||||||
| Acetaminophen | 2 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 50.0% | 50.0% |
| Calcium | 2 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Dextrose 50% | 6 | 0.0% | 50.0% | 0.0% | 0.0% | 0.0% | 50.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Fentanyl | 30 | 13.3% | 56.7% | 0.0% | 10.0% | 3.3% | 16.7% | 0.0% | 0.0% | 0.0% | 0.0% |
| Fosphenytoin | 1 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Insulin | 14 | 0.0% | 0.0% | 0.0% | 0.0% | 7.1% | 92.9% | 0.0% | 0.0% | 0.0% | 0.0% |
| Morphine | 1 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Phenytoin | 3 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 66.7% | 33.3% | 0.0% |
| Potassium | 2 | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Propofol | 8 | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Robert Witcher, New York University Langone Medical Centre, New York, NY, USA.
Amy L. Dzierba, New York-Presbyterian Hospital, New York, NY, USA
Catherine Kim, University of Pittsburgh, School of Pharmacy, Pittsburgh, PA, USA Department of Pharmacy, University of Pittsburgh Medical Centre, Pittsburgh, PA, USA.
Pamela L. Smithburger, University of Pittsburgh, School of Pharmacy, Pittsburgh, PA, USA Department of Pharmacy, University of Pittsburgh Medical Centre, Pittsburgh, PA, USA
Sandra L. Kane-Gill, School of Pharmacy, University of Pittsburgh, 918 Salk Hall, 3501 Terrace Street, Pittsburgh, PA 15261, USA.
References
- Al-Senani F., Graffagnino C., Grotta J., Saiki R., Wood D., Chung W., et al. (2004) A prospective, multicenter pilot study to evaluate the feasibility and safety of using the CoolGard™ System and Icy™ catheter following cardiac arrest. Resuscitation 62: 143–150. [DOI] [PubMed] [Google Scholar]
- Arrich J. (2007) Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med 35: 1041–1047. [DOI] [PubMed] [Google Scholar]
- Bell S. (2012) Effect of therapeutic hypothermia on drug metabolism. Neonatal Netw 31: 48–51. [DOI] [PubMed] [Google Scholar]
- Bernard S., Gray T., Buist M., Jones B., Silvester W., Gutteridge G., et al. (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346: 557–563. [DOI] [PubMed] [Google Scholar]
- Bjelland T., Klepstad P., Haugen B., Nilsen T., Dale O. (2013) Effects of hypothermia on the disposition of morphine, midazolam, fentanyl, and propofol in intensive care unit patients. Drug Metab Dispos 41: 214–223. [DOI] [PubMed] [Google Scholar]
- Bjelland T., Klepstad P., Haugen B., Nilsen T., Salvesen O., Dale O. (2014) Concentrations of remifentanil, propofol, fentanyl, and midazolam during rewarming from therapeutic hypothermia. Acta Anaesthesiol Scand 58: 709–715. [DOI] [PubMed] [Google Scholar]
- Chamorro C., Borrallo J., Romera M., Silva J., Balandín B. (2010) Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg 110: 1328–1335. [DOI] [PubMed] [Google Scholar]
- Dearing N., Norgard N. (2010) Rhabdomyolysis in a patient receiving high-dose simvastatin after the induction of therapeutic hypothermia. Ann Pharmacother 44: 1994–1997. [DOI] [PubMed] [Google Scholar]
- De Haan T., Bijleveld Y., Van Der Lee J., Groenendaal F., Van Den Broek M., Rademaker C., et al. (2012) Pharmacokinetics and pharmacodynamics of medication in asphyxiated newborns during controlled hypothermia: the PharmaCool multicenter study. BMC Pediatr 12: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Pestotnik S., Classen D., Bass S., Menlove R., Gardner R., et al. (1991) Development of a computerized adverse drug event monitor. Proc Annu Symp Comput Appl Med Care 23–27. [PMC free article] [PubMed] [Google Scholar]
- Goh C. (1989) An approach to the evaluation and documentation of adverse drug reaction. Singapore Med J 30: 285–289. [PubMed] [Google Scholar]
- Jones J. (1988) Definition of events associated with drugs: regulatory perspectives. J Rheumatol Suppl 17: 14–19. [PubMed] [Google Scholar]
- Kane-Gill S., Bellamy C., Verrico M., Handler S., Weber R. (2009) Evaluating the positive predictive values of antidote signals to detect potential adverse drug reactions (ADRs) in the medical intensive care unit (ICU). Pharmacoepidemiol Drug Saf 18: 1185–1191. [DOI] [PubMed] [Google Scholar]
- Kane-Gill S., Forsberg E., Verrico M., Handler S. (2012) Comparison of three pharmacovigilance algorithms in the ICU setting: a retrospective and prospective evaluation of ADRs. Drug Saf 35: 645–653. [DOI] [PubMed] [Google Scholar]
- Kane-Gill S., Visweswaran S., Saul M., Wong A., Penrod L., Handler S. (2011) Computerized detection of adverse drug reactions in the medical intensive care unit. Int J Med Inform 80: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp B., Erstad B., Allen M., Theodorou A., Priestley G. (2006) Medication errors and adverse drug events in an intensive care unit: direct observation approach for detection. Crit Care Med 34: 415–425. [DOI] [PubMed] [Google Scholar]
- Kramer M., Leventhal J., Hutchinson T., Feinstein A. (1979) An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA 242: 623–632. [PubMed] [Google Scholar]
- MacLaren R., Gallagher J., Shin J., Varnado S., Nguyen L. (2014) Assessment of adverse events and predictors of neurological recovery after therapeutic hypothermia. Ann Pharmacother 48: 17–25. [DOI] [PubMed] [Google Scholar]
- Members W., Lloyd-Jones D., Adams R., Carnethon M., Simone G., Ferguson T., et al. (2009) Heart disease and stroke statistics—2009 update a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119: e21–e181. [DOI] [PubMed] [Google Scholar]
- Michel D., Knodel L. (1986) Comparison of three algorithms used to evaluate adverse drug reactions. Am J Hosp Pharm 43: 1709–1714. [PubMed] [Google Scholar]
- Naranjo C., Busto U., Sellers E., Sandor P., Ruiz I., Roberts E., et al. (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30: 239–245. [DOI] [PubMed] [Google Scholar]
- Nielsen N., Hovdenes J., Nilsson F., Rubertsson S., Stammet P., Sunde K., et al. (2009) Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand 53: 926–934. [DOI] [PubMed] [Google Scholar]
- Nielsen N., Sunde K., Hovdenes J., Riker R., Rubertsson S., Stammet P., et al. (2011) Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med 39: 57–64. [DOI] [PubMed] [Google Scholar]
- Nielsen N., Wetterslev J., Cronberg T., Erlinge D., Gasche Y., Hassager C., et al. (2013) Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 369: 2197–2206. [DOI] [PubMed] [Google Scholar]
- Nunnally M., Jaeschke R., Bellingan G., Lacroix J., Mourvillier B., Rodriguez-Vega G., et al. (2011) Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit Care Med 39: 1113–1125. [DOI] [PubMed] [Google Scholar]
- Peberdy M., Callaway C., Neumar R., Geocadin R., Zimmerman J., Donnino M., et al. (2010) Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122: S768–S786. [DOI] [PubMed] [Google Scholar]
- Pichon N., Amiel J., François B., Dugard A., Etchecopar C., Vignon P. (2007) Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 11: R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloyac S. (2012) Altered drug metabolism in critically ill children: a significant source of adverse effects? Pediatr Crit Care Med 13: 118–119. [DOI] [PubMed] [Google Scholar]
- Rivkin A. (2007) Admissions to a medical intensive care unit related to adverse drug reactions. Am J Health Syst Pharm 64: 1840–1843. [DOI] [PubMed] [Google Scholar]
- Rothschild J., Landrigan C., Cronin J., Kaushal R., Lockley S., Burdick E., et al. (2005) The Critical Care Safety Study: the incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med 33: 1694–1700. [DOI] [PubMed] [Google Scholar]
- The Hypothermia after Cardiac Arrest Study Group. (2002) Mild hypothermia to improve neurologic outcome after cardiac arrest. N Engl J Med 346: 549–556. [DOI] [PubMed] [Google Scholar]
- Tortorici M., Kochanek P., Bies R., Poloyac S. (2006) Therapeutic hypothermia-induced pharmacokinetic alterations on CYP2E1 chlorzoxazone-mediated metabolism in a cardiac arrest rat model. Crit Care Med 34: 785–791. [DOI] [PubMed] [Google Scholar]
- Tortorici M., Kochanek P., Poloyac S. (2007) Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 35: 2196–2204. [DOI] [PubMed] [Google Scholar]
- Valentin A., Capuzzo M., Guidet B., Moreno R., Dolanski L., Bauer P., et al. (2006) Patient safety in intensive care: results from the multinational Sentinel Events Evaluation (SEE) study. Intensive Care Med 32: 1591–1598. [DOI] [PubMed] [Google Scholar]
- Van Den Broek M., Groenendaal F., Egberts A., Rademaker C. (2010) Effects of hypothermia on pharmacokinetics and pharmacodynamics: a systematic review of preclinical and clinical studies. Clin Pharmacokinet 49: 277–294. [DOI] [PubMed] [Google Scholar]
- Wahby K., Jhajhria S., Dalal B., Soubani A. (2014) Heparin dosing in critically ill patients undergoing therapeutic hypothermia following cardiac arrest. Resuscitation 85: 533–537. [DOI] [PubMed] [Google Scholar]
- Wilmer A., Louie K., Dodek P., Wong H., Ayas N. (2010) Incidence of medication errors and adverse drug events in the ICU: a systematic review. Qual Saf Health Care 19: e7. [DOI] [PubMed] [Google Scholar]
- Xiao G., Guo Q., Shu M., Xie X., Deng J., Zhu Y., et al. (2013) Safety profile and outcome of mild therapeutic hypothermia in patients following cardiac arrest: systematic review and meta-analysis. Emerg Med J 30: 91–100. [DOI] [PubMed] [Google Scholar]
- Zhou J., Empey P., Bies R., Kochanek P., Poloyac S. (2011) Cardiac arrest and therapeutic hypothermia decrease isoform-specific cytochrome P450 drug metabolism. Drug Metab Dispos 39: 2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Poloyac S. (2011) The effect of therapeutic hypothermia on drug metabolism and response: cellular mechanisms to organ function. Expert Opin Drug Metab Toxicol 7: 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.